Abstract
Objective
We wanted to evaluate the degree of conformity of papillary carcinoma and follicular carcinoma to the reported ultrasonographic findings of malignant thyroid tumor.
Materials and Methods
Between January 2003 and December 2004, fine needle aspiration biopsy was performed in 1,036 patients with palpable and nonpalpable thyroid lesions. We retrospectively reviewed the ultrasonographic findings of patients with papillary carcinomas (n = 127) and follicular carcinomas (n = 23) that were proven by operation or fine needle aspiration biopsy. We analyzed the ultrasonographic findings of these nodules based on the reported ultrasonographic findings of malignant thyroid tumor: hypoechogenicity, a taller than wide orientation, a microlobulated or irregular margin, a thick hypoechoic rim (halo sign), microcalcification and cystic change.
Results
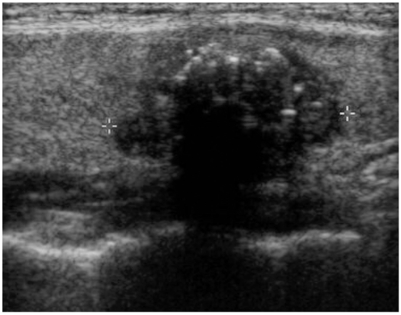
The echogenicity was hypoechoic in 72.4% (92/127) of the papillary carcinomas, but it was isoechoic in 65.2% (15/23) of the follicular carcinomas (p < 0.001). The nodule shape was tall or round in 74.1% of the papillary carcinomas, but it was flat in 72.7% of the follicular carcinomas (p < 0.001). The tumor margin was microlobulated or irregular in 92.9% of the papillary carcinomas and in 60.9% of the follicular carcinomas (p < 0.001). A hypoechoic rim was seen in 26% of the papillary carcinomas (thin rim: 13.4%, thick rim: 12.6%) and in 86.6% of the follicular carcinomas (thin rim: 39.1%, thick rim: 47.8%, p < 0.001). Microcalcifications were demonstrated in 33.9% of the papillary carcinomas and in none of the cases of follicular carcinoma (p < 0.001). A solid mass without cystic change were seen in 98.4% of the papillary carcinomas and in 82.6% of the follicular carcinomas (p < 0.001).
Conclusion
The previously reported ultrasonography findings of malignant thyroid tumor are in conformity with most of the papillary carcinomas, but not with follicular carcinomas. The current ultrasonographic features for thyroid malignancy should be cautiously applied as the indication for needle aspiration biopsy so that follicular carcinomas are not missed by too narrow and strict biopsy criteria.
Keywords: Thyroid gland, Ultrasonography, Papillary carcinoma, Follicular carcinoma
Thyroid nodules are present in up to 50% of the adult population, as determined by ultrasonography, and less than 15% of thyroid nodules are malignant (1-6). More than 70% of malignant nodules are papillary carcinoma, and about 10-20% of these are follicular carcinoma (1). The introduction of high resolution ultrasonography has enabled detection of many thyroid nodules. Criteria need to be established for fine needle aspiration biopsy to avoid unnecessary biopsy. The ultrasonographic findings of malignant thyroid nodules have been previously reported by many studies. These findings include completely solid or prominently solid nodule, microcalcification, an irregular or microlobulated margin, marked hypoechogenicity compared with the strap muscles, increased intranodular vascularity on Doppler imaging and a taller than wide orientation of the nodule (1-5,7-10). As the majority of papillary carcinomas have findings compatible to malignant thyroid nodules, they can be diagnosed by these ultrasonographic findings (11). However, the ultrasonographic findings of papillary carcinoma are different from those of follicular carcinoma (7, 8). So, we evaluated the degree of conformity of papillary carcinoma and follicular carcinoma to the reported ultrasonographic findings of malignant thyroid nodules.
MATERIALS AND METHODS
Between January 2003 and December 2004, fine needle aspiration biopsy was performed in 1,036 patients with palpable and non-palpable thyroid lesions. We retrospectively reviewed the ultrasonographic findings of patients with papillary carcinomas (n = 127, 109 females and 18 males, age range: 17-83 years, median age: 50 years) and the follicular carcinomas (n = 23, 17 females and 6 males, age range: 21-72 years, median age: 46 years) as was proven by operation or fine needle aspiration biopsy. All the follicular carcinomas were surgically confirmed.
Thyroid ultrasonography was performed with an HDI 3000 scanner (Advanced Technology Laboratories, Bothell, WA) and an HDI 5000 diagnostic sonography system (Philips Medical Systems, Bothell, WA) with a CL10-5 MHz compact linear array transducer.
We analyzed the ultrasonographic findings of these nodules based on the reported ultrasonographic findings of malignant thyroid nodules. These included the nodule shape (flat, round or tall), internal echogenicity as compared with that of the strap muscles (hypoechoic, isoechoic or hyperechoic), heterogenicity of the nodular echotexture (homogeneous or heterogeneous), the marginal character (smooth or irregular), calcification (no calcification, macrocalcifcaiton or microcalcification), hypoechoic rim (no rim, thin rim or thick rim) and the degree of cystic change (none, less than 50% or more than 50%).
We determined the nodule shape in accordance with the ratio of anteroposterior (AP) dimension to the transverse (T) dimension as round or tall (AP ≥ T) or flat (AP < T). We defined an irregular margin as the presence of more than three lobulations on the surface of nodules. Microcalcification was defined as tiny, punctuate hyperechoic foci that were usually less than 2 mm in size regardless of any posterior acoustic shadowing. The hypoechoic rim was defined as a low echoic rim surrounding the nodule. A greater than 2 mm rim was classified as a thick hyoechoic rim.
We compared the findings of papillary and follicular carcinomas by using the Chi-square test. A p-value of less than 0.01 was defined as statistically significant.
RESULTS
Seventy-four percents (94 patients among the 127 patients) of the papillary carcinomas had a round or tall shape, but about 74% (17 patients among the 23 patients) of the follicular carcinomas were flat (p < 0.001). About 72% (92 patients among the 127 patients) of the papillary carcinomas were hypoechoic, but about 65% (15 patients among the 23 patients) of the follicular carcinomas had iso- or hyperechogenicity (p < 0.001). The majority of both the papillary (52%) and follicular carcinomas (70%) showed heterogeneous echogenicity, and there was no discrete difference between the two carcinomas for the heterogeneity of the internal echoes. An irregular margin was seen in about 93% of 127 papillary carcinomas and in 61% of 23 follicular carcinomas (p < 0.001). About 64% (81 patients among the 127 patients) of the papillary carcinomas had calcifications of any shape and more than a half had microcalcifications that suggested malignant ultrasonographic findings. However, about 83% of the follicular carcinomas had no calcifications (p < 0.001). About 84% of the 23 patients with follicular carcinomas showed a hypoechoic rim, but this was not seen in the 74% of the papillary carcinomas (p < 0.001). 98.4% of the papillary carcinoma had no cystic change and 82.6% of the follicular carcinoma also had no cystic change (p < 0.001, Table 1).
Table 1.
Sonographic Findings in Thyroid Nodules with Papillary or Follicular Carcinoma
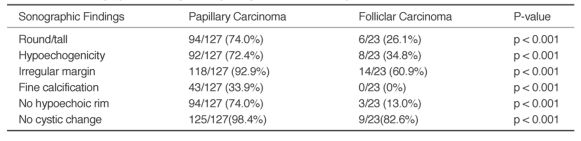
When we compared the ultrasonographic findings of the papillary carcinomas and follicular carcinomas based on the previously reported ultrasonographic findings of the malignant thyroid nodules, the majority of the papillary carcinomas (Fig. 2) showed conformity to those findings, but those findings also conformed with a few case of the follicular carcinomas (Table 2, Fig. 1).
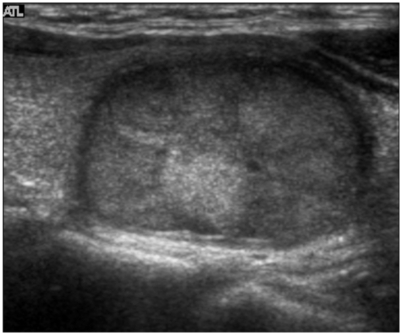
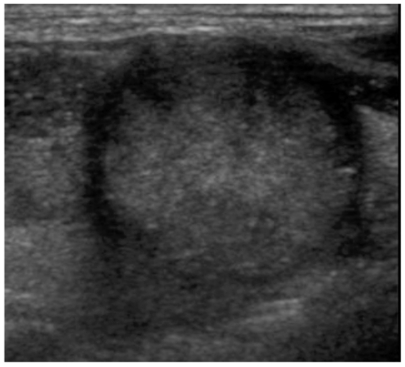
Fig. 2.
Sonography of a 35-year-old female with papillary carcinoma.
Sonography shows a hypoechoic thyroid nodule with fine calcifications and an irregular margin. This nodule demonstrates no cystic change and no surrounding hypoechoic rim.
Table 2.
Ultrasonographic Findings of Papillang and Follicular Thyroid Carcinomas
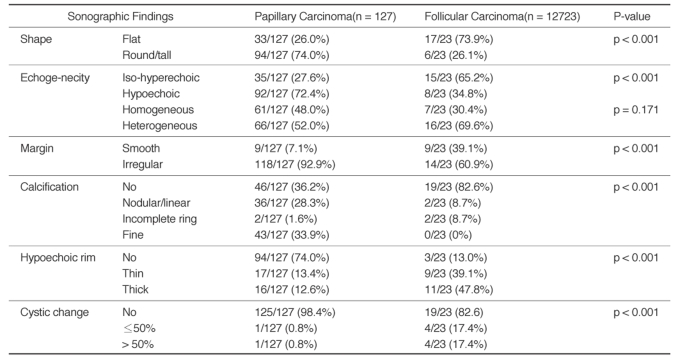
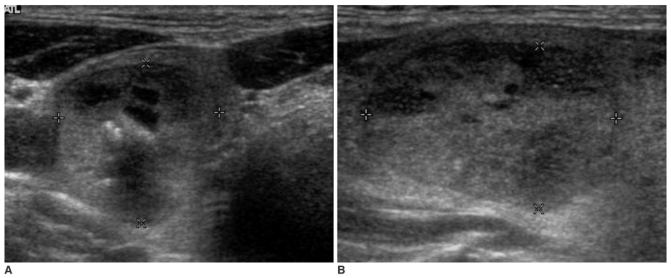
Fig. 1.
Sonography of a 54-year-old female with follicular carcinoma.
There is an isoechoic thyroid nodule with heterogeneous echogenicity. This nodule shows flat orientation and a smooth, thick hypoechoic rim without internal calcifications or any cystic changes.
DISCUSSION
Many reports have demonstrated the ultrasonographic findings of malignant thyroid nodules as completely solid or prominently solid nodule, hypoechogenicity compared with the strap muscles, an irregular margin, microcalcification in the nodule, a taller than wide (or antiparallel) orientation and increased intranodular vascularity (1-5, 7-10). These findings are usually used to differentiate papillary carcinomas from benign nodules (13). Nobuhiro et al. insisted that these findings had high accuracy for differentiating benign nodules from papillary carcinomas, which were the most common malignant thyroid neoplasm in that study, but the findings were not useful for detection of follicular carcinomas (8). This is because the two major types of thyroid carcinomas are quite dissimilar for their ultrasonographic findings. The reported ultrasonographic findings of follicular neoplasms are a solid mass with inhomogeneous internal echogenicity and a surrounding hypoechoic rim, and these are different from the known malignant sonographic findings. They infrequently showed an irregular margin and intranodular calcifications. Although irregularity of a hypoechoic rim, solid masses without cystic change and intranodular hypervascularity are more suggestive of follicular carcinomas, histological capsular and vascular invasion is required to diagnose follicular carcinomas. This is because the ultrasonographic findings of follicular adenomas are similar with those of follicular carcinomas (7, 8, 11). To the best of our knowledge, there are no papers about the ultrasonographic findings of follicular carcinoma compared with those of malignant thyroid nodules. We tried to identify the degree of conformity of the ultrasonographic findings of papillary and follicular carcinoma to the previously reported ultrasonographic findings of malignant thyroid nodules.
A nodule with a round or tall shape is due to tumor growth against the tissue plane, and an irregular margin of the nodule also signifies uneven infiltrating growth. Both findings have been considered as ultrasonographic findings of malignant thyroid nodules (2). In our study, round or tall shape findings were seen for 74% of the papillary carcinomas and for 26% of the follicular carcinomas. Irregular or microlobulated margins were seen for almost all cases (93%) of papillary carcinomas and for only 60% of the follicular carcinomas. Also, 98.4% of the papillary carcinomas were solid masses without an intranodular cystic portion and 82.6% of the follicular carcinomas were solid. Miyakawa et al. suggested that follicular carcinomas had irregular margin without a hypoechoic halo more frequently than did the follicular adenomas. They also suggested that the intranodular cystic degeneration was rarely observed in follicular carcinomas, but this was frequently seen in follicular adenomas (12). Therefore, more than half of the follicular carcinomas have marginal irregularity and they showed solid masses without intranodular cystic changes in our study.
In our study, hypoechoic rims were demonstrated more frequently for follicular carcinomas (87%) rather than for papillary carcinomas (26%). The presence of the hypoechoic surrounding rim is due to the compression of the normal thyroid tissue or capsule (6, 12). We thought that these findings of follicular carcinomas resulted from their growth parallel to the tissue plane and their compression of the normal tissue rather than infiltration into it.
Hypoechogenicity of the nodule, which suggests malignant findings, was thought to be due to nodule compaction and its high cellularity (7). This finding was shown in 35% of follicular carcinomas and in 72% of papillary carcinomas. The follicular neoplasm makes nests of multiple small follicles that mimic the normal thyroid gland and they contain variable amounts of colloid material. The echogenicity of follicular carcinomas might be dependent on the amount of the colloid material in the tumors, which is related to the gross color of a follicular neoplasm. Follicular carcinomas have high cellularity with smaller amounts of colloid material than do follicular adenomas (11).
Microcalcification, which was thought as the most sensitive criterion in many study (1, 2, 9), was a common finding of papillary carcinomas. In our study, few follicular neoplasms had calcifications (only 4 among the 23 cases), but no case had microcalcifications (Fig. 3). The microcalcifications in thyroid nodule are suggestive of psammoma bodies in papillary carcinomas (1, 2). Therefore microcalcifications could not be detected in follicular carcinomas because these tumors usually have no psammoma bodies.
Fig. 3.
A, B. Sonography of a follicular carcinoma in a 64-year-old male. Axial (A) and longitudinal (B) images of the ultrasonography. A nodule of the thyroid gland shows heterogeneous isoechogenicity with focal nodular macrocalcification, less than 50% of cystic change and a thin hypoechoic rim.
In our study, the size of follicular carcinomas (mean: 1.95 × 2.43 × 3.03 cm for the anteroposterior, transverse and craniocaudal diameters, respectively in respect) were larger than that of the papillary carcinomas (mean: 0.89 × 1.00 × 1.16 cm for the anteroposterior, transverse and craniocaudal diameters, respectively). We guessed that many follicular carcinomas might be found as a palpable mass or they were initially analyzed as benign nodules and then this was confirmed on the follow-up studies.
Overall, less than half of the follicular carcinomas in our study showed malignant features on ultrasonography, except for marginal irregularity and solid masses without cystic change.
In conclusion, the previously reported sonographic findings of malignant thyroid nodule are in conformity with those of most papillary carcinomas, but these findings do not conform to those of the follicular carcinomas in the present study. This suggests that many follicular carcinomas might be initially analyzed as non-malignant lesions. As reported by Chan et al., about 10% of papillary carcinomas showed uncommon ultrasound features such as hyperechogenicity and few or no peripheral calcifications, like our cases in Figure 4 (5). Less than 40% of the papillary carcinomas in the present study showed different findings from the known malignant ultrasonographic findings. Therefore, these reported, known ultrasonographic criteria of malignant thyroid nodules should be cautiously applied as the indications for needle aspiration biopsy so that follicular carcinomas and some atypical papillary carcinomas are not missed according to overly narrow and strict biopsy criteria. Also, since follicular adenomas showed similar findings with follicular carcinomas, further studies are required for determining the sonographic findings of those two lesions.
Fig. 4.
Sonography of a 45-year-old male with the atypical findings of papillary carcinoma.
The nodule of the thyroid gland shows the same iso-echogenicity as the normal thyroid gland without cystic change. An incomplete and irregular thick hypoechoic rim is seen in this nodule. Small foci without comet tail artifacts that are suggestive of microcalcifications are also seen.
References
- 1.Frates MC, Benson CB, Charbouneau JW, Cibas ES, Clark OH, Coleman BG, et al. Management of thyroid nodules detected at US: Society of Radiologists in Ultrasound consensus conference statement. Radiology. 2005;237:794–800. doi: 10.1148/radiol.2373050220. [DOI] [PubMed] [Google Scholar]
- 2.Kim EK, Park CS, Chung WY, Oh KK, Kim DI, Lee JT, et al. New sonographic criteria for recommending fine-needle aspiration biopsy of nonpalpable solid nodules of the thyroid. AJR Am J Roentgenol. 2002;178:687–691. doi: 10.2214/ajr.178.3.1780687. [DOI] [PubMed] [Google Scholar]
- 3.Frates MC, Benson CB, Doubilet PM, Cibas ES, Marqusee E. Can color Doppler sonography aid in prediction of malignancy of thyroid nodules? J Ultrasound Med. 2003;22:127–131. doi: 10.7863/jum.2003.22.2.127. [DOI] [PubMed] [Google Scholar]
- 4.Papini E, Guglielmi R, Bianchini A, Crescenzi A, Taccogna S, Nardi F, et al. Risk of malignancy in nonpalpable thyroid nodules: predictive value of ultrasound and color-Doppler features. J Clin Endocrinol Metab. 2002;87:1941–1946. doi: 10.1210/jcem.87.5.8504. [DOI] [PubMed] [Google Scholar]
- 5.Chan BK, Desser TS, McDougall IR, Weigel RJ, Jeffrey RB., Jr Common and uncommon sonographic features of papillary thyroid carcinoma. J Ultrasound Med. 2003;22:1083–1090. doi: 10.7863/jum.2003.22.10.1083. [DOI] [PubMed] [Google Scholar]
- 6.Nam-Goong IS, Kim HY, Gong G, Lee HK, Hong SJ, Kim WB, et al. Ultrasonography-guided fine-needle aspiration of thyroid incidentaloma: correlation with pathologic findings. Clin Endocrinol. 2004;60:21–28. doi: 10.1046/j.1365-2265.2003.01912.x. [DOI] [PubMed] [Google Scholar]
- 7.Rumack CM, Wilson SR, Charboneau JW. Diagnostic Ultrasound. 3rd ed. St. Louis: Mosby; 2005. pp. 735–770. [Google Scholar]
- 8.Fukunari N, Nagahama M, Sugino K, Mimura T, Ito K. Clinical evaluation of color Doppler imaging for the differential diagnosis of thyroid follicular lesions. World J Surg. 2004;28:1261–1265. doi: 10.1007/s00268-004-7597-8. [DOI] [PubMed] [Google Scholar]
- 9.Iannuccilli JD, Cronan JJ, Monchik JM. Risk for malignancy of thyroid nodules as assessed by sonographic criteria: the need for biopsy. J Ultrasound Med. 2004;23:1455–1464. doi: 10.7863/jum.2004.23.11.1455. [DOI] [PubMed] [Google Scholar]
- 10.Wienke JR, Chong WK, Fielding JR, Zou KH, Mittelstaedt CA. Sonographic features of benign thyroid nodules: interobserver reliability and overlap with malignancy. J Ultrasound Med. 2003;22:1027–1031. doi: 10.7863/jum.2003.22.10.1027. [DOI] [PubMed] [Google Scholar]
- 11.Pellitteri PK, McCaffrey TV. Tumor and tumor-like lesion in thyroid endocrine surgery of the head and neck. 1st ed. Pennsylvania: Singular publishing group; 2002. pp. 21–47. [Google Scholar]
- 12.Miyakawa M, Onoda N, Etoh M, Fukuda I, Takano K, Okamoto T, et al. Diagnosis of thyroid follicular carcinoma by the vascular pattern and velocimeteric parameters using high resolution pulsed and power Doppler ultrasonography. Endocr J. 2005;52:207–212. doi: 10.1507/endocrj.52.207. [DOI] [PubMed] [Google Scholar]
- 13.Shimura H, Haraguchi K, Hiejima Y, Fukunari N, Fujimoto Y, Katagiri M, et al. Distinct diagnositic criteria for ultrasonographic examination of papillary thyroid carcinoma: a multicenter study. Thyroid. 2005;15:251–258. doi: 10.1089/thy.2005.15.251. [DOI] [PubMed] [Google Scholar]