Abstract
Structure-activity studies centered on the naturally occurring antitumor agent dictyostatin have recently identified several highly active epimers and analogs. From these compounds, 6-epi-dictyostatin was selected for scaleup preparation and evaluation in animals. Here we describe a new total synthesis that produced more than 30 mg of 6-epi-dictyostatin. The compound was found to have potent antitumor activity in SCID mice bearing MDA-MB231 human breast cancer xenografts.
Since the approval of paclitaxel in the early 1990’s and docetaxel in the early 2000’s, interest in compounds that bind to and stabilize microtubules has continued to grow.1 Recently, a semisynthetic derivative of epothilone B, ixabepilone, was approved by the FDA for use either alone or in combination to combat certain types of breast tumors.2 Other potent microtubule stabilizing agents, including members of the discodermolide/dictyostatin class, have also generated significant interest as drug candidates.3
Dictyostatin is a rare macrocyclic lactone isolated from marine sponges4,5 that has recently become more available through total synthesis.6-10 Members of the dictyostatin family strongly inhibit cancer cell growth, have high affinities for the taxoid binding site, and competitively inhibit the binding of paclitaxel and epothilone B to microtubules.4,9,11-13 Among several potent analogs, including 7-epi-dictyostatin, 16-normethydictyostatin and 15(Z),16-normethyldictyostatin, 6-epi-dictyostatin 1 was the most potent inhibitor of binding of epothilone B to microtubules. It was also the most potent inhibitor of HeLa cell growth, and its activity was not affected by mutations that induce paclitaxel resistance in the taxoid binding site.9,13
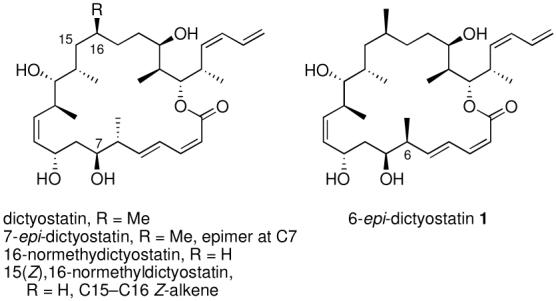
Accordingly, 6-epi-dictyostatin 1 was selected for a scaleup synthesis and in vivo evaluation of its antitumor properties. Here we report a new synthetic approach to the dictyostatin family that produced more that 30 mg of 6-epi-dictyostatin 1. In the first animal tests of any dictyostatin, epimer 1 showed high antitumor activity in SCIDa mice bearing xenografts of the MDA-MB231 human estrogen receptor negative breast cancer cell line. 6-epi-Dictyostatin 1 was significantly better than paclitaxel in inhibiting tumor growth.
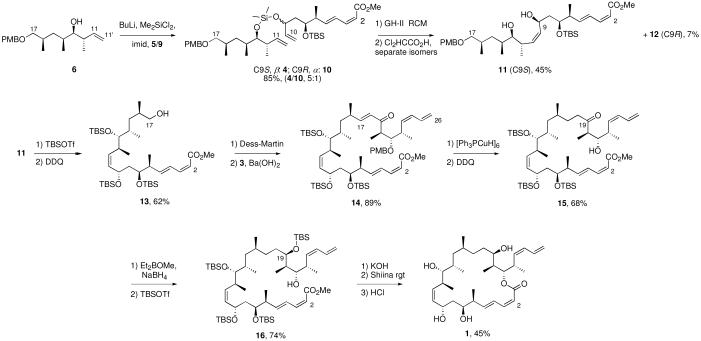
We adopted the retrosynthesis plan for 6-epi-dictyostatin shown in Figure 1. Compared to the original fluorous mixture synthesis of 1,12 the new route is more convergent because it features fully elaborated bottom 5, middle 6 and top 3 fragments. It also introduces a new strategy for uniting the bottom and middle fragments by a silicon-tethered ring-closing metathesis (RCM) reaction14 of 4 to form the critical C10-C11 Z-alkene.
Figure 1.
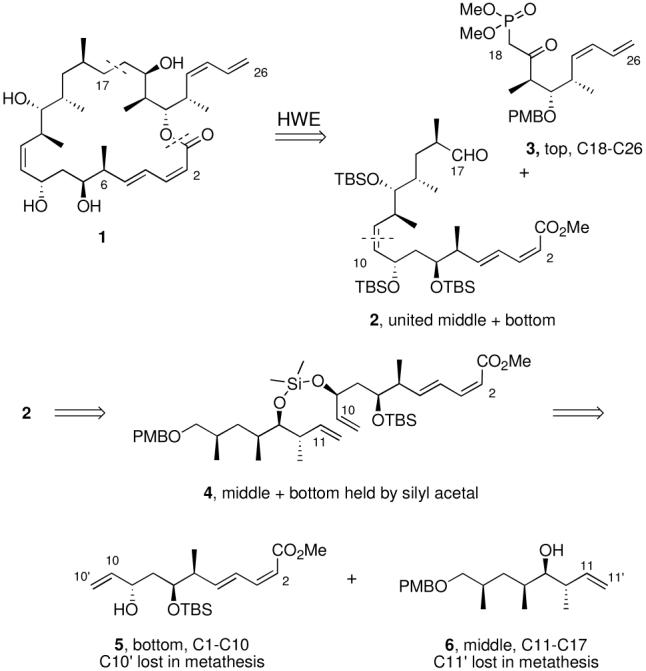
Retrosynthetic analysis of 6-epi-dictyostatin
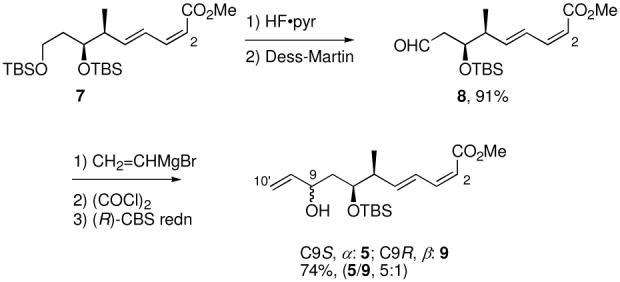
The C1-C10 fragment 5 was synthesized through five steps, starting from readily available intermediate 7,15 as shown below. Deprotection of TBS ether 7 with HF·pyr and Dess-Martin oxidation16 of the resulting alcohol provided the aldehyde 8 in 91% yield over two steps. Addition of vinyl magnesium bromide, Dess-Martin oxidation, and Corey-Bakshi-Shibata (CBS)17 reduction gave an inseparable mixture of two C9-epimers 5 (α) and 9 (β) in a 5:1 ratio favoring the needed alcohol 5 (74% yield, three steps).
The sequence of fragment couplings and macrolactonization is summarized in Scheme 1. The C11-C17 alcohol 6 was treated with BuLi/dimethyldichlorosilane, followed by addition of imidazole and the C1-C10 alcohol epimer mixture of 5 and 9. This provided another inseparable C9 mixture of the silylketals 4 and 10 in 85% yield, again in about 5:1 ratio. The RCM reaction of this silylketal mixture was mediated by a Grubbs-Hoveyda II catalyst to provide a third inseparable C9 mixture of the 8-membered disiloxanes in 57-69% yield. This mixture was deprotected with dichloroacetic acid and the crude product was purified by silica gel chromatography. This time, the C9 epimers separated, and the target diol 11 (C9S) was isolated in 45% yield alongside 7% of the C9R-epimer 12. Protection of the diol 11 as a TBS ether followed by treatment with DDQ gave the alcohol 13 in 62% yield over two steps. The alcohol 13 was oxidized by using the Dess-Martin periodinane to the aldehyde 2, which was coupled with the phosphonate 3 to provide the enone 14 possessing the full C1-C26 carbon skeleton of 6-epi-dictyostatin (89% yield over two steps).
Scheme 1.
Fragment couplings and macrolactonization
The C17-C18 alkene of the enone 14 was reduced using a Stryker reagent ([Ph3PCuH]6)18 to give a saturated ketone, then the C21 PMB group was removed with DDQ to provide the β-hydroxyketone 15 in 68% over two steps. The 1,3-syn-reduction of the hydroxyketone 15 with NaBH4 and Et2BOMe19 and then selective protection of the C19 hydroxy group of the resulting diol with TBSOTf furnished the alcohol 16 (74% yield over two steps).8,20
In the usual end game, the C1 methyl ester was hydrolyzed by using KOH to produce a seco-acid, which was used for the next reaction without further purification. The macrolactonization of the seco-acid using the Yamaguchi reagent (2,4,6-trichlorobenzoyl chloride)21 gave a mixture of the C2 E/Z isomers of the TBS-protected macrolactone in a varying ratio, but the use of a Shiina reagent (2,6-methylnitrobenzoyl anhydride)22 in toluene suppressed the isomerization of the C2Z-alkene (1:13, E/Z). Finally, global deprotection with HCl provided 33 mg of 6-epi-dictyostatin in 45% yield over three steps.23
6-epi-Dictyostatin 1 and paclitaxel were each administered intravenously in three doses of 20 mg/kg/dose spaced 7 days apart to ten C.B-17 SCID female mice bearing established MDA-MB231 human breast cancer xenografts. Mean tumor volumes (Figure 2) and body weight loss (Figure 3) were periodically measured. One of the mice receiving 6-epi-dictyostatin 1 was accidentally injured then euthanized between days 14 and 17, while the other nine mice in that group continued to be followed.
Figure 2.
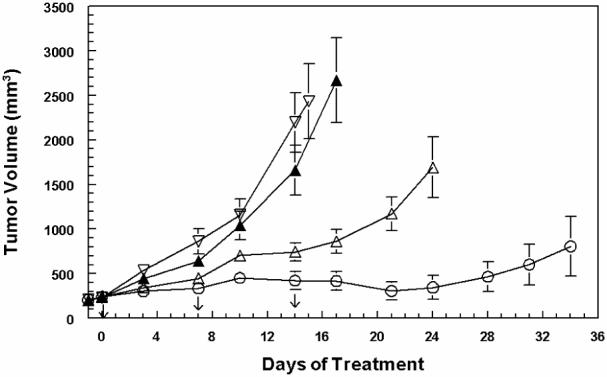
Antitumor activity in MDA-MB231 human breast cancer xenograft-bearing C.B-17 SCID female mice (n = 10 animals per group) treated with 20 mg/kg iv q7dx3 6-epi-dictyostatin 1 (○) or paclitaxel (△), vehicle (▲), or no treatment (▽). Arrows inidicate the days of dosing.
Figure 3.
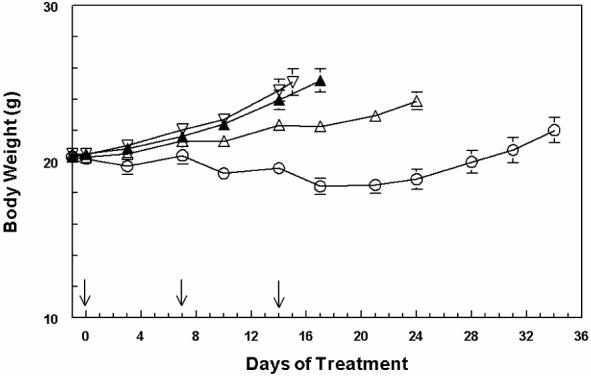
Body weight changes in MDA-MB231 human breast cancer xenograft-bearing C.B.-17 SCID female mice (n = 10 animals per group) over the course of treatment with 20 mg/kg iv q7dx3 6-epi-dictyostatin 1 (○) or paclitaxel (△), vehicle (▲), or no treatment (▽). Arrows inidicate the days of dosing.
Mean tumor volumes in the mice treated with 6-epi-dictyostatin 1 were significantly smaller than those in the control and vehicle-treated groups beginning on day 7 of treatment. And beginning on day 10, they were also smaller than those in the paclitaxel-treated group (Figure 2). Tumor regression was observed in six of the nine 6-epi-dictyostatin 1-treated mice on day 14 of study, and these tumors continued to regress until day 28 of study. In the remaining three 6-epi-dictyostatin 1-treated mice, tumor volumes did not increase until day 28, when tumor regrowth was observed. Tumors continued to grow in all the paclitaxel-treated mice, albeit at a slower rate than that observed for the tumors in the control and vehicle-treated groups. Paclitaxel-treated mice were euthanized between days 24 and 28 (Figure 2).
Body weight loss (Figure 3) was less than 10% in the 6-epi-dictyostatin 1-treated mice and their body weights were significantly lower than those in the other treatment groups. This difference in body weights may in part be due to the lack of tumor growth in the 6-epi-dictyostatin 1-treated mice.
Tumor doubling times, median optimal %T/C and median optimal %T/V for the various treatment groups are presented in Table 1. Tumors in the 6-epi-dictyostatin 1-treated mice did not double in volume at 28 days. The mean tumor doubling times for the paclitaxel-treated mice were significantly longer than the tumors in the control and vehicle-treated groups. Both the median optimal %T/C (day 14) and median optimal %T/V (day 17) were approximately 30% for the paclitaxel-treated mice, and 13% for the 6-epi-dictyostatin 1-treated mice, a significant difference.
Table 1.
Tumor doubling times, median optimal %T/C, and median optimal %T/V in C.B-17 SCID mice bearing MDA-MB231 xenografts
| Treatment Group N = 10 | Days to One Tumor Doubling | Days to Two Tumor Doublings | %T/C day 14 | %T/V day 17 |
|---|---|---|---|---|
| Paclitaxela | 7.2 ± 2.9c (7.9)d | 19.6 ± 7.4c (19.0)d | 33d | 31d |
| 6-epi-Dictyostatin 1a | 27.4 ± 18.7c (30.9)d,e | 38.1 ± 12.1c (35.0)d,e | 14d,e | 13d,e |
| Vehicleb | 4.9 ± 3.2 (4.0) | 9.8 ± 3.1 (8.9) | 61 | 100 |
| Control | 2.8 ± 1.7 (2.5) | 7.7 ± 2.4 (8.6) | 100 | — |
Tumors were implanted in C.B-17 SCID female mice as 25 mg fragments and mice were stratified on day 17 post-implantation, when tumors reached 101-463 mm3. Treatment began at day 0 (day 17 post-implantation). Results of time to one doubling and time to two doublings are presented as the mean ± std (median).
20 mg/kg iv q7dx3.
Cremophor EL-ethanol-saline 1:1:8 (v/v/v) 0.01 ml/g body weight iv q7dx3.
Value significantly different from control and vehicle, p ≤ 0.05 by Dunnett’s test.
Value significantly different from control and vehicle, p ≤ 0.05 by Mann-Whitney test.
Value significantly different from paclitaxel, p ≤ 0.05 by Mann-Whitney test.
In conclusion, we successfully executed an improved synthesis of 6-epi-dictyostatin 1 that yielded quantities sufficient for animal antitumor studies. 6-epi-Dictyostatin 1 was more effective than paclitaxel in mouse xeongraft studies, and excellent efficacy was observed at a dose that did not cause significant weight loss in the animals. Studies are ongoing to determine tissue distribution, metabolism of 1, and pharmacodynamic effects in tumor and normal tissue. The present results suggest that dictyostatins such as 1 hold promise as new microtubule-stabilizing chemotherapeutic agents.
Supplementary Material
Acknowledgements
We thank the NIH for grant CA78039 to support this work. We dedicate this paper to Dr. Ernest Hamel on the occasion of his 65th birthday.
Footnotes
- CBS
- Corey-Bakshi-Shibata
- DDQ
- dichlorodicyanoquinone GH-II, second-generation Grubbs-Hoveyda catalyst
- HWE
- Horner-Wadsworth-Emmons reaction
- PMB
- p-methoxybenzyl
- RCM
- ring-closing metathesis
- SCID
- severe combined immunodeficient
- T/C
- tumor volume of test agent-treated group divided by tumor volume of untreated control group
- T/V
- tumor volume of test agent-treated group divided by tumor volume of vehicle-treated group
References
- (1).Mooberry SL. Strategies for the development of novel Taxol-like agents. Methods Mol Med. 2007;137:289–302. doi: 10.1007/978-1-59745-442-1_20. [DOI] [PubMed] [Google Scholar]
- (2).Lee FY, Borzilleri R, Fairchild CR, Kamath A, Smykla R, Kramer R, Vite G. Preclinical discovery of ixabepilone, a highly active antineoplastic agent. Cancer Chemother. Pharmacol. 2008 doi: 10.1007/s00280-008-0724-8. published online 2008 Mar 18 [PMID: 18347795] [DOI] [PubMed] [Google Scholar]
- (3).Florence GJ, Gardner NM, Paterson I. Development of practical syntheses of the marine anticancer agents discodermolide and dictyostatin. Nat. Prod. Rep. 2008;25:342–375. doi: 10.1039/b705661n. [DOI] [PubMed] [Google Scholar]
- (4).Pettit GR, Cichacz ZA, Gao F, Boyd MR, Schmidt JM. Isolation and structure of the cancer cell growth inhibitor dictyostatin 1. J. Chem. Soc. Chem. Comm. 1994:1111–1112. [Google Scholar]
- (5).Isbrucker RA, Cummins J, Pomponi SA, Longley RA, Wright AE. Tubulin polymerizing activity of dictyostatin-1, a polyketide of marine sponge origin. Biochem. Pharmacol. 2003;66:75–82. doi: 10.1016/s0006-2952(03)00192-8. [DOI] [PubMed] [Google Scholar]
- (6).Paterson I, Britton R, Delgado O, Myer A, Poullennec KG. Total synthesis and configurational assignment of (-)-dictyostatin, a microtubule-stabilizing macrolide of marine sponge origin. Angew. Chem., Int. Ed. 2004;43:4629–4633. doi: 10.1002/anie.200460589. [DOI] [PubMed] [Google Scholar]
- (7).Shin Y, Fournier JH, Fukui Y, Brückner AM, Curran DP. Total synthesis of (-)-dictyostatin: Confirmation of relative and absolute configurations. Angew. Chem., Int. Ed. 2004;43:4634–4637. doi: 10.1002/anie.200460593. [DOI] [PubMed] [Google Scholar]
- (8).O’Neil GW, Phillips AJ. Total synthesis of (-)-dictyostatin. J. Am. Chem. Soc. 2006;128:5340–5341. doi: 10.1021/ja0609708. [DOI] [PubMed] [Google Scholar]
- (9).(a) Shin Y, Fournier JH, Fukui Y, Bruckner AM, Madiraju C, Edlerm MC, Vogt A, Balachandran R, Raccor BS, Zhum G, Hamel E, Day BW, Curran DP. Synthesis and biological evaluation of (-)-dictyostatin and stereoisomers. Tetrahedron. 2007;63:8537–8562. doi: 10.1016/j.tet.2007.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Jung W-H, Harrison C, Shin Y, Fournier JH, Balachandran R, Raccor BS, Sikorski RP, Vogt A, Curran DP, Day BW. Total synthesis and biological evaluation of C16 analogs of (-)-dictyostatin. J. Med. Chem. 2007;50:2951–2966. doi: 10.1021/jm061385k. [DOI] [PubMed] [Google Scholar]
- (10).Ramachandran P, Srivastava A, Hazra D. Total synthesis of potential antitumor agent, (-)-dictyostatin. Org. Lett. 2007;9:157–160. doi: 10.1021/ol062737k. V. [DOI] [PubMed] [Google Scholar]
- (11).Madiraju C, Edler MC, Hamel E, Raccor BS, Balachandran R, Zhu G, Giuliano KA, Vogt A, Shin Y, Fournier J-H, Fukui Y, Brückner AM, Curran DP, Day BW. Tubulin assembly, taxoid site binding, and cellular effects of the microtubule-stabilizing agent dictyostatin. Biochemistry. 2005;44:15053–15063. doi: 10.1021/bi050685l. [DOI] [PubMed] [Google Scholar]
- (12).Fukui Y, Brückner AM, Shin Y, Balachandran R, Day BW, Curran DP. Fluorous mixture synthesis of (-)-dictyostatin and three stereoisomers. Org. Lett. 2006;8:301–304. doi: 10.1021/ol0526827. [DOI] [PubMed] [Google Scholar]
- (13).Raccor BS, Vogt A, Sikorski RP, Madiraju C, Balachandran R, Montgomery K, Shin Y, Fukui Y, Jung W-H, Curran DP, Day BW. Cell-based and biochemical structure-activity analysis of analogs of the microtubule stabilizer dictyostatin. Mol. Pharmacol. 2008;73:718–726. doi: 10.1124/mol.107.042598. [DOI] [PubMed] [Google Scholar]
- (14).(a) Maier ME. Synthesis of medium-sized rings by the ring-closing metathesis reaction. Angew. Chem. Int. Ed. 2000;39:2073–2077. doi: 10.1002/1521-3773(20000616)39:12<2073::aid-anie2073>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]; (b) Boiteau J-G, Van de Weghe P, Eustache J. Formation of dissymmetric eight-membered silaketals by ring-closing metathesis and their conversion to spiroketals. Tetrahedron Lett. 2001;42:239–242. [Google Scholar]; (c) Harrison BA, Verdine GL. The synthesis of an exhaustively stereodiversified library of cis-1,5 enediols by silyl-tethered ring-closing metathesis. Org. Lett. 2001;3:2157–2159. doi: 10.1021/ol0159569. [DOI] [PubMed] [Google Scholar]; (d) Van de Weghe P, Bourg S, Eustache J. Electrophilic selenocyclization in 2-ene-1,5-diol systems: unexpected oxetane vs. tetrahydrofuran formation. Tetrahedron. 2003;59:7365–7376. [Google Scholar]; (e) Evans PA, Cui J, Gharpure SJ, Polosukhin A, Xhang H-R. Enantioselective total synthesis of the potent antitumor agent (-)-mucocin using a temporary silicontethered ring-closing metathesis cross-coupling reaction. J. Am. Chem. Soc. 2003;125:14702–14703. doi: 10.1021/ja0384734. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Hoye TR, Eklov BM, Jeon J, Khoroosi M. Sequencing of three-component olefin metatheses: total synthesis of either (+)-gigantecin or (+)-14-deoxy-9-oxygigantecin. Org. Lett. 2006;8:3383–3386. doi: 10.1021/ol061383u. [DOI] [PubMed] [Google Scholar]
- (15).Moura-Letts G, Curran DP. Selective synthesis of (2Z,4E)-dienyl esters by ene-diene cross metathesis. Org. Lett. 2007;9:5–8. doi: 10.1021/ol062017d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).(a) Dess DB, Martin JC. Readily accessible 12-I-5 oxidant for the conversion of primary and secondary alcohols to aldehydes and ketones. J. Org. Chem. 1983;48:4155–4156. [Google Scholar]; (b) Dess DB, Martin JC. A useful 12-I-5 triacetoxyperiodinane (the Dess-Martin periodinane) for the selective oxidation of primary or secondary alcohols and a variety of related 12-I-5 species. J. Am. Chem. Soc. 1991;113:7277–7287. [Google Scholar]
- (17).Corey EJ, Bakshi RK, Shibata S. Highly enantioselective borane redution of ketones catalyzed by chiral oxazaborolidines. Mechanism and synthetic implications. J. Am. Chem. Soc. 1987;109:5551–5553. [Google Scholar]
- (18).Mahoney WS, Brestensky DM, Stryker JM. Selective hydride-mediated conjugate reduction of a,β-unsaturated carbnyl compounds using [(Ph3P)CuH]6. J. Am. Chem. Soc. 1988;110:291–293. [Google Scholar]
- (19).Chen K-M, Hardtmann GE, Prasad K, Repic O, Shapiro MJ. 1,3-syn-Diastereoselective reduction of β-hydroxy ketones utilizing alkoxydialklylboranes. Tetrahedron Lett. 1987;28:155–158. [Google Scholar]
- (20).(a) Prusov E, Rohm H, Maier ME. Chemoenzymatic synthesis of the C10-C23 segment of dictyostatin. Org. Lett. 2006;8:1025–1028. doi: 10.1021/ol052917e. [DOI] [PubMed] [Google Scholar]; (b) Baba VS, Das P, Mukkanti K, Iqbal J. A cross metathesis approach to the synthesis of the C11-C23 fragment of (-)-16-normethyldictyostatin. Tetrahedron Lett. 2006;47:7927–7930. [Google Scholar]
- (21).Inanaga J, Hirata K, Hiroko S, Katsuki TJ, Yamaguchi M. A rapid esterification by means of mixed anhydride and its application to large-ring lactonization. Bull. Chem. Soc. Jpn. 1979;52:1989–1993. [Google Scholar]
- (22).(a) Shiina I, Ibuka R, Kubota M. A new condensation reaction for the synthesis of carboxylic esters from nearly equimolar amounts of carboxylic acids and alcohols using 2-methyl-6-nitrobenzoic anhydride. Chem. Lett. 2002:286–287. [Google Scholar]; (b) Shiina I, Kubota M, Ibuka R. A novel and efficient macrolactonization of w-hydroxycarboxylic acids using 2-methyl-6-nitrobenzoic anhydride (MBNA) Tetrahedron Lett. 2002;43:7535–7359. [Google Scholar]; (c) Shiina I, Oshiumi H, Hashizume M, Yamai YS, Ibuka R. Asymmetric total synthesis of octalactin B using a new and rapid lactonization. Tetrahedron Lett. 2004;45:543–547. [Google Scholar]; (d) Shiina I, Kubota M, Oshiumi H, Hashizume M. An effective use of benzoic anhydride and its derivatives for the synthesis of carboxylic esters and lactones: a powerful and convenient mixed anhydride method promoted by basic catalysts. J. Org. Chem. 2004;60:1822–1830. doi: 10.1021/jo030367x. [DOI] [PubMed] [Google Scholar]
- (23).This high level summary of the synthesis is supplemented by the discussions in the PhD theses ofJung W-H, Moura-Letts G. University of Pittsburgh; 2008. http://etd.library.pitt.edu/ETD/available/etd-03252008-125826/ http://etd.library.pitt.edu/ETD/available/etd-11162007-001904/
- (24).Kanick SC, Eiseman JL, Joseph E, Guo J, Parker RS. Noninvasive and nondestructive optical spectroscopic measurement of motexafin gadolinium in mouse tissues: comparison to high-performance liquid chromatography. J. Photochem. Photobiol. B. 2007;88:90–104. doi: 10.1016/j.jphotobiol.2007.05.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.