Abstract
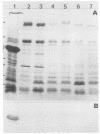
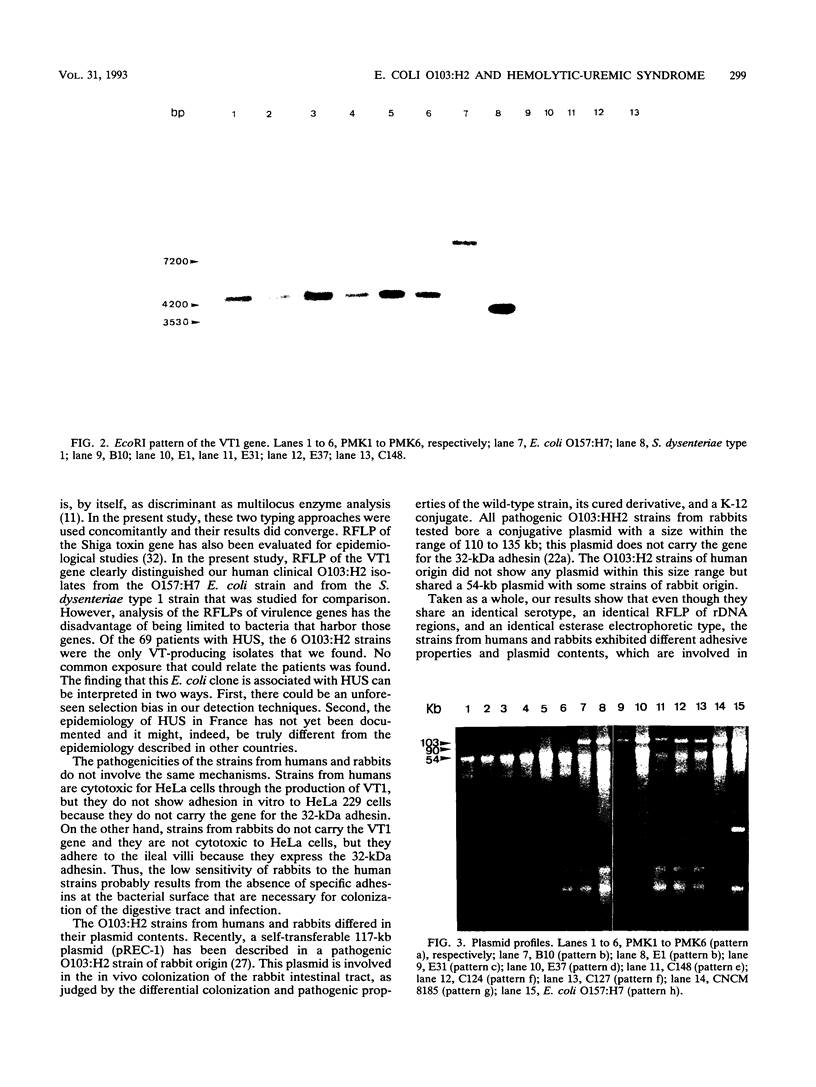
In a French multicenter study, six verocytotoxin-producing Escherichia coli strains were isolated from the stools of 6 of 69 children suffering from hemolytic-uremic syndrome. All strains belonged to serotype O103:H2, a serotype commonly associated with diarrhea in weaned rabbits in France. To determine whether the strains from humans and rabbits were genetically related, they were compared by analyzing their esterase electropherotypes and the restriction fragment length polymorphisms of the ribosomal DNA regions. A common clonal origin of these pathogenic strains was suggested by their identical esterase electropherotypes and their identical ribotypes, in addition to their identical serotypes. However, strains from humans, which are cytotoxic for HeLa cells through the production of verocytotoxin type 1, do not show adhesion in vitro to HeLa 229 cells and cannot infect rabbits. On the other hand, strains from rabbits do not carry the verocytotoxin type 1 gene, are not cytotoxic for Hela cells, and adhere to ileal villi and HeLa 229 cells because of the expression of their 32-kDa adhesin. Our results therefore identify a clone of verocytotoxin-producing E. coli O103:H2 as a potential agent of hemolytic uremic syndrome in France. They further suggest that clones from humans and rabbits probably have a common origin but that adaptation to the two species occurred by different mechanisms. Thus, they eliminate the hypothesis that the species is horizontally transmitted between rabbits and humans.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bingen E. H., Denamur E., Lambert-Zechovsky N. Y., Bourdois A., Mariani-Kurkdjian P., Cezard J. P., Navarro J., Elion J. DNA restriction fragment length polymorphism differentiates crossed from independent infections in nosocomial Xanthomonas maltophilia bacteremia. J Clin Microbiol. 1991 Jul;29(7):1348–1350. doi: 10.1128/jcm.29.7.1348-1350.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camguilhem R., Milon A. Biotypes and O serogroups of Escherichia coli involved in intestinal infections of weaned rabbits: clues to diagnosis of pathogenic strains. J Clin Microbiol. 1989 Apr;27(4):743–747. doi: 10.1128/jcm.27.4.743-747.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denamur E., Sayada C., Souriau A., Orfila J., Rodolakis A., Elion J. Restriction pattern of the major outer-membrane protein gene provides evidence for a homogeneous invasive group among ruminant isolates of Chlamydia psittaci. J Gen Microbiol. 1991 Nov;137(11):2525–2530. doi: 10.1099/00221287-137-11-2525. [DOI] [PubMed] [Google Scholar]
- Dorn C. R., Scotland S. M., Smith H. R., Willshaw G. A., Rowe B. Properties of Vero cytotoxin-producing Escherichia coli of human and animal origin belonging to serotypes other than O157:H7. Epidemiol Infect. 1989 Aug;103(1):83–95. doi: 10.1017/s0950268800030387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong J. S., de Chadarevian J. P., Kaplan B. S. Hemolytic-uremic syndrome. Current concepts and management. Pediatr Clin North Am. 1982 Aug;29(4):835–856. doi: 10.1016/s0031-3955(16)34216-x. [DOI] [PubMed] [Google Scholar]
- Fontaine A., Arondel J., Sansonetti P. J. Role of Shiga toxin in the pathogenesis of bacillary dysentery, studied by using a Tox- mutant of Shigella dysenteriae 1. Infect Immun. 1988 Dec;56(12):3099–3109. doi: 10.1128/iai.56.12.3099-3109.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentry M. K., Dalrymple J. M. Quantitative microtiter cytotoxicity assay for Shigella toxin. J Clin Microbiol. 1980 Sep;12(3):361–366. doi: 10.1128/jcm.12.3.361-366.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goullet P., Picard B. Characterization of enterobacteria by esterase specific-activity profiles. J Gen Microbiol. 1990 Mar;136(3):431–440. doi: 10.1099/00221287-136-3-431. [DOI] [PubMed] [Google Scholar]
- Goullet P., Picard B. Comparative electrophoretic polymorphism of esterases and other enzymes in Escherichia coli. J Gen Microbiol. 1989 Jan;135(1):135–143. doi: 10.1099/00221287-135-1-135. [DOI] [PubMed] [Google Scholar]
- Gransden W. R., Damm M. A., Anderson J. D., Carter J. E., Lior H. Further evidence associating hemolytic uremic syndrome with infection by Verotoxin-producing Escherichia coli O157:H7. J Infect Dis. 1986 Sep;154(3):522–524. doi: 10.1093/infdis/154.3.522. [DOI] [PubMed] [Google Scholar]
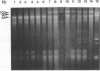
- Grimont F., Grimont P. A. Ribosomal ribonucleic acid gene restriction patterns as potential taxonomic tools. Ann Inst Pasteur Microbiol. 1986 Sep-Oct;137B(2):165–175. doi: 10.1016/s0769-2609(86)80105-3. [DOI] [PubMed] [Google Scholar]
- Gurwith M. J., Williams T. W. Gastroenteritis in children: a two-year review in Manitoba. I. Etiology. J Infect Dis. 1977 Aug;136(2):239–247. doi: 10.1093/infdis/136.2.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall G. A., Dorn C. R., Chanter N., Scotland S. M., Smith H. R., Rowe B. Attaching and effacing lesions in vivo and adhesion to tissue culture cells of Vero-cytotoxin-producing Escherichia coli belonging to serogroups O5 and O103. J Gen Microbiol. 1990 Apr;136(4):779–786. doi: 10.1099/00221287-136-4-779. [DOI] [PubMed] [Google Scholar]
- Kaplan B. S., Cleary T. G., Obrig T. G. Recent advances in understanding the pathogenesis of the hemolytic uremic syndromes. Pediatr Nephrol. 1990 May;4(3):276–283. doi: 10.1007/BF00857676. [DOI] [PubMed] [Google Scholar]
- Karmali M. A. Infection by verocytotoxin-producing Escherichia coli. Clin Microbiol Rev. 1989 Jan;2(1):15–38. doi: 10.1128/cmr.2.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karmali M. A., Petric M., Lim C., Fleming P. C., Arbus G. S., Lior H. The association between idiopathic hemolytic uremic syndrome and infection by verotoxin-producing Escherichia coli. J Infect Dis. 1985 May;151(5):775–782. doi: 10.1093/infdis/151.5.775. [DOI] [PubMed] [Google Scholar]
- Kleanthous H., Smith H. R., Scotland S. M., Gross R. J., Rowe B., Taylor C. M., Milford D. V. Haemolytic uraemic syndromes in the British Isles, 1985-8: association with verocytotoxin producing Escherichia coli. Part 2: Microbiological aspects. Arch Dis Child. 1990 Jul;65(7):722–727. doi: 10.1136/adc.65.7.722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Licois D., Reynaud A., Federighi M., Gaillard-Martinie B., Guillot J. F., Joly B. Scanning and transmission electron microscopic study of adherence of Escherichia coli O103 enteropathogenic and/or enterohemorrhagic strain GV in enteric infection in rabbits. Infect Immun. 1991 Oct;59(10):3796–3800. doi: 10.1128/iai.59.10.3796-3800.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milon A., Esslinger J., Camguilhem R. Adhesion of Escherichia coli strains isolated from diarrheic weaned rabbits to intestinal villi and HeLa cells. Infect Immun. 1990 Aug;58(8):2690–2695. doi: 10.1128/iai.58.8.2690-2695.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullis K. B. The polymerase chain reaction in an anemic mode: how to avoid cold oligodeoxyribonuclear fusion. PCR Methods Appl. 1991 Aug;1(1):1–4. doi: 10.1101/gr.1.1.1. [DOI] [PubMed] [Google Scholar]
- Picard B., Picard-Pasquier N., Krishnamoorthy R., Goullet P. Characterization of highly virulent Escherichia coli strains by ribosomal DNA restriction fragment length polymorphism. FEMS Microbiol Lett. 1991 Aug 1;66(2):183–188. doi: 10.1016/0378-1097(91)90330-d. [DOI] [PubMed] [Google Scholar]
- Pollard D. R., Johnson W. M., Lior H., Tyler S. D., Rozee K. R. Rapid and specific detection of verotoxin genes in Escherichia coli by the polymerase chain reaction. J Clin Microbiol. 1990 Mar;28(3):540–545. doi: 10.1128/jcm.28.3.540-545.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynaud A., Federighi M., Licois D., Guillot J. F., Joly B. R plasmid in Escherichia coli O103 coding for colonization of the rabbit intestinal tract. Infect Immun. 1991 Jun;59(6):1888–1892. doi: 10.1128/iai.59.6.1888-1892.1991. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Scaletsky I. C., Silva M. L., Trabulsi L. R. Distinctive patterns of adherence of enteropathogenic Escherichia coli to HeLa cells. Infect Immun. 1984 Aug;45(2):534–536. doi: 10.1128/iai.45.2.534-536.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Strockbine N. A., Marques L. R., Newland J. W., Smith H. W., Holmes R. K., O'Brien A. D. Two toxin-converting phages from Escherichia coli O157:H7 strain 933 encode antigenically distinct toxins with similar biologic activities. Infect Immun. 1986 Jul;53(1):135–140. doi: 10.1128/iai.53.1.135-140.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strockbine N. A., Parsonnet J., Greene K., Kiehlbauch J. A., Wachsmuth I. K. Molecular epidemiologic techniques in analysis of epidemic and endemic Shigella dysenteriae type 1 strains. J Infect Dis. 1991 Feb;163(2):406–409. doi: 10.1093/infdis/163.2.406. [DOI] [PubMed] [Google Scholar]
- Tarr P. I., Neill M. A., Clausen C. R., Watkins S. L., Christie D. L., Hickman R. O. Escherichia coli O157:H7 and the hemolytic uremic syndrome: importance of early cultures in establishing the etiology. J Infect Dis. 1990 Aug;162(2):553–556. doi: 10.1093/infdis/162.2.553. [DOI] [PubMed] [Google Scholar]
- Uriel J. Méthode d'électrophorèse dans des gels d'acrylamide-agarose. Bull Soc Chim Biol (Paris) 1966;48(8):969–982. [PubMed] [Google Scholar]
- Whittam T. S., Wachsmuth I. K., Wilson R. A. Genetic evidence of clonal descent of Escherichia coli O157:H7 associated with hemorrhagic colitis and hemolytic uremic syndrome. J Infect Dis. 1988 Jun;157(6):1124–1133. doi: 10.1093/infdis/157.6.1124. [DOI] [PubMed] [Google Scholar]
- de Graaf F. K., Klemm P., Gaastra W. Purification, characterization, and partial covalent structure of Escherichia coli adhesive antigen K99. Infect Immun. 1981 Sep;33(3):877–883. doi: 10.1128/iai.33.3.877-883.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]