Abstract
An evolutionary narrative explaining why organisms respond to inhaled anesthetics is proposed. It is conjectured that organisms today respond to inhaled anesthetics because their ion channels are sensitive to inhaled anesthetics by virtue of common descent from ancestral, anesthetic-sensitive ion channels in one-celled organisms (i.e., that the response to anesthetics did not arise as an adaptation of the nervous system, but rather of ion channels that preceded the origin of multicellularity). This sensitivity may have been refined by ongoing selection at synapses in multicellular organisms.
In particular, it is hypothesized that (1) the beneficial trait that was selected for in one-celled organisms was the coordinated response of ion channels to compounds that were present in the environment which influenced the conformational equilibrium of ion channels (2) that this coordinated response prevented the deleterious consequences of entry of positive charges into the cell, thereby increasing the fitness of the organism (3) that these compounds (which may have included organic anions, cations, and zwitterions as well as uncharged compounds) mimicked inhaled anesthetics in that they were interfacially active, and modulated ion channel function by altering bilayer properties coupled to channel function.
The proposed hypothesis is consistent with known properties of inhaled anesthetics. In addition, it leads to testable experimental predictions of nonvolatile compounds having anesthetic-like modulatory effects on ion channels and in animals, including that of endogenous compounds that may modulate ion channel function in health and disease. The latter included metabolites which are elevated in some types of end stage organ failure, and genetic metabolic diseases. Several of these predictions have been tested and proved to be correct.
Implications
A hypothesis for the origin and evolution of the response to inhaled anesthetics is offered, and experimental predictions of this theory are made.
Keywords: Anesthesia, Evolution, Natural Selection, Ion Channel
Introduction
“Nothing in biology makes sense, except in the light of evolution” (1), yet why organisms respond to inhaled anesthetics has rarely been considered. Two challenges confront any evolutionary narrative concerning responses to anesthetics. First, evolutionary narratives describe historical events that cannot be observed. Therefore, such narratives have value only if they generate testable hypotheses leading to new observations, beyond those used to develop the narrative. Second, processes that produce anesthesia in nature should be maladaptive (how does the anesthetized organism protect itself?), but selection favors beneficial traits. An evolutionary theory must identify these beneficial traits.
Several observations indicate that the response to inhaled anesthetics results from powerful natural selection and is ancient in origin.
Small Population Variability in Anesthetic Responses Implies Natural Selection
The variability in response to inhaled anesthetics is small as reflected in the steepness of anesthetic concentration-response curves (large Hill numbers) and small standard deviations in minimum alveolar concentration (MAC) (2) and other anesthetic phenotypes in diverse species (3, 4). This differs from the larger pharmacogenetic variability to many drugs (5-8). Compared to other drugs, it is also unusual that diverse molecules - ethers, alkanes, alcohols, ketones, cyclic and aromatic compounds, and noble gases (9, 10), compounds varying in physical properties and by an order of magnitude in size, all can cause anesthesia.
Phenotypic variability is determined by a balance between forces which increase variability, and forces which decrease variability. For inhaled anesthetics, this reduces to a balance between mutation, which increases variability, and natural selection, which decreases variability (11). [Other processes like genetic drift (random fluctuations in allele frequencies from generation to generation) and founder effects can affect population variability, but not in large populations or over long spans of time.] Accordingly, the small variability in anesthetic phenotypes results from natural selection. Selection could occur either directly as a response to anesthetic-like compounds, or indirectly as a byproduct of selection for another, related trait. Selection implies that either response increases fitness, adapts organisms to their environment, and confers a survival or reproductive advantage.
Ion Channels Respond to Anesthetics as a Result of Natural Selection
To a reasonable approximation, inhaled anesthetics enhance inhibitory ion channel function and/or block excitatory channel function (12-14) to produce net depression of the central nervous system. Inhibitory channels include γ-amino butyric acid type A (GABAA) (15) and glycine receptors (16) and several two-pore domain K channels (17); excitatory channels include neuronal nicotinic acetylcholine (nACh) (18) and N-methyl-D-aspartate (NMDA) receptors (19) and sodium channels (20). Minor exceptions, such as serotonin 5HT3 receptors (21), and the ρ1 subtype of GABAA receptors (22) expressed in the retina (23), are not thought to be important to anesthesia.
This observation is key because nonrandom patterns in biological processes are a hallmark of natural selection. Anesthetics act on ion channels to reduce or prevent membrane depolarization, suggesting that ion channels may be the target on which natural selection has acted, thereby rendering organisms sensitive to inhaled anesthetics.
The Response to Anesthetics Probably Arose in One-Celled Organisms
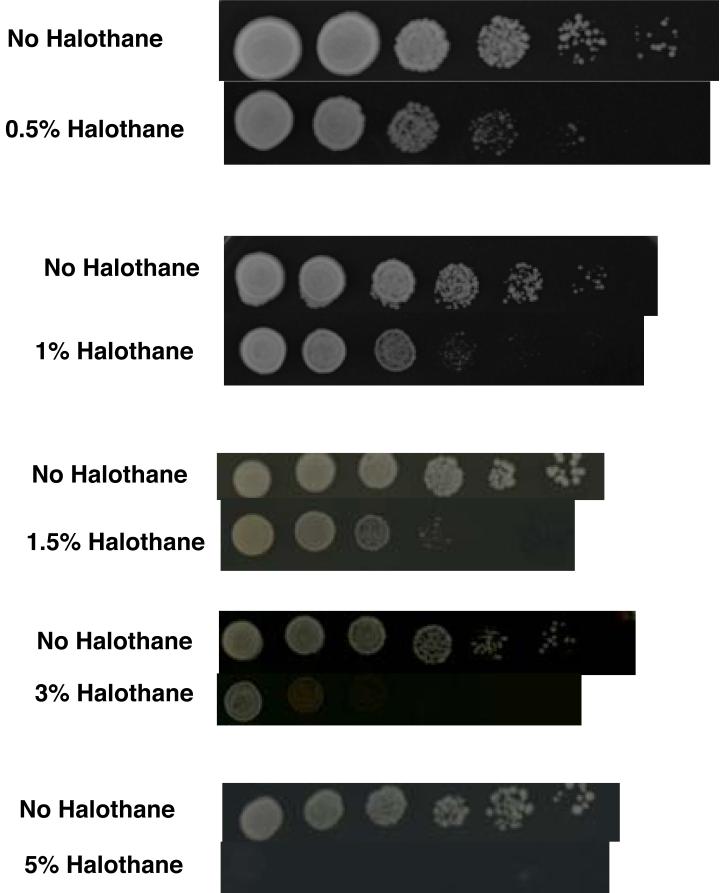
Inhaled anesthetics reversibly suppress evoked or spontaneous motor responses in vertebrates (24), invertebrates (24), tactile plants (25), and ciliated protists (26). Assuming common descent, the response to inhaled anesthetics arose in one-celled organisms. The one-celled organisms A. laidlawii (27, 28), E. coli (29) and tetrahymena (30, 31) alter the composition of their cell membranes when exposed to volatile anesthetics, suggesting the biochemical response to inhaled anesthetics originated in a prokaryote ancestral to these bacteria and protist. Fig 1 shows that the in spite of the distant common ancestry between humans and the one-celled eukaryote S. cerevisiae (baker's yeast), this organism is affected by halothane in clinical concentrations.
Fig 1.
Yeast are inhibited by halothane in clinical concentrations. The results of yeast spot tests on agar plates are shown. This is a semi-quantitative measure of growth. Wild type homozygous diploid strain BY4743 was studied. Each column represents a five-fold dilutions in concentration from the previous column. Halothane concentrations from 0.5% to 5% were investigated. A concurrent control without halothane is shown for each concentration of halothane. There is a concentration-dependent inhibition of growth, with inhibition at clinical concentrations. Yeast were grown at 26°C in a gas tight chamber through which halothane flowed continuously. Halothane was delivered via a calibrated vaporizer.
Origin of the anesthetic state: one-celled organisms
Why might such organisms or their ion channels respond to volatile anesthetics? Table 1 provides a clue: cationic, anionic, and zwitterionic surfactants (detergents) (32) act like volatile anesthetics on such channels, as do amino acids (33), ketoacids (34), organic acids (unpublished data, Y. Weng, PhD and J. Zhao, MD), and ammonia (35). Like volatile anesthetics (36, 37), surfactants (38) and amino acids (39, 40) are interfacially active (i.e., accumulate at the boundary between immiscible condensed phases, such as the bilayer/water interface, where they alter interfacial properties). Such diverse interfacially active compounds, which are plausibly present in the environment, may exert a selective pressure on microorganisms bearing homologs of anesthetic-sensitive channels (41-43). But why selection for responding to these compounds? Here it is proposed that natural selection solved a chemical problem faced by one-celled organisms in a manner that led to sensitivity to these compounds, and consequently to volatile anesthetics.
Table 1.
Volatile anesthetics, alcohols and nonvolatile surfactants show similar modulation of three anesthetic-sensitive receptors.
| Effect on currents through: | ||||
|---|---|---|---|---|
| Compound | Type | GABAAR | GlycineR | NMDAR |
| Isoflurane | anesthetic | I | I | D |
| Halothane | anesthetic | I | I | D |
| Ethanol | anesthetic alcohol |
I | I | D |
| Octanol | Surfactant alcohol |
I | I | D |
| Dodecanol | Surfactant alcohol |
I | I | D |
| SOS | Anionic surfactant |
I | I | D |
| OTABR | Cationic surfactant |
I | I | D |
| SDS | Anionic surfactant |
I | D | D |
| DAPS | Zwitterionic surfactant |
I | I | I |
| DTACL | Cationic surfactant |
I | I | D |
SDS = sodium dodecyl sulfate
DAPS = 3-(dodecyldimethylammonio)propanesulfonate
DTACL = (1dodecyl)trimethylammonium chloride
SOS = sodium n-octyl sulfate
OTABR = (1-octyl)trimethylammonium bromide
GABAAR = γ-amino butyric acid type A receptor
GlycineR = Glycine receptor
NMDAR = N-methyl-D-aspartate receptor
D indicates a decrease inhibition) in currents through the receptor.
I indicates an increase (enhancement) in currents through the receptor
Data for surfactants are from reference (32). Results for isoflurane, halothane, and ethanol are from references (13, 52, 72, 73)
Microorganisms change bilayer composition when exposed to detergents or volatile anesthetics (27-31), suggesting that microorganisms sense and respond to these compounds. Since composition determines many bilayer physical properties (44), changing bilayer composition could limit the effect of these compounds on bilayer properties. Because membrane proteins undergo the conformational changes that underlie their function within the bilayer [which affects protein function (45)], sensing and maintaining bilayer properties would assure optimal functioning of membrane proteins.
Successful microorganisms may have benefitted from two adaptations to changes in bilayer properties produced by interfacially active compounds encountered in the environment. On a short (seconds, or less) time scale, favored organisms would have ion channels that prevented the deleterious effects of entry of positive charges into the cell (e.g., depolarization leading to spurious motile responses, short circuiting of electrochemical potentials), by shifting their conformational equilibria to enhance inhibitory channel function or inhibit excitatory channel function or both - as inhaled anesthetics do today. On a longer time scale (minutes), membrane composition changes that restored bilayer properties to normal might provide the definitive adaptation.
Selection for anesthetic responses in multicellular organisms
Why do multicellular animals respond to inhaled anesthetics?
Common descent from anesthetic-sensitive channels originating in one-celled organisms and used in the construction of early nervous systems by ancestral multicellular organisms may provide part of the explanation. In addition, it has been proposed that ion channels at synapses may confront a situation similar to that of membrane proteins of microorganisms faced with a changing chemical environment (46). The synaptic architecture of the nervous system demands intermittent, high concentrations of neurotransmitters in the synapse. At synapses, it has been hypothesized that in addition to rapid binding to receptors, there is a second, slower, membrane-mediated effect of neurotransmitters to which particular receptors must be adapted. This hypothesis accurately models many characteristics of deactivation and desensitization for both excitatory and inhibitory channels (46). Like their peers in single-celled organisms, neuronal ligand-gated ion channels may be adapted to changes in their chemical environment, in this case produced by neurotransmitters on bilayer properties. A byproduct of this process is a selective pressure to respond to anesthetics.
Properties of anesthetics
These ideas are consistent with known properties of inhaled anesthetics:
Additivity
Bilayer-mediated theories of anesthesia predict anesthetic additivity (47) where one anesthetic molecule simply replaces another in the bilayer, at concentrations far below binding saturation.
Sensitivity of non-neural proteins to anesthetics
Membrane proteins important to fitness (not just channels) should be sensitive to inhaled anesthetics. Supporting this hypothesis, inhaled anesthetics affect energy production, including photosynthesis (48), electron transport (49) and the function of bacteriorhodopsin (50).
Sensitivity of ligand-gated ion channels to anesthetics
Inhaled anesthetics more readily depress synaptic transmission than axonal conduction (51), perhaps because receptors at synapses operate in a milieu of fluctuating concentrations of neurotransmitters providing a selective pressure for anesthesia (46) which is absent for voltage gated channels that are not at synapses.
Location of mutations
Receptor mutations affecting anesthetic sensitivity lie in transmembrane domains where they can interact with the bilayer (52).
Forward genetics
Many ion channels should be affected by the bilayer mediated actions of inhaled anesthetics, perhaps explaining why modulators affecting the function of multiple channels are found in forward genetic screens (53).
Predictions
Theories are made to fit existing data, but their value lies in the new predictions they make. What new predictions do these ideas make, which can be compared to predictions made by other theories?
Metabolic diseases that cause “anesthesia”
Ion channels are exposed to high concentrations of nonvolatile metabolites (e.g., ammonium ions, amino acids, and ketoacids) which can cross the blood/brain barrier, in several diseases which produce “anesthesia” [e.g., coma in liver failure (54, 55), kidney failure (56), diabetic ketoacidosis (57)]. Clearance of these metabolites by transplantation, dialysis, or insulin administration reverses the anesthesia.
Anesthesia from metabolites puts organisms at risk and should be selected against. That organisms respond to endogenous compounds in a deleterious manner is evidence of positive selection for some other beneficial trait, such as the adaptation to neurotransmitters discussed above. Sickle cell trait provides a parallel example. This is a harmful blood disorder selected for because it protects against the malaria parasite.
Neurotransmitter corelease
Two neurotransmitters are coreleased from the same vesicle at various synapses (58, 59). If neurotransmitters modulate bilayer properties which influence channel function, organisms may use corelease of a second neurotransmitter to modulate the function of the native neurotransmitter. Consistent with this prediction, GABA potentiates glycine receptor function, acetylcholine inhibits NMDA receptor function (35), and a glycine receptor mutation (α1 S267I) that decreases sensitivity to alcohols and volatile anesthetics (52), also decreases sensitivity to GABA (33).
Ion channels in one-celled organisms
which are related to mammalian anesthetic-sensitive channels are predicted to be sensitive to inhaled anesthetics.
What About Volatile Anesthetics as Ligands?
A current view holds that volatile anesthetics bind to sites on proteins. Natural selection, however, cannot act on random processes. Theories that posit random binding sites must make the assumption that, unlike other traits, natural selection does not shape the response of organisms to anesthetics. In theories where the postulated sites do not occur at random, several questions about selection for these sites deserve attention: What is the selective pressure for the binding sites? Are there endogenous ligands for these sites (other than water, which nonspecifically and ubiquitously solvates surfaces of proteins in contact with water)? Why are there no conserved binding sites among diverse anesthetic-sensitive proteins? Whether binding sites are postulated to occur at random or via natural selection, the following questions arise: Why are proteins from the same family affected differently by inhaled agents? For example, nACh receptor currents are decreased (18) while GABAA receptors are increased (15) by volatile anesthetics. Because these are homologous receptors from the same family, having similar protein folds and pockets, if anesthetic binding to folds or pockets is important, then anesthetics might be expected to have similar--not opposite—effects on these channels. Most importantly, what new anesthetic-like compounds do these theories predict, based on knowledge of these binding sites?
The difficulty of reconciling the need for conserved binding sites with the absence of these sites on diverse ion channels suggests a reexamination of evidence for binding models may be in order. The following analysis of four lines of evidence cited to support these models is offered as an example.
The weak stereoselective actions of inhaled anesthetics in animals (60, 61) and on ion channels (62, 63) is said to indicate inhaled anesthetics bind to proteins. However, this finding is as consistent with a bilayer as with a protein site of action, because cholesterol and phospholipids are chiral. A single study claimed to show otherwise, by demonstrating that isoflurane's enantiomers did not act stereoselectively with artificial bilayers (64), but this study was flawed. It used an unvalidated method for measuring stereoselectivity. No control, in this or other studies, demonstrated that their method could detect chiral interactions of isoflurane with any molecule. And, the investigation assumed that equal concentrations of enantiomers in a bilayer would have equal functional effects. This assumption is incorrect, as a counter example proves: equal concentrations of cholesterol enantiomers have different effects on bilayer properties (65). No control with isoflurane's enantiomers has been performed.
High resolution X-ray diffraction crystal structures have been obtained for anesthetics bound to soluble proteins (e.g., albumin, firefly luciferase) which are not related to any anesthetic-sensitive ion channel and are not themselves involved in the neural effects of anesthetics (66). These soluble proteins are used as models for anesthetic action because atomic-scale structures are known for them. However, a recent search for common binding motifs in several of these structures found merely “polar and nonpolar interactions within an amphiphilic binding cavity” (66). These general physical characteristics of anesthetics have long been known from other measurements, such as partition coefficients (47). In contrast to X-ray crystal structures of drug binding to other proteins (67), it would appear that X-ray crystal structures of anesthetic binding to these soluble proteins do not provide new physical insights or predict new anesthetics. This calls into question the hypothesis that these proteins can model the anesthetic site of action.
Anesthetics competitively inhibit the enzyme firefly luciferase (68). A recent investigation shows why the X-ray crystal structure of this enzyme (69) does not provide the anticipated atomic-scale general understanding of anesthetic binding described above: anesthetics affect the global motion of the protein in a manner that reduces enzyme function, giving the appearance of competitive inhibition (70).
Amino acids in a receptor's putative anesthetic binding site have been chemically modified to resemble covalent linkage of anesthetic to the amino acid (71). Permanent anesthetic-like modulation of receptor function was reported. This was attributed to permanent occupancy of a specific anesthetic binding site. However, to interpret these results as specific binding, a control ruling out nonspecific effects is essential. If linking a compound that is not an anesthetic to this site showed anesthetic-like modulation of channel function, then no conclusion about anesthetic binding would be possible. The results would only show that changes in channel structure affect channel function. This control has never been done.
Conclusions
A narrative for the origin and evolution of the response to inhaled anesthetics is proposed. It is hypothesized that the capacity to respond to inhaled anesthetics arose in one-celled organisms, as an adaptation of ion channels to changing environmental conditions which perturbed bilayer properties coupled to channel function. Several hypotheses flowing from this model have led to predictions and to experiments supporting the model. In particular, nonvolatile compounds having anesthetic-like modulatory effects on ion channels and in animals, including that of endogenous compounds that may modulate ion channel function in health and disease, have been identified.
Acknowledgements
The author is indebted to Robert Cantor for discussions on anesthetic mechanisms and evolution extending over many years. The author thanks Howard Nash for his initial review of this manuscript, and Edmond I Eger for his tireless advice and suggestions. The author is grateful to Martha Cyert and the members of her lab for providing a welcoming and open-minded environment for investigating anesthetic effects on yeast.
This work was supported in part by NIGMS R01 GM069379
Footnotes
Reprints will not be available
Presented in part at the International Society of Anesthetic Pharmacology meeting in Atlanta, GA (October 2005)
References
- 1.Mayr E. What evolution is. Basic Books; New York: 2001. [Google Scholar]
- 2.Sonner JM. Issues in the design and interpretation of minimum alveolar anesthetic concentration (MAC) studies. Anesth Analg. 2002;95(3):609–14. doi: 10.1097/00000539-200209000-00021. [DOI] [PubMed] [Google Scholar]
- 3.Sonner JM, Gong D, Eger EI., 2nd Naturally occurring variability in anesthetic potency among inbred mouse strains. Anesth Analg. 2000;91(3):720–6. doi: 10.1097/00000539-200009000-00042. [DOI] [PubMed] [Google Scholar]
- 4.Takasaki M, Tatara T, Suezaki Y, Shirahama K, Kamaya H, Ueda I, Totoki T. Effect of inhalation anesthetics on swimming activity of artemia salina. J Anesth. 1991;5:287–93. doi: 10.1007/s0054010050287. [DOI] [PubMed] [Google Scholar]
- 5.Leabman MK, Giacomini KM. Estimating the contribution of genes and environment to variation in renal drug clearance. Pharmacogenetics. 2003;13(9):581–4. doi: 10.1097/00008571-200309000-00007. [DOI] [PubMed] [Google Scholar]
- 6.Holleman A, Cheok MH, den Boer ML, Yang W, Veerman AJ, Kazemier KM, Pei D, Cheng C, Pui CH, Relling MV, Janka-Schaub GE, Pieters R, Evans WE. Gene-expression patterns in drug-resistant acute lymphoblastic leukemia cells and response to treatment. N Engl J Med. 2004;351:533–42. doi: 10.1056/NEJMoa033513. [DOI] [PubMed] [Google Scholar]
- 7.Kampf-Sherf O, Zlotogorski Z, Gilboa A, Speedie L, Lereya J, Rosca P, Shavit Y. Neuropsychological functioning in major depression and responsiveness to selective serotonin reuptake inhibitors antidepressants. J Affect Disord. 2004;82:453–9. doi: 10.1016/j.jad.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 8.Brewster LM, van Montfrans GA, Kleijnen J. Systematic review: antihypertensive drug therapy in black patients. Ann Intern Med. 2004;141(8):614–27. doi: 10.7326/0003-4819-141-8-200410190-00009. [DOI] [PubMed] [Google Scholar]
- 9.Sewell JC, Sear JW. Derivation of preliminary three-dimensional pharmacophores for nonhalogenated volatile anesthetics. Anesth Analg. 2004;99(3):744–51. doi: 10.1213/01.ANE.0000129978.92936.A2. [DOI] [PubMed] [Google Scholar]
- 10.Sewell JC, Sear JW. Determinants of volatile general anesthetic potency: a preliminary three-dimensional pharmacophore for halogenated anesthetics. Anesth Analg. 2006;102(3):764–71. doi: 10.1213/01.ane.0000195421.46107.d0. [DOI] [PubMed] [Google Scholar]
- 11.Lynch M, Walsh B. Genetics and analysis of quantitative traits. Sinauer Associates, Inc.; Sunderland, MA: 1998. [Google Scholar]
- 12.Miller KW. The nature of sites of general anaesthetic action. Br J Anaesth. 2002;89(1):17–31. doi: 10.1093/bja/aef167. [DOI] [PubMed] [Google Scholar]
- 13.Krasowski MD, Harrison NL. General anaesthetic actions on ligand-gated ion channels. Cell Mol Life Sci. 1999;55(10):1278–303. doi: 10.1007/s000180050371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dilger JP. The effects of general anaesthetics on ligand-gated ion channels. Br J Anaesth. 2002;89(1):41–51. doi: 10.1093/bja/aef161. [DOI] [PubMed] [Google Scholar]
- 15.Franks NP, Lieb WR. Molecular and cellular mechanisms of general anaesthesia. Nature. 1994;367(6464):607–14. doi: 10.1038/367607a0. [DOI] [PubMed] [Google Scholar]
- 16.Harrison NL, Kugler JL, Jones MV, Greenblatt EP, Pritchett DB. Positive modulation of human gamma-aminobutyric acid type A and glycine receptors by the inhalation anesthetic isoflurane. Mol Pharmacol. 1993;44(3):628–32. [PubMed] [Google Scholar]
- 17.Franks NP, Honore E. The TREK K2P channels and their role in general anaesthesia and neuroprotection. Trends Pharmacol Sci. 2004;25(11):601–8. doi: 10.1016/j.tips.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 18.Flood P, Role LW. Neuronal nicotinic acetylcholine receptor modulation by general anesthetics. Toxicol Lett. 1998;100-101:149–53. doi: 10.1016/s0378-4274(98)00179-9. [DOI] [PubMed] [Google Scholar]
- 19.Raines DE, Gioia F, Claycomb RJ, Stevens RJ. The N-methyl-D-aspartate receptor inhibitory potencies of aromatic inhaled drugs of abuse: evidence for modulation by cation-pi interactions. J Pharmacol Exp Ther. 2004;311(1):14–21. doi: 10.1124/jpet.104.069930. [DOI] [PubMed] [Google Scholar]
- 20.Shiraishi M, Harris RA. Effects of alcohols and anesthetics on recombinant voltage-gated Na+ channels. J Pharmacol Exp Ther. 2004;309(3):987–94. doi: 10.1124/jpet.103.064063. [DOI] [PubMed] [Google Scholar]
- 21.Harris RA, Mihic SJ, Dildy-Mayfield JE, Machu TK. Actions of anesthetics on ligand-gated ion channels: role of receptor subunit composition. Faseb J. 1995;9(14):1454–62. doi: 10.1096/fasebj.9.14.7589987. [DOI] [PubMed] [Google Scholar]
- 22.Mihic SJ, Harris RA. Inhibition of rho1 receptor GABAergic currents by alcohols and volatile anesthetics. J Pharmacol Exp Ther. 1996;277(1):411–6. [PubMed] [Google Scholar]
- 23.Cutting GR, Lu L, O'Hara BF, Kasch LM, Montrose-Rafizadeh C, Donovan DM, Shimada S, Antonarakis, SE, Guggino WB, Uhl GR. Cloning of the gamma-aminobutyric acid (GABA) rho 1 cDNA: a GABA receptor subunit highly expressed in the retina. Proc Natl Acad Sci U S A. 1991;88:2673–7. doi: 10.1073/pnas.88.7.2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oliver AE, Deamer DW. Sensitivity to anesthesia by pregnanolone appears late in evolution. In: Rubin E, Miller KW, Roth SH, editors. Molecular and Cellular Mechanisms of Alcohol and Anesthetics. Annals of the New York Academy of Sciences; New York: 1991. pp. 561–65. [DOI] [PubMed] [Google Scholar]
- 25.Milne A, Beamish T. Inhalational and local anesthetics reduce tactile and thermal responses in mimosa pudica. Can J Anaesth. 1999;46(3):287–9. doi: 10.1007/BF03012612. [DOI] [PubMed] [Google Scholar]
- 26.Nunn JF, Sturrock JE, Wills EJ, Richmond JE, McPherson CK. The effect of inhalational anaesthetics on the swimming velocity of Tetrahymena pyriformis. J Cell Sci. 1974;15(3):537–54. doi: 10.1242/jcs.15.3.537. [DOI] [PubMed] [Google Scholar]
- 27.Wieslander A, Rilfors L, Lindblom G. Metabolic changes of membrane lipid composition in Acholeplasma laidlawii by hydrocarbons, alcohols, and detergents: arguments for effects on lipid packing. Biochemistry. 1986;25(23):7511–7. doi: 10.1021/bi00371a038. [DOI] [PubMed] [Google Scholar]
- 28.Koblin DD, Wang HH. Chronic exposure to inhaled anesthetics increases cholesterol content in Acholeplasma laidlawii. Biochim Biophys Acta. 1981;649(3):717–25. doi: 10.1016/0005-2736(81)90176-0. [DOI] [PubMed] [Google Scholar]
- 29.Ingram LO. Adaptation of membrane lipids to alcohols. J Bacteriol. 1976;125(2):670–8. doi: 10.1128/jb.125.2.670-678.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nandini-Kishore SG, Mattox SM, Martin CE, Thompson GA., Jr Membrane changes during growth of Tetrahymena in the presence of ethanol. Biochim Biophys Acta. 1979;551(2):315–27. doi: 10.1016/0005-2736(89)90009-6. [DOI] [PubMed] [Google Scholar]
- 31.Nandini-Kishore SG, Kitajima Y, Thompson GA., Jr Membrane fluidizing effects of the general anesthetic methoxyflurane elicit an acclimation response in Tetrahymena. Biochim Biophys Acta. 1977;471(1):157–61. doi: 10.1016/0005-2736(77)90403-5. [DOI] [PubMed] [Google Scholar]
- 32.Yang L, Sonner JM. Anesthetic-like modulation of receptor function by surfactants: A test of the interfacial theory of anesthesia. Submitted Anesth Analg MS #07-1248. [DOI] [PMC free article] [PubMed]
- 33.Milutinovic PS, Yang L, Cantor RS, Eger EI, 2nd, Sonner JM. Anesthetic-like modulation of a gamma-aminobutyric acid type A, strychnine-sensitive glycine, and N-methyl-d-aspartate receptors by coreleased neurotransmitters. Anesth Analg. 2007;105(2):386–92. doi: 10.1213/01.ane.0000267258.17197.7d. [DOI] [PubMed] [Google Scholar]
- 34.Yang L, Zhao J, Milutinovic PS, Brosnan RJ, Eger EI, 2nd, Sonner JM. Anesthetic properties of the ketone bodies beta-hydroxybutyric acid and acetone. Anesth Analg. 2007;105(3):673–9. doi: 10.1213/01.ane.0000278127.68312.dc. [DOI] [PubMed] [Google Scholar]
- 35.Brosnan RJ, Yang L, Milutinovic PS, Zhao J, Laster MJ, Eger EI, 2nd, Sonner JM. Ammonia has anesthetic properties. Anesth Analg. 2007;104:1430–3. doi: 10.1213/01.ane.0000264072.97705.0f. [DOI] [PubMed] [Google Scholar]
- 36.Chipot C, Wilson MA, Pohorille A. Interactions of anesthetics with the water-hexane interface. A molecular dynamics study. J Phys Chem B Condens Matter Mater Surf Interfaces Biophys. 1997;101(5):782–91. doi: 10.1021/jp961513o. [DOI] [PubMed] [Google Scholar]
- 37.Koubi L, Tarek M, Klein ML, Scharf D. Distribution of halothane in a dipalmitoylphosphatidylcholine bilayer from molecular dynamics calculations. Biophys J. 2000;78(2):800–11. doi: 10.1016/S0006-3495(00)76637-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rosen MJ. Surfactants and Interfacial Phenomena. Third ed Wiley-Interscience; 2004. [Google Scholar]
- 39.Chipot C, Pohorille A. Conformational equilibria of terminally blocked single amino acids at the water-hexane interface. A molecular dynamics study. J Phys Chem B Condens Matter Mater Surf Interfaces Biophys. 1998;102(1):281–90. doi: 10.1021/jp970938n. [DOI] [PubMed] [Google Scholar]
- 40.Pico C, Pons A, Palou A. In vitro adsorption of amino acids onto isolated rat erythrocyte membranes. Int J Biochem Cell Biol. 1995;27(8):761–5. doi: 10.1016/1357-2725(95)00049-u. [DOI] [PubMed] [Google Scholar]
- 41.Ouyang W, Jih TY, Zhang TT, Correa AM, Hemmings HC., Jr Isoflurane Inhibits NaChBac, a Prokaryotic Voltage-Gated Sodium Channel. J Pharmacol Exp Ther. 2007;322(3):1076–83. doi: 10.1124/jpet.107.122929. [DOI] [PubMed] [Google Scholar]
- 42.Gray AT, Winegar BD, Leonoudakis DJ, Forsayeth JR, Yost CS. TOK1 is a volatile anesthetic stimulated K+ channel. Anesthesiology. 1998;88(4):1076–84. doi: 10.1097/00000542-199804000-00029. [DOI] [PubMed] [Google Scholar]
- 43.Bocquet N, Prado de Carvalho L, Cartaud J, Neyton J, Le Poupon C, Taly A, Grutter T, Changeux JP, Corringer PJ. A prokaryotic proton-gated ion channel from the nicotinic acetylcholine receptor family. Nature. 2007;445:116–9. doi: 10.1038/nature05371. [DOI] [PubMed] [Google Scholar]
- 44.Gruner S, Shyamsunder E. Is the mechanism of general anesthesia related to lipid membrane spontaneous curvature? Annals of the New York Academy of Sciences. 1991;625(1):685–697. doi: 10.1111/j.1749-6632.1991.tb33902.x. [DOI] [PubMed] [Google Scholar]
- 45.McIntosh TJ, Simon SA. Roles of bilayer material properties in function and distribution of membrane proteins. Annu Rev Biophys Biomol Struct. 2006;35:177–98. doi: 10.1146/annurev.biophys.35.040405.102022. [DOI] [PubMed] [Google Scholar]
- 46.Cantor R. Receptor desensitization by neurotransmitters in membranes: are neurotransmitters the endogenous anesthetics? Biochemistry. 2003;42(41):11891–7. doi: 10.1021/bi034534z. [DOI] [PubMed] [Google Scholar]
- 47.Fang Z, Ionescu P, Chortkoff BS, Kandel L, Sonner J, Laster MJ, et al. Anesthetic potencies of n-alkanols: results of additivity and solubility studies suggest a mechanism of action similar to that for conventional inhaled anesthetics. Anesth Analg. 1997;84(5):1042–8. doi: 10.1097/00000539-199705000-00017. [DOI] [PubMed] [Google Scholar]
- 48.Nakao H, Ogli K, Yokono S, Ono J, Miyatake A. The effect of volatile anesthetics on light-induced phosphorylation in spinach chloroplasts. Toxicol Lett. 1998;100-101:135–8. doi: 10.1016/s0378-4274(98)00177-5. [DOI] [PubMed] [Google Scholar]
- 49.Brunner EA, Cheng SC, Berman ML. Effects of anesthesia on intermediary metabolism. Annu Rev Med. 1975;26:391–401. doi: 10.1146/annurev.me.26.020175.002135. [DOI] [PubMed] [Google Scholar]
- 50.Gao MM, Boucher F. The uncoupling of bacteriorhodopsin by high temperature and anaesthetics. Toxicol Lett. 1998;100-101:393–6. doi: 10.1016/s0378-4274(98)00212-4. [DOI] [PubMed] [Google Scholar]
- 51.Richards CD. Anaesthetic modulation of synaptic transmission in the mammalian CNS. Br J Anaesth. 2002;89(1):79–90. doi: 10.1093/bja/aef162. [DOI] [PubMed] [Google Scholar]
- 52.Mihic SJ, Ye Q, Wick MJ, Koltchine VV, Krasowski MD, Finn SE, Mascia MP, Valenzuela CF, Hanson KK, Greenblatt EP, Harris RA, Harrison NL. Sites of alcohol and volatile anaesthetic action on GABA(A) and glycine receptors. Nature. 1997;389:385–9. doi: 10.1038/38738. [DOI] [PubMed] [Google Scholar]
- 53.Hawasli AH, Saifee O, Liu C, Nonet ML, Crowder CM. Resistance to volatile anesthetics by mutations enhancing excitatory neurotransmitter release in Caenorhabditis elegans. Genetics. 2004;168(2):831–43. doi: 10.1534/genetics.104.030502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sass DA, Shakil AO. Fulminant hepatic failure. Liver Transpl. 2005;11(6):594–605. doi: 10.1002/lt.20435. [DOI] [PubMed] [Google Scholar]
- 55.Yokoyama T, Banta S, Berthiaume F, Nagrath D, Tompkins RG, Yarmush ML. Evolution of intrahepatic carbon, nitrogen, and energy metabolism in a D-galactosamine-induced rat liver failure model. Metab Eng. 2005;7(2):88–103. doi: 10.1016/j.ymben.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 56.Brouns R, De Deyn PP. Neurological complications in renal failure: a review. Clin Neurol Neurosurg. 2004;107(1):1–16. doi: 10.1016/j.clineuro.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 57.Warrell D, Cox T, Firth J, Benz E, editors. Oxford Textbood of Medicine. Fourth ed Oxford University Press; Oxford: 2003. [Google Scholar]
- 58.Jonas P, Bischofberger J, Sandkuhler J. Corelease of two fast neurotransmitters at a central synapse. Science. 1998;281(5375):419–24. doi: 10.1126/science.281.5375.419. [DOI] [PubMed] [Google Scholar]
- 59.Dugue GP, Dumoulin A, Triller A, Dieudonne S. Target-dependent use of coreleased inhibitory transmitters at central synapses. J Neurosci. 2005;25(28):6490–8. doi: 10.1523/JNEUROSCI.1500-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dickinson R, White I, Lieb WR, Franks NP. Stereoselective loss of righting reflex in rats by isoflurane. Anesthesiology. 2000;93(3):837–43. doi: 10.1097/00000542-200009000-00035. [DOI] [PubMed] [Google Scholar]
- 61.Won A, Oh I, Laster MJ, Popovich J, Eger EI, 2nd, Sonner JM. Chirality in anesthesia I: minimum alveolar concentration of secondary alcohol enantiomers. Anesth Analg. 2006;103(1):81–4. doi: 10.1213/01.ane.0000217199.90426.7d. [DOI] [PubMed] [Google Scholar]
- 62.Hall AC, Lieb WR, Franks NP. Stereoselective and non-stereoselective actions of isoflurane on the GABAA receptor. Br J Pharmacol. 1994;112(3):906–10. doi: 10.1111/j.1476-5381.1994.tb13166.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Brosnan R, Gong D, Cotten J, Keshavaprasad B, Yost CS, Eger EI, 2nd, Sonner JM. Chirality in anesthesia II: stereoselective modulation of ion channel function by secondary alcohol enantiomers. Anesth Analg. 2006;103:86–91. doi: 10.1213/01.ane.0000221437.87338.af. [DOI] [PubMed] [Google Scholar]
- 64.Dickinson R, Franks NP, Lieb WR. Can the stereoselective effects of the anesthetic isoflurane be accounted for by lipid solubility? Biophys J. 1994;66(6):2019–23. doi: 10.1016/S0006-3495(94)80994-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lalitha S, Kumar SS, Stine KJ, Covey DF. Chirality in membranes: first evidence that enantioselective interactions between cholesterol and cell membrane lipids can be a determinant of membrane physical properties. Journal of Supramolecular Chemistry. 2001;1:53–61. [Google Scholar]
- 66.Bertaccini EJ, Trudell JR, Franks NP. The common chemical motifs within anesthetic binding sites. Anesth Analg. 2007;104(2):318–24. doi: 10.1213/01.ane.0000253029.67331.8d. [DOI] [PubMed] [Google Scholar]
- 67.Powers RA, Morandi F, Shoichet BK. Structure-based discovery of a novel, noncovalent inhibitor of AmpC beta-lactamase. Structure. 2002;10(7):1013–23. doi: 10.1016/s0969-2126(02)00799-2. [DOI] [PubMed] [Google Scholar]
- 68.Franks NP, Lieb WR. Do general anaesthetics act by competitive binding to specific receptors? Nature. 1984;310(5978):599–601. doi: 10.1038/310599a0. [DOI] [PubMed] [Google Scholar]
- 69.Franks NP, Jenkins A, Conti E, Lieb WR, Brick P. Structural basis for the inhibition of firefly luciferase by a general anesthetic. Biophys J. 1998;75(5):2205–11. doi: 10.1016/S0006-3495(98)77664-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Szarecka A, Xu Y, Tang P. Dynamics of firefly luciferase inhibition by general anesthetics: Gaussian and anisotropic network analyses. Biophys J. 2007;93(6):1895–905. doi: 10.1529/biophysj.106.102780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mascia MP, Trudell JR, Harris RA. Specific binding sites for alcohols and anesthetics on ligand-gated ion channels. Proc Natl Acad Sci U S A. 2000;97(16):9305–10. doi: 10.1073/pnas.160128797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ogata J, Shiraishi M, Namba T, Smothers CT, Woodward JJ, Harris RA. Effects of anesthetics on mutant N-methyl-D-aspartate receptors expressed in Xenopus oocytes. J Pharmacol Exp Ther. 2006;318(1):434–43. doi: 10.1124/jpet.106.101691. [DOI] [PubMed] [Google Scholar]
- 73.Ronald KM, Mirshahi T, Woodward JJ. Ethanol inhibition of N-methyl-D-aspartate receptors is reduced by site-directed mutagenesis of a transmembrane domain phenylalanine residue. J Biol Chem. 2001;276(48):44729–35. doi: 10.1074/jbc.M102800200. [DOI] [PubMed] [Google Scholar]