Abstract
Background
The helpfulness of bedside assessment of gastric residual volume in the prediction of aspiration has been questioned, as has the volume that signals increased risk of aspiration.
Objective
To describe the association between gastric residual volumes and aspiration of gastric contents.
Methods
In a prospective study of 206 critically ill patients receiving gastric tube feedings for 3 consecutive days, gastric residual volumes were measured with 60-mL syringes every 4 hours. Measured volumes were categorized into 3 overlapping groups: at least 150 mL, at least 200 mL, and at least 250 mL. Patients were categorized as frequent aspirators if 40% or more of their tracheal secretions were positive for pepsin and as infrequent aspirators if less than 40% of their secretions were positive for pepsin. Gastric residual volumes were compared between the 2 aspiration groups.
Results
Approximately 39% of the 206 patients had 1 or more gastric residual volumes of at least 150 mL, 27% had 1 or more volumes of at least 200 mL, and 17% had 1 or more volumes of at least 250 mL. Large-bore tubes identified most of the high volumes. Eighty-nine patients were frequent aspirators. Volumes less than 150 mL were common in both aspiration groups. However, the frequent aspirators had a significantly greater frequency of 2 or more volumes of at least 200 mL and 1 or more volumes of at least 250 mL.
Conclusions
No consistent relationship was found between aspiration and gastric residual volumes. Although aspiration occurs without high gastric residual volumes, it occurs significantly more often when volumes are high.
Notice to CE enrollees
A closed-book, multiple-choice examination following this article tests your understanding of the following objectives:
Describe the association between gastric residual volumes (GRVs) and aspiration of gastric contents.
Recognize that measurement error is a significant problem in assessing GRVs.
Understand that aspiration risk is significantly increased when GRVs are high.
To read this article and take the CE test online, visit www.ajcconline.org and click “CE Articles in This Issue.” No CE test fee for AACN members.
Measurement of gastric residual volume (GRV) is often recommended to determine tolerance to gastric tube feedings.1–7 An underlying assumption is that high GRVs increase the risk for gastroesophageal reflux and associated aspiration. However, the extent to which bedside assessment of GRVs can help predict aspiration risk has been questioned,8 as has the amount of GRV that signals increased risk of aspiration.9–13 Values as low as 50 mL and higher than 500 mL have been reported.14–19
Objective
The objective of this prospective study was to describe the association between GRV and aspiration of gastric contents in a group of critically ill patients receiving gastric tube feedings.
Methods
Setting and Subjects
The study was conducted at Saint Louis University Hospital in St Louis, Missouri. Table 1 specifies demographic information for the 206 patients, and Table 2 has a description of their treatment conditions. The work was done in accordance with the appropriate institutional review body and carried out within the ethical standards set forth in the Helsinki Declaration of 1975. Written informed consent was obtained from the patients or their legal guardians. Because of reports1,6–19 that high GRVs are more likely to occur during the first few days of tube feedings, attempts were made to enroll patients on the day that feedings began.
Table 1.
Demographic information
| Variable | Findingsa |
|---|---|
| Total sample | 206 |
|
| |
| Service from which enrolled | |
| Trauma/surgery | 101 (49.0) |
| General medicine | 46 (22.3) |
| Neuromedicine/neurosurgery | 46 (22.3) |
| Cardiac services | 13 (6.3) |
|
| |
| Sex | |
| Male | 126 (61.2) |
| Female | 80 (38.8) |
|
| |
| Age, mean (SD, range), y | 51.9 (18.1, 18–88) |
|
| |
| Acute Physiology and Chronic Health Evaluation score on admission, mean (SD, range) | 23.3 (6.3, 10–40) |
Values are number (%) of patients unless otherwise indicated. Because of rounding, not all percentages total 100.
Table 2.
Description of treatment conditions
| Variable | Findingsa |
|---|---|
| Gastric feeding site | 206 (100) |
|
| |
| Feeding tubes | |
| 10F polyurethane nasogastric or orogastric tube | 91 (44.2) |
| 14F polyvinyl chloride nasogastric or orogastric sump tube | 11 (5.3) |
| 16F polyvinyl chloride nasogastric or orogastric sump tube | 3 (1.5) |
| 18F polyvinyl chloride nasogastric or orogastric sump tube | 85 (41.3) |
| 20F gastrostomy tube | 16 (7.8) |
|
| |
| Enteral formula administration | |
| No. of days feedings in use at entry into the study, mean (SD, range) | 1.2 (1.9, 0–10) |
| Rate of formula administered, mean(SD, range), mL/h | 54.3 (17.4, 10–90) |
|
| |
| Mean backrest elevation | |
| ≥30° | 72 (35.0) |
| ≥45° | 2 (1.0) |
|
| |
| Use of stress ulcer prophylaxis | |
| H2-receptor antagonist | 144 (69.9) |
| Proton pump inhibitor | 62 (30.1) |
|
| |
| Use of prokinetics | 107 (51.9) |
|
| |
| Use of opioids | 154 (74.8) |
|
| |
| Use of dopamine | 21 (10.2) |
Values are number (%) of patients unless otherwise indicated. Because of rounding, not all percentages total 100.
To be included in the study, patients must have been admitted to 1 of 5 intensive care units (ICUs) at Saint Louis University Hospital, be receiving continuous gastric tube feedings, have a tracheal intubation, be at least 18 years old, and provide informed consent (or have a legal guardian provide informed consent). Patients were excluded from the study if tube feedings were discontinued or tracheal extubation occurred before completion of the 3-day study.
Data Collection
Table 3 is a summary of measurements made during the 3-day study period. All data were collected by registered nurse research assistants who were present in the involved ICUs from 8 AM through midnight, 7 days per week, for 24 months. Each patient participated in the study for 3 consecutive days. Most (n = 155) patients were admitted to the study within 24 hours of the start of gastric feeding; the remaining 51 patients had been fed a mean of 3.8 (SD, 2.3) days before entry into the study (range, 2–10 days).
Table 3.
Summary of measurements
| Measurement | Frequency |
|---|---|
| Assay of sputum for pepsin | Samples collected at time of routine suctioning between 8 am and midnight for 3 consecutive days |
| Residual volume from feeding tube | |
| Level of consciousness | |
| Level of sedation | Every 4 hours between 8 am and midnight for 3 consecutive days |
| Presence of vomiting | |
| Use of medications (opioids, dopamine, prokinetics, stress ulcer prophylaxis) | |
| Volume of formula administered | |
| Acute Physiology and Chronic Health Evaluation II score | At time of admission to intensive care unit |
| Angle of backrest elevation | Hourly from 8 am through midnight |
Measurements
Pepsin Assay to Assess for Aspiration
An immunoassay was used to detect pepsin in the suctioned tracheal secretions. The procedure for the assay has been described previously20; in an animal model, the assay had a sensitivity of 93% and a specificity of 100%. Within an hour after collection, each secretion was cryopreserved for later analysis. The assay can detect pepsin in a concentration as low as 1 μg/mL. Gels were read by the same biochemist, who did not know the patients’ clinical statuses; results were recorded as positive or negative. Pepsin-positive tracheal secretions served as a proxy for the aspiration of gastric contents.
Gastric Residual Volume
As shown in Table 2, 44.2% (n = 91) of the patients enrolled in the study had 10F polyurethane tubes; the rest had either polyvinyl chloride sump tubes (n = 99) or gastrostomy tubes (n = 16). Formula flow was turned off and the tube was flushed with 30 mL of air to force the tube’s ports away from the mucosal folds. A 60-mL syringe was used to withdraw as much fluid as possible from the stomach. If 60 mL was withdrawn into the syringe, the fluid was emptied into a calibrated container, and the procedure was repeated until no more fluid could be withdrawn. Any amount less than 200 mL was reinstilled into the feeding tube. The tube was then flushed with 30 mL of water before being reconnected to the feeding pump. The GRV measurements were made in whatever position the patient was in at the time; no attempt was made to control this variable.
Glasgow Coma Scale
The Glasgow Coma Scale (GCS) is used to assess level of consciousness by evaluating eye opening, motor response, and verbal response. Because all patients were intubated, the best verbal response was estimated as appears able to converse, ability to converse is in question, or generally unresponsive. Possible scores ranged from 3 (worst) to 15 (best).
Sedation Score
The level of sedation was assessed by using the Vancouver Interaction and Calmness Scale.21 This scale was specifically developed for use with adult, critically ill patients receiving mechanical ventilation and consists of two 5-item subscales for quantifying interaction along a continuum from 5 to 30 points. Possible total scores ranged between 10 (worst) to 60 (best).
Acute Physiology and Chronic Health Evaluation II
The score on the Acute Physiology and Chronic Health Evaluation (APACHE) II was calculated for each patient when the patient was admitted to the ICU. Parameters used to calculate the score included body temperature; mean arterial pressure; heart rate; respiratory rate; oxygenation; serum levels of sodium, potassium, and creatinine; hematocrit; white blood cell count; GCS score; chronic health points; and age. Possible scores were from 0 (best) to 71 (worst).
Vomiting and Use of Medications
The registered nurses who were the research assistants reviewed patients’ medical records to determine if vomiting had occurred and to see if specific medications (stress ulcer prophylaxis, prokinetics, opioids, and dopamine) had been administered. These data were recorded at 4-hour intervals on the data collection forms for comparison with other clinical data.
Data Analysis
Descriptive statistics (frequencies, percentages, and means and standard deviations) were used to report the data. A 1-way analysis of variance was used to compare variation in the percentage of aspiration according to changes in GRVs (categorized in 50-mL intervals from 0 to >250 mL). A χ2 test was used to evaluate the association between dichotomized variables. A backward logistic regression was used to compare the relationship between aspiration and GRVs in context with other risk factors for aspiration.22
Results
As indicated in Table 1, most patients were recruited from the trauma/surgery ICU; the next most frequently represented ICUs were general medicine and neurosurgery/neuromedicine. A higher percentage of men than women (61.2% vs 38.8%) participated in the project, primarily because the study site is a level I trauma center. A total of 86 patients (41.7%) had head injuries or cranial neurosurgical conditions. The mean GCS score was 7.1 (SD, 3.0); almost three-fourths (72.3%) of the patients had a mean GCS score less than 9. Further, the score on the Vancouver Interaction and Calmness Scale (mean, 35.9; SD, 4.8) indicated that the patients were heavily sedated; 71.2% had a mean score of 35 or less. Only a small percentage (6.8%) of the patients vomited during the 3-day study period. Although all 206 patients were receiving gastric feedings at the start of data collection, 14 patients (6.8%) had their tubes moved to the small bowel shortly before the end of the data collection period. In 12 of these patients, the tubes were repositioned to the small bowel because of persistent high GRVs or hypoactive bowel sounds; in the remaining 2, large-bore nasogastric tubes were replaced with small-bore nasointestinal tubes.
Frequency of Aspiration
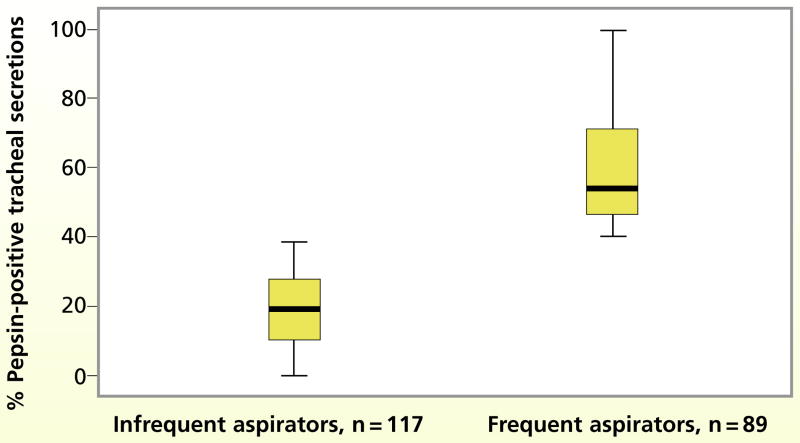
A total of 3203 tracheal secretions were assayed for pepsin. The mean percentage of tracheal secretions ppositive for pepsin in the entire sample was 36.2% (SD, 24.7%; range, 0%–100%). Most (92.7%) of the 206 patients had at least 1 tracheal secretion positive for pepsin during the 3-day study period. Because of this result, all patients had to be categorized according to the frequency of aspiration. Patients whose secretions were positive for pepsin in 40% or more of the observations were classified as frequent aspirators; patients with pepsin detected in less than 40% of observations were classified as infrequent aspirators. The median percentage of pepsin-positive tracheal secretions among the 89 frequent aspirators was 53.8%, as opposed to 19.2% among the 117 infrequent aspirators (Figure 1).
Figure 1.
Median, upper and lower quartiles, and range of percentages of pepsin-positive tracheal secretions in the frequent and infrequent aspiration groups.
Frequency of High GRVs
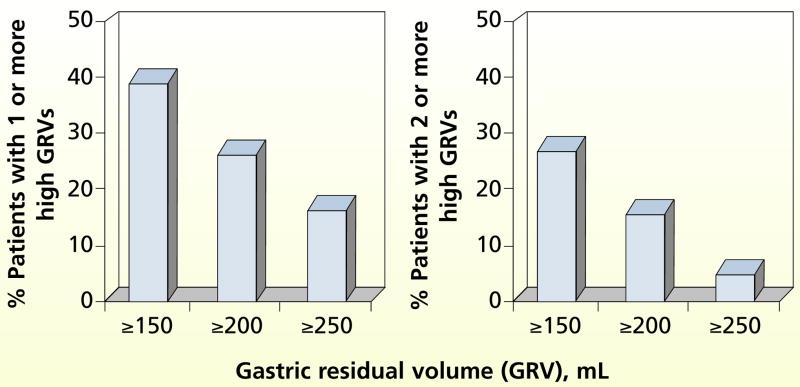
A total of 3286 GRVs were measured (mean, 37.1 mL; SD, 36.6 mL). In an attempt to evaluate cutoff points for high GRVs commonly referred to in the literature,4,23–25 we categorized high GRVs into 3 overlapping groups: at least 150 mL, at least 200 mL, and at least 250 mL. The frequency with which these GRV categories were identified is shown in Figure 2. Overall, 72.8% of the GRVs of at least 150 mL, 74.5% of the GRVs of at least 200 mL, and 80% of the GRVs of at least 250 mL were in patients with large-bore tubes; the rest of the high GRVs were in patients with small-bore tubes.
Figure 2.
Frequency of high gastric residual volumes (N = 206)
Patients admitted to the study within 24 hours of the start of gastric feeding were more likely to have at least 1 high GRV in the subsequent 3-day period. For example, 69 of the 81 patients with 1 or more GRVs of at least 150 mL were enrolled within 24 hours of the start of feeding (P = .008).
Relationship Between Aspiration and Gastric Residual Volume
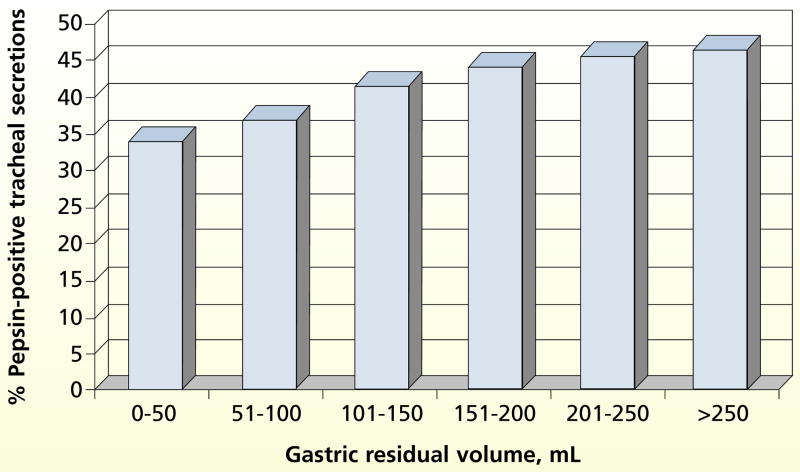
Figure 3 depicts the percentage of secretions indicating aspiration associated with a continuum of GRVs (categorized in 50-mL intervals from 0 to >250 mL). The percentage of secretions indicating aspiration that occurred when GRVs were between 0 and 50 mL was relatively high (33.7%). However, the percentage of aspiration increased as GRVs increased (F = 7.7, P < .001).
Figure 3.
Mean percentage of pepsin-positive tracheal secretions according to gastric residual volume. Data are from 3286 aspirates from small- and large-bore tubes. By 1-way analysis of variance, P < .001.
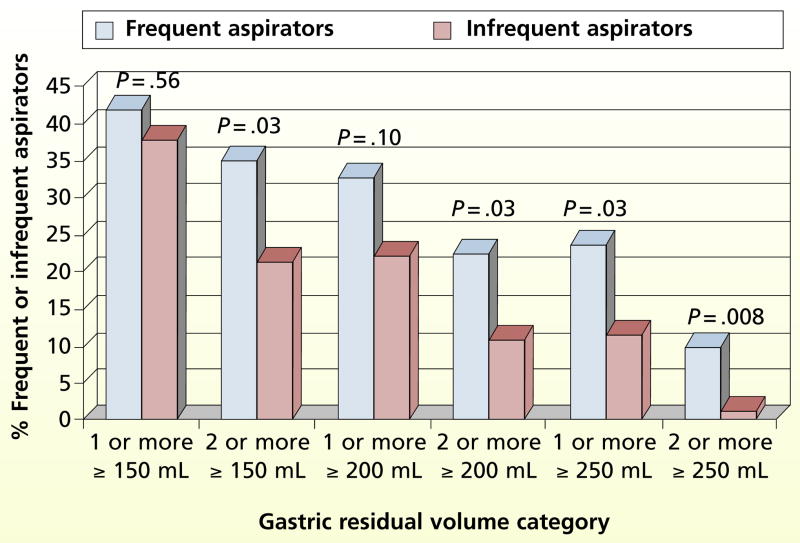
Chi-square tests were done to determine the relationship between the high GRV categories and the 2 aspiration groups. The frequent and infrequent aspirators did not differ significantly in the following categories: 1 or more GRVs of at least 150 mL (χ2 = 0.3, P = .56) and 1 or more GRVs of at least 200 mL (χ2 = 2.8, P = .10; Figure 4). However, the aspiration groups differed significantly in the following GRV categories: 2 or more GRVs of at least 150 mL (χ2 = 4.6, P = .03), 2 or more GRVs of at least 200 mL (χ2=4.9, P = .03), 1 or more GRVs of at least 250 mL (χ2 = 4.9, P = .03), and 2 or more GRVs of at least 250 mL (χ2 = 7.1, P = .008).
Figure 4.
Distribution of gastric residual volume categories in frequent and infrequent aspirators (univariate analysis, χ2 analysis).
Because of the patients’ high acuity level, we evaluated the association between the various GRV categories and aspiration in context with other risk factors for aspiration. To predict the aspiration group, each of the GRV categories (1 or more GRVs ≥150 mL, 2 or more GRVs ≥150 mL, 1 or more GRVs ≥200 mL, 2 or more GRVs ≥200 mL, 1 or more GRVs ≥250 mL, and 2 or more GRVs ≥250 mL) were entered separately into a backward logistic regression equation with the following variables: a mean GCS score less than 9, heavy sedation (defined as a mean Vancouver Interaction and Calmness Score ≤35), vomiting, a mean head-of-bed elevation <30°, and severity of illness (APACHE II score). Only the categories of 2 or more GRVs of at least 200 mL, 1 or more GRVs of at least 250 mL, and 2 or more GRVs of at least 250 mL remained in the model at a P value less than .05 (Table 4). In the logistic regression analysis, the risk associated with having 2 or more GRVs of at least 200 mL was 2.3 (95% confidence interval [CI], 1.1–5.1), the risk associated with having 1 or more GRVs of at least 250 mL was 2.2 (95% CI, 1.0–4.6), and the risk associated with having 2 or more GRVs of at least 250 mL was 5.4 (95% CI, 1.1–26.4). Additional variables that were significant in 1 or more of the analyses were a mean GCS score less than 9 and a mean head-of-bed elevation less than 30° (Table 4).
Table 4.
Prediction of aspiration group (frequent versus infrequent) according to multiple risk factors for aspirationa
| Analyses | Retained variables | Risk (95% CI) | P |
|---|---|---|---|
| Analysis 1 | Head of bed <30° | 1.9 (1.0–3.5) | .04b |
| 1 or more GRVs ≥150 mL | GCS score <9 | 2.0 (1.0–3.8) | .04b |
|
| |||
| Analysis 2 | Head of bed <30° | 1.8 (0.9–3.3) | .08 |
| 2 or more GRVs ≥150 mL | GCS score <9 | 2.0 (1.0–3.9) | .04b |
| 2 or more GRVs ≥150 mL | 1.8 (0.9–3.5) | .06 | |
|
| |||
| Analysis 3 | Head of bed <30° | 1.9 (1.0–3.5) | .04b |
| 1 or more GRVs ≥200 mL | GCS score <9 | 2.0 (1.0–3.8) | .04b |
|
| |||
| Analysis 4 | APACHE II score | 1.1 (0.9–1.1) | .06 |
| 2 or more GRVs ≥200 mL | GCS score <9 | 2.0 (1.0–3.9) | .04b |
| 2 or more GRVs ≥200 mL | 2.3 (1.1–5.1) | .03b | |
|
| |||
| Analysis 5 | Head of bed <30° | 1.9 (1.0–3.5) | .048b |
| 1 or more GRVs ≥250 mL | GCS score <9 | 1.9 (0.9–3.7) | .06 |
| 1 or more GRVs ≥250 mL | 2.2 (1.0–4.6) | .047b | |
|
| |||
| Analysis 6 | Head of bed <30° | 1.9 (1.0–3.5) | .04b |
| 2 or more GRVs ≥250 mL | GCS score <9 | 1.7 (0.9–3.5) | .10 |
| 2 or more GRVs ≥250 mL | 5.4 (1.1–26.4) | .04b | |
Abbreviations: APACHE, Acute Physiology and Chronic Health Evaluation; CI, confidence interval; GCS, Glasgow Coma Scale; GRV, gastric residual volume.
Variables in model include dichotomized mean elevation of head of bed, dichotomized mean score on GCS, dichotomized mean sedation score, presence or absence of vomiting, and the mean APACHE II score.
P < .05.
Discussion
Frequency of Aspiration
The high rate of aspiration in the study most likely reflects the high acuity level of the patients and the sensitive assay used to detect aspiration. Other investigators8 have reported similar findings in critically ill, tube-fed patients when a sensitive laboratory method was used to detect aspiration.
Frequency of High GRVs
Our ability to identify 1 or more GRVs of at least 150 mL in 81 of the 206 patients (39.3%) was most likely related to the use of large-bore tubes in 55.8% of the patients. In a similar study, Elpern et al25 were able to identify 1 or more GRVs of at least 150 mL in 28.2% of 39 patients (more than three-fourths of whom had 18F multiport tubes). Although the percentage of high GRVs in patients with small-bore tubes in our study was relatively low, we most likely achieved a higher success rate than would have occurred if we had not used the air insufflation technique during the procedure.26
Two major factors affect the ability to withdraw fluid blindly from a feeding tube via a syringe. First, more fluid can generally be withdrawn from large-bore tubes with multiple ports than from small-bore feeding tubes with only 1 or 2 ports. In a study27 in which 645 concurrent measurements of GRV were made in 62 critically ill patients with 2 types of gastric tubes (10F polyurethane tubes and 14F–18F sump tubes), high volumes were detected 2 to 3 times more often in patients with large-bore sump tubes. Second, the tubes’ ports must be resting in a pool of gastric fluid. For example, Taylor et al28 reported that a single blind aspiration of GRV in 10 supine obese adults removed only 53% of the total gastric contents. In comparison, Cook-Sather et al29 were successful in removing 98% of the gastric contents from 17 fasting pediatric patients by using large-diameter (16F–18F) multiport tubes during 3 passes (once while the patients were supine, then again when they were in alternate side-lying positions).
As indicated earlier, 69 of the 81 patients (85.2%) identified as having 1 or more GRVs of at least 150 mL were fed within the first 24 hours of admission to the study. This finding supports the view that high GRVs tend to be more prevalent during the first few days of tube feeding.19
Relationship Between Aspiration and High GRVs
We found no consistent relationship between aspiration and GRVs; that is, as reported by other investigators,8 aspiration occurred relatively often when GRVs were consistently low. However, we found that the frequency of aspiration increased significantly as GRVs increased (perhaps indicating a greater level of gastroesophageal reflux; Figure 3). Xin et al30 found a significant positive correlation (0.932) between gastroesophageal reflux and GRVs measured by blind aspiration in 19 critically ill patients.
Although high GRVs may simply coincide with other risk factors for poor outcomes,31 our findings suggest that high GRVs have an independent effect on risk for aspiration when entwined with other known risk factors. Additional risk factors (a mean GCS score <9 and a mean head-of-bed elevation <30°) were significant in some of the analyses (Table 4). It is not surprising that a low level of consciousness increased the risk for aspiration, because low levels of consciousness interfere with patients’ ability to protect the airway from regurgitated gastric contents.32–35 Other investigators36 have shown that a low head-of-bed elevation predisposes to aspiration.
Strengths and Limitations of the Study
A total of 206 critically ill patients participated in the study for a total of 618 patient days, allowing us to measure 3286 GRVs and to collect 3203 tracheal specimens for pepsin analysis. Registered nurses serving as research assistants collected all of the data; thus, the data collection methods were consistent. In addition, the assay used to detect aspiration was specific for the aspiration of gastric contents (the type of aspiration that is of greatest concern in tube-fed patients). However, because this study was descriptive, we had no control over the types of tubes used or other treatment conditions. Further, the highly sensitive pepsin assay could be viewed as a limitation, because small-volume aspirations could be detected. We attempted to deal with this limitation by separating the subjects into 2 distinct groups; as described earlier, we defined frequent aspirators as patients whose tracheal secretions were positive for pepsin in at least 40% of the observations.
Conclusions
No consistent relationship exists between aspiration and GRVs. In our sample of 206 patients, aspiration occurred fairly often when GRVs were consistently low; however, it occurred significantly more often when GRVs were high. Measurement error is a major problem when attempting to detect high GRVs; we were able to detect proportionately more high GRVs in patients with large-bore tubes than in patients with small-bore tubes. Aspiration risk associated with GRVs probably should be evaluated in context with other risk factors (eg, level of consciousness, position of the head of the bed, sedation, vomiting, severity of illness). In a logistic regression analysis that included these factors, GRV categories identified as significant were having 2 or more GRVs of at least 200 mL, having 1 or more GRVs of at least 250 mL, and especially having 2 or more GRVs of at least 250 mL.
The descriptive nature of our study does not allow us to project significant GRV cutoff points for other adult populations. However, our findings tend to coincide with opinions expressed by several expert panels. For example, in the guidelines for enteral feeding in adult hospital patients, Stroud et al37 recommended that patients with questionable gastrointestinal motility have GRV measurements every 4 hours and that the feeding policy be reviewed if the volume exceeds 200 mL. The consensus statement38 of the North American Summit on Aspiration in the Critically Ill Patient indicated that residual volumes of 200 to 500 mL should prompt careful bedside evaluation and initiation of an algorithmic approach to reduce risk. Also, the statement indicated that although GRVs less than 200 mL appear to be well tolerated, evaluation of risk should be ongoing.
Our findings suggest that it is prudent to measure GRVs at 4-hour intervals in critically ill patients in an effort to determine which patients are at greatest risk for aspiration. Our findings also suggest that to increase the probability of detecting high GRVs, it may be wise to use large-bore multiport tubes during the first few days of tube feedings when high GRVs are most likely to occur. Although only about one-fifth to one-fourth of the high GRVs detected in our study were in patients with small-bore tubes, this number is of sufficient clinical concern to warrant measurement of GRVs in patients with small-bore tubes.
Acknowledgments
The authors dedicate this article to the memory of Dr Ray E. Clouse, who died shortly after the manuscript was completed. Dr Clouse was a quintessential physician and colleague as well as a beloved friend.
FINANCIAL DISCLOSURES
Funding for the study was provided by grant R01-NR0-5007 from the National Institute of Nursing Research.
To purchase electronic or print reprints, contact The InnoVision Group, 101 Columbia, Aliso Viejo, CA 92656. Phone, (800) 809-2273 or (949) 362-2050 (ext 532); fax, (949) 362-2049; e-mail, reprints@aacn.org
eLetters
Now that you’ve read the article, create or contribute to an online discussion about this topic using eLetters. Just visit www.ajcconline.org and click “Respond to This Article” in either the full-text or PDF view of the article.
Contributor Information
Norma A. Metheny, Professor of nursing at Saint Louis University School of Nursing, St Louis, Missouri
Lynn Schallom, Clinical nurse specialist at Barnes-Jewish Hospital, St Louis, Missouri.
Dana A. Oliver, Biostatistician at the Cancer Center of Saint Louis University Medical Center, St Louis, Missouri
Ray E. Clouse, Professor of medicine at Washington University School of Medicine, St Louis, Missouri
References
- 1.Guenter P, Silkroski M. Tube Feeding: Practical Guidelines and Nursing Protocols. Gaithersburg, MD: Aspen Publishers; 2001. [Google Scholar]
- 2.Pullen RL., Jr Measuring gastric residual volume. Nursing. 2004;34(4):18. doi: 10.1097/00152193-200404000-00017. [DOI] [PubMed] [Google Scholar]
- 3.Mayer AP, Durward A, Turner C, et al. Amylin is associated with delayed gastric emptying in critically ill children. Intensive Care Med. 2002;28(3):336–340. doi: 10.1007/s00134-002-1224-7. [DOI] [PubMed] [Google Scholar]
- 4.Kompan L, Vidmar G, Spindler-Vesel A, Pecar J. Is early enteral nutrition a risk factor for gastric intolerance and pneumonia? Clin Nutr. 2004;23(4):527–532. doi: 10.1016/j.clnu.2003.09.013. [DOI] [PubMed] [Google Scholar]
- 5.Mihatsch WA, von Schoenaich P, Fahnenstich H, et al. The significance of gastric residuals in the early enteral feeding advancement of extremely low birth weight infants. Pediatrics. 2002;109(3):457–459. doi: 10.1542/peds.109.3.457. [DOI] [PubMed] [Google Scholar]
- 6.Lord L, Harrington M The A.S.P.E.N. Nutrition Support Practice Manual. 2. Silver Spring, MD: The American Society for Parenteral and Enteral Nutrition; 2005. [Google Scholar]
- 7.Potter P, Perry AG. Fundamentals of Nursing. 6. St Louis, MO: Mosby; 2005. [Google Scholar]
- 8.McClave SA, Lukan JK, Stefater JA, et al. Poor validity of residual volumes as a marker for risk of aspiration in critically ill patients. Crit Care Med. 2005;33(2):324–330. doi: 10.1097/01.ccm.0000153413.46627.3a. [DOI] [PubMed] [Google Scholar]
- 9.Chang WK, McClave SA, Hsieh CB, Chao YC. Gastric residual volume (GRV) and gastric contents measurement by refractometry. JPEN J Parenter Enteral Nutr. 2007;31(1):63–68. doi: 10.1177/014860710703100163. [DOI] [PubMed] [Google Scholar]
- 10.Lin HC, Van Citters GW. Stopping enteral feeding for arbitrary gastric residual volume may not be physiologically sound: results of a computer simulation model. JPEN J Parenter Enteral Nutr. 1997;21(5):286–289. doi: 10.1177/0148607197021005286. [DOI] [PubMed] [Google Scholar]
- 11.Tarling MM, Toner CC, Withington PS, Baxter MK, Whelpton R, Goldhill DR. A model of gastric emptying using paracetamol absorption in intensive care patients. Intensive Care Med. 1997;23(3):256–260. doi: 10.1007/s001340050325. [DOI] [PubMed] [Google Scholar]
- 12.Goldhill DR, Toner CC, Tarling MM, Baxter K, Withington PS, Whelpton R. Double-blind, randomized study of the effect of cisapride on gastric emptying in critically ill patients. Crit Care Med. 1997;25(3):447–451. doi: 10.1097/00003246-199703000-00013. [DOI] [PubMed] [Google Scholar]
- 13.Hardman JG, O’Connor PJ. Predicting gastric contents following trauma: an evaluation of current practice. Eur J Anaesthesiol. 1999;16(6):404–409. doi: 10.1046/j.1365-2346.1999.00513.x. [DOI] [PubMed] [Google Scholar]
- 14.Johnson JL, Hirsch CS. Aspiration pneumonia: recognizing and managing a potentially growing disorder. Postgrad Med. 2003;113(3):99–102. 105–106, 111–112. doi: 10.3810/pgm.2003.03.1390. [DOI] [PubMed] [Google Scholar]
- 15.Lee JS, Auyeung TW. A comparison of two feeding methods in the alleviation of diarrhoea in older tube-fed patients: a randomised controlled trial. Age Ageing. 2003;32(4):388–393. doi: 10.1093/ageing/32.4.388. [DOI] [PubMed] [Google Scholar]
- 16.Davies AR, Froomes PR, French CJ, et al. Randomized comparison of nasojejunal and nasogastric feeding in critically ill patients. Crit Care Med. 2002;30(3):586–590. doi: 10.1097/00003246-200203000-00016. [DOI] [PubMed] [Google Scholar]
- 17.Pruitt B, Jacobs M. Best-practice interventions: how can you prevent ventilator-associated pneumonia? Nursing. 2006;36(2):36–41. [PubMed] [Google Scholar]
- 18.Nguyen NQ, Fraser RJ, Chapman MJ, et al. Feed intolerance in critical illness is associated with increased basal and nutrient-stimulated plasma cholecystokinin concentrations. Crit Care Med. 2007;35(1):82–88. doi: 10.1097/01.CCM.0000250317.10791.6C. [DOI] [PubMed] [Google Scholar]
- 19.Mentec H, Dupont H, Bocchetti M, Cani P, Ponche F, Bleichner G. Upper digestive intolerance during enteral nutrition in critically ill patients: frequency, risk factors, and complications. Crit Care Med. 2001;29(10):1955–1961. doi: 10.1097/00003246-200110000-00018. [DOI] [PubMed] [Google Scholar]
- 20.Metheny NA, Dahms TE, Chang YH, Stewart BJ, Frank PA, Clouse RE. Detection of pepsin in tracheal secretions after forced small-volume aspirations of gastric juice. JPEN J Parenter Enteral Nutr. 2004;28(2):79–84. doi: 10.1177/014860710402800279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Lemos J, Tweeddale M, Chittock D. Measuring quality of sedation in adult mechanically ventilated critically ill patients: The Vancouver Interaction and Calmness Scale. Sedation Focus Group. J Clin Epidemiol. 2000;53(9):908–919. doi: 10.1016/s0895-4356(00)00208-0. [DOI] [PubMed] [Google Scholar]
- 22.Metheny NA, Clouse RE, Chang YH, Stewart BJ, Oliver DA, Kollef MH. Tracheobronchial aspiration of gastric contents in critically ill tube-fed patients: frequency, outcomes, and risk factors. Crit Care Med. 2006;34(4):1–9. doi: 10.1097/01.CCM.0000206106.65220.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van der Voort PH, Zandstra DF. Enteral feeding in the critically ill: comparison between the supine and prone positions: a prospective crossover study in mechanically ventilated patients. Crit Care. 2001;5(4):216–220. doi: 10.1186/cc1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pinilla JC, Samphire J, Arnold C, Liu L, Thiessen B. Comparison of gastrointestinal tolerance to two enteral feeding protocols in critically ill patients: a prospective, randomized controlled trial. JPEN J Parenter Enteral Nutr. 2001;25(2):81–86. doi: 10.1177/014860710102500281. [DOI] [PubMed] [Google Scholar]
- 25.Elpern EH, Stutz L, Peterson S, Gurka DP, Skipper A. Outcomes associated with enteral tube feedings in a medical intensive care unit. Am J Crit Care. 2004;13(3):221–227. [PubMed] [Google Scholar]
- 26.Metheny N, Reed L, Worseck M, Clark J. How to aspirate fluid from small-bore feeding tubes. Am J Nurs. 1993;93(5):86–88. [PubMed] [Google Scholar]
- 27.Metheny NA, Stewart J, Nuetzel G, Oliver D, Clouse RE. Effect of feeding-tube properties on residual volume measurements in tube-fed patients. JPEN J Parenter Enteral Nutr. 2005;29(3):192–197. doi: 10.1177/0148607105029003192. [DOI] [PubMed] [Google Scholar]
- 28.Taylor WJ, Champion MC, Barry AW, Hurtig JB. Measuring gastric contents during general anaesthesia: evaluation of blind gastric aspiration. Can J Anaesth. 1989;36(1):51–54. doi: 10.1007/BF03010887. [DOI] [PubMed] [Google Scholar]
- 29.Cook-Sather SD, Liacouras CA, Previte JP, Markakis DA, Schreiner MS. Gastric fluid measurement by blind aspiration in paediatric patients: a gastroscopic evaluation. Can J Anaesth. 1997;44(2):168–172. doi: 10.1007/BF03013006. [DOI] [PubMed] [Google Scholar]
- 30.Xin Y, Dai N, Zhao L, Wang JG, Si JM. The effect of famotidine on gastroesophageal and duodeno-gastro-esophageal refluxes in critically ill patients. World J Gastroenterol. 2003;9(2):356–358. doi: 10.3748/wjg.v9.i2.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wolf SE, Jeschke MG, Rose JK, Desai MH, Herndon DN. Enteral feeding intolerance: an indicator of sepsis-associated mortality in burned children. Arch Surg. 1997;132(12):1310–1313. doi: 10.1001/archsurg.1997.01430360056010. [DOI] [PubMed] [Google Scholar]
- 32.Moulton C, Pennycook AG. Relation between Glasgow coma score and cough reflex. Lancet. 1994;343(8908):1261–1262. doi: 10.1016/s0140-6736(94)92155-5. [DOI] [PubMed] [Google Scholar]
- 33.Smith Hammond CA, Goldstein LB, Zajac DJ, Gray L, Davenport PW, Bolser DC. Assessment of aspiration risk in stroke patients with quantification of voluntary cough. Neurology. 2001;56(4):502–506. doi: 10.1212/wnl.56.4.502. [DOI] [PubMed] [Google Scholar]
- 34.Nishino T. Physiological and pathophysiological implications of upper airway reflexes in humans. Jpn J Physiol. 2000;50(1):3–14. doi: 10.2170/jjphysiol.50.3. [DOI] [PubMed] [Google Scholar]
- 35.Adnet F, Baud F. Relation between Glasgow Coma Scale and aspiration pneumonia. Lancet. 1996;348(9020):123–124. doi: 10.1016/s0140-6736(05)64630-2. [DOI] [PubMed] [Google Scholar]
- 36.Ibanez J, Penafiel A, Raurich JM, Marse P, Jorda R, Mata F. Gastroesophageal reflux in intubated patients receiving enteral nutrition: effect of supine and semirecumbent positions. JPEN J Parenter Enteral Nutr. 1992;16(5):419–422. doi: 10.1177/0148607192016005419. [DOI] [PubMed] [Google Scholar]
- 37.Stroud M, Duncan H, Nightingale J British Society of Gastroenterology. Guidelines for enteral feeding in adult hospital patients. Gut. 2003;52(suppl 7):vii1–vii12. doi: 10.1136/gut.52.suppl_7.vii1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McClave SA, DeMeo MT, DeLegge MH, et al. North American Summit on Aspiration in the Critically Ill Patient: consensus statement. JPEN J Parenter Enteral Nutr. 2002;26(6 suppl):S80–S85. doi: 10.1177/014860710202600613. [DOI] [PubMed] [Google Scholar]