Abstract
Introduction
Tolerance is observed for a variety of central nervous system depressants including ethanol, which is an anesthetic, but has not been convincingly demonstrated for a potent halogenated volatile anesthetic. Failure to demonstrate tolerance to these agents may be the result of inadequate exposure to anesthetic. In this study, we exposed Xenopus laevis tadpoles to surgical anesthetic concentrations of isoflurane for one week.
Methods
Xenopus laevis tadpoles were produced by in vitro fertilization, and exposed to isoflurane (0.59%, 0.98%, 1.52%) or oxygen for one week starting from the time of fertilization.
Results
Changes in anesthetic EC50 were small and not in a consistent direction. Control animals had an anesthetic EC50 of 0.594% ± 0.003% isoflurane. Tadpoles exposed to 1.52% isoflurane had a lower EC50 than controls (by 16%), while tadpoles raised under 0.59 and 0.98% isoflurane had higher EC50s than control (by 4.7% and 7.4%, respectively).
Conclusion
We provide the first description of week-long exposures of vertebrates to surgical anesthetic concentrations of isoflurane, and the first report of such exposures in developing vertebrates. Tolerance to isoflurane does not occur in developing Xenopus laevis tadpoles. Taken together with studies in other organisms, the development of tolerance to ethanol but not isoflurane suggests that mechanisms these drugs share probably do not account for the development of tolerance.
Keywords: Anesthesia, tolerance, isoflurane, Xenopus laevis, teratogen
Introduction
Tolerance, defined as the diminished effect of a drug with time, is observed for several central nervous system depressants, including barbiturates(1), ethanol(2), nitrous oxide(3), and benzodiazepines(4). Potent halogenated clinical anesthetics such as isoflurane and desflurane are thought to share certain mechanism with these drugs. For example, barbiturates (5), ethanol (6), benzodiazepines (7), and potent halogenated agents (6) all positively modulate GABA type A (GABAA) receptor function. Nitrous oxide, ethanol, and isoflurane inhibit N-methyl-D-aspartate (NMDA) receptor function (8, 9). There is additional evidence of a common mechanism between potent halogenated agents and alcohol. Desflurane is additive in its effects with ethanol (10). The similar modulation of NMDA (9), GABAA and glycine (6) receptor function by isoflurane and ethanol is attenuated by the same point mutations on those receptors(6, 9). Indeed, it has been proposed that ethanol and isoflurane bind to the same site on glycine receptors (11). Despite this similarity in mechanism, unlike ethanol, tolerance to potent halogenated anesthetics has not been convincingly demonstrated.
Attempts to demonstrate tolerance to potent inhaled agents have generally focused on short (minutes to hours) exposure to surgical anesthetic concentrations of anesthetics in order to mimic clinical situations, or longer exposures (days) to lower, subanesthetic concentrations in order to address mechanistic questions. For example, burst suppression on EEG produced by approximately 2 MAC desflurane has been reported to change to continuous electrical activity after approximately 30 minutes in dogs (12). However, a similar protocol showed no EEG evidence for tolerance to desflurane in swine (13). MAC to halothane does not change over 500 minutes in dogs (14). Mice continuously exposed to 0.15% or 0.30% atm isoflurane for 21 or 42 days showed small (2-9%) but insignificant increases in the righting reflex EC50.
Lack of development of tolerance to a potent inhaled anesthetic would be of considerable mechanistic significance, because it would indicate a difference in mechanism of action with other, tolerance-producing agents,(15) and in particular would suggest that common molecular mechanisms are unlikely to be involved in producing tolerance. However, lengthy exposures over the course of days to high concentrations of inhaled anesthetic have not been attempted in efforts to produce tolerance, perhaps because of the difficulty in caring for animals anesthetized for these longer spans of time. In the present report, we studied this concentration range. We exposed animals to concentrations of isoflurane greater than MAC for a week to determine whether tolerance developed. To circumvent the husbandry issues associated with long term exposure of most animals to immobilizing concentrations of anesthetic, we exposed developing tadpoles to isoflurane. In their first week of life, tadpoles subsist on their yolk sac and do not require food. Tadpoles were produced by in vitro fertilization, and exposed to isoflurane from the moment of fertilization until approximately one week of age.
In humans, more than fifty percent of acutely intoxicated patients have a blood alcohol concentration of 150 mg/dl (15). By contrast, patients who chronically ingest alcohol can have more than twice this blood alcohol concentration (300-400 mg/dl) without sedation, a concentration that approaches the lethal concentration in a nontolerant individual (15). Thus, EC50s more than double with tolerance to ethanol in humans. In this study of isoflurane, we regarded a 25% increase in EC50 as the lowest increase indicative of tolerance.
Methods
All studies in animals were approved by the UCSF institutional animal care and use committee. Tadpoles were raised by in vitro fertilization (16). A sexually mature, 9 cm Xenopus laevis female (Nasco, Modesto, CA) was primed with 50 IU human chorionic gonadotropin (hCG) (1 IU/μL) (Sigma, St. Louis, MO) injected into the dorsal lymph sac. Seven days later ovulation was induced by injecting 800 IU hCG into the dorsal lymph sac. After induction, the frog was kept in high salt modified Barth's solution (high salt MBS) (108mM NaCl, 1mM KCl, 1mM MgSO4, 5mM HEPES, 2.5mM NaHCO3, 0.7 mM CaCl2, adjusted to pH 7.0). Egg laying began 6-12 hours later. A male frog was euthanized; the testes were removed and kept in 1X MBS (88mM NaCl,1mM KCl, 1mM MgSO4, 5mM HEPES, 2.5mM NaHCO3, adjusted to pH 7.0) on ice. A sperm slurry was made by crushing testes with a razor blade in a small amount of 0.1X Marc's modified ringer's (MMR) + gentamicin (10mM NaCl, 0.18mM KCl, 0.2mM CaCl2, 0.1mM MgCl2, 0.5 mM HEPES, 50 mcg/mL gentamicin, adjusted to pH 7.0); this solution was kept on ice until the fertilization step.
Eggs were collected within 10 minutes of laying, and placed in 100 × 15 mM Petri dishes (Fisher Scientific, Pittsburgh, PA). The high salt MBS was decanted, and the eggs were covered with the sperm slurry. Following successful fertilization, as indicated by upward rotation of the animal pole, eggs were placed in 0.1X MMR equilibrated with the isoflurane concentration under study. The eggs were kept in batches of up to 30-40 in Petri dishes in 0.1X MMR + gentamicin for the first two days; subsequently, the developing embryos were kept in 0.1X MMR without gentamicin. Medium was changed every day.
Petri dishes were kept in gas tight plastic cylinders with rubber stoppers at both ends. Air (control) or varying concentrations of isoflurane in air delivered by vaporizer was passed through the cylinders at 60 mL/minute. Dry gasses passed through a humidifier before being introduced into the cylinders.
Anesthetic potency was tested in tadpoles at 7-8 days post fertilization using established, validated methods (17). 112, 25, 26, and 25 fertilized eggs, respectively, were initially placed in 0.00%, 0.59%, 0.98%, and 1.52% isoflurane. However, only 30 control tadpoles (0% isoflurane group) were used for testing of anesthetic response. Tadpoles were scored for survival and gross morphological abnormalities on days 4, 6, and 7 or 8. Those with gross morphological abnormalities were not tested for anesthetic response.
To test anesthetic potency, tadpoles were placed in 100 mL cylindrical glass containers containing 0.1X MMR with isoflurane. Isoflurane was bubbled into this solution at a rate of 120 mL/min. The system was covered with a plastic top and allowed to equilibrate for 20 minutes. A small sample of gas was drawn with a gas-tight syringe through a line hanging just above the liquid and analyzed by gas chromatography.
Tadpoles were prodded on their side for thirty seconds with a glass rod, or until they moved, whichever occurred first. Tadpoles that responded to any amount of prodding were classified as moving. Tadpoles that did not respond to prodding were classified as non-moving. Non-moving tadpoles were removed and placed in isoflurane-free 0.1X MMR for recovery. The concentration of isoflurane was increased by approximately 20% for the tadpoles that moved, and the procedure of equilibrating the 0.1X MMR and testing for movement in response to prodding was repeated until a concentration of isoflurane was found at which no tadpole moved.
Sample size
Sample size was calculated based on pilot data which suggested that under control conditions, the isoflurane EC50 in tadpoles would be approximately 0.6 % atm with a Hill coefficient of 12. This Hill coefficient corresponds to a standard deviation in the underlying distribution of EC50s of about 0.08 % atm (18). As noted previously, we regarded tolerance resulting in an increase in EC50 by at least 25%, from 0.6 to 0.75% atm, as biologically important. To have a 90% probability of detecting an effect of this size at the 1% significance threshold using an unpaired t-test required at least 10 tadpoles per group.
Statistics
The fraction of non-moves as a function of isoflurane concentration was analyzed by nonlinear regression to a Hill equation. EC50s and Hill coefficients were calculated and used to compare the curves. Comparisons of means between groups was done using a Student's t-test, or an ANOVA with the Student-Newman-Keuls test used for post-hoc multiple comparison testing. We considered tolerance to develop if there was a concentration-dependent increase in anesthetic EC50 with exposure to increasing concentrations of isoflurane during development. We regarded tolerance resulting in an increase in EC50 by at least 25% as biologically important and amenable to further study. Data on abnormalities and mortality were compared using a chi-square test. Where appropriate, a Bonferroni correction was used. P < 0.05 was considered significant.
Results
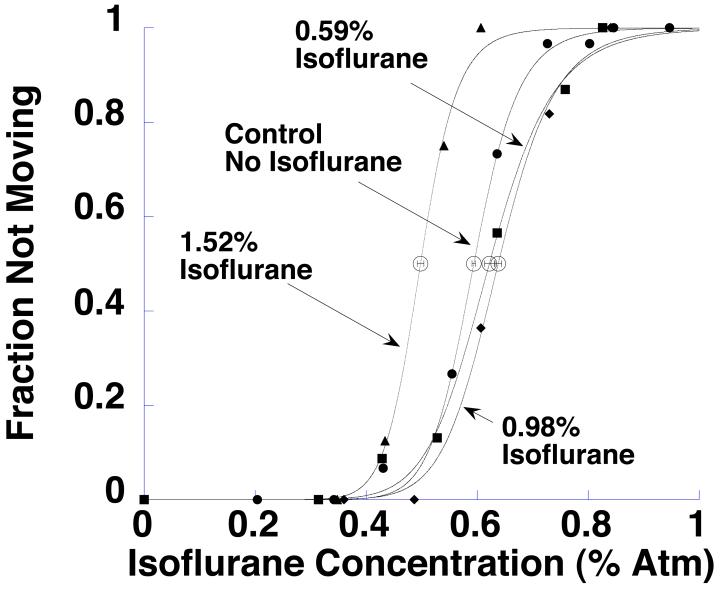
The concentration-response curves in Fig 1 show the relation between isoflurane exposure and evoked movement in tadpoles. The EC50 and Hill coefficient for each of these curves is tabulated in Table 1.
Fig 1.
Xenopus laevis tadpoles were produced by in vitro fertilization and raised from conception until one week of age in air (control), or in air with 0.59, 0.98, or 1.52 % atmospheres (% atm) isoflurane. Isoflurane EC50 was determined at one week of age. This figure shows the fraction of tadpoles moving in response to prodding as a function of isoflurane concentration. EC50s are open circles, with horizontal error bars representing the standard error in the EC50.
Table 1.
EC50 and Hill coefficient for no move in response to isoflurane in tadpoles raised in varying concentrations of isoflurane.
| Treatment* (% isoflurane in air) | EC50(% isoflurane) | Hill Coefficient | N |
|---|---|---|---|
| 0.00 | 0.594 ± 0.003 | 14.6 ± 1.0 | 30 |
| 0.59 | 0.622 ± 0.009 | 10.7 ± 1.4 | 23 |
| 0.98 | 0.638 ± 0.006 | 12.4 ± 1.3 | 11 |
| 1.52 | 0.498 ± 0.007 | 15.0 ±1.9 | 8 |
EC50 and Hill Coefficient are presented as mean ± SE
N is the number of tadpoles studies for each treatment
Tadpoles were exposed to these concentration of isoflurane for 7 or 8 days
There was a statistically significant difference in the EC50s (one-way ANOVA F3,68 = 46.50, p < 0.001). A post-hoc Student-Newman-Keuls test showed that all of the groups differed at the 0.05 significance threshold from control, with tadpoles treated with 1.52% isoflurane having a lower EC50 than controls (by 16.2%), and tadpoles raised under 0.59 and 0.98% isoflurane having higher EC50s than control (by 4.7% and 7.4%, respectively). There was no significant difference in Hill coefficients (F3,68 = 2.29, p = 0.087).
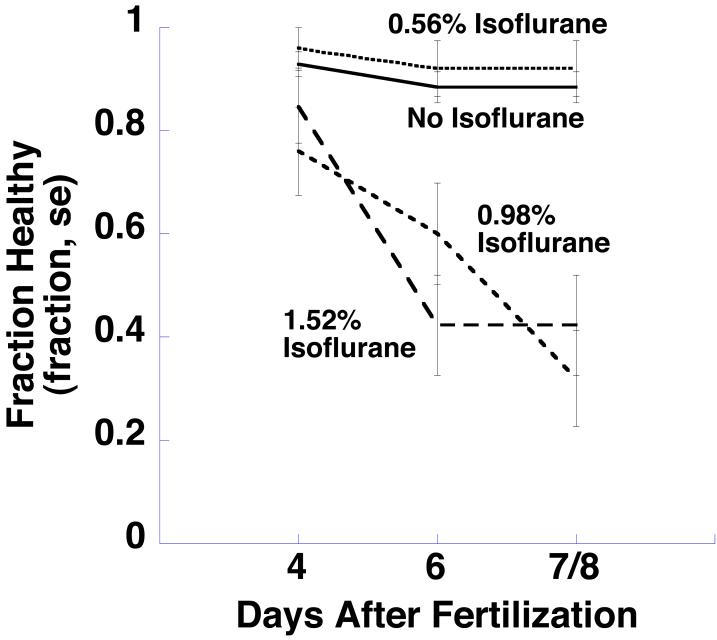
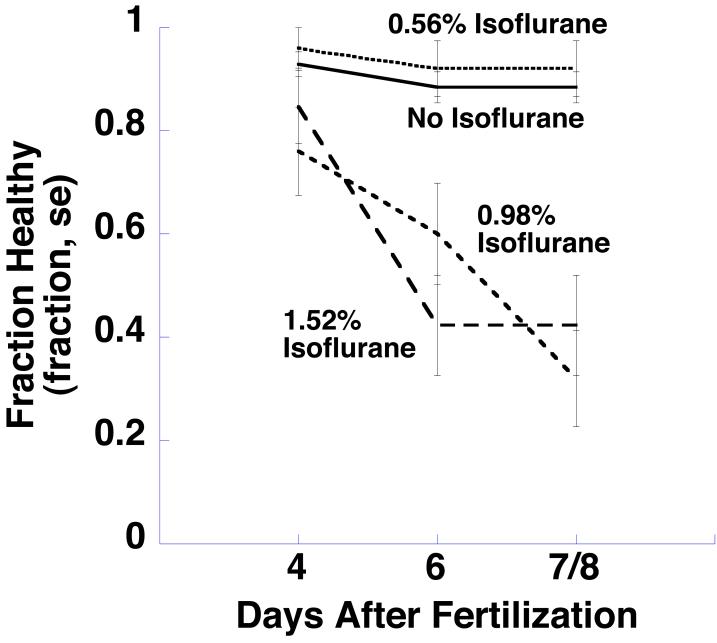
Fig 2 shows the fraction of tadpoles that were healthy (i.e., did not die and were not morphologically abnormal) at 4, 6 and 7 or 8 (denoted 7/8) days after fertilization as a function of their isoflurane exposure. At 4 days, there was no difference in survival among control animals or animals raised in any concentration of isoflurane (p > 0.05). At days 6 and 7/8 healthy survival was high and unchanged in the control and 0.56% isoflurane groups. However, animals exposed to 0.98% isoflurane and 1.52% isoflurane began to die or develop morphological abnormalities by day 6 (p < 0.005 for both groups). Table 2 lists the number of animals that died or developed morphological abnormalities in each treatment group at these times.
Fig 2.
Xenopus laevis tadpoles were raised from zygotes to one week old tadpoles in air, or in air with 0.59, 0.98, or 1.52 % atmospheres isoflurane. The fraction of tadpoles that were healthly (did not die and had no morphological abnormalities) on days 4, 6, and 7/8 is shown here (some animals were studied on day 7, and some on day 8; these results were pooled and are denoted 7/8). At 4 days, there was no difference in survival among control animals or animals raised in any concentration of isoflurane. Animals exposed to 0.98% isoflurane and 1.52% isoflurane began to die or develop morphological abnormalities by day 6.
Table 2.
Discussion
We used tadpoles to address the question of whether isoflurane could produce tolerance in a vertebrate. We chose to use tadpoles because week-long exposures to high concentrations of anesthetic are possible in this animal, they have many of the same neurotransmitters (e.g., γ-aminobutyric acid type A, glycine, glutamate, acetylcholine) and anatomic substrates (forebrain, hindbrain, spinal cord) as more complex vertebrates, and most importantly tadpoles have been used as a model organism to study mechanisms of anesthetic action (17, 19, 20).
The isoflurane concentrations we used were equivalent to 0.4 to 1 MAC in mice or rats. Keeping sufficient numbers of rats or mice healthy while exposed continuously to these concentrations of isoflurane for 7 days would have posed significant husbandry (and ethical) issues. For example, the animals would need to be fed and hydrated while anesthetized, and body temperature and airway patency would have to be monitored and maintained around the clock. These issues were not present in developing tadpoles. Although the isoflurane concentration we used reduced spontaneous movement in tadpoles, in their first week of development tadpoles derive nutrition from their yolk sac and have none of the aforementioned care requirements.
Our tadpoles were raised in an aqueous environment at room temperature (averaging 22°C in our laboratory). Because the potency of volatile anesthetics delivered in the gas phase increases as temperature decreases (21), the gas phase concentrations we report place a lower limit on the MAC fraction of isoflurane we applied. However, even without a correction for temperature, ours are the highest concentrations that have been used in any study of anesthetic tolerance in vertebrates. At the highest exposure, tadpoles were exposed to approximately three times their anesthetic EC50 during development.
Although we found statistically significant effects of prolonged treatment with isoflurane on the concentration of isoflurane required to prevent movement in Xenopus laevis tadpoles, this effect was small and varied from at most a 7.4% increase in EC50 (with exposure to 0.98% isoflurane for one week) to a 16.2% decrease in EC50 (with exposure to 1.52% isoflurane). This decrease is in the opposite direction expected for tolerance. Possibly, the presence of isoflurane throughout development led to compensation for the presence of isoflurane. Because the observed effects are small and there was no consistent direction to the change in EC50 over a range of isoflurane concentrations, these effects are probably of little biological significance. Given the small and inconsistent effects we observed at one week, it is doubtful that shorter exposures, which might correspond to the duration of surgical procedures would produce measurable effects. We conclude that tolerance does not develop in tadpoles with exposure to isoflurane. Our results are in accord with other published studies that fail to show tolerance to halogenated clinical anesthetics at lower concentrations or with shorter exposures.
At the two higher concentrations of isoflurane, some animals died in the course of the study. Might the loss of these animals have biased our results, by selecting for animals with isoflurane EC50s that promoted survival? Although we cannot exclude this, we think selection, if present, would have biased the data in favor of producing tolerance. We reasoned that animals that were particularly sensitive to isoflurane, with lower EC50s, should have succumbed to exposure to isoflurane, rather than ones that were more resistant to isoflurane and had higher EC50s. This would have enriched the remaining population in animals with higher EC50s. That there was no increase in EC50 at the highest concentration, and only small increases at lower concentrations, in the face of possible selection, strengthens our conclusion that tolerance did not develop.
We did not design our study to evaluate possible toxic effects of isoflurane during development; however, developing Xenopus laevis tadpoles are an established model for these effects. The Frog Embryo Teratogenesis Assay-Xenopus (FETAX) model, and modifications thereof (22), use procedures similar to ours. The FETAX test concludes on day 4, at which time the number of abnormalities and deaths are totaled and compared between treatment groups. We determined the cumulative number of abnormalities and deaths in each group by day 4 and asked whether there were differences between groups in this measure. Although at the two higher concentrations, some morphological abnormalities were present while none were present at the two lower concentrations, and there was a greater frequency of deaths in the control (no isoflurane) group, the total number of deaths and abnormalities combined did not differ between any of the isoflurane exposure groups and the control group over this span of time (P > 0.05). However, by day 6 and day 7/8, there were significantly more abnormalities and deaths in the two highest isoflurane exposure groups compared to control, but not in the lowest isoflurane exposure group. While this might suggest a toxic or teratogenic effect to high or prolonged exposures to isoflurane, the clinical relevance of this finding is questionable since surgical procedures during preganancy do not expose developing human fetuses to isoflurane over such a large fraction of their development. The finding of toxicity from isoflurane in developing Xenopus laevis tadpoles would be of concern if there were a short but crucial time when exposure to isoflurane is deleterious in humans, but this is a situation for which there is currently no evidence.
A variety of microorganisms respond to long term exposure to anesthetics by changing the composition of their membranes (23-25). This is presumably part of a general response to changing environmental conditions, which allows the organism to compensate for perturbations in membrane properties produced by chemicals in the environment. This response might be considered to produce “tolerance” in these organisms. We did not investigate membrane compositional changes in tadpoles reasoning that because vertebrates regulate their internal milieu, there would be no selection to maintain such a mechanism. The lack of tolerance of Xenopus laevis tadpoles to isoflurane in this study suggests either that we were correct in thinking that this mechanism does not occur in Xenopus laevis, or that the mechanism occurs but has little functional effect.
In summary, we provide the first description of week-long exposures of vertebrates to surgical anesthetic concentrations of isoflurane, and the first report of such exposures in developing vertebrates. We conclude that tolerance to isoflurane does not occur in developing Xenopus laevis tadpoles. Taken together with studies in other organisms, the development of tolerance to ethanol but not isoflurane indicates that molecular mechanisms shared with isoflurane probably cannot account for the development of tolerance to ethanol.
Acknowledgments
This work was supported in part by NIGMS R01 GM069379
References
- 1.Okamoto M, Hinman DJ, Aaronson LM. Comparison of ethanol and barbiturate physical dependence. J Pharmacol Exp Ther. 1981;218(3):701–8. [PubMed] [Google Scholar]
- 2.Chandler LJ, Harris RA, Crews FT. Ethanol tolerance and synaptic plasticity. Trends Pharmacol Sci. 1998;19(12):491–5. doi: 10.1016/s0165-6147(98)01268-1. [DOI] [PubMed] [Google Scholar]
- 3.Ramsay DS, Leroux BG, Rothen M, Prall CW, Fiset LO, Woods SC. Nitrous oxide analgesia in humans: acute and chronic tolerance. Pain. 2005;114(12):19–28. doi: 10.1016/j.pain.2004.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hutchinson MA, Smith PF, Darlington CL. The behavioural and neuronal effects of the chronic administration of benzodiazepine anxiolytic and hypnotic drugs. Prog Neurobiol. 1996;49(1):73–97. doi: 10.1016/0301-0082(96)00011-1. [DOI] [PubMed] [Google Scholar]
- 5.Ito T, Suzuki T, Wellman SE, Ho IK. Pharmacology of barbiturate tolerance/dependence: GABAA receptors and molecular aspects. Life Sci. 1996;59(3):169–95. doi: 10.1016/0024-3205(96)00199-3. [DOI] [PubMed] [Google Scholar]
- 6.Mihic SJ, Ye Q, Wick MJ, Koltchine VV, Krasowski MD, Finn SE, Mascia MP, Valenzuela CF, Hanson KK, Greenblatt EP, Harris RA, Harrison NL. Sites of alcohol and volatile anaesthetic action on GABA(A) and glycine receptors. Nature. 1997;389(6649):385–9. doi: 10.1038/38738. [DOI] [PubMed] [Google Scholar]
- 7.Rudolph U, Crestani F, Benke D, Brunig I, Benson JA, Fritschy JM, Martin JR, Bluethmann H, Mohler H. Benzodiazepine actions mediated by specific gamma-aminobutyric acid(A) receptor subtypes. Nature. 1999;401(6755):796–800. doi: 10.1038/44579. [DOI] [PubMed] [Google Scholar]
- 8.Yamakura T, Harris RA. Effects of gaseous anesthetics nitrous oxide and xenon on ligand-gated ion channels. Comparison with isoflurane and ethanol. Anesthesiology. 2000;93(4):1095–101. doi: 10.1097/00000542-200010000-00034. [DOI] [PubMed] [Google Scholar]
- 9.Ogata J, Shiraishi M, Namba T, Smothers CT, Woodward JJ, Harris RA. Effects of anesthetics on mutant N-methyl-D-aspartate receptors expressed in Xenopus oocytes. J Pharmacol Exp Ther. 2006;318(1):434–43. doi: 10.1124/jpet.106.101691. [DOI] [PubMed] [Google Scholar]
- 10.Fang Z, Ionescu P, Chortkoff BS, Kandel L, Sonner J, Laster MJ, Eger EI., 2nd Anesthetic potencies of n-alkanols: results of additivity and solubility studies suggest a mechanism of action similar to that for conventional inhaled anesthetics. Anesth Analg. 1997;84(5):1042–8. doi: 10.1097/00000539-199705000-00017. [DOI] [PubMed] [Google Scholar]
- 11.Roberts MT, Phelan R, Erlichman BS, Pillai RN, Ma L, Lopreato GF, et al. Occupancy of a single anesthetic binding pocket is sufficient to enhance glycine receptor function. J Biol Chem. 2006;281(6):3305–11. doi: 10.1074/jbc.M502000200. [DOI] [PubMed] [Google Scholar]
- 12.Lutz LJ, Milde JH, Milde LN. The cerebral functional, metabolic, and hemodynamic effects of desflurane in dogs. Anesthesiology. 1990;73(1):125–31. doi: 10.1097/00000542-199007000-00018. [DOI] [PubMed] [Google Scholar]
- 13.Rampil IJ, Laster M, Dwyer RC, Taheri S, Eger EI., 2nd No EEG evidence of acute tolerance to desflurane in swine. Anesthesiology. 1991;74(5):889–92. doi: 10.1097/00000542-199105000-00014. [DOI] [PubMed] [Google Scholar]
- 14.Eger EI., 2nd . MAC, Anesthetic Uptake and Action. Williams and Wilkins; Balitmore: 1974. [Google Scholar]
- 15.Brunton L, Lazo J, Parker K. Goodman & Gilman's The Pharmacological Basis of Therapeutics. McGraw-Hll Professional; 2005. [Google Scholar]
- 16.Sive H, Grainger R, Harland R. Early Development of Xenopus laevis. Cold Spring Harbor Press; Cold Spring Harbor, New York: 2000. [Google Scholar]
- 17.Mohr JT, Gribble GW, Lin SS, Eckenhoff RG, Cantor RS. Anesthetic potency of two novel synthetic polyhydric alkanols longer than the n-alkanol cutoff: evidence for a bilayer-mediated mechanism of anesthesia? J Med Chem. 2005;48(12):4172–6. doi: 10.1021/jm049459k. [DOI] [PubMed] [Google Scholar]
- 18.Sonner JM. Issues in the design and interpretation of minimum alveolar anesthetic concentration (MAC) studies. Anesth Analg. 2002;95(3):609–14. doi: 10.1097/00000539-200209000-00021. [DOI] [PubMed] [Google Scholar]
- 19.Alifimoff JK, Firestone LL, Miller KW. Anesthetic potencies of secondary alcohol enantiomers. Anesthesiology. 1987;66(1):55–9. doi: 10.1097/00000542-198701000-00010. [DOI] [PubMed] [Google Scholar]
- 20.Halsey MJ, Wardley-Smith B. Pressure reversal of narocsis produced by anaesthetics, narcotics and tranquillisers. Nature. 1975;257(5529):811–3. doi: 10.1038/257811a0. [DOI] [PubMed] [Google Scholar]
- 21.Franks NP, Lieb WR. Temperature dependence of the potency of volatile general anesthetics: implications for in vitro experiments. Anesthesiology. 1996;84(3):716–20. doi: 10.1097/00000542-199603000-00027. [DOI] [PubMed] [Google Scholar]
- 22.Fort DJ, Rogers RL, Thomas JH, Buzzard BO, Noll AM, Spaulding CD. Comparative sensitivity of Xenopus tropicalis and Xenopus laevis as test species for the FETAX model. J Appl Toxicol. 2004;24(6):443–57. doi: 10.1002/jat.997. [DOI] [PubMed] [Google Scholar]
- 23.Nandini-Kishore SG, Mattox SM, Martin CE, Thompson GA., Jr Membrane changes during growth of Tetrahymena in the presence of ethanol. Biochim Biophys Acta. 1979;551(2):315–27. doi: 10.1016/0005-2736(89)90009-6. [DOI] [PubMed] [Google Scholar]
- 24.Koblin DD, Wang HH. Chronic exposure to inhaled anesthetics increases cholesterol content in Acholeplasma laidlawii. Biochim Biophys Acta. 1981;649(3):717–25. doi: 10.1016/0005-2736(81)90176-0. [DOI] [PubMed] [Google Scholar]
- 25.Ingram LO. Adaptation of membrane lipids to alcohols. J Bacteriol. 1976;125(2):670–8. doi: 10.1128/jb.125.2.670-678.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]