Abstract
The corneal epithelium is exposed to reactive oxygen species that are potentially deleterious to nuclear DNA. However, our previous studies show that corneal epithelial cells have a novel, developmentally-regulated mechanism for protection from such damage that involves having the iron-sequestering molecule, ferritin, in the nucleus. Nuclear localization of ferritin is achieved through the action of a tissue-specific nuclear transporter, ferritoid, which is itself a ferritin family member. Here we show that during development ferritoid appears prior to ferritin. At this time ferritoid is cytoplasmic suggesting that its nuclear transport function requires an interaction with ferritin. To examine the developmental regulation of these two interacting components, cultured corneas were treated with the iron chelator deferoxamine. The results show that while iron-mediated translational regulation is involved in the synthesis of both molecules, ferritoid is also transcriptionally regulated, demonstrating that these family members – whose functions depend upon one another – are regulated differently.
Keywords: ferritoid, ferritin, cornea, iron, nucleus
Introduction
The corneal epithelium (CE) is constantly exposed to reactive oxygen species (ROS) such as superoxide (O2−), hydrogen peroxide (H2O2), and the hydroxyl radical (˙OH). In the embryo, ROS can arise from endogenous sources such as cellular oxidative metabolism, and in the adult they can arise from environmental sources such as molecular oxygen and UV light. During development, the CE is exposed to amniotic fluid, which has sufficient ROS that their activity can be detected, for example, by the lipid-peroxidation products they produce (Longini et al., 2007). In the adult, the CE is constantly exposed to UV light that, through the generation of ROS, can damage a wide variety of macromolecules including DNA. Damage to DNA is a major factor in epidermal cancers (Hart et al., 1977); however, CE cells seem to be refractory to such damage, as primary cancers of these cells are extraordinarily rare (Smolin and Thoft, 1987).
For CE cells, our studies using the chicken embryo suggest that one form of protection from oxidative damage to DNA involves having the iron-sequestering molecule, ferritin, in a nuclear location rather than the cytoplasmic localization it has in other cell types (Cai et al., 1997). This nuclear ferritin has been demonstrated to protect against both H2O2-mediated damage (Cai et al., 2008), which would occur in the embryo, and UV-induced oxidative damage, which would occur in the adult (Cai et al., 1998). The protection is likely to be afforded, at least in part, by the sequestration of iron, as free iron greatly exacerbates the deleterious effects of UV and H2O2-induced ROS by catalyzing the Fenton reaction (Stohs and Bagchi, 1995).
In the Fenton reaction, Fe2+ catalyzes the conversion of H2O2 in the presence of UV light to ˙OH which, although it acts over a short distance, is the most active ROS (Janssen et al., 1993). Therefore, some of the damage to nuclear DNA is likely to result from the presence of free iron in the nucleus, although the exact function of iron in the nucleus is unknown (Meneghini, 1997). It has been reported that free iron can bind to specific sites on DNA, and that this iron can generate Fenton-derived ˙OH that specifically cleave the DNA at these sites (Henle et al., 1996;Henle et al., 1999;Luo et al., 1996).
One parameter required for the protective function of ferritin is that the molecule itself undergoes nuclear transport. The nuclear ferritin in the CE cells is composed of the same ferritin heavy chain as the cytoplasmic ferritin found in other cell types (Cai et al., 1997). Thus it has no consensus nuclear localization signal (NLS). Instead, other of our studies suggest that CE cells have a tissue-specific transporter for ferritin (Millholland et al., 2003). We have termed this transporter ferritoid for its similarities to ferritin in amino acid sequence and in structure, as predicted by molecular modelling. In situ hybridization shows that ferritoid mRNA is tissue-specific for the CE; and from work done largely with transfected COS-1 cells, ferritoid meets all of the functional criteria for a nuclear transporter of ferritin: it contains a functional SV40-type NLS, and it is capable of binding to ferritin and transporting it into the nucleus (Millholland et al., 2003).
Another parameter required for this protective mechanism is that ferritoid and ferritin be regulated in a manner ensuring that they are capable of interacting with one another. For ferritin, our previous studies suggest that in CE cells its developmental regulation is largely at the translational level – as ferritin mRNA is present at least four days before the protein is detectable. Also, at least one factor involved in this regulation is iron, as the iron chelator deferoxamine (DFX) can block the appearance of ferritin (Cai et al., 1997). These observations are consistent with the involvement of an iron response element (IRE) located in the 5’untranslated region (UTR) of the ferritin mRNA – whose role in the translational regulation of cytoplasmic ferritin has been extensively characterized (Harrison and Arosio, 1996;Hentze et al., 1987).
For the regulation of ferritoid, our previous information suggests, indirectly, that its developmental appearance occurs either concomitant with, or prior to, the synthesis of ferritin. This is based on the observation that when ferritin first becomes detectable in the CE, it is nuclear – suggesting that the transporter, ferritoid, is also present at this time (Cai et al., 1997).
In the present study, we examined the developmental relationships between the expression of ferritoid and ferritin in corneas from precisely staged embryos – using immunofluorescence to evaluate the expression of the proteins and quantitative RT-PCR for their mRNAs. We also compared the expression pattern of ferritoid to that of a marker of CE differentiation (cytokeratin K3) and determined the subcellular localization of ferritoid when (1) the differentiation of CE cells progresses in vivo, and (2) its synthesis, and that of ferritin, is experimentally manipulated using DFX. Our results show that during normal development in vivo ferritoid appears slightly before ferritin, and at this early time point, ferritoid (in the absence of ferritin) is cytoplasmic, suggesting that for nuclear transport of either protein to occur an interaction between both is necessary. Furthermore, experimental manipulation of CE cells in culture using DFX treatment shows that iron is a necessary regulatory component for ferritoid as well as for ferritin, as neither protein appears in the presence of DFX. This co-regulation appears to be translationally-mediated, as the mRNAs for both ferritoid and ferritin remain constant in the presence of DFX. Upon release from DFX inhibition, the sequence of appearance of ferritoid and ferritin proteins recapitulates what is observed during development (i.e., ferritoid before ferritin), and further supports the requirement for both proteins in effecting nuclear translocation.
Experimental Procedures
Cell and organ culture
Chicken eggs (white leghorn) were obtained from Hyline (Elizabethtown, PA) and incubated at 38°C. Embryos were removed, rinsed in Hank's balanced saline solution (Invitrogen), and staged both by chronological time of incubation and by the criteria of Hamburger and Hamilton (Hamburger and Hamilton, 1951).
Primary cultures of CE cells were initiated as described previously (Cai et al., 1997). For corneas <E10, epithelia and stroma were separated using trypsin (0.25% at 4°C, 20 minutes), and the cells of the CE were then liberated by gentle pipetting. For embryos >E10, the corneas were separated into epithelia and stroma by treatment with 0.5% Dispase in PBS (4°C, 1 hour), and then the epithelia were rinsed with PBS and digested further (0.25% trypsin at 37°C, 5 minutes) before dissociation by pipetting.
CE cells were cultured in a 1:1 mixture of Dulbecco’s Modified Eagle Medium (DMEM) and F-12 (Invitrogen), supplemented with 10% fetal bovine serum (heat-inactivated at 65°C, 30 minutes; Hyclone), 1% chicken serum (Invitrogen), 5 µg/mL insulin (Sigma), and 10 ng/mL each of human recombinant EGF, penicillin and streptomycin (Invitrogen).
For organ culture, whole corneas were removed from E8 or E12 embryos, rinsed in Hank’s balanced saline solution, and placed into wells of 12-well tissue culture treated plates (BD Biosciences), 2 corneas per well, endothelium side down for 48 hours.
For in vitro studies on the involvement of iron on the expression of ferritoid and ferritin, the iron chelator DFX (100 µM; Sigma) was added to the medium. As a control for possible toxicity, equimolar concentrations of DFX and ferrous sulfate (Sigma) were used. To assess the effects of lysosomal degradation in vitro, the medium was supplemented with the lysosomal inhibitors Leupeptin (5 µg/mL; Sigma) or Chloroquine (100 µM; Sigma).
Immunofluorescence analyses
Anterior eyes or cultured corneas were removed, fixed in 4% paraformaldehyde (20 minutes on ice), and rinsed with PBS. They were then cryoprotected in 8% sucrose (30 minutes to overnight at 4°C) and embedded in Tissue Tek OCT. Frozen sections (10 µm) were cut using a cryostat (Microm), and were mounted on 12-spot slides (ThermoShandon Scientific) coated with BIOBOND (Electron Microscopy Sciences).
Cultured cells were fixed in 4% paraformaldehyde (10 minutes on ice), rinsed with PBS, and permeabilized (100% methanol for 10 minutes at −20°C).
Indirect immunofluorescence was performed as described previously (Cai et al., 1997). Fixed cells or tissue sections were rinsed with PBS, free aldehydes were quenched (50 mM lysine, 50 mM glycine in PBS, 10 minutes at room temperature), and samples were treated with blocking solution (1% BSA and 10% chicken serum in PBS, 30 minutes at room temperature). Samples were then incubated in primary antibody (overnight at 4°C), rinsed with PBS (4 times for 5 minutes each at room temperature), and incubated in secondary antibody (1.5 hours at room temperature). For double-labelling, samples were incubated in each primary antibody separately (16–18 hours at 4°C), and rinsed with PBS between antibodies. Then samples were incubated with a mixture of secondary antibodies against the primary antibodies or streptavidin for the anti-ferritoid antibody (see below) for 1.5 hours at room temperature. Samples were then rinsed with PBS and mounted in 95% glycerol in PBS containing Hoechst 33258 to stain nuclei (100 ng/mL; Sigma).
The primary antibodies were: a monoclonal antibody against chicken ferritin (6D11, undiluted) (Zak and Linsenmayer, 1983), a monoclonal antibody against keratin K3 (AE5, 1:10 dilution), a gift from Dr. T.T. Sun, a monoclonal antibody against type X collagen (X-AC9, undiluted) (Schmid and Linsenmayer, 1985), and a biotinylated IgY antibody against a synthetic peptide of ferritoid (1:250 dilution) (Aves Laboratories see Experimental Results). The secondary antibodies used for the monoclonal antibodies were either rhodamine or FITC-conjugated goat anti-mouse IgG (Pierce), and for the biotinylated IgY antibody, TRITC-conjugated NeutrAvidin (Molecular Probes).
The samples were visualized by conventional immunofluorescence using a Nikon Fluophot microscope, equipped with a SPOT RT real time CCD camera (Diagnostic Instruments, Inc.), and by confocal microscopy using a Zeiss LSM510 laser scanning microscope and detector.
Conventional and quantitative RT-PCR
Freshly dissected tissues were rinsed with DEPC-treated PBS and stored at −80°C. mRNA was isolated using a Micro FastTrack kit (Invitrogen) and single-stranded cDNA was synthesized with SuperScript III reverse transcriptase (Invitrogen; 5 ng mRNA). PCR amplification was performed for 28 cycles using an MJ Research thermocycler with the following primers: for chicken ferritoid, primers GAGTTCAGAACAGGCATAGAGC (positions 592–613) and GTGGCAGCGTGGCAGT (positions 752–766); for chicken ferritin, primers ACATCAAGAAACCGGATCGTG (positions 402–422) and CCAATTTGTGCAGCTCTAACA (positions 492–512) ; and for chicken β-actin, primers ATATTGCTGCGCTCGTTGTTGTTGAC (positions 79–99) and GCATGGGGGAGGGCGTAG (positions 572–589).
Real-time quantitative RT-PCR was performed using a QuantiTect SYBR Green PCR kit (Qiagen). Reactions were done in triplicate for each pair of primers, using a Stratagene Mx4000 sequence detector, and the results were analyzed using Microsoft Excel.
Preparation of protein lysates
Total protein lysates
CE tissue was lysed for 60 minutes in ice-cold RIPA buffer (10mM Tris pH 7.4, 300 mM NaCl, 1% sodium deoxycholate, 1% Triton X-100), containing EDTA-free Complete Protease Inhibitors (Roche). DNA was sheared by passing the lysate through an 18 gauge needle, and cell debris was removed by centrifugation (10 minutes at 14400 rpm). The supernatant was stored at −20°C.
Protein lysates enriched for ferritin/ferritoid
Tissue lysates were enriched for the ferritoid and ferritin using a heat treatment procedure [adapted from (Mete et al., 2005)]. The frozen tissues were thawed on ice for approximately 30 minutes. Then the CE tissue from 4 dozen corneas was resuspended in 200 µL of 50 mM Hepes buffer, pH 7.4, and homogenized by sonication on ice (2 × 10 seconds). Samples were then heated to 70°C for 10 minutes, cooled on ice for 30 minutes, and centrifuged twice at 16000 rpm for 30 minutes each. The supernatant containing the enriched ferritin/ferritoid complexes was collected and stored at −20°C.
Immunoprecipitation and Western blot
Lysates were incubated with the primary antibody (either 1µg of purified hybridoma 6D11 or anti-ferritoid IgY per 100 µg protein lysate) for 1 hour at 4°C with rocking. Then 20µL of either Protein A/G+ Agarose (for 6D11; EMD Biosciences) or donkey anti-IgY-coupled agarose (for IgY; Gallus Immunotech, Inc.) was added per 100 µg lysate and incubated overnight at 4°C with rocking. The immunoprecipitates were collected by centrifugation (2500 rpm for 5 minutes at 4°C), rinsed twice with RIPA and twice with PBS, and resuspended in 40µl of PBS plus 8 µL 6x Laemmli’s sample buffer (375mM Tris pH 6.8, 6% SDS, 48% glycerol, 9% MSH, 0.03% Bromophenol). Samples were heated (95°C, 5 minutes) to denature proteins, spun briefly, and electrophoresed (12% SDS-PAGE).
Proteins separated by SDS-PAGE were transferred to polyvinylidene difluoride (PVDF) membranes (BioRad). Western blots were performed using the following protocol: PVDF membranes were treated with blocking solution (5% non-fat dry milk in PBS containing 0.1% Tween-20) for one hour at room temperature with shaking. Primary antibodies were diluted in 25% blocking solution (1:10000 for IgY; 1:500 for purified 6D11), and incubated overnight at 4°C with shaking. Membranes were then washed (4 × 10 minutes) with PBST (PBS plus 0.1% Tween-20) to remove unbound antibody. Secondary antibodies [HRP-conjugated streptavidin (Sigma) at 1:5000 for IgY or HRP-conjugated goat anti-mouse IgG (Pierce) at 1:10000 for 6D11] were diluted in PBST and placed onto membranes for 1 hour at room temperature. Membranes were washed again in PBST as above, and incubated in chemiluminescent substrate (Denville Scientific) for 5 minutes prior to exposure to photographic film.
Results
Generation and characterization of an anti-ferritoid antibody
Previously we (Millholland et al., 2003) obtained results suggesting that ferritoid was the nuclear transporter for ferritin in CE cells. As we did not have an antibody against ferritoid, many of these studies were performed using COS-1 cells co-transfected with epitope-tagged constructs for both ferritoid and ferritin. This approach, however, was limited in that it could not provide information on the behavior of the endogenous ferritoid protein in the CE cells themselves – either in vivo during corneal development or experimentally in cell and organ cultures in vitro.
The anti-ferritoid antibody was generated in chickens from a sequence in ferritoid that shares little homology with ferritin (Fig. 1A, yellow box). This peptide was used to immunize hens, with the resulting IgY antibody purified from the yolks of eggs (performed by Aves Laboratories). For immunoprecipation (IP) the antibody was used directly. For immunofluorescence and Western blot (WB), however, to eliminate potential non-specific staining from endogenous chicken immunoglobulin within the samples, the anti-ferritoid antibody was biotinylated, and visualized using fluorescently-labelled or HRP-labelled streptavidin.
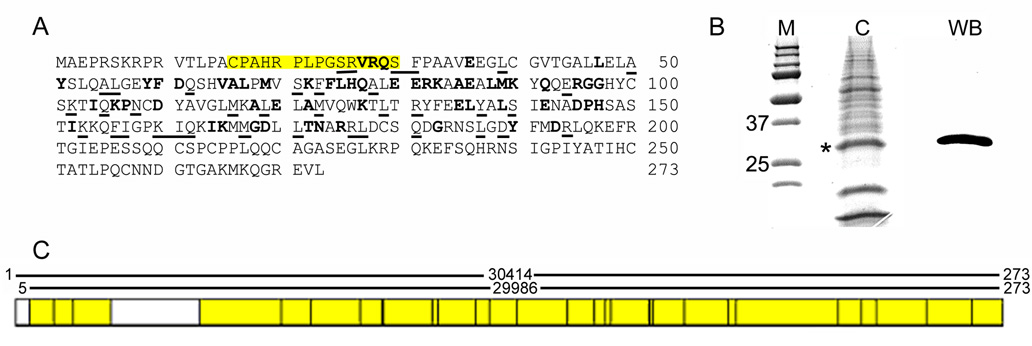
Figure 1. Characterization of an anti-ferritoid antibody.
(A) Ferritoid sequence showing location of peptide used to immunize hens (yellow box) and conserved residues between ferritoid AAN70987 and ferritin P08267 (bold = identity; underline = conserved); (B) Coomassie stain (lane C) and Western blot for ferritoid (lane WB) of E15 CE tissue lysates enriched for ferritin complex; (C) Mass spectroscopy results of the 30 kD band. Regions of the ferritoid sequence identified by mass spectroscopy are highlighted in yellow.
The specificity of the antibody for ferritoid was determined by Western blot of a protein lysate from E15 CE tissue, a time when the CE has initiated transcription of ferritoid mRNA (Millholland et al., 2003). One of the analytical procedures we planned to use was mass spectroscopy of material from SDS-PAGE gels; therefore, to decrease the extraneous non-ferritoid and ferritin proteins in the samples, we used lysates that had been subjected to a heat denaturation procedure designed to enrich for ferritin [(Mete et al., 2005), see M&M], and which we have observed can also be used to enrich for ferritoid/ferritin complexes (Nurminskaya et al., unpublished observations). Denaturing PAGE of the enriched lysate, when analyzed by Western blot with the anti-ferritoid antibody (Fig. 1B, lane WB), shows a single strong band at 30 kD.
The 30 kD band is the molecular weight predicted for ferritoid from the cDNA sequence, and is also the molecular weight of the protein synthesized by COS-1 cells transfected with an epitope-tagged construct for ferritoid (Millholland et al., 2003). In addition, to definitively identify the 30 kD band in the enriched lysate as ferritoid, the corresponding band from a Coomassie-stained gel (asterisk in Fig. 1B, lane C) was excised and subjected to HPLC - mass spectroscopic analysis. A total of 97 peptides covering 89% of the ferritoid amino acid sequence, were identified (Fig. 1C, yellow boxes).
Ferritoid and ferritin expression in the CE of mature embryos
As described previously (Cai et al., 1997), in CE cells of embryos older than E11, ferritin has a nuclear localization. If ferritoid is responsible for the nuclear transport of ferritin, then ferritoid should also be detected at this time. To examine this – and to determine if and how during embryonic development ferritoid and ferritin are temporally and spatially related to one another – we looked for the expression of both molecules in sectioned corneas from embryos at closely-spaced temporal intervals between E10 and E11. We also examined the behavior of these molecules in cultures of CE cells, in which their synthesis can be manipulated using DFX (see below). For comparison we analyzed the expression of keratin 3 (K3), a marker of differentiated CE cells (Schermer et al., 1986).
Initial analyses were performed on sections of E15 anterior eyes (which include cornea, sclera, iris, and ciliary body), when nuclear ferritin has been shown to be clearly detectable in CE cells (Cai et al., 1997). These analyses employed double-label immunofluorescence for ferritoid and ferritin (Fig. 2 A–C), and for ferritoid and K3 (Fig. 2 E–G). For comparison, nuclei are stained with Hoechst (Fig. 2D and H).
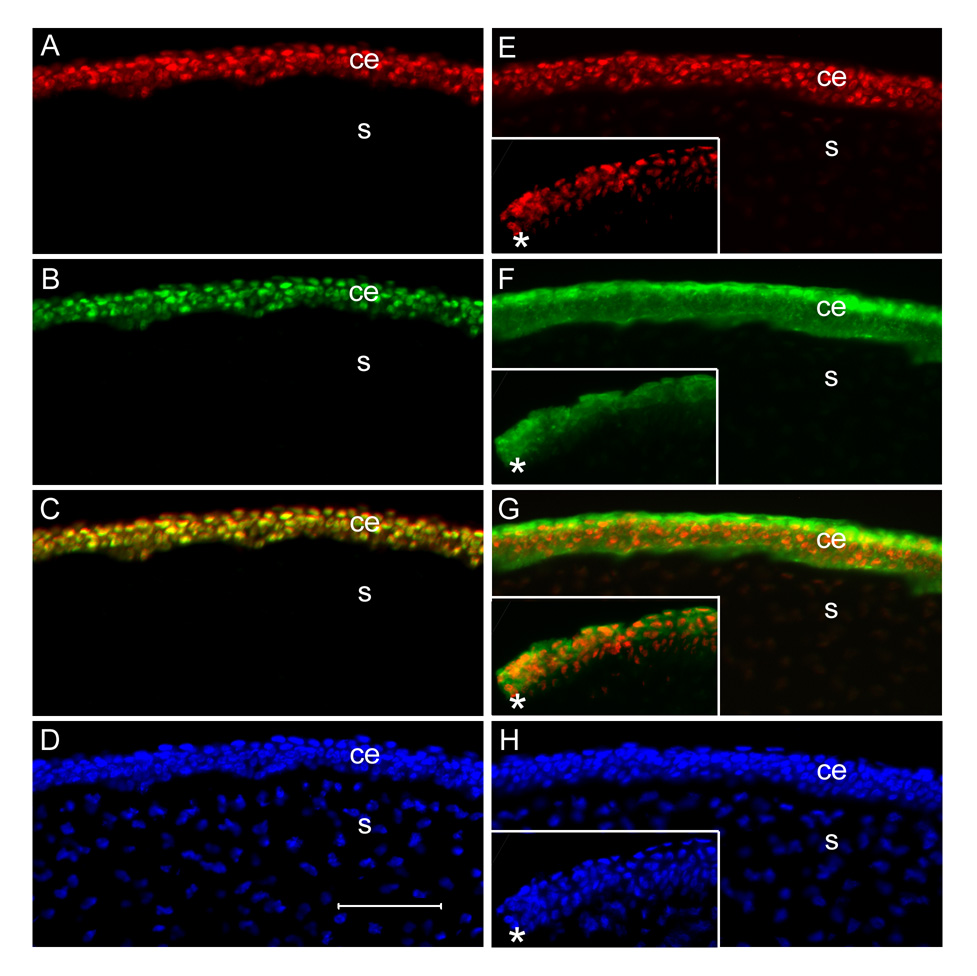
Figure 2. Immunofluorescence micrographs of sections of E15 central corneas showing the CE (ce) and stroma (s).
A–D are double-labelled for ferritoid (A) and ferritin (B), with a merged image shown in C and Hoechst staining in D. E–H are double-labelled for ferritoid (E) and cytokeratin K3 (F) with a merged image shown in G and Hoechst staining in H. The inserts in E–H show staining with these antibodies at the extreme periphery of the cornea with the cornea-scleral junction demarcated by an asterisk. Scale bar in D = 50 µm.
As can be seen in figures 2 A–D – which are micrographs of the central cornea – throughout the length of the CE, all of the cells contain nuclear ferritoid (Fig. 2A) and ferritin (Fig. 2B). Consistent with ferritoid being obligatory for nuclear transport to occur, there are no CE cells that have nuclear ferritin that do not also have nuclear ferritoid (Fig. 2C).
That ferritoid is restricted to the CE was verified by comparing its expression (Fig. 2E) to that of the CE-specific cytokeratin, K3 (Fig. 2F). As can be seen in these figures of the central cornea, and also the figure in which they are merged (Fig. 2G), both ferritoid and K3 are present in the CE (ce) but are not in the stroma (s). Consistent with ferritoid being exclusive to the CE, its expression ends at the cornea-scleral junction, as does that of K3 – shown by the asterisks in the insets in figures 2E–2G.
Temporal acquisition of ferritoid and ferritin during early development
Our previous immunohistochemical observations show that when ferritin protein first becomes detectable in the CE at E11 (the earliest time examined), it is nuclear (Cai et al., 1997). Therefore, the nuclear transporter ferritoid should also be present at this time. This could result either from the initiation of synthesis of ferritoid occurring before that of ferritin, or by the coordinated initiation of synthesis of both proteins. To distinguish between these possibilities, we performed immunofluorescence analyses on corneas harvested at six-hour intervals from E10 to E11, coupled with staging (Hamburger and Hamilton, 1951). In these studies – as well as in others described later – to obtain an overview of the immuno-labelling we first employed visualization with conventional fluorescence microscopy. Then for areas considered to be of interest, and for those requiring increased resolution, we employed laser confocal microscopy.
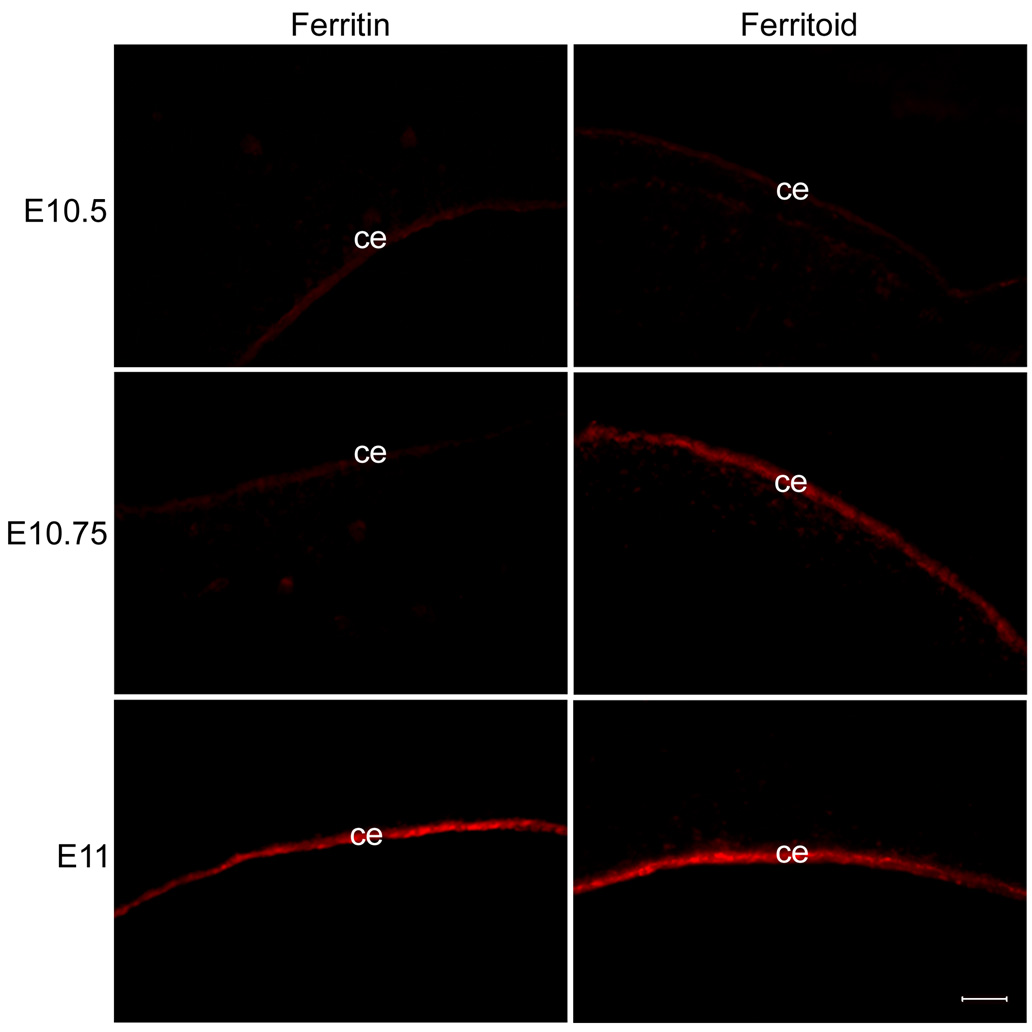
By conventional fluorescence microscopy (Fig. 3), at E10.5 neither ferritoid nor ferritin is detectable in the CE, with the only fluorescence being the low level of background autofluorescence that is always observed in the CE (data not shown). Six hours later (Fig. 3, E10.75), there is now a strong signal for ferritoid in the CE, but still no detectable ferritin. The signal for ferritin does not appear for another six hours (Fig. 3, E11), at which time both proteins are strongly expressed. Thus, at the time ferritin is expressed in the CE, ferritoid is already present to facilitate its nuclear transport.
Figure 3. Staining of corneas for ferritoid and ferritin at 6-hour intervals from E10.5 to E11.
Ferritoid is present at E10.75, but ferritin is not detected until E11. The very light fluorescence seen in the CE stained for ferritin at E10.5 and E10.75 and for ferritoid at E10.5 is non-specific background. Scale bar in bottom right panel = 10 µm.
An additional observation from these analyses is that when ferritoid is first detectable, but ferritin has not yet appeared (Fig. 3, E10.75), ferritoid appears to be cytoplasmic rather than nuclear. However, once ferritoid and ferritin are both present, both appear to be nuclear (Fig. 3, E11). To examine this more closely, we employed laser confocal microscopy (Fig. 4).
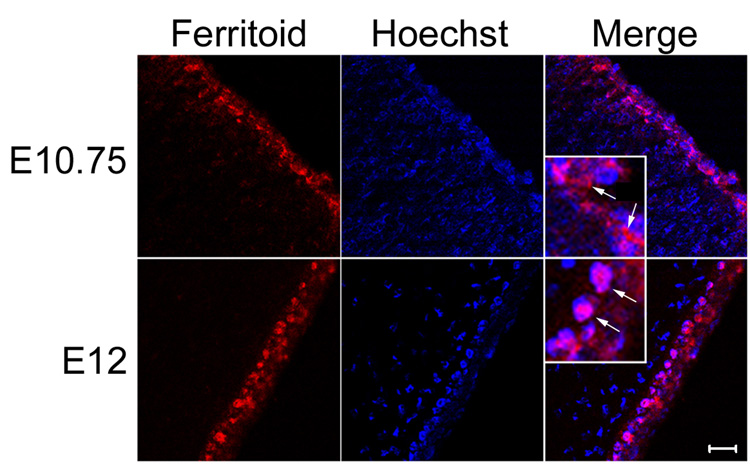
Figure 4. Confocal micrographs of ferritoid at stages before the appearance of ferritin (E10.75), and after the appearance of ferritin (E12).
When these images are merged with that of Hoechst staining, it can be seen that at E10.75 the ferritoid in CE cells is cytoplasmic and at E12 it is nuclear (the inserts are enlarged images in which the arrows point to cytoplasmic staining at E10.75 and nuclear staining at E12). Scale bar in bottom right panel = 10 µm.
At the time when only ferritoid is expressed (Fig. 4, E10.75), it is cytoplasmic, as compared to the Hoechst staining used to visualize nuclei. This localization is seen more advantageously in the merged image shown in figure 4 (E10.75), and its inset, in which the cytoplasmic ferritoid is indicated by arrows. However, when both ferritoid and ferritin are present (Fig. 4, E12), ferritoid is now nuclear, co-localizing with the Hoechst staining. This can be seen in the merged image (E12) and in its inset, where arrows indicate nuclear ferritoid. These results suggest that to activate the nuclear localization capability of ferritoid, an interaction between ferritoid and ferritin is necessary; and then ferritoid, in turn, mediates the transport of ferritin into the nucleus (see below and Discussion).
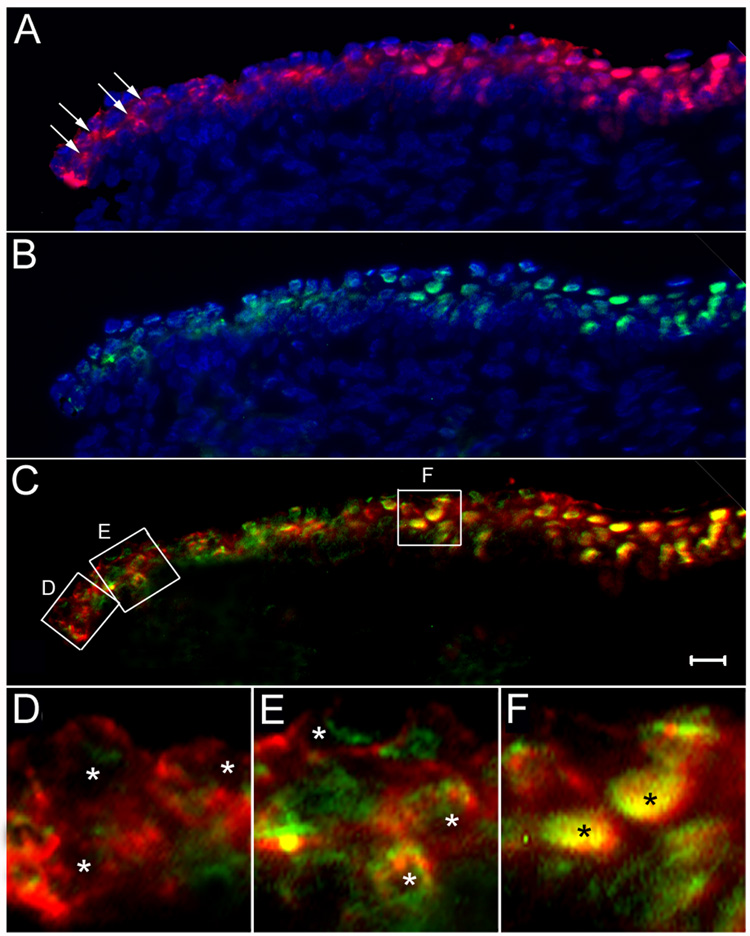
Spatial acquisition of ferritoid and ferritin at the limbal/corneal junction
The mature CE undergoes constant renewal, during which cells from the pericorneal, limbal region migrate centripetally into the CE proper as they undergo differentiation into mature CE cells (Schermer et al., 1986). To determine whether the expression of ferritoid precedes that of ferritin – as was observed during development – also occurs during this process of CE differentiation in mature corneas, we examined the periphery of the CE in mature, E15 corneas using double-label immunofluorescence for ferritoid (red) and ferritin (green) (figure 5). Figure 5A shows that although ferritoid has a nuclear localization throughout most of the CE, in the most peripheral region (seen at the left of the figure) it is cytoplasmic and predominantly perinuclear (arrows). Likewise, ferritin is nuclear throughout most of the CE (Fig. 5B), but in these peripheral CE cells it is cytoplasmic and perinuclear. A merged image for ferritoid and ferritin (Fig. 5C) indicates that ferritoid is expressed more peripherally to ferritin and that there appears to be a region in which both proteins are expressed but remain cytoplasmic.
Figure 5. Immunofluorescence micrographs of sections of E15 cornea at the corneal-scleral junction.
(A–C) are conventional fluorescence micrographs immunostained for ferritoid (A) and ferritin (B), and counterstained with Hoechst for nuclei. (C) is a merged image of the ferritoid (A) and the ferritin (B). (D–F) are confocal micrographs of the regions demarcated by the corresponding boxes in figure (C), enlarged to show nuclei (designated by asterisks). Scale bar in C = 10 µm.
These observations were confirmed by laser confocal microscopy in figures 5D–F, which correspond to the boxed regions (D–F) in figure 5C. Starting at the periphery of the cornea (Fig. 5D), there are cells that have synthesized ferritoid, but have little if any detectable ferritin. (The small amount of green signal present at the extreme periphery likely represents autofluorescence of the CE.) In these cells, ferritoid is cytoplasmic with none detectable in the nuclei (marked by asterisks). Progressing toward the center of the cornea, cells are observed that have both ferritoid and ferritin (Fig. 5E), but neither of these is nuclear (asterisks mark the nuclei). Instead both molecules have a perinuclear, cytoplasmic location. Progressing even more centrally (Fig. 5F) both ferritoid and ferritin the CE cells are now localized to the nucleus (asterisks in Fig. 5F). Taken together, these data suggest that as limbal cells migrate into the CE they first acquire ferritoid, which remains cytoplasmic; then when ferritin becomes expressed both proteins translocate to the nucleus.
Ferritoid / ferritin interaction
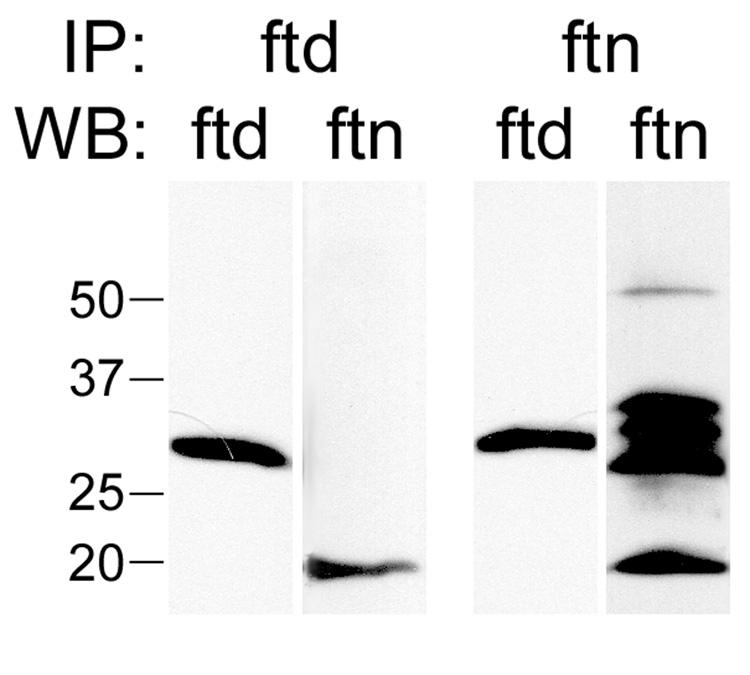
Our immunofluorescence observations are consistent with the hypothesis that ferritoid is the nuclear transporter for ferritin in the CE. In addition, for transport to occur, our model requires that ferritoid must bind to ferritin. To determine whether such binding occurs in CE cells, we performed co-immunoprecipitations for ferritoid and ferritin using a total protein lysate from E15 CE tissue (Fig. 6). Immunoprecipitations were carried out using an antibody to either ferritoid or ferritin, and were then analyzed by Western blot for both ferritoid and ferritin.
Figure 6. Co-immunoprecipitations of lysates of E15 CE tissue.
The lysates were immunoprecipitated with antibodies against either ferritoid (IP: ftd) or ferritin (IP: ftn), followed by SDS-PAGE and Western blot (WB) for ferritoid (ftd) or ferritin (ftn). Immunoprecipitates for both ferritoid and ferritin contained a 30 kD band corresponding to ferritoid and a 19 kD band corresponding to ferritin. A molecular weight marker (in kD) is shown on the left.
Following immunoprecipitation for ferritoid (Fig. 6, IP: ftd), a 30 kD band was detected by Western blot for ferritoid (Fig. 6, WB: ftd) and a 19 kD band was detected by Western blot for ferritin (Fig. 6, WB: ftn). Similarly, following immunoprecipitation for ferritin (Fig. 6, IP: ftn), both ferritoid and ferritin were detected by Western blot. The results of these immunoprecipitations demonstrate that ferritoid and ferritin bind to one another in CE cells. (The additional bands present in the Western blot for ferritin following immunoprecipitation with the ferritin antibody are IgG heavy and light chain bands. These bands are not seen in fractions in which the ferritoid antibody was used either for the immunoprecipitations or the Western blot as streptavidin was employed for detection rather than a secondary antibody.)
Co-regulation of ferritoid and ferritin expression in the developing CE
For cytoplasmic ferritin in other systems, it is well established that the chief mode of regulation is translational and that this regulation involves iron (Harrison and Arosio, 1996). Similarly, our previous observations (Cai et al., 1997) suggest that the developmental appearance of the nuclear ferritin in CE cells also involves translational regulation, as ferritin mRNA is detected as early as E6, but the protein is not detected until E11. That iron is involved in this regulation is suggested by the additional observation that in cultures of pre-ferritin CE cells (e.g. E8), the initiation of ferritin synthesis can be inhibited by addition of the iron chelator DFX (Cai et al., 1997). We have now expanded these studies on CE ferritin and extended them to include ferritoid.
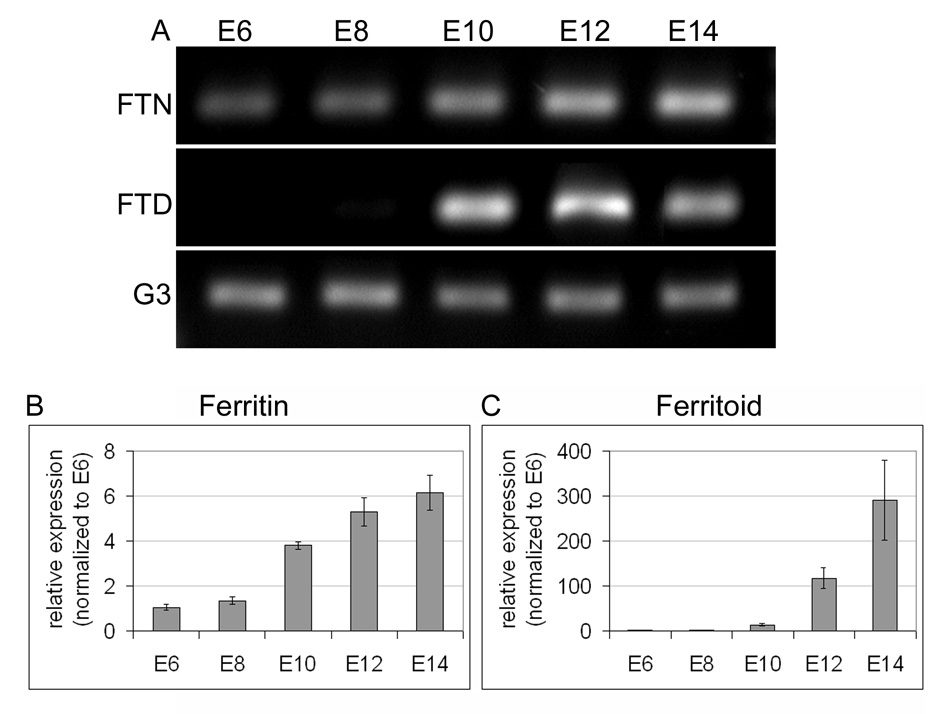
To determine when ferritoid mRNA becomes expressed – relative to ferritin mRNA – we first performed RT-PCR for each of these using CE from progressive stages of embryonic development (from E6 to E14, Fig. 7A), and we then quantified these data by real-time PCR (qRT-PCR) (Fig. 7B–C). Consistent with our previous results for ferritin, RT-PCR shows that ferritin mRNA is present by E6 (the earliest time examined) and increases with time through E14 (the oldest time examined) (Fig. 7A, FTN). qRT-PCR analysis shows that the increase in ferritin mRNA over this time period is 6-fold (Fig. 7B). These results, taken together with those for the appearance of ferritin protein (see Fig. 3), which does not occur until E11, are consistent with translation being the primary form of regulation for ferritin.
Figure 7. Expression of ferritoid and ferritin mRNA in developing CE.
(A) RT-PCR for ferritin (FTN) and ferritoid (FTD), performed on mRNA isolated from CE at 2-day intervals from E6–E14, with G3PDH (G3) used as a loading control. (B–C) Real-time RT-PCR for ferritin (B) and ferritoid (C) performed on the same mRNAs as in (A). The values at each time point (calculated from the ratio of ferritin or ferritoid to β-actin) represent the fold increase relative to E6 (the earliest time examined and arbitrarily assigned a value of 1).
Conversely, ferritoid mRNA is not detectable by RT-PCR until E10 (Fig. 7A, FTD), the time at which the protein also appears (Fig. 3). At this time, qRT-PCR shows that the mRNA for ferritoid is approximately 13-fold greater than at E6, and this increase continues rapidly until E14 when it is almost 300-fold higher (Fig. 7C). Therefore, for ferritoid a major form of regulation – and perhaps the major form – is transcriptional. However, other of our studies suggest that translational regulation of ferritoid also occurs (see below).
Co-regulation of ferritoid and ferritin proteins by iron
The data already presented demonstrate that (1) for nuclear localization to occur ferritoid and ferritin must interact with one another and, (2) the production of these two proteins is coordinately regulated, as developmentally they both appear within at most six hours of one another.
In addition, other of our studies have shown that one factor involved in the regulation of ferritin synthesis is iron (Cai et al., 1997). For example, when CE cells from pre-ferritin stage embryos (e.g., E8) are cultured in vitro, ferritin appears precociously (in a time as short as 16 hours). However, the initiation of ferritin synthesis can be inhibited by removing iron from the medium through the addition of the iron chelator DFX. This inhibition is reversible; when these cells are transferred to iron-containing medium (without DFX) they initiate ferritin synthesis (Cai et al., 1998).
We have now used this approach to examine experimentally: (1) whether DFX also blocks the appearance of ferritoid – thus implicating iron as a common factor in the regulation of both ferritoid and ferritin, and (2) if so, whether the release from inhibition by DFX results in the appearance of ferritoid and ferritin in the same temporal sequence that occurs during development (i.e., initially ferritoid and then ferritin).
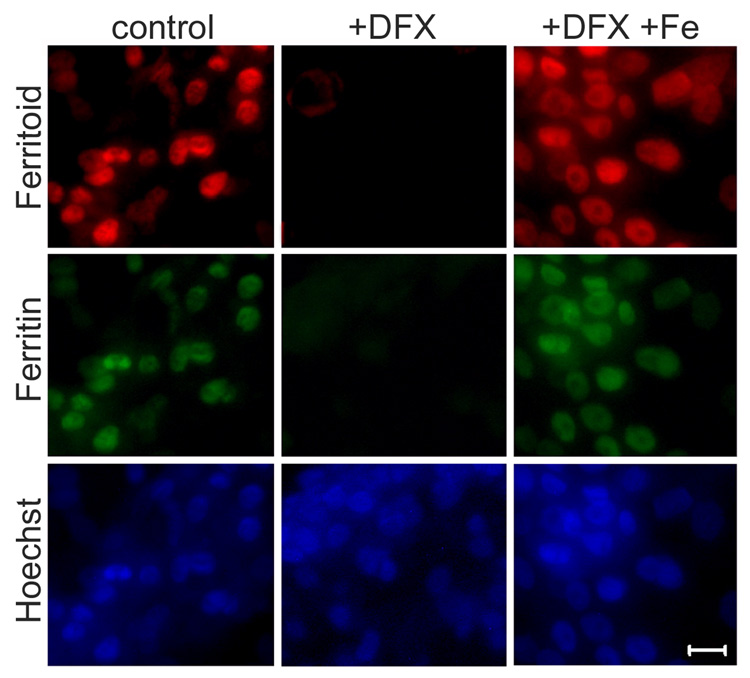
When pre-ferritin stage, E8 CE cells are cultured in normal medium (containing iron) both ferritoid and ferritin are precociously expressed within 24 hours (Fig. 8, control column), and both proteins are localized to the nucleus, as compared with Hoechst staining. However, when pre-ferritin CE cells are cultured in the presence of DFX (Fig. 8, +DFX) neither ferritoid nor ferritin are produced. To verify that this inhibition is not due to any toxic side-effects of the DFX, cells were also cultured in equimolar concentrations of DFX and iron. In this combination the iron counteracts the effects of the DFX, and the cells initiate the expression of both ferritoid and ferritin (Fig. 8, +DFX +Fe).
Figure 8. Fluorescence micrographs of E8 CE cell cultures.
CE cells were cultured for 48 hours in normal medium (control), or medium supplemented with either 100 µM deferoxamine (+DFX) or with equimolar concentrations of deferoxamine and ferrous sulfate (+DFX+FeSO4) and then stained for ferritoid and ferritin (nuclei counterstained with Hoechst). Scale bar = 10 µm.
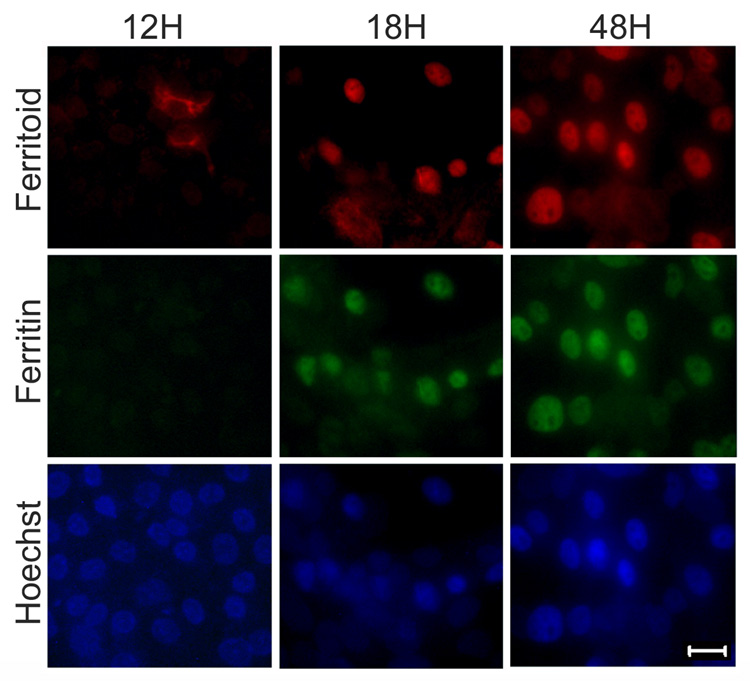
We further studied the temporal sequence of the appearance of ferritoid and ferritin in CE cell cultures by examining, at six-hour intervals, cells released from DFX inhibition by transfer to iron-containing medium (without DFX) (Fig. 9). By 12 hours after release from DFX inhibition (Fig. 9, 12H), some cells had initiated synthesis of ferritoid, but none had detectable ferritin. This ferritoid was cytoplasmic and was largely perinuclear. By 18 hours (Fig. 9, 18H) most cells contained both ferritoid and ferritin, and both proteins were nuclear (as compared to Hoechst staining); and by 48 hours almost all cells showed both ferritoid and ferritin in a nuclear location – appearing identical to cells cultured in the control medium (compare Fig. 9, 48H and Fig. 8, control). These results show that initiation of ferritoid and ferritin synthesis in cell culture recapitulates the events that occur in vivo and implicate iron as a common factor involved in the regulation of both ferritoid and ferritin.
Figure 9. Staining for ferritoid and ferritin in cultured CE cells during recovery from DFX treatment.
Immunofluorescence micrographs of E8 CE cells cultured in DFX-containing medium for 24 hours, and then transferred to high iron medium for 12 hours (12H), 18 hours (18H), or 48 hours (48H) before immunostaining for ferritoid and ferritin (nuclei counterstained with Hoechst). Scale bar = 10 µm.
Translational regulation of ferritoid and ferritin
Studies on ferritin in a number of cell types (Harrison and Arosio, 1996) have demonstrated that iron-mediated regulation is largely translational. Consistent with this, the ferritin mRNA in CE cells contains a canonical IRE in its 5’ UTR (Cai et al., 1997). Although we have detected no consensus IRE in the ferritoid mRNA, the above experiments suggest that iron is involved in the regulation of ferritoid.
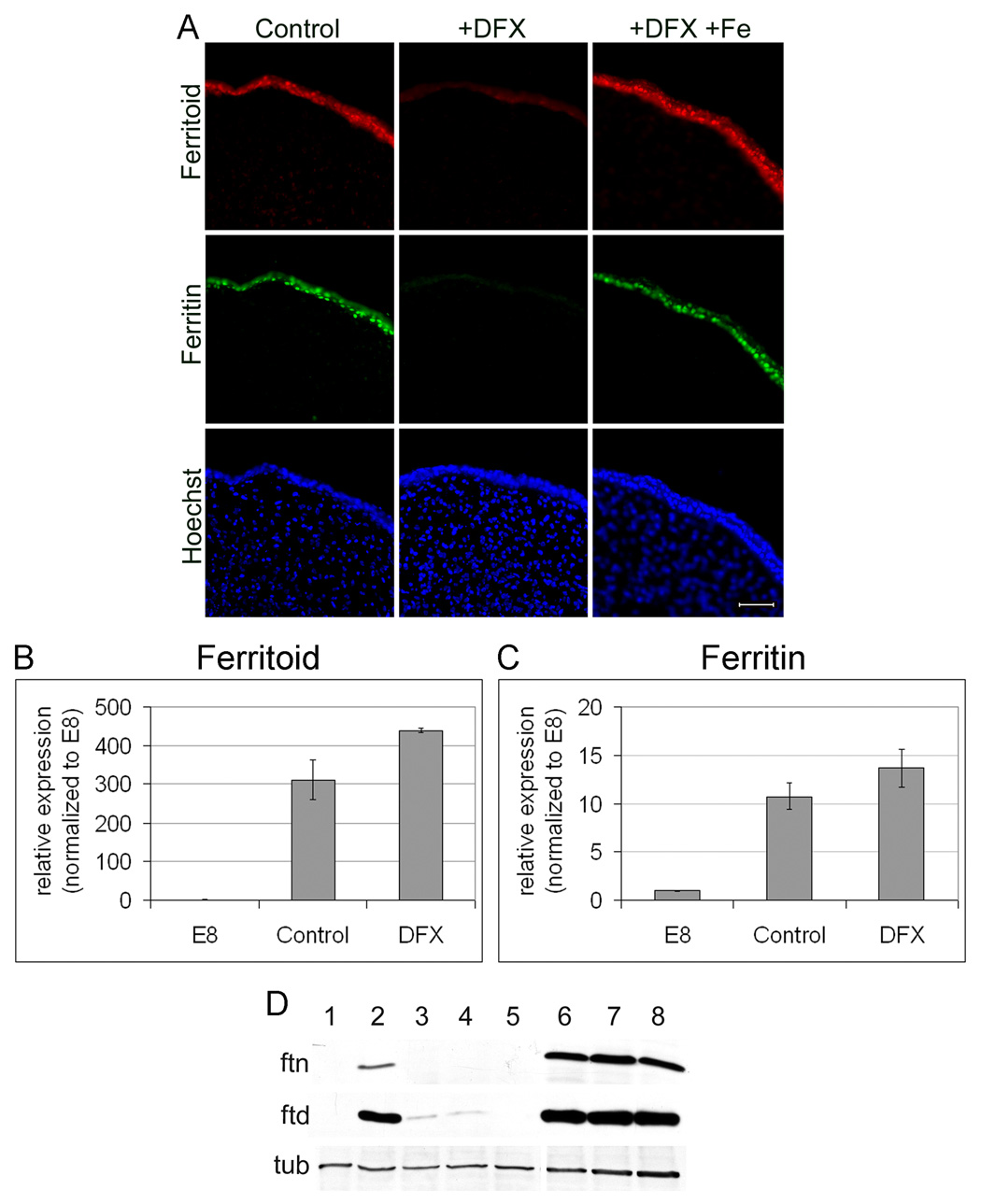
We therefore examined further the regulation of ferritoid by iron – as well as that of ferritin – both at the mRNA and protein levels using DFX to manipulate iron in culture (Fig. 10). To obtain sufficient tissue to perform the required analyses, including those by real time qRT-PCR, we devised an organ culture system utilizing whole corneas, rather than the primary cultures of CE cells just described. To determine whether the CE of these organ-cultured corneas behaved similar to the cultured CE cells, we performed immunofluorescence analysis for ferritoid and ferritin on E8 corneas cultured in the presence or absence of DFX for 48 hours. The CE of corneas cultured in control medium show ferritoid and ferritin in a nuclear localization (Fig. 10A, control; compare with Hoechst staining for nuclei). However, corneas cultured in medium containing DFX show only background autofluorescence in the CE (Fig. 10A, DFX). This lack of expression is not due to any toxicity of DFX, as the corneas cultured in medium containing equimolar DFX and iron (Fig. 10A, +DFX+Fe) have nuclear ferritoid and ferritin identical to that of the control.
Figure 10. Regulation of ferritoid and ferritin by iron in E8 corneal organ cultures.
(A) Immunofluorescence micrographs of E8 corneas cultured for 48 hours in normal medium (control) or medium supplemented with DFX (+DFX) or DFX plus equimolar ferrous sulfate (+DFX+Fe), and then immuno-labelled for ferritoid and ferritin. Nuclei are stained with Hoechst. Scale bar = 10 µm. (B–C) Real-time PCR for ferritoid (B) and ferritin (C) from E8 corneas (E8), or E8 corneas cultured for 48 hours in control medium (control) or in DFX-containing medium (DFX). (D) Western blots for ferritin (ftn) and ferritoid (ftd) of E8 corneas (lane 1); E8 corneas cultured for 48 hours in the following media: control medium (lane 2), control medium containing DFX (lane 3), or control medium containing DFX plus leupeptin (lane 4) or DFX plus chloroquine (lane 5); E12 corneas (lane 6); or E12 corneas cultured in control medium (lane 7) or control medium containing DFX (lane 8). Tubulin (tub) was used as a loading control.
We then analyzed the CE of these cultured corneas for ferritoid and ferritin mRNAs by qRT-PCR (Fig. 10B–C), using E8 CE tissue as a control. For the CE of corneas cultured in control medium, the increase in the mRNA for ferritoid (Fig. 10B) is approximately 300-fold greater than at the time of initiation of the cultures (E8) and the increase for ferritin is approximately 11-fold greater (Fig. 10 C). For the CE of corneas cultured in DFX-containing medium, the increases in the mRNAs are even larger than in the control medium, with that for ferritoid being 450-fold and that for ferritin being 14-fold. The observation that DFX increases the mRNAs for ferritoid and ferritin, while inhibiting the synthesis of the proteins, shows that regulation by iron in both cases is effected at the translational level.
For ferritin, studies by others have shown that in addition to inhibiting ferritin translation by removing iron, DFX can also stimulate the degradation of ferritin protein through the lysosomal pathway (Kidane et al., 2006). We therefore examined by Western blot whether the observed effects of DFX on ferritoid and ferritin protein synthesis was effected through lysosomal degradation (Fig. 10D). The control Western blots confirmed the results observed by immunofluorescence in figure 10A: (1) E8 corneas have synthesized neither ferritin (ftn) nor ferritoid (ftd) (lane 1); (2) when cultured in control medium for 48 hours, both ferritoid and ferritin are synthesized precociously (lane2), and (3) the appearance of both proteins is largely, if not completely, inhibited by DFX (lane 3). The effect of DFX on the inhibition of protein synthesis is not altered by the lysosomal inhibitors leupeptin or chloroquine (lanes 4 and 5), confirming that DFX acts by inhibiting the synthesis of these proteins rather than promoting their degradation via the lysosomal pathway.
To further determine whether DFX inhibits the expression of ferritoid and ferritin by promoting their degradation, we cultured E12 corneas for 48 hours in the presence or absence of DFX (Fig. 10D). Whereas E8 corneas have not yet synthesized ferritoid and ferritin (lane 1), E12 corneas already express both proteins (lane 6). E12 corneas cultured either in control medium (lane 7) or in medium containing DFX (lane 8) have both ferritoid and ferritin, demonstrating that DFX does not destabilize these proteins once they are already synthesized.
Discussion
Our previous studies show that in CE cells the iron-storage molecule, ferritin, is a nuclear protein (Cai et al., 1997), and that in this location it plays a role in protecting CE cells from oxidative damage to DNA, both from UV light (Cai et al., 1998) and from H2O2 (Cai et al., 2008). Instrumental in this nuclear localization is the action of a tissue-specific nuclear transporter, ferritoid, which itself is a ferritin family member. In the present study we examined the relationships between ferritoid and ferritin during corneal development in vivo and in vitro, including (1) their developmental appearance at the protein and mRNA levels and (2) their subcellular localizations. We also determined that iron is a factor common to the co-regulation of both proteins.
Co-regulation of ferritoid and ferritin expression in the CE
Double-label immunofluorescence analysis for ferritoid and ferritin from a timed series of precisely-staged corneas shows that during development of the CE, ferritoid is synthesized prior to ferritin – but marginally so since the time differential is six hours or less. It is unlikely that this simply reflects differences in the rates of transcription and translation. At the time of initiation of ferritin synthesis, only translation is required, as the mRNA has already been present for at least five days (see Fig. 7). For ferritoid, however, both transcription and translation are required as no mRNA is detectable until about the time that the protein is synthesized. Despite this, ferritoid is the protein that is synthesized first. This also suggests that some form of integrated regulation exists between the production of ferritoid and ferritin.
Consistent with this finding, the sequence of ferritoid being synthesized before ferritin is also observed experimentally when pre-ferritin stage CE cells, cultured in the presence of the iron chelator DFX (which inhibits the synthesis of both proteins), are released from this inhibition by their transference into iron-containing medium. We currently do not know the details of the regulation that is responsible for this sequential appearance. However, that this temporal sequence occurs in CE cells that are cultured in a uniform in vitro environment eliminates a requirement for external cues in this progression. Instead, it is likely that the regulation occurs within the CE cells themselves.
The studies employing DFX also implicate iron as a common factor involved in controlling the synthesis of both ferritoid and ferritin. In pre-ferritin stage corneas cultured in the presence of DFX, which depletes iron from the medium, initiation of the synthesis of both proteins in the CE is reversibly inhibited; however, expression of their mRNAs is unimpeded. This suggests that regulation by iron for both of these proteins is most likely at the translational/post-transcriptional level.
Other studies have suggested that DFX may promote degradation of ferritin protein, and that this degradation occurs specifically via the lysosomal pathway with no involvement of the proteosomal pathway (Kidane et al., 2006). If such degradation is responsible for the absence of ferritoid and ferritin in the CE of DFX-treated cultures, then inhibitors of the lysosomal pathway should result in the presence of both proteins in these cultures (due to an inhibition of their degradation). However, when two different lysosomal inhibitors were included in the DFX-treated cornea cultures, ferritoid and ferritin were still undetectable in the CE. Therefore it is unlikely that post-synthetic lysosomal degradation is responsible for the absence of ferritoid and ferritin expression in the DFX-treated cultures. Additionally, in E12 corneas, which have already initiated synthesis of both ferritoid and ferritin, both proteins continue to be present when the corneas are cultured in the presence of DFX-containing medium for at least two days, demonstrating that DFX does not noticeably affect the stability of these proteins once they are already synthesized. It also suggests that these proteins are relatively long-lived.
For ferritin, iron-mediated translational regulation has been extensively studied and is known to involve an interaction between a cytosolic iron responsive protein and an IRE located in the 5’ UTR of the ferritin mRNA (Harrison and Arosio, 1996). In analyses of the ferritoid mRNA sequence, we have not found a consensus IRE. However, the possibility that ferritoid does contain a non-canonical IRE cannot be ruled out. Also, as we have not yet determined whether our ferritoid cDNA contains a complete 5’ UTR, another possibility is that iron-mediated regulation could occur through an IRE located elsewhere within the ferritoid 5’ UTR.
Regulation of ferritoid nuclear transport by ferritin
As the cornea develops, a window in time exists when CE cells are synthesizing ferritoid but not ferritin. In these cells, ferritoid is cytoplasmic, and it remains so until ferritin is also synthesized – at which time both proteins undergo nuclear transport. Likewise, when pre-ferritin stage CE cells are cultured in the presence of DFX – to inhibit ferritoid and ferritin synthesis – and are then transferred to iron containing medium, they go through a stage when ferritoid is synthesized but ferritin is not. During this period the ferritoid remains cytoplasmic; then, when ferritin is subsequently produced, both proteins undergo nuclear transport.
Although ferritoid contains a canonical NLS, the above results suggest that the nuclear transport capability of ferritoid, to become functional, requires an interaction with ferritin. NLSs have been classically defined by primary amino acid sequence alone, but there is increasing evidence that additional regulation is involved in mediating their activity. For example, for some NLS-containing proteins, nuclear localization is regulated by inter-molecular interactions – similar to those that occur between ferritoid and ferritin – that result in the masking or unmasking of the NLS [reviewed in (Terry et al., 2007)].
However, another observation suggests that an interaction between ferritoid and ferritin per se is not sufficient for transport to occur. At the periphery of the mature cornea, pericorneal limbal cells migrate into the cornea where they differentiate into CE. We have observed that as these cells differentiate they also undergo the sequence of ferritoid being synthesized first and ferritin later. Within this cell population, however, there is a distinct stage in which both ferritoid and ferritin are present, but both remain cytoplasmic. This raises the possibility that at least one additional regulatory step is involved – such as a post-translational modification, a variety of which are known to affect the activity of NLSs [reviewed in (Terry et al., 2007)]. These include phosphorylation, which other of our studies suggest may occur in ferritoid, and which, in turn, results in functional changes in its NLS (unpublished observations).
Our previous studies using both COS-1 cells co-transfected with ferritoid and ferritin (Millholland et al., 2003), and the yeast two-hybrid system (unpublished observations), show that ferritoid and ferritin are capable of binding to one another; here we have observed that this does, in fact, occur in CE cells. Also, based on comparison between the amino acid sequences of ferritoid and ferritin, molecular modelling predicts (1) that ferritoid has a conformation similar to that of ferritin (Millholland et al., 2003) and (2) that many residues in ferritin, previously shown to be critical for ferritin supramolecular complex assembly (Santambrogio et al., 1997), are conserved in ferritoid. These observations raise the possibility that ferritoid can also participate in supramolecular assembly. Consistent with this possibility, we have recently isolated and characterized high molecular weight, stable complexes from the nuclear extracts of CE cells that contain both ferritoid and ferritin (Nurminskaya et al., unpublished observations). Whether it is such a pre-formed complex that undergoes nuclear transport remains to be determined.
Acknowledgements
We kindly thank Dr. T.T. Sun for providing the monoclonal antibody to the chicken cytokeratin K3. This work was supported by National Institutes of Health grant EY13127.
National Institutes of Health: EY13127 (T.F.L.)
Reference List
- Cai CX, Birk DE, Linsenmayer TF. Ferritin is a developmentally-regulated nuclear protein of avian corneal epithelial cells. J. Biol. Chem. 1997;272:12831–12839. doi: 10.1074/jbc.272.19.12831. [DOI] [PubMed] [Google Scholar]
- Cai CX, Birk DE, Linsenmayer TF. Nuclear ferritin protects DNA from UV damage in corneal epithelial cells. Mol. Biol. Cell. 1998;9:1037–1051. doi: 10.1091/mbc.9.5.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai CX, Ching A, Lagace C, Linsenmayer T. Nuclear ferritin-mediated protection of corneal epithelial cells from oxidative damage to DNA. Dev. Dyn. 2008 doi: 10.1002/dvdy.21494. In Press. [DOI] [PubMed] [Google Scholar]
- Hamburger V, Hamilton HL. A series of normal stages in the development of the chick embryo. J. Morphol. 1951;88:49–92. [PubMed] [Google Scholar]
- Harrison PM, Arosio P. The ferritins: molecular properties, iron storage function and cellular regulation. Biochim. Biophys. Acta. 1996;1275:161–203. doi: 10.1016/0005-2728(96)00022-9. [DOI] [PubMed] [Google Scholar]
- Hart RW, Setlow RB, Woodhead AD. Evidence that pyrimidine dimers in DNA can give rise to tumors. Proc. Natl. Acad. Sci. USA. 1977;74:5574–5578. doi: 10.1073/pnas.74.12.5574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henle ES, Han Z, Tang N, Rai P, Luo Y, Linn S. Sequence-specific DNA cleavage by Fe2+-mediated fenton reactions has possible biological implications. J. Biol. Chem. 1999;274:962–971. doi: 10.1074/jbc.274.2.962. [DOI] [PubMed] [Google Scholar]
- Henle ES, Luo Y, Gassmann W, Linn S. Oxidative damage to DNA constituents by iron-mediated fenton reactions. The deoxyguanosine family. J. Biol. Chem. 1996;271:21177–21186. [PubMed] [Google Scholar]
- Hentze MW, Caughman SW, Rouault TA, Barriocanal JG, Dancis A, Harford JB, Klausner RD. Identification of the iron-responsive element for the translational regulation of human ferritin mRNA. Science. 1987;238:1570–1573. doi: 10.1126/science.3685996. [DOI] [PubMed] [Google Scholar]
- Janssen YM, Van Houten B, Borm PJ, Mossman BT. Cell and tissue responses to oxidative damage. Lab. Invest. 1993;69:261–274. [PubMed] [Google Scholar]
- Kidane TZ, Sauble E, Linder MC. Release of iron from ferritin requires lysosomal activity. Am. J Physiol Cell Physiol. 2006;291:C445–C455. doi: 10.1152/ajpcell.00505.2005. [DOI] [PubMed] [Google Scholar]
- Longini M, Perrone S, Vezzosi P, Marzocchi B, Kenanidis A, Centini G, Rosignoli L, Buonocore G. Association between oxidative stress in pregnancy and preterm premature rupture of membranes. Clin. Biochem. 2007;40:793–797. doi: 10.1016/j.clinbiochem.2007.03.004. [DOI] [PubMed] [Google Scholar]
- Luo Y, Henle ES, Linn S. Oxidative damage to DNA constituents by iron-mediated fenton reactions. The deoxycytidine family. J. Biol. Chem. 1996;271:21167–21176. [PubMed] [Google Scholar]
- Meneghini R. Iron homeostasis, oxidative stress, and DNA damage [Review] [74 refs] Free Radical Biology & Medicine. 1997;23:783–792. doi: 10.1016/s0891-5849(97)00016-6. [DOI] [PubMed] [Google Scholar]
- Mete A, Van Zeeland YRA, Vaandrager AB, Van Dijk JE, Marx JJM, Dorrestein GM. Partial purification and characterization of ferritin from, the liver and intestinal mucosa of chickens, turtledoves and mynahs. Avian Pathol. 2005;34:430–434. doi: 10.1080/03079450500267908. [DOI] [PubMed] [Google Scholar]
- Millholland JM, Fitch JM, Cai CX, Gibney EP, Beazley KE, Linsenmayer TF. Ferritoid, a tissue-specific nuclear transport protein for ferritin in corneal epithelial cells. J. Biol. Chem. 2003;278:23963–23970. doi: 10.1074/jbc.M210050200. [DOI] [PubMed] [Google Scholar]
- Santambrogio P, Pinto P, Levi S, Cozzi A, Rovida E, Albertini A, Artymiuk P, Harrison PM, Arosio P. Effects of modifications near the 2-, 3- and 4-fold symmetry axes an human ferritin renaturation. Biochem. J. 1997;322:461–468. doi: 10.1042/bj3220461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schermer A, Galvin S, Sun T. Differentiation-related expression of a major 64K corneal keratin in vivo and in culture suggests limbal location of corneal epithelial stem cells. J. Cell Biol. 1986;103:49–62. doi: 10.1083/jcb.103.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid TM, Linsenmayer TF. Immunohistochemical localization of short chain cartilage collagen (type X) in avian tissues. J. Cell Biol. 1985;100:598–605. doi: 10.1083/jcb.100.2.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smolin G, Thoft RA. The Cornea: Scientific Foundations and Clinical Practice. Boston/Toronto: Little, Brown and Company; 1987. [Google Scholar]
- Stohs SJ, Bagchi D. Oxidative mechanisms in the toxicity of metal ions. Free Radical Biol. & Med. 1995;18:321–336. doi: 10.1016/0891-5849(94)00159-h. [DOI] [PubMed] [Google Scholar]
- Terry LJ, Shows EB, Wente SR. Crossing the nuclear envelope: hierarchical regulation of nucleocytoplasmic transport. Science. 2007;318:1412–1416. doi: 10.1126/science.1142204. [DOI] [PubMed] [Google Scholar]
- Zak NB, Linsenmayer TF. Monoclonal antibodies against developmentally regulated corneal antigens. Dev. Biol. 1983;99:373–381. doi: 10.1016/0012-1606(83)90287-7. [DOI] [PubMed] [Google Scholar]