Abstract
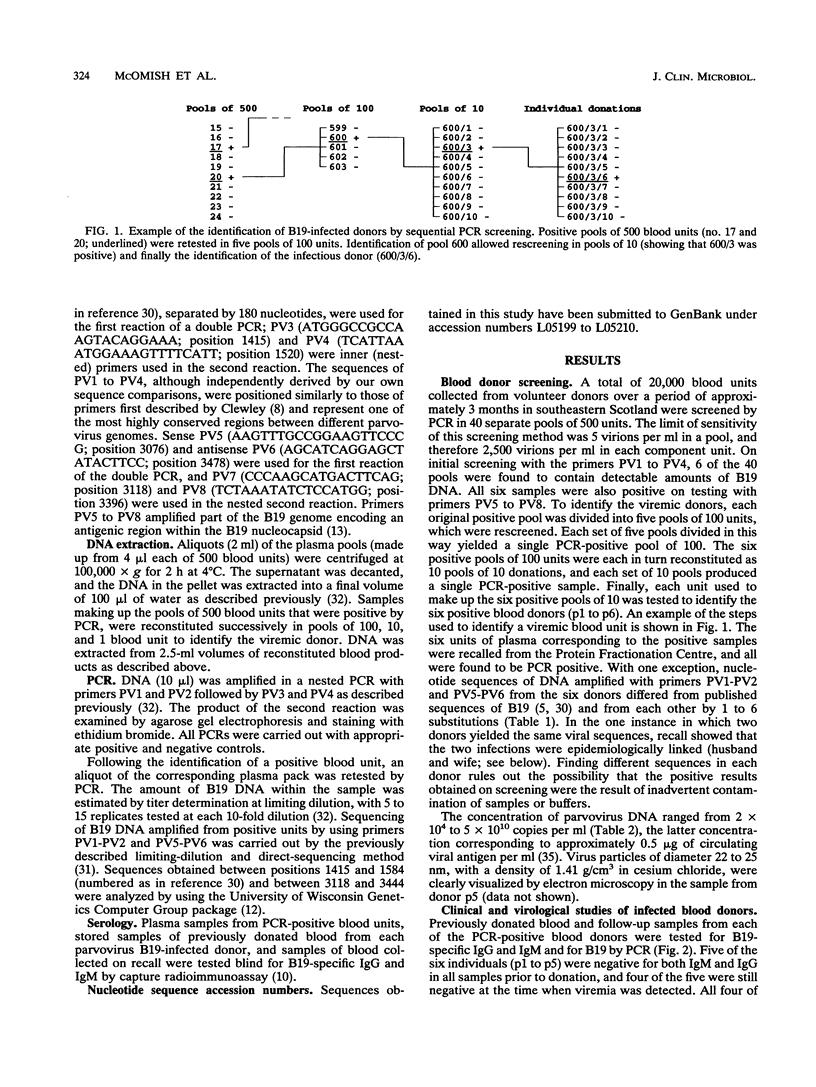
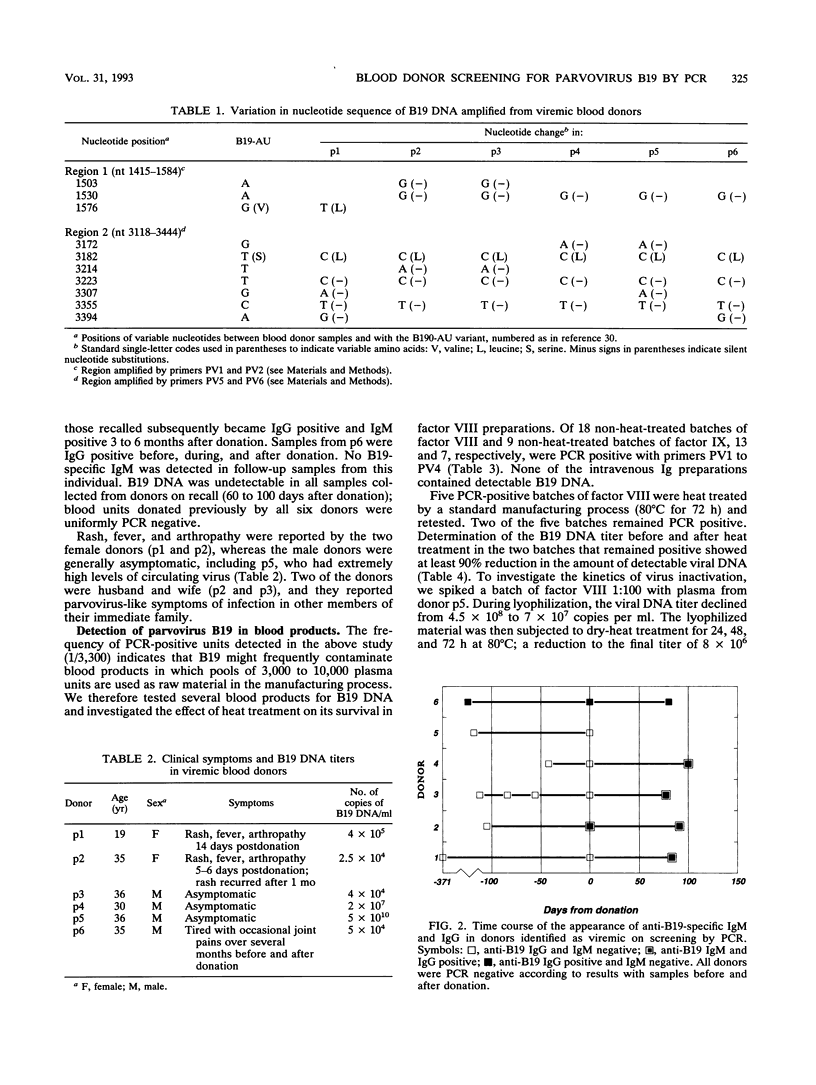
A highly sensitive and rapid method for routinely screening large numbers of donated blood units for parvovirus B19 by the polymerase chain reaction (PCR) was developed. Over a 3-month trial period in Edinburgh, B19 DNA was detected in 6 of 20,000 consecutive units of blood (0.03%), in concentrations ranging from 2.4 x 10(4) to 5 x 10(10) copies of viral DNA per ml. Seroconversion for B19-specific immunoglobulin M and immunoglobulin G and disappearance of circulating B19 DNA occurred in the interval between donation and recall in four of the five implicated donors who could be recalled. B19 DNA was detected in 18 of 27 separate batches of non-heat-treated factor VIII and IX concentrate manufactured from donated plasma unscreened for B19 DNA. Dry-heat treatment at 80 degrees C for 72 h reduced but did not always eliminate detectable B19 from factor VIII concentrates, consistent with recent observations that current methods for virus inactivation during blood product manufacture are insufficient to entirely eliminate B19 infectivity. The methods developed in this study for PCR screening could be applied routinely to prevent transfusion of B19 in blood and blood products and could play an important role in the prevention of iatrogenic transmission of infection. PCR screening could also be used for detection and exclusion of a range of other transmission-associated viruses for which current serological detection methods are only partially effective.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson M. J., Higgins P. G., Davis L. R., Willman J. S., Jones S. E., Kidd I. M., Pattison J. R., Tyrrell D. A. Experimental parvoviral infection in humans. J Infect Dis. 1985 Aug;152(2):257–265. doi: 10.1093/infdis/152.2.257. [DOI] [PubMed] [Google Scholar]
- Astell C. R., Thomson M., Merchlinsky M., Ward D. C. The complete DNA sequence of minute virus of mice, an autonomous parvovirus. Nucleic Acids Res. 1983 Feb 25;11(4):999–1018. doi: 10.1093/nar/11.4.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azzi A., Ciappi S., Zakvrzewska K., Morfini M., Mariani G., Mannucci P. M. Human parvovirus B19 infection in hemophiliacs first infused with two high-purity, virally attenuated factor VIII concentrates. Am J Hematol. 1992 Mar;39(3):228–230. doi: 10.1002/ajh.2830390315. [DOI] [PubMed] [Google Scholar]
- Bartolomei Corsi O., Azzi A., Morfini M., Fanci R., Rossi Ferrini P. Human parvovirus infection in haemophiliacs first infused with treated clotting factor concentrates. J Med Virol. 1988 Jun;25(2):165–170. doi: 10.1002/jmv.1890250206. [DOI] [PubMed] [Google Scholar]
- Blundell M. C., Beard C., Astell C. R. In vitro identification of a B19 parvovirus promoter. Virology. 1987 Apr;157(2):534–538. doi: 10.1016/0042-6822(87)90296-0. [DOI] [PubMed] [Google Scholar]
- Brown K. E., Mori J., Cohen B. J., Field A. M. In vitro propagation of parvovirus B19 in primary foetal liver culture. J Gen Virol. 1991 Mar;72(Pt 3):741–745. doi: 10.1099/0022-1317-72-3-741. [DOI] [PubMed] [Google Scholar]
- Carlson J., Rushlow K., Maxwell I., Maxwell F., Winston S., Hahn W. Cloning and sequence of DNA encoding structural proteins of the autonomous parvovirus feline panleukopenia virus. J Virol. 1985 Sep;55(3):574–582. doi: 10.1128/jvi.55.3.574-582.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewley J. P. Polymerase chain reaction assay of parvovirus B19 DNA in clinical specimens. J Clin Microbiol. 1989 Dec;27(12):2647–2651. doi: 10.1128/jcm.27.12.2647-2651.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen B. J., Field A. M., Gudnadottir S., Beard S., Barbara J. A. Blood donor screening for parvovirus B19. J Virol Methods. 1990 Dec;30(3):233–238. doi: 10.1016/0166-0934(90)90065-n. [DOI] [PubMed] [Google Scholar]
- Cohen B. J., Mortimer P. P., Pereira M. S. Diagnostic assays with monoclonal antibodies for the human serum parvovirus-like virus (SPLV). J Hyg (Lond) 1983 Aug;91(1):113–130. doi: 10.1017/s0022172400060095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham D. A., Pattison J. R., Craig R. K. Detection of parvovirus DNA in human serum using biotinylated RNA hybridisation probes. J Virol Methods. 1988 Mar-Apr;19(3-4):279–288. doi: 10.1016/0166-0934(88)90022-5. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridell E., Trojnar J., Wahren B. A new peptide for human parvovirus B19 antibody detection. Scand J Infect Dis. 1989;21(6):597–603. doi: 10.3109/00365548909021686. [DOI] [PubMed] [Google Scholar]
- Garson J. A., Preston F. E., Makris M., Tuke P., Ring C., Machin S. J., Tedder R. S. Detection by PCR of hepatitis C virus in factor VIII concentrates. Lancet. 1990 Jun 16;335(8703):1473–1473. doi: 10.1016/0140-6736(90)91510-h. [DOI] [PubMed] [Google Scholar]
- Harris J. W. Parvovirus B19 for the hematologist. Am J Hematol. 1992 Feb;39(2):119–130. doi: 10.1002/ajh.2830390209. [DOI] [PubMed] [Google Scholar]
- Koch W. C., Adler S. P. Detection of human parvovirus B19 DNA by using the polymerase chain reaction. J Clin Microbiol. 1990 Jan;28(1):65–69. doi: 10.1128/jcm.28.1.65-69.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtzman G. J., Cohen B. J., Field A. M., Oseas R., Blaese R. M., Young N. S. Immune response to B19 parvovirus and an antibody defect in persistent viral infection. J Clin Invest. 1989 Oct;84(4):1114–1123. doi: 10.1172/JCI114274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtzman G. J., Cohen B., Meyers P., Amunullah A., Young N. S. Persistent B19 parvovirus infection as a cause of severe chronic anaemia in children with acute lymphocytic leukaemia. Lancet. 1988 Nov 19;2(8621):1159–1162. doi: 10.1016/s0140-6736(88)90233-4. [DOI] [PubMed] [Google Scholar]
- Lyon D. J., Chapman C. S., Martin C., Brown K. E., Clewley J. P., Flower A. J., Mitchell V. E. Symptomatic parvovirus B19 infection and heat-treated factor IX concentrate. Lancet. 1989 May 13;1(8646):1085–1085. doi: 10.1016/s0140-6736(89)92488-4. [DOI] [PubMed] [Google Scholar]
- Morfini M., Longo G., Rossi Ferrini P., Azzi A., Zakrewska C., Ciappi S., Kolumban P. Hypoplastic anemia in a hemophiliac first infused with a solvent/detergent treated factor VIII concentrate: the role of human B19 parvovirus. Am J Hematol. 1992 Feb;39(2):149–150. doi: 10.1002/ajh.2830390217. [DOI] [PubMed] [Google Scholar]
- Mori J., Field A. M., Clewley J. P., Cohen B. J. Dot blot hybridization assay of B19 virus DNA in clinical specimens. J Clin Microbiol. 1989 Mar;27(3):459–464. doi: 10.1128/jcm.27.3.459-464.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortimer P. P., Luban N. L., Kelleher J. F., Cohen B. J. Transmission of serum parvovirus-like virus by clotting-factor concentrates. Lancet. 1983 Aug 27;2(8348):482–484. doi: 10.1016/s0140-6736(83)90512-3. [DOI] [PubMed] [Google Scholar]
- Musiani M., Zerbini M., Gibellini D., Gentilomi G., Venturoli S., Gallinella G., Ferri E., Girotti S. Chemiluminescence dot blot hybridization assay for detection of B19 parvovirus DNA in human sera. J Clin Microbiol. 1991 Sep;29(9):2047–2050. doi: 10.1128/jcm.29.9.2047-2050.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhode S. L., 3rd, Paradiso P. R. Parvovirus genome: nucleotide sequence of H-1 and mapping of its genes by hybrid-arrested translation. J Virol. 1983 Jan;45(1):173–184. doi: 10.1128/jvi.45.1.173-184.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollag H., Patou G., Pattison J. R., Degré M., Evensen S. A., Fröland S. S., Glomstein A. Prevalence of antibodies against parvovirus B19 in Norwegians with congenital coagulation factor defects treated with plasma products from small donor pools. Scand J Infect Dis. 1991;23(6):675–679. doi: 10.3109/00365549109024292. [DOI] [PubMed] [Google Scholar]
- Salimans M. M., Holsappel S., van de Rijke F. M., Jiwa N. M., Raap A. K., Weiland H. T. Rapid detection of human parvovirus B19 DNA by dot-hybridization and the polymerase chain reaction. J Virol Methods. 1989 Jan;23(1):19–28. doi: 10.1016/0166-0934(89)90085-2. [DOI] [PubMed] [Google Scholar]
- Schramm W., Roggendorf M., Rommel F., Kammerer R., Pohlmann H., Rasshofer R., Gürtler L., Deinhardt F. Prevalence of antibodies to hepatitis C virus (HCV) in haemophiliacs. Blut. 1989 Oct;59(4):390–392. doi: 10.1007/BF00321210. [DOI] [PubMed] [Google Scholar]
- Schwarz T. F., Roggendorf M., Hottenträger B., Stolz W., Schwinn H. Removal of parvovirus B19 from contaminated factor VIII during fractionation. J Med Virol. 1991 Sep;35(1):28–31. doi: 10.1002/jmv.1890350107. [DOI] [PubMed] [Google Scholar]
- Shade R. O., Blundell M. C., Cotmore S. F., Tattersall P., Astell C. R. Nucleotide sequence and genome organization of human parvovirus B19 isolated from the serum of a child during aplastic crisis. J Virol. 1986 Jun;58(3):921–936. doi: 10.1128/jvi.58.3.921-936.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmonds P., Balfe P., Ludlam C. A., Bishop J. O., Brown A. J. Analysis of sequence diversity in hypervariable regions of the external glycoprotein of human immunodeficiency virus type 1. J Virol. 1990 Dec;64(12):5840–5850. doi: 10.1128/jvi.64.12.5840-5850.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmonds P., Balfe P., Peutherer J. F., Ludlam C. A., Bishop J. O., Brown A. J. Human immunodeficiency virus-infected individuals contain provirus in small numbers of peripheral mononuclear cells and at low copy numbers. J Virol. 1990 Feb;64(2):864–872. doi: 10.1128/jvi.64.2.864-872.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava A., Lusby E. W., Berns K. I. Nucleotide sequence and organization of the adeno-associated virus 2 genome. J Virol. 1983 Feb;45(2):555–564. doi: 10.1128/jvi.45.2.555-564.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umene K., Nunoue T. Genetic diversity of human parvovirus B19 determined using a set of restriction endonucleases recognizing four or five base pairs and partial nucleotide sequencing: use of sequence variability in virus classification. J Gen Virol. 1991 Aug;72(Pt 8):1997–2001. doi: 10.1099/0022-1317-72-8-1997. [DOI] [PubMed] [Google Scholar]
- Williams M. D., Cohen B. J., Beddall A. C., Pasi K. J., Mortimer P. P., Hill F. G. Transmission of human parvovirus B19 by coagulation factor concentrates. Vox Sang. 1990;58(3):177–181. doi: 10.1111/j.1423-0410.1990.tb02086.x. [DOI] [PubMed] [Google Scholar]
- Woolf A. D., Campion G. V., Chishick A., Wise S., Cohen B. J., Klouda P. T., Caul O., Dieppe P. A. Clinical manifestations of human parvovirus B19 in adults. Arch Intern Med. 1989 May;149(5):1153–1156. [PubMed] [Google Scholar]


