Abstract
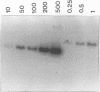
Amplification of DNA sequences from ribosomal DNA (rDNA) was tested as a specific and sensitive method for the detection of small numbers of Toxoplasma gondii tachyzoite cells. We applied the polymerase chain reaction (PCR) on the basis of detection of the 110-fold repetitive rDNA as a target by using (i) DNA sequences within the small ribosomal subunit known to be universal and conserved in all eukaryotes and (ii) small ribosomal subunit and intergenic spacer rDNA sequences known to be T. gondii species specific. The level of sensitivity obtained from a crude cell lysate allowed the detection of as few as one parasite visualized directly as a specific PCR product in agarose gels. By using a combination of universal and T. gondii species-specific primers, we propose a comultiplex-based PCR approach as a new diagnostic tool. The combination of sensitivity, specificity, and built-in positive and negative PCR controls should make detection of the rDNA sequences by comultiplex PCR a useful clinical test for the diagnosis of toxoplasmosis and for epidemiological studies. Finally, the idea of a built-in positive control to support or counter the T. gondii-specific PCR result is novel and is a notable advance.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burg J. L., Grover C. M., Pouletty P., Boothroyd J. C. Direct and sensitive detection of a pathogenic protozoan, Toxoplasma gondii, by polymerase chain reaction. J Clin Microbiol. 1989 Aug;27(8):1787–1792. doi: 10.1128/jcm.27.8.1787-1792.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cazenave J., Cheyrou A., Blouin P., Johnson A. M., Begueret J. Use of polymerase chain reaction to detect Toxoplasma. J Clin Pathol. 1991 Dec;44(12):1037–1037. doi: 10.1136/jcp.44.12.1037-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain J. S., Gibbs R. A., Ranier J. E., Nguyen P. N., Caskey C. T. Deletion screening of the Duchenne muscular dystrophy locus via multiplex DNA amplification. Nucleic Acids Res. 1988 Dec 9;16(23):11141–11156. doi: 10.1093/nar/16.23.11141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberland S., Current W. L. Use of mouse macrophage cell lines for in vitro propagation of Toxoplasma gondii RH tachyzoites. Proc Soc Exp Biol Med. 1991 Jun;197(2):150–157. doi: 10.3181/00379727-197-43237. [DOI] [PubMed] [Google Scholar]
- Cornelissen A. W., Overdulve J. P., van der Ploeg M. Determination of nuclear DNA of five eucoccidian parasites, Isospora (Toxoplasma) gondii, Sarcocystis cruzi, Eimeria tenella, E. acervulina and Plasmodium berghei, with special reference to gamontogenesis and meiosis in I. (T.) gondii. Parasitology. 1984 Jun;88(Pt 3):531–553. doi: 10.1017/s0031182000054792. [DOI] [PubMed] [Google Scholar]
- Cristina N., Derouin F., Pelloux H., Pierce R., Cesbron-Delauwn M. F., Ambroise-Thomas P. Détection de Toxoplasma gondii chez des patients sidéens par la technique de "Polymerase Chain Reaction" (PCR), à l'aide de la séquence répétée TGR1E. Pathol Biol (Paris) 1992 Jan;40(1):52–55. [PubMed] [Google Scholar]
- Dahl R. J., Johnson A. M. Purification of Toxoplasma gondii from host cells. J Clin Pathol. 1983 May;36(5):602–604. doi: 10.1136/jcp.36.5.602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovannoni S. J., DeLong E. F., Olsen G. J., Pace N. R. Phylogenetic group-specific oligodeoxynucleotide probes for identification of single microbial cells. J Bacteriol. 1988 Feb;170(2):720–726. doi: 10.1128/jb.170.2.720-726.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grover C. M., Thulliez P., Remington J. S., Boothroyd J. C. Rapid prenatal diagnosis of congenital Toxoplasma infection by using polymerase chain reaction and amniotic fluid. J Clin Microbiol. 1990 Oct;28(10):2297–2301. doi: 10.1128/jcm.28.10.2297-2301.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
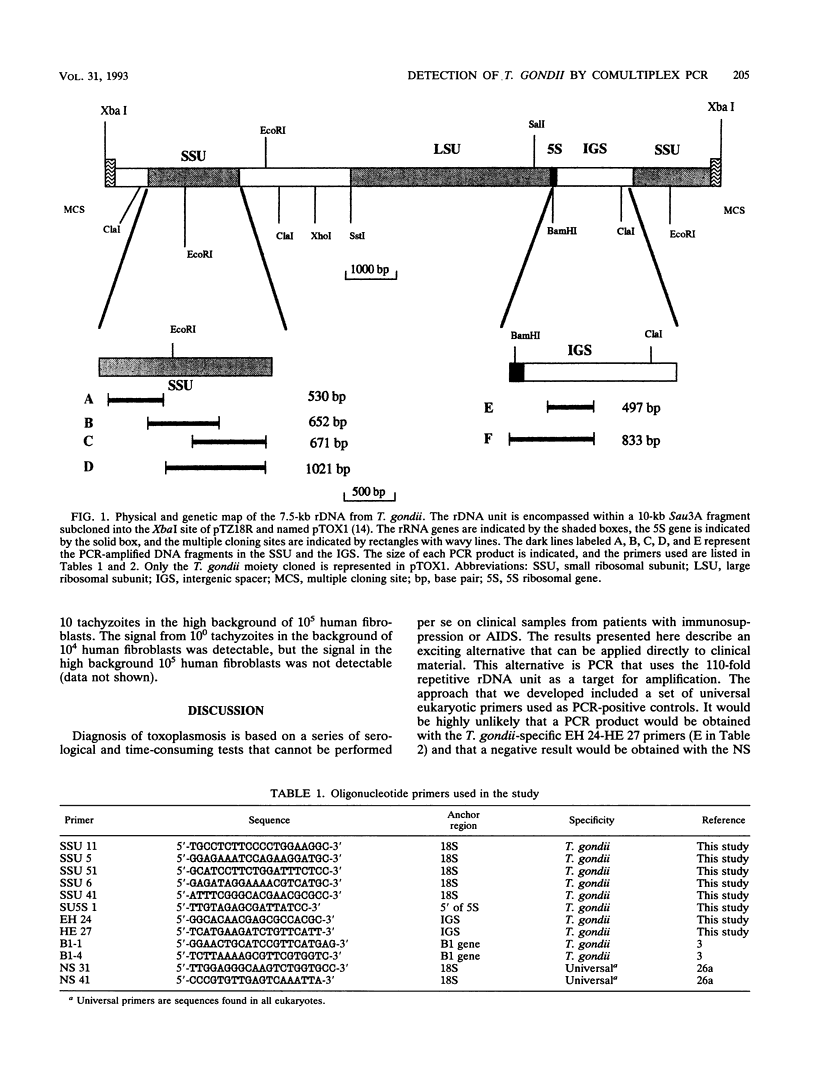
- Guay J. M., Huot A., Gagnon S., Tremblay A., Levesque R. C. Physical and genetic mapping of cloned ribosomal DNA from Toxoplasma gondii: primary and secondary structure of the 5S gene. Gene. 1992 May 15;114(2):165–171. doi: 10.1016/0378-1119(92)90570-f. [DOI] [PubMed] [Google Scholar]
- Herbrink P., van Loon A. M., Rotmans J. P., van Knapen F., van Dijk W. C. Interlaboratory evaluation of indirect enzyme-linked immunosorbent assay, antibody capture enzyme-linked immunosorbent assay, and immunoblotting for detection of immunoglobulin M antibodies to Toxoplasma gondii. J Clin Microbiol. 1987 Jan;25(1):100–105. doi: 10.1128/jcm.25.1.100-105.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes H. P., Hudson L., Fleck D. G. In vitro culture of Toxoplasma gondii in primary and established cell lines. Int J Parasitol. 1986 Aug;16(4):317–322. doi: 10.1016/0020-7519(86)90109-8. [DOI] [PubMed] [Google Scholar]
- Luft B. J., Conley F., Remington J. S., Laverdiere M., Wagner K. F., Levine J. F., Craven P. C., Strandberg D. A., File T. M., Rice N. Outbreak of central-nervous-system toxoplasmosis in western Europe and North America. Lancet. 1983 Apr 9;1(8328):781–784. doi: 10.1016/s0140-6736(83)91847-0. [DOI] [PubMed] [Google Scholar]
- Reeder R. H. Enhancers and ribosomal gene spacers. Cell. 1984 Sep;38(2):349–351. doi: 10.1016/0092-8674(84)90489-6. [DOI] [PubMed] [Google Scholar]
- Remington J. S., Cavanaugh E. N. Isolation of the encysted form of Toxoplasma gondii from human skeletal muscle and brain. N Engl J Med. 1965 Dec 9;273(24):1308–1310. doi: 10.1056/NEJM196512092732404. [DOI] [PubMed] [Google Scholar]
- Sarkar G., Kapelner S., Sommer S. S. Formamide can dramatically improve the specificity of PCR. Nucleic Acids Res. 1990 Dec 25;18(24):7465–7465. doi: 10.1093/nar/18.24.7465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar G., Sommer S. S. Shedding light on PCR contamination. Nature. 1990 Jan 4;343(6253):27–27. doi: 10.1038/343027a0. [DOI] [PubMed] [Google Scholar]
- Snider W. D., Simpson D. M., Nielsen S., Gold J. W., Metroka C. E., Posner J. B. Neurological complications of acquired immune deficiency syndrome: analysis of 50 patients. Ann Neurol. 1983 Oct;14(4):403–418. doi: 10.1002/ana.410140404. [DOI] [PubMed] [Google Scholar]
- Weiss L. M., Chen Y. Y., Berry G. J., Strickler J. G., Dorfman R. F., Warnke R. A. Infrequent detection of Toxoplasma gondii genome in toxoplasmic lymphadenitis: a polymerase chain reaction study. Hum Pathol. 1992 Feb;23(2):154–158. doi: 10.1016/0046-8177(92)90236-v. [DOI] [PubMed] [Google Scholar]