Abstract
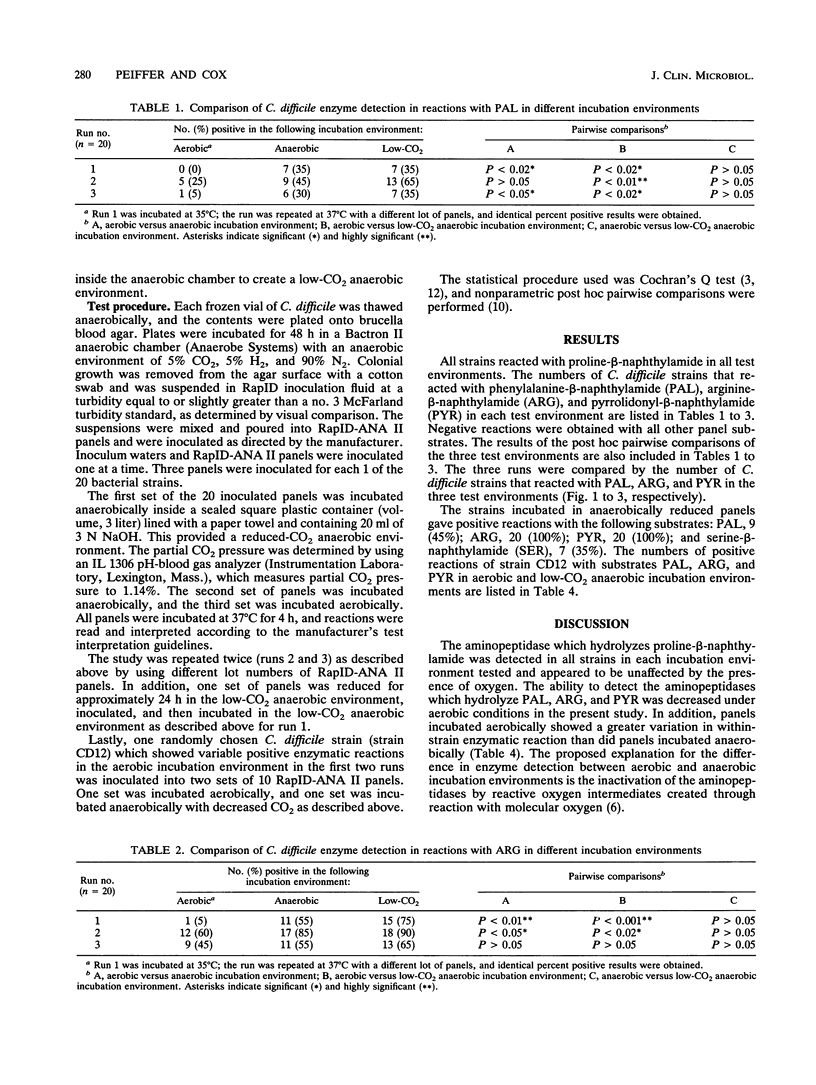
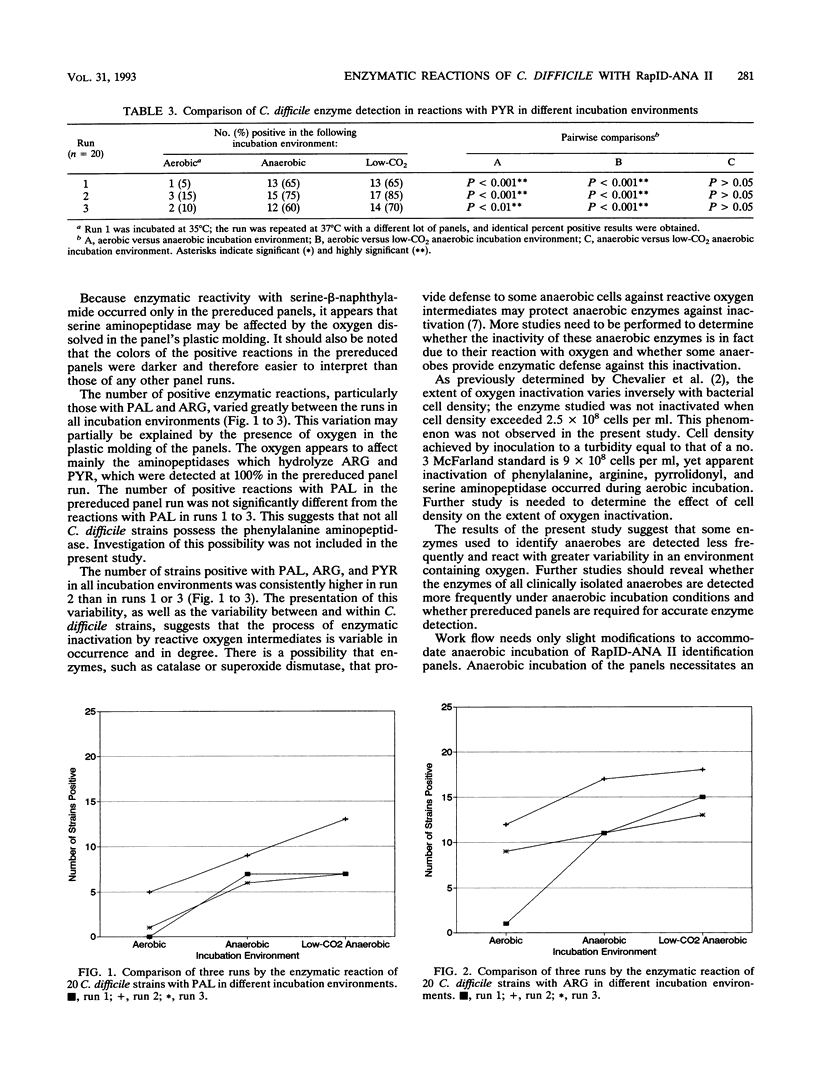
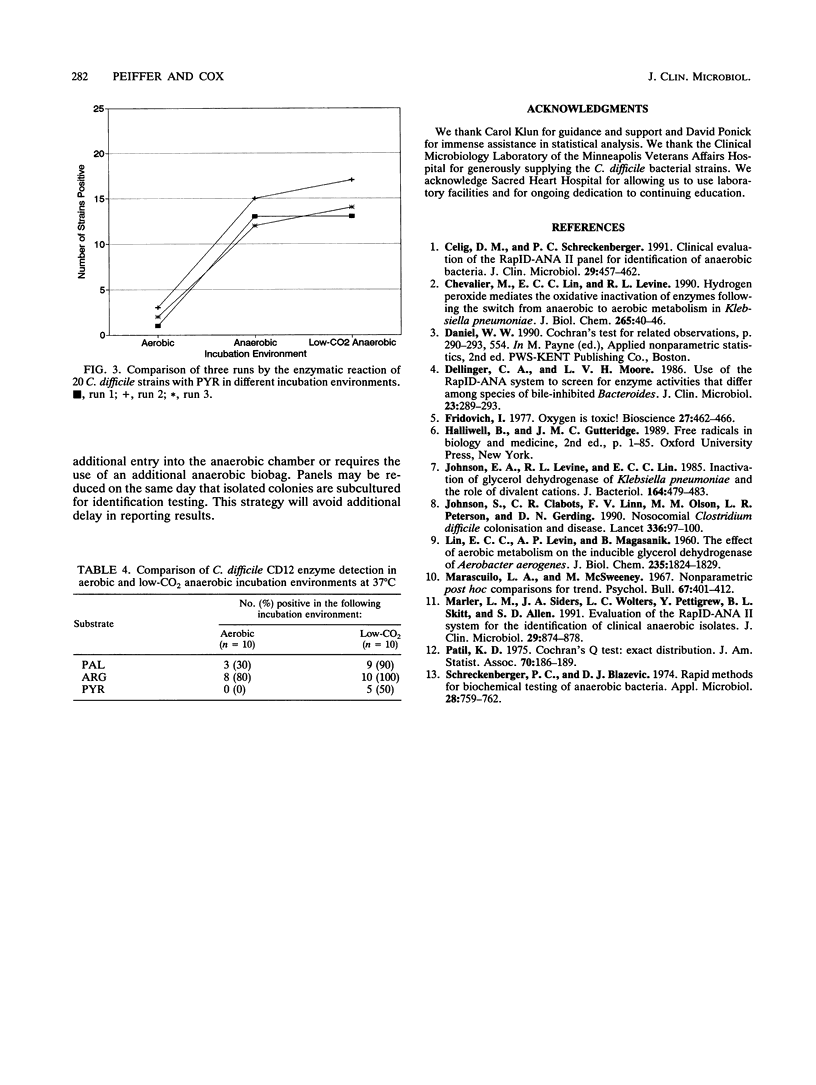
The RapID-ANA II anaerobic identification system (Innovative Diagnostic Systems, Inc., Atlanta, Ga.) was used to determine whether the incubation environment affects enzyme detection. Twenty strains of Clostridium difficile were tested in aerobic, anaerobic, and low-CO2 anaerobic incubation environments. The percentages of enzymes detected in reactions with the following substrates were noted in the three incubation environments: phenylalanine-beta-naphthylamide, aerobic, 0%; anaerobic, 35%; low-CO2 anaerobic, 35%; arginine-beta-naphthylamide, aerobic, 5%; anaerobic, 55%; low-CO2 anaerobic, 75%; pyrrolidonyl-beta-naphthylamide, aerobic, 5%; anaerobic, 65%; low-CO2 anaerobic, 65%. When the aerobic incubation environment was compared with either the anaerobic or the low-CO2 anaerobic incubation environments, the results were statistically different with respect to enzyme detection in reactions with the substrates listed above. The results for the anaerobic and low-CO2 anaerobic environments were not statistically different. The study was repeated twice. Statistical comparisons between the three environments were consistent with the results presented above, with the following exceptions. The aerobic and the anaerobic environments were not different in a reaction with phenylalanine-beta-naphthylamide in one of the runs, and there was no significant difference between the three environments in a reaction with arginine-beta-naphthylamide in another run. These results suggest that some of the enzymes used in the identification of clinical anaerobes appear to be inactive in an environment containing oxygen.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Celig D. M., Schreckenberger P. C. Clinical evaluation of the RapID-ANA II panel for identification of anaerobic bacteria. J Clin Microbiol. 1991 Mar;29(3):457–462. doi: 10.1128/jcm.29.3.457-462.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevalier M., Lin E. C., Levine R. L. Hydrogen peroxide mediates the oxidative inactivation of enzymes following the switch from anaerobic to aerobic metabolism in Klebsiella pneumoniae. J Biol Chem. 1990 Jan 5;265(1):42–46. [PubMed] [Google Scholar]
- Dellinger C. A., Moore L. V. Use of the RapID-ANA System to screen for enzyme activities that differ among species of bile-inhibited Bacteroides. J Clin Microbiol. 1986 Feb;23(2):289–293. doi: 10.1128/jcm.23.2.289-293.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson E. A., Levine R. L., Lin E. C. Inactivation of glycerol dehydrogenase of Klebsiella pneumoniae and the role of divalent cations. J Bacteriol. 1985 Oct;164(1):479–483. doi: 10.1128/jb.164.1.479-483.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson S., Clabots C. R., Linn F. V., Olson M. M., Peterson L. R., Gerding D. N. Nosocomial Clostridium difficile colonisation and disease. Lancet. 1990 Jul 14;336(8707):97–100. doi: 10.1016/0140-6736(90)91605-a. [DOI] [PubMed] [Google Scholar]
- LIN E. C., LEVIN A. P., MAGASANIK B. The effect of aerobic metabolism on the inducible glycerol dehydrogenase of Aerobacter aerogenes. J Biol Chem. 1960 Jun;235:1824–1829. [PubMed] [Google Scholar]
- Marascuilo L. A., McSweeney M. Nonparametric post hoc comparisons for trend. Psychol Bull. 1967 Jun;67(6):401–412. doi: 10.1037/h0020421. [DOI] [PubMed] [Google Scholar]
- Marler L. M., Siders J. A., Wolters L. C., Pettigrew Y., Skitt B. L., Allen S. D. Evaluation of the new RapID-ANA II system for the identification of clinical anaerobic isolates. J Clin Microbiol. 1991 May;29(5):874–878. doi: 10.1128/jcm.29.5.874-878.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreckenberger P. C., Blazevic D. J. Rapid methods for biochemical testing of anaerobic bacteria. Appl Microbiol. 1974 Nov;28(5):759–762. doi: 10.1128/am.28.5.759-762.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]


