Abstract
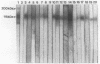
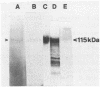
A 115-kDa exoantigen produced by Cryptococcus neoformans recognized by the previously described murine monoclonal antibody 7C9 has been purified from culture filtrate by a combination of membrane ultrafiltration, isoelectric focusing, and preparative gel electrophoresis. It is produced in late-log-phase cultures and is present in greater amounts in cultures grown at 25 degrees C than in those grown at 37 degrees C. Recognition of the antigen by 7C9 on immunoblots is abolished by the proteolytic enzymes papain and trypsin. The antigen is a glycoprotein bearing N-linked oligosaccharides, of which mannose is an important constituent. It does not appear to have proteolytic activity and is acidic, with a pI of 3 to 3.2. Its relationship to previously described C. neoformans mannoprotein is unclear since 7C9 shows only very weak cross-reactivity with a purified sample of the latter. Sera from patients infected with C. neoformans exhibited strong recognition of the glycoprotein as shown by immunoenzyme development of Western immunoblots, indicating its possible significance as a marker of disease.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Breen J. F., Lee I. C., Vogel F. R., Friedman H. Cryptococcal capsular polysaccharide-induced modulation of murine immune responses. Infect Immun. 1982 Apr;36(1):47–51. doi: 10.1128/iai.36.1.47-51.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherniak R., Morris L. C., Anderson B. C., Meyer S. A. Facilitated isolation, purification, and analysis of glucuronoxylomannan of Cryptococcus neoformans. Infect Immun. 1991 Jan;59(1):59–64. doi: 10.1128/iai.59.1.59-64.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherniak R. Soluble polysaccharides of Cryptococcus neoformans. Curr Top Med Mycol. 1988;2:40–54. doi: 10.1007/978-1-4612-3730-3_2. [DOI] [PubMed] [Google Scholar]
- Dismukes W. E. Cryptococcal meningitis in patients with AIDS. J Infect Dis. 1988 Apr;157(4):624–628. doi: 10.1093/infdis/157.4.624. [DOI] [PubMed] [Google Scholar]
- Hamilton A. J., Bartholomew M. A., Figueroa J., Fenelon L. E., Hay R. J. Production of species-specific murine monoclonal antibodies against Cryptococcus neoformans which recognize a noncapsular exoantigen. J Clin Microbiol. 1991 May;29(5):980–984. doi: 10.1128/jcm.29.5.980-984.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton A. J., Jeavons L., Hobby P., Hay R. J. A 34- to 38-kilodalton Cryptococcus neoformans glycoprotein produced as an exoantigen bearing a glycosylated species-specific epitope. Infect Immun. 1992 Jan;60(1):143–149. doi: 10.1128/iai.60.1.143-149.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heussen C., Dowdle E. B. Electrophoretic analysis of plasminogen activators in polyacrylamide gels containing sodium dodecyl sulfate and copolymerized substrates. Anal Biochem. 1980 Feb;102(1):196–202. doi: 10.1016/0003-2697(80)90338-3. [DOI] [PubMed] [Google Scholar]
- James P. G., Cherniak R. Galactoxylomannans of Cryptococcus neoformans. Infect Immun. 1992 Mar;60(3):1084–1088. doi: 10.1128/iai.60.3.1084-1088.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozel T. R., Gotschlich E. C. The capsule of cryptococcus neoformans passively inhibits phagocytosis of the yeast by macrophages. J Immunol. 1982 Oct;129(4):1675–1680. [PubMed] [Google Scholar]
- Mandal B. AIDS and fungal infections. J Infect. 1989 Nov;19(3):199–205. doi: 10.1016/s0163-4453(89)90649-x. [DOI] [PubMed] [Google Scholar]
- Murphy J. W., Mosley R. L., Cherniak R., Reyes G. H., Kozel T. R., Reiss E. Serological, electrophoretic, and biological properties of Cryptococcus neoformans antigens. Infect Immun. 1988 Feb;56(2):424–431. doi: 10.1128/iai.56.2.424-431.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prevost E., Newell R. Commercial cryptococcal latex kit: clinical evaluation in a medical center hospital. J Clin Microbiol. 1978 Nov;8(5):529–533. doi: 10.1128/jcm.8.5.529-533.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read S. M., Northcote D. H. Minimization of variation in the response to different proteins of the Coomassie blue G dye-binding assay for protein. Anal Biochem. 1981 Sep 1;116(1):53–64. doi: 10.1016/0003-2697(81)90321-3. [DOI] [PubMed] [Google Scholar]
- Reiss E., Cherniak R., Eby R., Kaufman L. Enzyme immunoassay detection of IgM to galactoxylomannan of Cryptococcus neoformans. Diagn Immunol. 1984;2(2):109–115. [PubMed] [Google Scholar]
- Turner S. H., Cherniak R., Reiss E. Fractionation and characterization of galactoxylomannan from Cryptococcus neoformans. Carbohydr Res. 1984 Feb 15;125(2):343–349. doi: 10.1016/0008-6215(84)85172-1. [DOI] [PubMed] [Google Scholar]
- Vartivarian S. E., Reyes G. H., Jacobson E. S., James P. G., Cherniak R., Mumaw V. R., Tingler M. J. Localization of mannoprotein in Cryptococcus neoformans. J Bacteriol. 1989 Dec;171(12):6850–6852. doi: 10.1128/jb.171.12.6850-6852.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]