Abstract
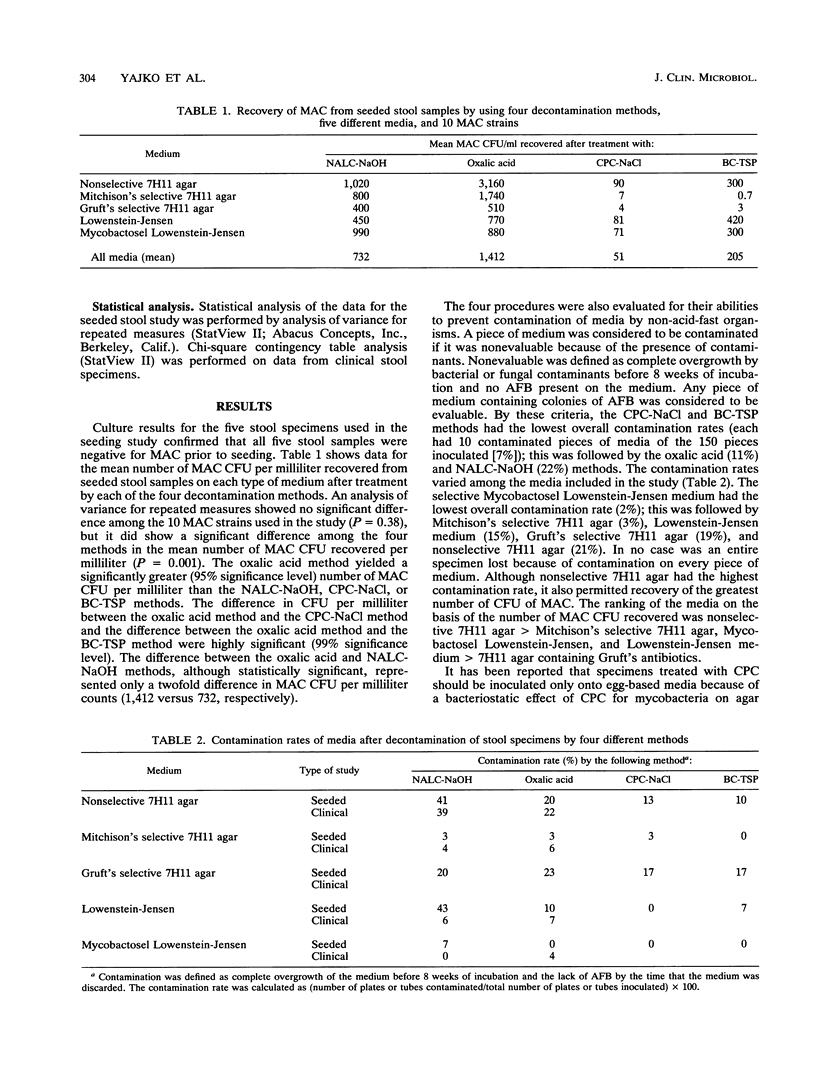
The presence of Mycobacterium avium complex (MAC) in stool specimens may be a predictor of disseminated MAC infection, yet the methods for decontaminating stools have not been evaluated for their usefulness in recovering MAC organisms. In the present study, four decontamination methods commonly used to recover acid-fast bacteria from respiratory specimens were compared for their utility in recovering MAC from stool specimens. Ten strains of MAC were used at a level of 10(4) to 10(6) CFU to seed the stool specimens. Specimens were divided into four portions and were decontaminated by using the following treatments: (i) N-acetyl-L-cysteine-sodium hydroxide (NALC-NaOH), (ii) cetylpyridinium chloride-sodium chloride (CPC-NaCl), (iii) oxalic acid, or (iv) benzalkonium chloride-trisodium phosphate (BC-TSP). The specimens were then plated onto a total of five pieces of selective and nonselective egg- and agar-based media. The oxalic acid method yielded the greatest number of MAC CFU from seeded stool samples; this was followed by NALC-NaOH, BC-TSP, and CPC-NaCl. The difference between the oxalic acid method and each of the other methods was statistically significant (analysis of variance at the 95% significance level). Although more MAC CFU was recovered from seeded stool samples by using oxalic acid than NALC-NaOH, no difference in culture positivity rates was observed when the two methods were used to test 368 clinical stool specimens processed with either oxalic acid (164 specimens) or NALC-NaOH (204 specimens) (P = 0.07) or 67 specimens processed by both methods (P = 0.77). The oxalic acid and NALC-NaOH decontamination methods both appear to be useful for the recovery of MAC organisms from stool specimens.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Conlon C. P., Banda H. M., Luo N. P., Namaambo M. K., Perera C. U., Sikweze J. Faecal mycobacteria and their relationship to HIV-related enteritis in Lusaka, Zambia. AIDS. 1989 Aug;3(8):539–541. doi: 10.1097/00002030-198908000-00009. [DOI] [PubMed] [Google Scholar]
- Gold J. W. Mycobacterial infections in immunosuppressed patients. Semin Respir Infect. 1986 Sep;1(3):160–165. [PubMed] [Google Scholar]
- Hawkins C. C., Gold J. W., Whimbey E., Kiehn T. E., Brannon P., Cammarata R., Brown A. E., Armstrong D. Mycobacterium avium complex infections in patients with the acquired immunodeficiency syndrome. Ann Intern Med. 1986 Aug;105(2):184–188. doi: 10.7326/0003-4819-105-2-184. [DOI] [PubMed] [Google Scholar]
- Horsburgh C. R., Jr, Metchock B. G., McGowan J. E., Jr, Thompson S. E. Clinical implications of recovery of Mycobacterium avium complex from the stool or respiratory tract of HIV-infected individuals. AIDS. 1992 May;6(5):512–514. [PubMed] [Google Scholar]
- Jacobson M. A., Hopewell P. C., Yajko D. M., Hadley W. K., Lazarus E., Mohanty P. K., Modin G. W., Feigal D. W., Cusick P. S., Sande M. A. Natural history of disseminated Mycobacterium avium complex infection in AIDS. J Infect Dis. 1991 Nov;164(5):994–998. doi: 10.1093/infdis/164.5.994. [DOI] [PubMed] [Google Scholar]
- KUBICA G. P., DYE W. E., COHN M. L., MIDDLEBROOK G. Sputum digestion and decontamination with N-acetyl-L-cysteine-sodium hydroxide for culture of mycobacteria. Am Rev Respir Dis. 1963 May;87:775–779. doi: 10.1164/arrd.1963.87.5.775. [DOI] [PubMed] [Google Scholar]
- KUBICA G. P., KAUFMANN A. J., DYE W. E. COMMENTS ON THE USE OF THE NEW MUCOLYTIC AGENT, N-ACETYL-L-CYSTEINE, AS A SPUTUM DIGESTANT FOR THE ISOLATION OF MYCOBACTERIA. Am Rev Respir Dis. 1964 Feb;89:284–286. doi: 10.1164/arrd.1964.89.2.284. [DOI] [PubMed] [Google Scholar]
- Kiehn T. E., Edwards F. F., Brannon P., Tsang A. Y., Maio M., Gold J. W., Whimbey E., Wong B., McClatchy J. K., Armstrong D. Infections caused by Mycobacterium avium complex in immunocompromised patients: diagnosis by blood culture and fecal examination, antimicrobial susceptibility tests, and morphological and seroagglutination characteristics. J Clin Microbiol. 1985 Feb;21(2):168–173. doi: 10.1128/jcm.21.2.168-173.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portaels F., Larsson L., Smeets P. Isolation of mycobacteria from healthy persons' stools. Int J Lepr Other Mycobact Dis. 1988 Sep;56(3):468–471. [PubMed] [Google Scholar]
- Roth R. I., Owen R. L., Keren D. F., Volberding P. A. Intestinal infection with Mycobacterium avium in acquired immune deficiency syndrome (AIDS). Histological and clinical comparison with Whipple's disease. Dig Dis Sci. 1985 May;30(5):497–504. doi: 10.1007/BF01318186. [DOI] [PubMed] [Google Scholar]
- Smithwick R. W., Stratigos C. B., David H. L. Use of cetylpyridinium chloride and sodium chloride for the decontamination of sputum specimens that are transported to the laboratory for the isolation of Mycobacterium tuberculosis. J Clin Microbiol. 1975 May;1(5):411–413. doi: 10.1128/jcm.1.5.411-413.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WAYNE L. G., KRASNOW I., KIDD G. Finding the "hidden positive" in tuberculosis eradication programs. The role of the sensitive trisodium phosphate-benzalkonium (zephiran) culture technique. Am Rev Respir Dis. 1962 Oct;86:537–541. doi: 10.1164/arrd.1962.86.4.537. [DOI] [PubMed] [Google Scholar]
- Yajko D. M., Nassos P. S., Hadley W. K. Broth microdilution testing of susceptibilities to 30 antimicrobial agents of Mycobacterium avium strains from patients with acquired immune deficiency syndrome. Antimicrob Agents Chemother. 1987 Oct;31(10):1579–1584. doi: 10.1128/aac.31.10.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]


