Abstract
Objective
To investigate the diagnostic value of CT colonography for the detection of colorectal polyps.
Materials and Methods
From December 2004 to December 2005, 399 patients underwent CT colonography and follow-up conventional colonoscopy. We excluded cases of advanced colorectal cancer. We retrospectively analyzed the CT colonography findings and follow-up conventional colonoscopy findings of 113 patients who had polyps more than 6 mm in diameter. Radiologists using 3D and 2D computer generated displays interpreted the CT colonography images. The colonoscopists were aware of the CT colonography findings before the procedure.
Results
CT colonography detected 132 polyps in 107 of the 113 patients and conventional colonoscopy detected 114 colorectal polyps more than 6 mm in diameter in 87 of the 113 patients. The sensitivity of CT colonography analyzed per polyp was 91% (41/45) for polyps more than 10 mm in diameter and 89% (101/114) for polyps more than 6 mm in diameter. Thirteen polyps were missed by CT colonography and were detected on follow-up conventional colonoscopy.
Conclusion
CT colonography is a sensitive diagnostic tool for the detection of colorectal polyps and adequate bowel preparation, optimal bowel distention and clinical experience are needed to reduce the rate of missing appropriate lesions.
Keywords: Colon, CT; Colon neoplasm; Computed tomography (CT) colonography; Virtual colonoscopy
Increased awareness of colorectal cancer has made conventional colonoscopy a common screening procedure. Previously, a barium colon study was the primary screening tool, but conventional colonoscopy has replaced the barium colon study because of its higher sensitivity for small polyps and the ability to simultaneously obtain a biopsy. However, controversy remains about conventional colonoscopy as a screening tool in asymptomatic patients because it is relatively invasive, potentially painful, and is dependent on the technique of the colonoscopist.
In recent years, patients have considered the use of CT colonography as a preferred evaluation tool for colorectal disorders. Moreover, when a patient complains of vague abdominal symptoms, a clinician may prefer an evaluation by CT colonography, which can give both a colonic and extra-colonic evaluation simultaneously.
Earlier CT colonography studies have shown variable sensitivity and specificity (1-12). One study reported CT colonography sensitivity as high as 88.7% for polyps more than 6 mm in diameter (1), while other studies have shown lower sensitivities (29-57%) for polyps 5-9 mm in diameter (2).
We evaluated the diagnostic value of CT colonography for the detection of colorectal polyps and determined the nature of false-positive and false-negative lesions.
MATERIALS AND METHODS
Patients and Setting
Between December 1, 2004 and December 31, 2005, 2,343 patients underwent CT colonography. Asymptomatic patients, symptomatic patients (anal bleeding, hematochezia, bowel habit change, etc.), and high-risk patients for developing a colorectal neoplasm were all recruited for undertaking CT colonography. Of the 2,343 patients, 399 underwent follow-up conventional colonoscopy. We retrospectively reviewed the results of CT colonography and compared the findings with the follow-up conventional colonoscopy results. We excluded cases of advanced colorectal cancer, and polyps less than 5 mm in diameter. In total, 113 of 399 patients who underwent both procedures were found to have polyps more than 6 mm in diameter. The mean age of the 113 patients was 52 years (22-81 years). The mean interval between CT colonography and follow-up colonoscopy was 47 days (0-334 days).
Bowel Preparation
We divided the 113 patients who underwent both studies into two groups according to the method of bowel preparation. The first group performed bowel cleansing with fecal tagging while the second group had only bowel cleansing. The fecal tagging group was comprised of 50 patients, and non-fecal tagging group had a total of 63 patients.
In the fecal tagged group, patients consumed 250 ml of a barium solution (2% by weight) three times after every meal for fecal tagging, and 90 ml of sodium phosphate (Colclean; Taejoon Pharmaceuticals, Seoul, Korea) one day before the examination. In the non-fecal tagged group, patients consumed only the 90 ml of sodium phosphate prior to the examination.
CT Colonography
All patients received a 10 mg intramuscular injection of scopolamine N-butylbromide (Buscopan; Boehringer Ingelheim, Ingelheim, Germany), unless contraindicated, prior to CT acquisition. After the insertion of a small flexible rectal catheter without a balloon, pneumocolon was achieved by the use of an automatic inflator (Teleflator; Kaigen, Osaka, Japan). Approximately 1,400-1,600 ml of room air was insufflated into the colon. CT scanning was performed with a 16 channel multi-detector row CT scanner (Mx8000 IDT, Philips Medical Systems, Best, The Netherlands). Scanning parameters for CT colonography were as follows: beam collimation, 16×1.5 mm; reconstruction interval, 1 mm; pitch, 1.2; gantry rotation time, 0.75 second; table speed, 36 mm/sec; 120 kV; and 180 mA. Acquisition time ranged from 12 to 14 seconds.
No oral contrast agent was administered. Patients were scanned in the prone position without contrast media enhancement and in the supine position after intravenous injection with 150 ml of iopromide (Ultravist 320 Schering, Berlin, Germany). Iopromide was injected with a power injector at a rate of 3 ml/sec through an 18-gauge needle inserted in the antecubital vein. After a scan in the supine position was obtained, an additional scan was obtained in the right or left decubitus position in most patients depending on the presence of any collapsed segment. The decubitus position is helpful in case that bowel distention is not adequate in the supine or prone position.
Image processing and interpretation were performed with the use of a CT colonographic system (Rapidia, version 2.8, Infinitt, Seoul, Korea). This software program generates automatic navigation and shows three-dimensional endoluminal images and two-dimensional axial, coronal and sagittal images on a single screen.
Three radiologists interpreted the CT colonography and the CT colonography was interpreted with 3D and 2D images. We detected polypoid lesions primarily on 3D images and then differentiated true polyps from fecal material on the 2D images. Polyps were recorded according to segment location in the colon (cecum, ascending colon, hepatic flexure, transverse colon, splenic flexure, descending colon, sigmoid colon, and rectum). Each of the segments was subdivided into proximal, mid and distal portions. The diameter of detected polyps was measured in the 3D images by use of an electrical caliper. The size, number and location of individual polyps were recorded, although the shape of the polyp was not described (except for flat lesions).
Conventional Colonoscopy
Gastroenterologists performed conventional colonoscopies. A standard video-compatible colonoscope (EC 3831M, Pentax, Japan) was used. The gastroenterologists were aware of the preceding CT colonography results before performing a colonoscopy. All patients had a complete colonoscopy completed within 20 minutes without sedation. Polyps were photographed and were measured with the use of a calibrated linear probe or by visual estimation. The colonoscopist scored polyps with respect to size, number, and location (cecum, ascending colon, transverse colon, descending colon, sigmoid colon and rectum). Each of the segments (except for the cecum) were subdivided into proximal, mid and distal portions, and the morphology was categorized as pedunculated (Ip), sessile (Is), sub-pedunculated (Isp), or flat (II- a, b, c). Based on the criteria of the Japanese Research Society Classification (JRSC), Ip lesions were defined as those with a distinct pedicle, Isp lesions had a clearly defined neck but no distinct stalk. Sessile lesions were defined as having no stalk or pedicle evidence and having a diameter less than or equal to twice the height. Flat lesions were defined as having a mucosal change with a flat or rounded surface combined with a height of less than one-half the diameter of the lesion (13, 14).
Analysis
The results of conventional colonoscopy that followed CT colonography (and benefited from its use) were considered as the reference standard. The polyp sizes were subdivided into polyps 5 mm or less in diameter, and polyps more than 6 mm in diameter. Polyps more than 6 mm in diameter were individually sized by use of the CT colonography electrical caliper if the polyp was detected by use of both procedures, but when a polyp was detected only by conventional colonoscopy, the colonoscopic measurement was accepted.
The polyps detected by CT colonography and by conventional colonoscopy were considered as the same lesion if their sizes were within 50% and if they were in the same or adjacent segments.
The sensitivities of CT colonography with regard to identifying polyps were evaluated in all 113 patients. To evaluate whether there was a significant difference in diagnostic criteria (sensitivity and positive predictive value) within the fecal tagging group and non-fecal tagging group, the independent samples t test was used. For this test, a p value of 0.05 or less was considered statistically significant. For the false positive and false negative results, radiologists retrospectively reassessed the CT colonography.
RESULTS
Table 1 summarizes the total polyps discovered by conventional colonoscopy in the study population. A total of 113 of the 399 patients who underwent both procedures were found to have polyps more than 6 mm in diameter. In 87 of the 113 patients, a total of 114 polyps more than 6 mm in diameter were detected by conventional colonoscopy. In 107 of the 113 patients, 132 polyps more than 6 mm in diameter were detected by CT colonography.
Table 1.
CT Colonography by Polyp Size
In 84 of the 87 patients, a polypectomy was performed for 105 of the 114 polyps detected by conventional colonoscopy. Three patients with polyps refused to undergo the polypectomy. The pathological findings for the 105 polyps removed included: adenocarcinoma (n = 9), adenomatous polyps (n = 78), hyperplastic polyps (n = 6), hamartoma (n = 3), serrated adenomatous polyps (n = 2), chronic inflammation (n = 3), mucosal tag (n = 1), villoglandular polyp (n = 1), carcinoid (n = 1) and lipoma (n = 1). One patient was found to have a hepatoma as a major extracolonic disease.
Sensitivity and the Positive Predictive Value of CT Colonography for Polyp Identification in the 113 Patients
Table 2 summarizes the results of the CT colonography, including the sensitivities and positive predictive values per polyp. The sensitivity and positive predictive value of CT colonography for polyps more than 6 mm in diameter were 89% (101/114) and 77% (101/132), respectively. The sensitivity and positive predictive value of CT colonography for polyps more than 10 mm in diameter were 91% (41/45) and 87% (41/47), respectively.
Table 2.
Sensitivity of CT Colonography for Polyp Detection
Sensitivity and Positive Predictive Value of CT Colonography for Identification of Polyps in the Fecal Tagging Group and Non-fecal Tagging Group
Table 3 summarizes the results of the CT colonography in the fecal tagging group and non-fecal tagging group. For the fecal tagging group, the sensitivity and positive predictive value of CT colonography for polyps more than 6 mm in diameter were 79% (45/57) and 82% (45/55), respectively; for polyps more than 10 mm, the sensitivity and positive predictive value were 82% (14/17) and 93% (14/15), respectively.
Table 3.
Sensitivity of CT Colonography for Polyp Detection in the Fecal Tagging Group and Non-fecal Tagging Group
Note.-FT = fecal tagging, non-FT = non-fecal tagging
In the non-fecal tagging group, the sensitivity and positive predictive value of CT colonography for polyps more than 6 mm in diameter were 98% (56/57) and 73% (56/77), respectively; for polyps more than 10 mm, the sensitivity and positive predictive value were 96% (27/28) and 84% (27/32), respectively.
A comparison of the sensitivities for the fecal tagging and non-fecal tagging groups showed that sensitivity was improved in the non-fecal tagging group and statistically significant (p < 0.05). However, a comparison of the positive predictive values between the two groups showed that the difference was not statistically significant (p > 0.05).
Morphology of the Polyps
The morphology of 114 polyps were as follows: 21 pedunculated polyps, 40 subpedunculated polyps, 39 sessile polyps, 12 flat-elevated lesions, and two flat-elevated lesions with central depression. The missing rates according to morphology were polypoid (5%, 1/21), subpedunculated (8%, 3/38), sessile (17%, 7/41), and flat elevated or flat-elevated with central depression (14%, 2/14). Two of the 14 flat lesions were adenocarcinomas (Fig. 1).
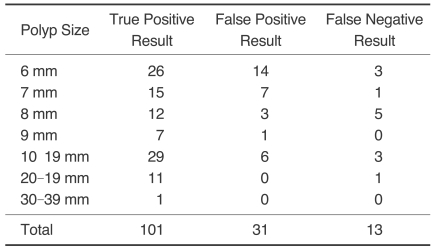
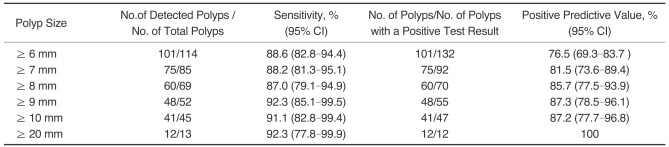
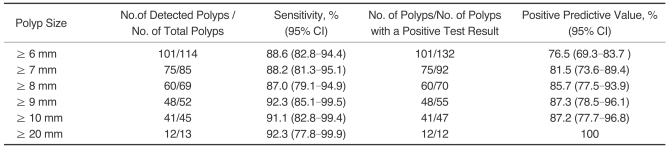
Fig. 1.
An 8 mm flat-elevated rectal lesion in a 50-year-old man.
A. A three-dimensional lumen view of the proximal rectum revealing an 8 mm, flat-elevated lesion with central depression (arrow) on CT colonography.
B. A contrast-enhanced axial CT image at the level of the proximal rectum shows a small enhancing flat-elevated lesion (arrow).
C. A photograph from conventional colonoscopy displays a flat-elevated lesion with central depression in the proximal rectum. Histological examination revealed that the lesion was an adenocarcinoma.
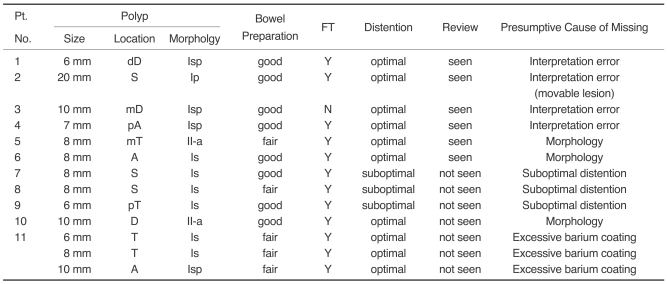
Retrospective Analysis of False Negative Lesions seen on CT Colonography
Table 4 reviews a follow-up of the false negative findings of the CT colonography findings for polyps more than 6 mm in diameter. Thirteen polyps more than 6 mm in diameter were not initially detected by CT colonography in 11 patients. Upon careful review of the digitally recorded CT colonographies, six polyps were detectable, which represented interpretation errors. One of the six polyps was 2.0 cm in diameter and peduculated, causing movement during positional changes. Even with the large size, pedunculated polyps could be misinterpreted as fecal material or fluid due to their ability to move. Two of the six polyps were a flat-elevated lesion and a sessile lesion, which were not easily perceptible lesions identified on retrospective review. The remaining three lesions were easily detectable on retrospective review, which represents purely an interpretation error.
Table 4.
Retrospective Analysis of the Missed Lesions by CT Colonography
Note.-A = ascending colon, D = descending colon, T = transverse colon, S = sigmoid colon, p = proximal, d = distal, m = mid, Isp = subpeduculated, Ip = polypoid, Is = sessile, II-a = flat-elevated, FT = fecal tagging
Seven polyps were not detectable during the retrospective review. The presumptive causes were as follows: suboptimal bowel distention (n = 3), excessive barium coating (n = 3), and flat characteristics of the lesions (n = 1). In one patient, three polyps were missed due to an excessive barium coating of the ascending and transverse colon and were not detected in the retrospective review. One flat lesion was not detected in the retrospective review of the CT colonography without any other reasonable cause.
Retrospective Analysis of False Positive Lesions seen by CT Colonography
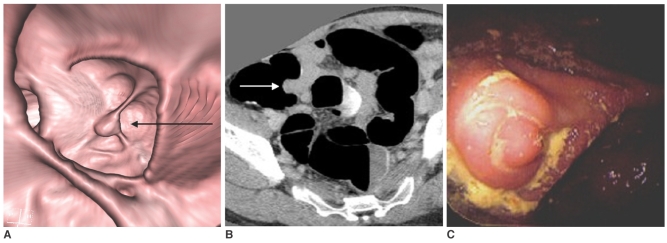
Thirty-one pseudopolyps more than 6 mm were detected in 27 patients. The causes for false positive findings on CT colonography were as follows: appendiceal base elevation (n = 1), seed (n = 1), fecal materials (n = 29). An elevation of the appendiceal base due to pelvic adhesion was misinterpreted as a cecal polyp (Fig. 2).
Fig. 2.
A pseudopolyp at the appendiceal orifice in 35-year-old asymptomatic woman.
A. A three-dimensional view of the cecal lumen revealing a 15 mm, polypoid lesion (arrow) at the appendiceal orifice. This lesion was misinterpreted as a cecal polyp on CT colonography.
B. A contrast-enhanced axial CT image at the level of the cecum, shows an enhancing polypoid lesion (arrow) at the appendiceal orifice. This lesion was constant and non-movable in the prone, supine, and decubitus positions.
C. A photograph from a conventional colonoscopy displaying the nodular endoluminal protrusion at the appendiceal base (not an intussusception of the appendix). The woman underwent pelvic surgery for a cesarean section and pelvic endometriosis a few years earlier. The endoluminal protrusion at the appendiceal base into the air-inflated cecum is thought to be due to pelvic adhesions.
DISCUSSION
The development of multi-detector row CT has spawned a new era of the use of CT colonography as a promising tool for the evaluation of colorectal polyps and cancer. However, CT colonography has not yet been proven as a sensitive and specific method for the identification of colorectal polyps and cancer detection. Many studies have been performed in an effort to validate the accuracy of CT colonography, but the reported sensitivity and accuracy of CT colonography has been quite variable. Consequently, the usefulness of CT colonography is still controversial.
A study by Van Gelder et al. reported that the sensitivity of polyp detection by CT colonography was 76% for large polyps (more than 10 mm), 70% for medium sized polyps (6-9 mm), and sensitivities per patient for large and medium polyps was 84% and 78%, respectively (3). Cotton and his colleagues reported disappointing results for CT colonscopy, in which polyp sensitivity was only 55% for polyps more than 10 mm (4). For lesions more than 6-9 mm in diameter, the sensitivity was merely 39% (4). However, a recent multi-center study reported by Pickhardt et al. (1) found a higher sensitivity and specificity with CT colonography than in previous reports.
Pickhardt reported CT colonography sensitivities of 89% and 94% for adenomatous polyps more than 6 mm and more than 10 mm in diameter, respectively (1). Contrary to the previous studies, non-adenomatous polyps were excluded from the statistical analysis, and bowel preparation was done with laxatives followed by solid fecal tagging by barium and liquid opacification with diatrizoate meglumine and diatrizoate sodium (Gastrografin) (1).
In our study, polyp sensitivities for polyps more than 10 mm in diameter and polyps more than 6 mm in diameter were 91% and 89%, respectively. We included both adenomatous and non-adenomatous polyps and only stratified the lesions by the size of the polyp.
Four reasonable explanations for the false negative results found in our study were as follows: interpretation errors, the bowel preparation methods utilized, suboptimal distention during examination, and the morphologic characteristics of the polyps.
In early CT colonography studies, fecal residue was the major cause of false positive findings, limiting the clinical application of CT colonography. To overcome this problem, fecal tagging with barium was introduced and has since been widely used. Lefere et al. reported that the fecal tagging with barium reduced false positive results and improved specificity (15, 16). In one report, a comparison of the overall sensitivity and negative predictive value in the non-fecal tagging group versus the fecal tagging group indicated that the observed differences were minimal and not statistically significant (15). In our study, 50 of 113 patients had undergone bowel cleansing with fecal tagging and 63 patients had only undergone bowel cleansing. For polyps more than 10 mm, the sensitivity was increased in the non-fecal tagging group (96%) than in the fecal tagging group (82%) and was found to be statistically significant (p < 0.05). However, the positive predictive value for polyps more than 10 mm was higher in the fecal tagging group (93%) than in the non-fecal tagging group (84%), but the difference was not statistically significant (p > 0.05). By use of the fecal tagging technique in our study, false-negative results were greater.
Limited fluid intake and fecal tagging with barium causes a "dry-dirty bowel", with so-called "dry-dirty" cleansing. Dry cleansing for CT colonography allows for sufficient bowel insufflation and eliminates "blind" areas caused by intra-luminal fluid. We did not subtract the electrical signal caused by intra-luminal fluid. Positioning of the patient usually removes the fluid and compensates for the blind spots. Colonoscopists generally favor "wet cleansing" to dry cleansing because sufficient fluid intake on the day of examination leads to more complete bowel cleansing and intra-luminal fluid can be easily aspirated out during colonoscopy. In our opinion, wet cleansing without barium fecal tagging would likely provide more effective bowel cleansing.
In CT colonography, fecal tagging can produce false positive and false negative results. This can occur during the post-contrast enhanced scans, and when the colonic segment is collapsed in the pre-contrast enhanced scans. During these conditions, it is difficult to differentiate the barium-containing fecal material from an enhancing polyp. In some cases, excessive barium coating of the colon can obscure the image of small polyps. In fact, one patient in our study had three polyps (6-10 mm in diameter) in the ascending and transverse colons that were obscured on CT colonography by barium coating of the endoluminal surface. We believe it is easier to differentiate polyps from fecal material by contrast enhancement for polyps more than 6 mm in diameter without using fecal tagging.
Recently, CT colonography without cathartic preparation has been studied and has been reported by several groups (17-19). One report by Iannaccone et al. showed that the sensitivity of CT colonography without cathartic preparation was 96% for polyps more than 8 mm and 86% for polyps more than 6 mm in diameter (17). In the study, only an oral contrast agent (diatrizoate meglumine with diatrizoate sodium, total dose of 200 mL) was used for fecal tagging. Lefere and his colleagues have reported that fecal tagging with 50 ml of barium (40% w/v) and a low dose cathartic agent are effective for CT colonography (16). However, more studies are needed before clinical application of non-cathartic CT colonography is accepted.
The "miss rate" of CT colonography and conventional colonoscopy for colorectal polyps and cancer have been closely examined and studied by radiologists and colonoscopists (20-24). Many colonoscopists are interested in flat and depressed lesions as a larger number of these lesions have high-grade dysplasia and focal cancer (13, 25, 26). Rembacken et al. reported that a total 327 adenomas were found in 1,000 patients who underwent colonoscopy (25). Their study found 119 (36%) flat lesions and four (1%) depressed lesions, while 20 (16%) of 123 flat or depressed lesions had severe dysplasia or Duke-A carcinoma foci (25). Park et al. reported that flat and small polyps are the two main causes for missed (non-detected) lesions on CT colonography (20). In their study, 29 of 63 lesions were missed (46%) and all three flat lesions more than 10 mm in diameter were missed (20). In our study, nine polyps (69%) of 13 missed lesions were sessile (n = 7) or flat-elevated lesions (n = 2). Sessile and flat lesions are not easily detectable despite thorough bowel cleansing or adequate distention and barium or fecal materials or fluid can easily obscure them. One flat lesion was not detectable in the retrospective review of CT colonography without any other cause such as inadequate bowel preparation or suboptimal bowel distention. Two sessile polyps abutting the colonic folds were initially missed on CT colonography and were not easily detectable even on the retrospective review.
It is worthy to note that colonoscopists normally have difficulty detecting small flat lesions. Regardless of technique, careful examination and experience is helpful for detection of the flat lesions.
Our study has several limitations. We adopted conventional colonoscopy as a reference standard, so possible false negative results from conventional colonoscopy could be considered as false positive results for CT colonography. Because we retrospectively analyzed CT colonographies and follow-up colonoscopies in patients who had polyps more than 6 mm in diameter, there may have been some bias in patient selection. In most cases, the colonoscopist did not use an endoscopic ruler, so measurements of polyps only detected by conventional colonoscopy may have been misrepresented.
In conclusion, CT colonography appears to be a valuable screening method. Adequate bowel preparation and distention, along with clinical experience are needed to reduce the rate of missing lesions.
References
- 1.Pickhardt PJ, Choi JR, Hwang I, Butler JA, Puckett ML, Hildebrandt HA, et al. Computed tomography virtual colonoscopy to screen for colorectal neoplasia in asymptomatic adults. N Engl J Med. 2003;349:2191–2200. doi: 10.1056/NEJMoa031618. [DOI] [PubMed] [Google Scholar]
- 2.Johnson CD, Harmsen WS, Wilson LA, Maccarty RL, Welch TJ, Ilstrup DM, et al. Prospective blinded evaluation of computed tomographic colonography for screen detection of colorectal polyps. Gastroenterology. 2003;125:311–319. doi: 10.1016/s0016-5085(03)00894-1. [DOI] [PubMed] [Google Scholar]
- 3.Van Gelder RE, Nio CY, Florie J, Bartelsman JF, Snel P, De Jager SW, et al. Computed tomographic colonography compared with colonoscopy in patients at increased risk for colorectal cancer. Gastroenterology. 2004;127:41–48. doi: 10.1053/j.gastro.2004.03.055. [DOI] [PubMed] [Google Scholar]
- 4.Cotton PB, Durkalski VL, Pineau BC, Palesch YY, Mauldin PD, Hoffman B, et al. Computed tomographic colonography: a multicenter comparison with standard colonoscopy for detection of colorectal neoplasia. JAMA. 2004;291:1713–1719. doi: 10.1001/jama.291.14.1713. [DOI] [PubMed] [Google Scholar]
- 5.Pineau BC, Paskett ED, Chen GJ, Espeland MA, Phillips K, Han JP, et al. Virtual colonoscopy using oral contrast compared with colonoscopy for the detection of patients with colorectal polyps. Gastroenterology. 2003;125:304–310. doi: 10.1016/s0016-5085(03)00885-0. [DOI] [PubMed] [Google Scholar]
- 6.Yee J, Akerkar GA, Hung RK, Steinauer-Gebauer AM, Wall SD, McQuaid KR. Colorectal neoplasia: performance characteristics of CT colonography for detection in 300 patients. Radiology. 2001;219:685–692. doi: 10.1148/radiology.219.3.r01jn40685. [DOI] [PubMed] [Google Scholar]
- 7.Johnson CD, Toledano AY, Herman BA, Dachman AH, McFarland EG, Barish MA, et al. Computerized tomograhpic colonography: performance evaluation in a retrospective multicenter setting. Gastroenterology. 2003;125:688–695. doi: 10.1016/s0016-5085(03)01058-8. [DOI] [PubMed] [Google Scholar]
- 8.Spinzi G, Belloni G, Martegani A, Sangiovanni A, Favero CD, Minoli G. Computed tomographic colonography and conventional colonoscopy for colon diseases: a prospective, blinded study. Am J Gastroenterol. 2001;96:394–400. doi: 10.1111/j.1572-0241.2001.03550.x. [DOI] [PubMed] [Google Scholar]
- 9.Fenlon HM, Nunes DP, Schroy PC, 3rd, Barish MA, Clarke PD, Ferrucci JT. A comparison of virtual and conventional colonoscopy for the detection of colorectal polyps. N Engl J Med. 1999;341:1496–1503. doi: 10.1056/NEJM199911113412003. [DOI] [PubMed] [Google Scholar]
- 10.Iannaccone R, Laghi A, Catalano C, Brink JA, Mangiapane F, Trenna S, et al. Detection of Colorectal lesions: lower-dose multi-detector row helical CT colonography compared with conventional colonoscopy. Radiology. 2003;229:775–781. doi: 10.1148/radiol.2293021399. [DOI] [PubMed] [Google Scholar]
- 11.Macari M, Bini EJ, Xue X, Milano A, Katz SS, Resnick D, et al. Colorectal neoplasm: prospective comparison of thin-section low-dose multi-detector row CT colonography and conventional colonoscopy for detection. Radiology. 2002;224:383–392. doi: 10.1148/radiol.2242011382. [DOI] [PubMed] [Google Scholar]
- 12.Durkalski VL, Palesch YY, Pineau BC, Vining DJ, Cotton PB. The virtual colonoscopy study: A large multicenter clinical trial designed to compare two diagnostic screening procedures. Control Clin Trials. 2002;23:570–583. doi: 10.1016/s0197-2456(02)00232-5. [DOI] [PubMed] [Google Scholar]
- 13.Kudo S, Kashida H, Tamura T, Koqure E, Imai Y, Yamano H, et al. Colonoscopic diagnosis and management of nonpolypoid early colorectal cancer. World J Surg. 2000;24:1081–1090. doi: 10.1007/s002680010154. [DOI] [PubMed] [Google Scholar]
- 14.Muto T, Kamiya J, Sawada T, Morioka Y. Morphogenesis of human colonic cancer. Dis Colon Rectum. 1983;26:257–262. doi: 10.1007/BF02562493. [DOI] [PubMed] [Google Scholar]
- 15.Lefere PA, Gryspeerdt SS, Dewyspelaere J, Baekelandt M, Van Holsbeeck BG. Dietary fecal tagging as a cleansing method before CT colonography: Initial results-polyp detection and patient acceptance. Radiology. 2002;224:393–403. doi: 10.1148/radiol.2241011222. [DOI] [PubMed] [Google Scholar]
- 16.Lefere P, Gryspeerdt S, Marrannes J, Baekelandt M, Van Holsbeeck B. CT colonography after fecal tagging with a reduced cathartic cleansing and a reduced volume of barium. AJR Am J Roentgenol. 2005;184:1836–1842. doi: 10.2214/ajr.184.6.01841836. [DOI] [PubMed] [Google Scholar]
- 17.Iannaccone R, Laghi A, Catalano C, Mangiapane F, Lamazza A, Schillaci A, et al. Computed tomographic colonography without cathartic preparation for the detection of colorectal polyps. Gastroenterology. 2004;127:1300–1311. doi: 10.1053/j.gastro.2004.08.025. [DOI] [PubMed] [Google Scholar]
- 18.Callstrom MR, Johnson CD, Fletcher JG, Reed JE, Ahlquist DA, Harmsen WS, et al. CT colonography without cathartic preparation: feasibility study. Radiology. 2001;219:693–698. doi: 10.1148/radiology.219.3.r01jn22693. [DOI] [PubMed] [Google Scholar]
- 19.Lefere P, Gryspeerdt S, Baekelandt M, Van Holsbeeck B. Laxative-free CT colonography. AJR Am J Roentgenol. 2004;183:945–948. doi: 10.2214/ajr.183.4.1830945. [DOI] [PubMed] [Google Scholar]
- 20.Park SH, Ha HK, Kim MJ, Kim KW, Kim AY, Yang DH, et al. False-negative results at multi-defector row CT colonography: Multivariate analysis of causes for missed lesions. Radiology. 2005;235:495–502. doi: 10.1148/radiol.2352040606. [DOI] [PubMed] [Google Scholar]
- 21.Postic G, Lewin D, Bickerstaff C, Wallace MB. Colonoscopic miss rates determined by direct comparison of colonoscopy with colon resection specimens. Am J Gastroenterol. 2002;97:3182–3185. doi: 10.1111/j.1572-0241.2002.07128.x. [DOI] [PubMed] [Google Scholar]
- 22.Rex DK, Cutler CS, Lemmel GT, Rahmani EY, Clark DW, Helper DJ, et al. Colonoscopic miss rates of adenomas determined by back-to-back colonoscopies. Gastroenterology. 1997;112:24–28. doi: 10.1016/s0016-5085(97)70214-2. [DOI] [PubMed] [Google Scholar]
- 23.Macari M, Bini EJ, Jacobs SL, Lui YW, Laks S, Milano A, et al. Significance of missed polyps at CT colonography. AJR Am J Roentgenol. 2004;183:127–134. doi: 10.2214/ajr.183.1.1830127. [DOI] [PubMed] [Google Scholar]
- 24.Gluecker TM, Fletcher JG, Welch TJ, MacCarty RL, Harmsen WS, Harrington JR, et al. Characterization of lesions missed on interpretation of CT colonography using a 2D search method. AJR Am J Roentgenol. 2004;182:881–889. doi: 10.2214/ajr.182.4.1820881. [DOI] [PubMed] [Google Scholar]
- 25.Rembacken BJ, Fujii T, Cairns A, Dixon MF, Yoshida S, Chalmers DM, et al. Flat and depressed colonic neoplasms: a prospective study of 1000 colonoscopies in the UK. Lancet. 2000;355:1211–1214. doi: 10.1016/s0140-6736(00)02086-9. [DOI] [PubMed] [Google Scholar]
- 26.Hurlstone DP, Cross SS, Adam I, Shorthouse AJ, Brown S, Sanders DS, et al. A prospective clinicopathological and endoscopic evaluation of flat and depressed colorectal lesions in the United Kingdom. Am J Gastroenterol. 2003;98:2543–2549. doi: 10.1111/j.1572-0241.2003.07679.x. [DOI] [PubMed] [Google Scholar]