Abstract
Objective
We have used diffusion tensor tractography (DTT) for the evaluation of the somatotopic organization of corticospinal tracts (CSTs) in the posterior limb of the internal capsule (PLIC) and cerebral peduncle (CP).
Materials and Methods
We imaged the brains of nine healthy right-handed subjects. We used a spin-echo echo-planar imaging (EPI) sequence with 12 diffusion-sensitized directions. DTT was calculated with an angular threshold of 35 degrees and a fractional anistropy (FA) threshold of 0.25. We determined the location of the CSTs by using two regions of interest (ROI) at expected areas of the pons and expected areas of the lateral half of the PLIC, in the left hemisphere of the brain. Fiber tracts crossing these two ROIs and the precentral gyrus (PCG) were defined as CSTs. Four new ROIs were then defined for the PCG, from the medial to lateral direction, as ROI 1 (medial) to ROI 4 (lateral). Finally, we defined each fiber tract of the CSTs between the pons and each ROI in the PCG by using two ROIs methods.
Results
In all subjects, the CSTs were organized along the long axis of the PLIC, and the hand fibers were located anterior to the foot fibers. The CSTs showed transverse orientation in the CP, and the hand fibers were located usually medial to the foot fibers.
Conclusion
Corticospinal tracts are organized along the long axis of the PLIC and the horizontal direction of the CP.
Keywords: Brain, MR; MR, technology
Diffusion tensor MR imaging (DTI) and tractography (DTT) can depict white matter tracts of the brain in vivo. Recent DTT studies have demonstrated relatively unanimous results regarding the location of the corticospinal tract (CST) in the posterior limb of the internal capsule (PLIC) and the brain stem as determined by the use of DTI (1-4). However, controversy remains about the somatotopic organization of the pyramidal tract at PLIC. A recent DTT study demonstrated the somatotopic organization of the CST in the PLIC, in which the hand fibers were located anterolateral to the foot fibers, and the somatotopic organization showed transverse orientation at the PLIC (1). Additional DTT studies showed results suggesting an anteroposterior orientation of the pyramidal fibers at the PLIC (4, 5). Until now, there have been no remarkable DTT results about the organization of the pyramidal tract at the cerebral peduncle (CP). The goal of this study is to evaluate the somatotopic organization of the CSTs in the PLIC and CP, and to demonstrate the relationship of the organization among the precentral gyrus (PCG), PLIC, and CP by the use of DTT.
MATERIALS AND METHODS
We imaged the brains of nine healthy right-handed subjects. Four male and five female volunteers (mean age: 34 ± 5.4 years) were included in the study and all had normal results on physical and neurological examinations. Informed consent was obtained from all the subjects after the nature of the MR examination had been fully explained.
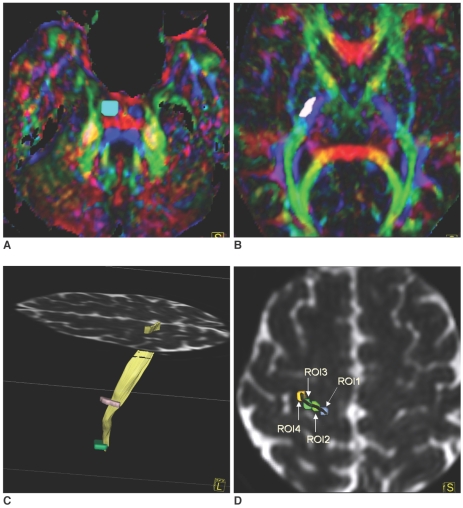
The DTI data was obtained with a 1.5 T MR unit (Magnetom Sonata, Siemens Medical Solutions, Erlangen, Germany). We used a spin-echo EPI sequence with 12 diffusion-sensitized directions (TR/TE = 5300/100 msec, number of excitations = 3, b-value = 0, 1000 s/mm2, field of view = 24 cm, 128 × 128 matrix, 3 mm thickness with no gap, parallel to anterior commissure-posterior commissure (AC-PC) line, covering from the pons to the high convexity). We also obtained anatomical T1 and T2-weighted images and magnetization-prepared rapid-acquisition gradient-echo (MPRAGE) images, which were correlated with the DTI images. The DTI map and tractography were calculated using DTI task card version 1.7 (Magnetic Resonance Center, Massachusetts General Hospital, Boston, MA) on a Siemens scanner. An angular threshold of 35 degrees and a fractional anistropy (FA) threshold of 0.25 were used. We defined the CSTs by using a two region of interest (ROI) approach. The first ROI was placed on the left side of the pons, which was an expected area for a CST on the DTI color map (Fig. 1A). The second ROI was placed on the left side of the lateral half of the PLIC (Fig. 1B). The fiber tracts crossing these two ROIs and the PCG, which was identified by using known anatomic landmarks such as the cerebral sulci, including the superior frontal, precentral and central sulci, and the omega shaped posterior knob (6, 7), were defined as CSTs (Fig. 1C). Four new ROIs were then drawn at the PCG from the medial to lateral direction as ROI 1 (most medial) to ROI 4 (most lateral) (Fig. 1D). Finally, we defined each fiber tract of the CSTs between the pons and each ROI in the PCG by using two ROIs methods. The tractography results were assigned a different color for the each of the CSTs from the medial to lateral direction (ROI 1-pink, ROI 2-blue, ROI 3-yellow, ROI 4-red). The location and somatotopic organization of the CSTs at the level of the CP and PLIC were evaluated by using multiple projection views. The reconstruction time for tractography was less than twenty minutes per each subject.
Fig. 1.
Region of interest selection for diffusion tensor tractography at pons, posterior limb of internal capsule and precentral gyrus.
A. Region of interest for pons was selected at expected corticopinal tract area on diffusion tensor MR imaging color map. Left side is left.
B. Region of interest for corticospinal tract was selecteed at lateral half of posterior limb of internal capsule on diffusion tensor MR imaging color map.
C. Precentral gyrus was determined by fibers crossing pons and posterior limb of internal capsule regions of interest, and it was identified by using anatomical landmarks such as posterior knob and superior frontal and precentral sulci on a B0 image.
D. Four precentral gyrus regions of interest were drawn from medial (ROI 1) to lateral precentral gyrus (ROI 4) on B0 image. ROI 3 and ROI 4 were drawn near to posterior knob of precentral gyrus.
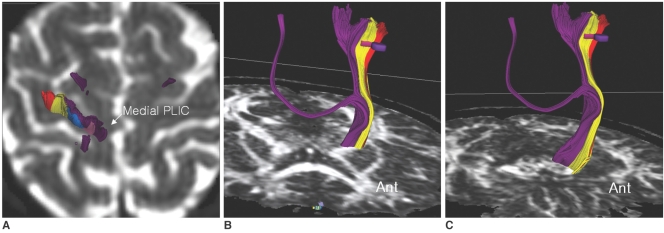
We initially drew an ROI for the CST of the whole PLIC, but some fibers descended posterior to the CP, near to the medial lemniscus.
By using two ROI methods, we could not find any fiber tracts between the pons ROI and the medial half of the PLIC. By single ROI tracking at the medial PLIC, the fiber tracts from the medial half of the PLIC descended near to the medial lemniscus of the midbrain and they never traversed the CP, but they ascended to the medial portion of the PCG, supplementary motor area, and to the postcentral gyrus area (Fig. 2).
Fig. 2.
Fiber tracts from medial half of posterior limb of internal capsule by single region of interest tracking.
A-C. Fibers from medial posterior limb of internal capsule (purple color) descended to midbrain, which is posterior to cerebral peduncle. Fibers from medial posterior limb of internal capsule terminated at medial portion of precentral gyrus (medial to precentral gyrus regions of interest for corticospinal tracts), postcentral gyrus, and supplementary motor area.
RESULTS
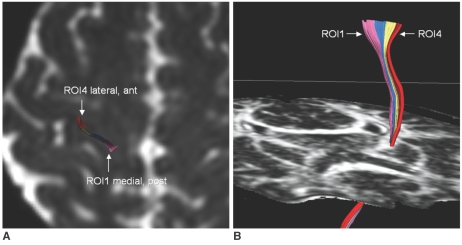
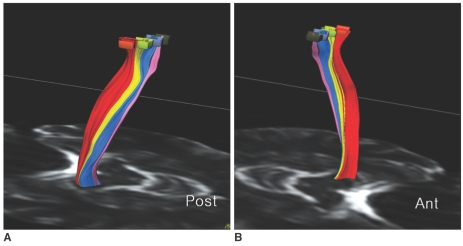
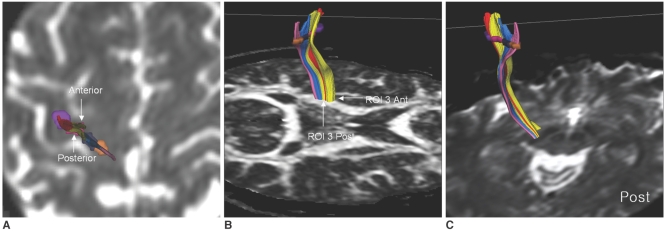
In all cases, the two medial ROIs (ROI 1 and ROI 2) were located slightly posterior to the two lateral ROIs (ROI 3 and ROI 4) at the PCG. In the all subjects, the CSTs traversed the mid to posterior portion of the lateral half of the PLIC, and showed somatotopic organization along the long axis (anterior-posterior) of the PLIC. The CSTs from ROI 1 and ROI 2 (foot fibers) were located posterior and medial to the CSTs from ROI 3 and ROI 4 (hand fibers) in the PLIC (Fig. 3). In all subjects, the CSTs traversed the mid to lateral portion of the CP, and they showed a transverse orientation (right to left) of the somatotopic organization in the CP. In the left hemisphere, the CSTs looked like they were rotating in a clockwise direction descending from the PCG to the CP and the foot fibers were located lateral to the hand fibers in the CP (Fig. 4). In four cases, intermixing of the fibers was present within the hand fibers. The CSTs from ROI 3 were divided as anterior and posterior bundles, and the CSTs from ROI 4 were located between the anterior and posterior bundles of ROI 3 at the PLIC and the CP (Fig. 5).
Fig. 3.
Typical pattern of somatotopic organization of corticospinal tracts at posterior limb of internal capsule.
A. Fiber tracts from lateral two regions of interest were located anterior to fiber tracts from medial two regions of interest at precentral gyrus as seen on B0 image (ROI 1-pink, ROI 2-blue, ROI 3-yellow, ROI 4-red).
B. Corticospinal tract fibers were organized mainly along long axis of posterior limb of internal capsule. Medial fibers (ROI 1 and ROI 2, presumed foot fibers) were located more posterior to lateral fibers (ROI 3 and ROI 4, presumed hand fibers).
Fig. 4.
Typical pattern of somatotopic organization of corticospinal tracts at cerebral peduncle.
A, B. Corticospinal tract fibers were organized mainly along horizontal (left-to-right) axis of cerebral peduncle. Medial fibers (ROI 1 and ROI 2, presumed foot fibers) were located more lateral to lateral fibers (ROI 3 and ROI 4, presumed hand fibers).
Fig. 5.
Intermixing of fibers from lateral regions of interest.
A. ROI 3 fibers divided anterior and posterior division at precentral gyrus (B0 image).
B, C. ROI 4 fibers were located between anterior and posterior divisions at posterior limb of internal capsule and cerebral peduncle.
DISCUSSION
Despite of the limited spatial resolution of DTT for the tracking of human axonal fibers, two recent DTT studies have described the somatotopic organization of the CSTs and sensory tracts (1, 5). There is controversy concerning these two studies about the somatotopic organization of the CSTs. In one study, the hand-foot fibers were organized along the short axis of the PLIC like the hand-foot homunculus in the PCG, and rotation of pyramidal tract was not mentioned (1). In the other study, the hand-foot fibers were organized along the long axis of the PLIC and had an approximate 90° rotation as they descended from the PCG (5). Our DTT results were similar with the latter study and showed antero-posterior alignment of the CSTs and a clockwise rotation descending from the PCG to the CP in the left hemisphere. Recent clinical symptoms based studies also support the antero-posterior orientation of the somatotopic organization of the CSTs in the PLIC (4, 8). In these two studies, a more anterior located lacunar infarction in the PLIC and corona radiata was correlated with symptoms of the face and arm.
The CP of the midbrain, where the CSTs are densely concentrated, was less sensitive to a susceptibility artifact of DTI and DTT may reliably visualize white matter tracts. The CSTs were located at the mid to lateral portion of the CP, according to previous DTT studies (2, 9). Our DTT results also showed a similar location of the CSTs at the CP. There have been no DTT reports about the somatotopic organization of the pyramidal tracts at the midbrain level. The CSTs were organized along the horizontal (mediolateral) direction at the CP in our study. The CSTs had about an 180° rotation as they descended from the PCG, and the hand fibers were located medial to the foot fibers.
We drew only the lateral half of the PLIC for tracking of the CSTs as the fiber tracts made by tracking a single ROI from the medial half of the PLIC did not go through the CP in the midbrain. In previous DTT reports, it was noted that the PLIC contains part of the thalamocortical pathway (3, 10). These thalamic radiations included pathways between the ventrolateral, ventral anterior, ventral posterior nuclei of the thalamus and premotor, and the motor cortex (10). In our study, the fiber tracts from the medial half of the PLIC showed a similar course of these thalamic radiations, thus we considered the fiber tracts from the medial PLIC as part of thalamic radiations. In our opinion, it is better to select one of the seed ROIs at only the lateral half of the PLIC for determining accurate tractography of the CSTs.
We used DTT software based on the streamline methods in our study; so an inherent limitation for the tracking of crossing fibers within a voxel was present (11, 12). The fiber tracts evaluated in the present study included only the fibers from the hand, trunk and leg area of the PCG due to this limitation of the DTT method (5, 13). Our study showed intermixing of some fibers within the CSTs as in a previous study (1). However, there has been no anatomic specimen based study describing intermixed fibers within the CSTs. This intermixing of fibers may be true or erroneous and we could not exclude the possibility of an error related with the crossing fibers within a voxel.
In conclusion, the CSTs from the anterior PCG ROI (hand fibers) were located anterior in the PLIC and medially in the CP. The CSTs were organized along the long axis of the PLIC and the horizontal (mediolateral) direction at the CP. The orientation of the somatotopic organization of the CSTs was rotated in a clockwise direction descending from the PCG to the CP in the left hemisphere. Knowledge of the exact location, orientation, and organization of CSTs through internal capsule, CP provided in our investigation may help in the performance of delicate functional neurosurgery such as deep brain stimulation and neurosurgery for brain tumors. In addition, the findings are be helpful in understanding the emerging clinical sequelae that present in the motor domain following a localized trauma or a lacunar infarction.
Abbreviations
- AC-PC
anterior commissure-posterior commissure
- CP
cerebral peduncle
- CSTs
corticospinal tracts
- DTI
diffusion tensor MR imaging
- EPI
echo-planar imaging
- FA
fractional anistropy
- MPRAGE
magnetization-prepared rapid-acquisition gradient-echo
- PCG
precentral gyrus
- PLIC
posterior limb of the internal capsule
- ROI
region of interest
References
- 1.Holodny AI, Gor DM, Watts R, Gutin PH, Ulug AM. Diffusion-tensor MR tractography of somatotopic organization of corticospinal tracts in the internal capsule: initial anatomic results in contradistinction to prior reports. Radiology. 2005;234:649–653. doi: 10.1148/radiol.2343032087. [DOI] [PubMed] [Google Scholar]
- 2.Mori S, van Zijl PC. Fiber tracking: Principles and strategies - a technical review. NMR Biomed. 2002;15:468–480. doi: 10.1002/nbm.781. [DOI] [PubMed] [Google Scholar]
- 3.Wakana S, Jiang H, Nagae-Poetscher LM, van Zijl PC, Mori S. Fiber tract-based atlas of human white matter anatomy. Radiology. 2004;230:77–87. doi: 10.1148/radiol.2301021640. [DOI] [PubMed] [Google Scholar]
- 4.Lee JS, Han MK, Kim SH, Kwon OK, Kim JH. Fiber tracking by diffusion tensor imaging in corticospinal tract stroke: Topographical correlation with clinical symptoms. Neuroimage. 2005;26:771–776. doi: 10.1016/j.neuroimage.2005.02.036. [DOI] [PubMed] [Google Scholar]
- 5.Yamada K, Nagakane Y, Yoshikawa K, Kizu O, Ito H, Kubota T, et al. Somatotopic organization of thalamocortical projection fibers as assessed with MR tractography. Radiology. 2007;242:840–845. doi: 10.1148/radiol.2423060297. [DOI] [PubMed] [Google Scholar]
- 6.Kido DK, LeMay M, Levinson AW, Benson WE. Computed tomographic localization of the precentral gyrus. Radiology. 1980;135:373–377. doi: 10.1148/radiology.135.2.7367629. [DOI] [PubMed] [Google Scholar]
- 7.Yousry TA, Schmid UD, Alkadhi H, Schmidt D, Peraud A, Buettner A, et al. Localization of the motor hand area to a knob on the precentral gyrus. A new landmark. Brain. 1997;120:141–157. doi: 10.1093/brain/120.1.141. [DOI] [PubMed] [Google Scholar]
- 8.Kim JS, Pope A. Somatotopically located motor fibers in corona radiata: evidence from subcortical small infarcts. Neurology. 2005;64:1438–1440. doi: 10.1212/01.WNL.0000158656.09335.E7. [DOI] [PubMed] [Google Scholar]
- 9.Newton JM, Ward NS, Parker GJ, Deichmann R, Alexander DC, Friston KJ, et al. Non-invasive mapping of corticofugal fibres from multiple motor areas-relevance to stroke recovery. Brain. 2006;129:1844–1858. doi: 10.1093/brain/awl106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Behrens TE, Johansen-Berg H, Woolrich MW, Smith SM, Wheeler-Kingshott CA, Boulby PA, et al. Non-invasive mapping of connections between human thalamus and cortex using diffusion imaging. Nat Neurosci. 2003;6:750–757. doi: 10.1038/nn1075. [DOI] [PubMed] [Google Scholar]
- 11.Parker GJ, Alexander DC. Probabilistic Monte Carlo based mapping of cerebral connections utilizing whole-brain crossing fibre information. Inf Process Med Imaging. 2003;18:684–695. doi: 10.1007/978-3-540-45087-0_57. [DOI] [PubMed] [Google Scholar]
- 12.Parker GJ, Haroon HA, Wheeler-Kingshott CA. A framework for a streamline-based probabilistic index of connectivity (PICo) using a structural interpretation of MRI diffusion measurements. J Magn Reson Imaging. 2003;18:242–254. doi: 10.1002/jmri.10350. [DOI] [PubMed] [Google Scholar]
- 13.Holodny AI, Watts R, Korneinko VN, Pronin IN, Zhukovskiy ME, Gor DM, et al. Diffusion tensor tractography of the motor white matter tracts in man: Current controversies and future directions. Ann N Y Acad Sci. 2005;1064:88–97. doi: 10.1196/annals.1340.016. [DOI] [PubMed] [Google Scholar]