Abstract
Human immunodeficiency virus (HIV) infection of the central nervous system (CNS) can lead to cognitive dysfunction, even in individuals treated with highly active antiretroviral therapy. Using an established simian immunodeficiency virus (SIV)/macaque model of HIV CNS disease, we previously reported that infection shifts the balance of activation of mitogen-activated protein kinase (MAPK) signaling pathways in the brain, resulting in increased activation of the neurodegenerative MAPKs p38 and JNK. Minocycline treatment of SIV-infected macaques reduced the incidence and severity of SIV encephalitis in this model, and suppressed the activation of p38 in the brain. The purpose of this study was to further examine the effects of minocycline on neurodegenerative MAPK signaling. We first demonstrated that minocycline also decreases JNK activation in the brain and levels of the inflammatory mediator nitric oxide (NO). We next used NO to activate these MAPK pathways in vitro, and demonstrated that minocycline suppresses p38 and JNK activation by reducing intracellular levels, and hence, activation of apoptosis signal-regulating kinase 1 (ASK1), a MAPKKK capable of selectively activating both pathways. We then demonstrated that ASK1 activation in the brain during SIV infection is suppressed by minocycline. By suppressing p38 and JNK activation pathways, which are important for the production of and responses to inflammatory mediators, minocycline may interrupt the vicious cycle of inflammation that both results from, and promotes, virus replication in SIV and HIV CNS disease.
Keywords: HIV, MAPK, CNS, signal transduction
Introduction
Minocycline, a second generation tetracycline derivative introduced in the 1960’s, has proven effective not only for the conventional treatment of bacterial infections, but also for treatment of inflammatory conditions (Sapadin and Fleischmajer, 2006). Recent studies have revealed that minocycline can penetrate the blood brain barrier and exhibits neuroprotective properties in many animal models (Elewa et al, 2006; Stirling et al, 2005), prompting its evaluation in clinical trials for the treatment of Huntington’s disease, multiple sclerosis, amyotrophic lateral sclerosis, stroke, and autism (Brigham and Women’s Hospital; EMD Serono; Gordon et al, 2007; Huntington Study Group; National Institute of Mental Health). Using a simian immunodeficiency virus (SIV) model of human immunodeficiency virus (HIV)-associated neurological disease (HAND), we recently demonstrated that minocycline reduced the incidence and severity of encephalitis in SIV-infected macaques (Zink et al, 2005). This study provided strong preclinical support for a multi-center clinical trial now underway for the treatment of decreased cognitive function in HIV-infected adults in the United States (National Institute of Allergy and Infectious Diseases), and a clinical trial in Uganda (personal communication, Dr. Ned Sacktor).
Neurocognitive dysfunction continues to be a problem for HIV-infected individuals, even those treated with highly active antiretroviral therapy (Ances and Ellis, 2007; Dore et al, 2003; Robertson et al, 2007; Sacktor et al, 2002; Tozzi et al, 2007). One hypothesis for this observation is that persistent HIV infection in the brain participates in a chronic self-perpetuating inflammatory cycle leading to progressive damage to the CNS. In this model, localized virus replication in the brain leads to activation of resident macrophages/microglia and astrocytes and the recruitment and activation of monocyte/macrophages and lymphocytes. These cells collectively produce pro-inflammatory mediators, which further activate infected cells, resulting in the amplification of virus replication. These processes ultimately establish a neurotoxic environment consisting of dying cells, viral proteins and nucleic acids, cytokines and chemokines, as well as reactive oxygen and nitrogen species.
In the CNS milieu of virus replication and inflammation observed during the development of SIV CNS disease, a shift occurs in the balance of activated mitogen activated protein kinases (MAPKs) from a marked increase in ERK activation early after infection to a predominance of JNK and p38 activation during late stage encephalitis (Barber et al, 2004). Typically, ERK is activated primarily by mitogens and is associated with cell growth and survival, whereas JNK and p38 are activated by diverse stress stimuli and are associated with apoptosis and neurodegeneration, although these roles are dynamic (Harper and LoGrasso, 2001; Mielke and Herdegen, 2000). In a previous study, minocycline treatment of SIV-infected macaques in our model not only resulted in decreased virus replication and less inflammation in the CNS (as evidenced by decreased levels of CD68+ and TIA-1+ cells in the brain, and reduced levels of the chemokine [C-C motif] ligand 2 [CCL2] in cerebrospinal fluid), but also decreased activation of p38 in neurons and astrocytes in the brain at terminal infection (Zink et al, 2005).
Since that initial report, we have continued to explore how this antibiotic mediates such pleiotropic effects. In this study we performed parallel in vitro and in vivo experiments to dissect the effects of minocycline on MAPK signaling pathways. We demonstrated the ability of minocycline to suppress JNK activation and lower nitric oxide (NO) levels in the brain in our SIV model and then focused on the mechanism by which minocycline is able to suppress the activation not only of p38, but also JNK. We selected an activator of p38 and JNK in vitro that is common to many neurodegenerative pathological processes including SIV encephalitis, nitric oxide (NO). Minocycline inhibited NO-induced activation of p38 and JNK by suppressing levels of activated apoptosis signal-regulating kinase 1 (ASK1). In parallel in vivo studies, we demonstrated suppression of ASK1 activation in the brains of infected macaques by minocycline. Since p38 and JNK participate in the generation of, and response to, inflammatory mediators, these results suggest that suppression of ASK1 activation contributes to the neuroprotective efficacy of minocycline.
Results
Minocycline Ameliorates the Pro-Inflammatory, Neurodegenerative Signaling Environment in the SIV-Infected Brain
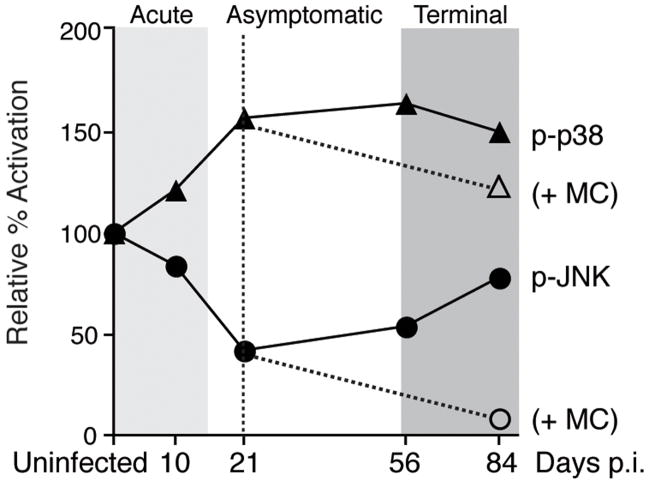
Our initial report demonstrated the ability of minocycline to reduce the increased activation of p38 in the brain that is characteristic of SIV encephalitis (Zink et al, 2005). We previously showed that during early asymptomatic infection at 21 days post-infection, when we initiated minocycline treatment, active phospho-p38 levels in the brain were significantly elevated from basal levels and were continuing to increase (Barber et al, 2004) (Figure 1). Thus intervention with minocycline at this time is able to suppress this process that is already in progress in the infected brain. Concomitant with increased p38 activation during the development of SIV encephalitis (84 days), a rebound to basal levels of JNK activation also was seen following significant suppression during the asymptomatic stage of infection (Barber et al, 2004).
Figure 1.
Schematic of changes in p38 and JNK activation seen in the brain longitudinally throughout SIV infection, as determined by quantitative immunohistochemistry in subcortical white matter. Solid lines depict mean immunohistochemical data obtained from multiple SIV-infected macaques at each time point and presented as percent activation relative to mean uninfected levels (data adapted from Barber et al, 2004). Dashed lines indicate decreased levels of active phoshpo-p38 (Zink et al, 2005) and phospho-JNK observed terminally at 84 days post-infection (p.i.) in minocycline-treated SIV-infected animals (+MC; treatment initiated at 21 days p.i.).
Here we examined the effect of minocycline on JNK activation in the brain of SIV-infected macaques using quantitative immunohistochemical analysis in subcortical white matter. Levels of activated phospho-JNK in the brain of SIV-infected minocycline-treated macaques were significantly decreased as compared to those of SIV-infected untreated animals (p = 0.004; Mann-Whitney test), which were not different than those in uninfected control macaques (p = 0.171; Mann-Whitney test) (Figure 2A). A predominance of the activated JNK was observed in neuronal axons in the terminal SIV-infected animals (Figure 2B); detection of activated phospho-JNK in neurons was substantially decreased in minocycline treated animals (Figure 2C). These findings suggest that minocycline inhibits the rebound of JNK activation observed in the brain during the transition from asymptomatic to terminal disease, extending the initial suppression of JNK activation observed during early asymptomatic infection. Thus, minocycline suppresses activation of both p38 and JNK in our SIV macaque model of HIV CNS disease.
Figure 2.
Quantitative immunohistochemical detection of phospho-JNK in macaque brain sections. (A) Staining for phospho-JNK in subcortical white matter was quantitated in samples from uninfected control animals (n = 4), SIV-infected animals (n = 6), and SIV-infected minocycline-treated animals (n = 5). Each data point represents the mean of 20 repeated measures of phospho-JNK staining in adjacent fields (bars represent group medians). Levels of active phospho-JNK in control animals were not significantly different from levels in SIV-infected untreated animals (p = 0.171; Mann-Whitney test), while SIV-infected minocycline-treated animals had significantly lower levels of phospho-JNK (p = 0.004; Mann-Whitney test). Staining for phospho-JNK with hematoxylin counterstaining to aid visualization (top) is shown in representative brain sections from an SIV-infected untreated animal (B) and an SIV-infected minocycline-treated animal (C). Representative staining present in subcortical white matter, without counterstain, as quantitated for (A) is shown at increased magnification (bottom). Prominent axonal staining is seen in untreated animals, and this is diminished in minocycline-treated animals. Original magnifications 100x and 200x.
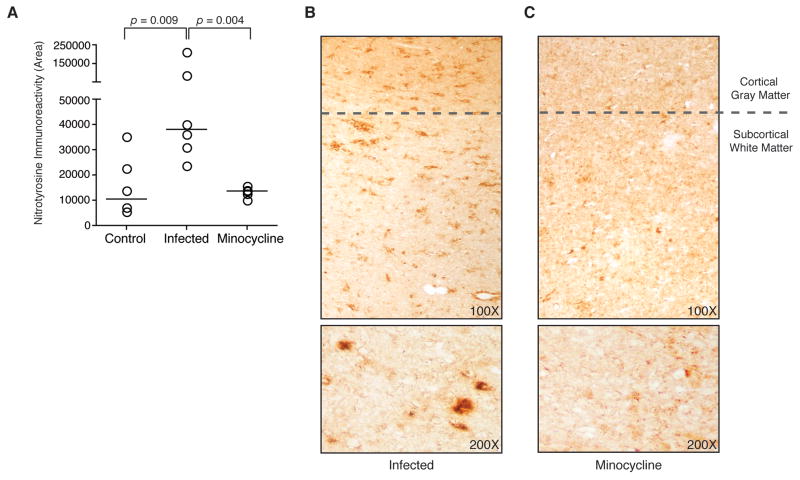
To examine potential mechanisms by which minocycline might suppress the activation of p38 and JNK, we needed to select an activator of both kinases that is associated with CNS disease in our model. Because excessive NO production has been observed in HIV CNS pathology (Boven et al, 1999; Bukrinsky et al, 1995; Vincent et al, 1999), and is common to the degenerative processes associated with a number of other neurological disorders such as Parkinson’s disease, Alzheimer’s disease, amyotrophic lateral sclerosis, and multiple sclerosis among others (Duncan and Heales, 2005; Sarchielli et al, 2003), we examined expression of NO in the brains of SIV-infected macaques. We used quantitative immunohistochemical analysis to detect nitrotyrosine as a stable indicator of the presence of elevated levels of NO and the resulting production of reactive nitrogen species (Halliwell, 1997; Pacher et al, 2007). Adjacent sections of the same region of subcortical white matter examined for phospho-JNK were immunohistochemically stained for nitrotyrosine. Nitrotyrosine levels were significantly increased at the terminal stage of infection in SIV-infected macaques as compared to uninfected control animals (p = 0.009; Mann-Whitney test; Figure 3A). Immunoreactivity for nitrotyrosine was detected throughout the subcortical white matter, and was seen frequently in perivascular areas, which have been previously associated with inflammation and virus replication in this model (Zink et al, 1999). In contrast, SIV-infected macaques treated with minocycline exhibited decreased levels of nitrotyrosine in the brain as compared to SIV-infected untreated macaques (p = 0.004; Mann-Whitney test; Figure 3B, C). As reactive nitrogen species are known to activate p38 and JNK, these findings indicate that NO may contribute to the shifting pattern of MAPK activation observed in this model during terminal infection and represents a relevant activator of both kinases in mechanistic in vitro studies.
Figure 3.
Quantitative immunohistochemical detection of nitrotyrosine in macaque brain sections. (A) Staining for nitrotyrosine in subcortical white matter was quantitated in samples from uninfected control animals (n = 6), SIV-infected untreated animals (n = 6), and SIV-infected minocycline-treated animals (n = 5) (bars represent group medians). Samples evaluated are serial sections of the same tissue from each animal stained for phospho-JNK in Figure 2. Each data point represents the mean of 20 repeated measures of nitrotyrosine staining in adjacent fields from one sample. Levels of nitrotyrosine staining were significantly increased from control at terminal infection (p = 0.009; Mann-Whitney test). SIV-infected minocycline-treated animals had significantly lower levels of nitrotyrosine immunopositive staining (p = 0.004; Mann-Whitney test). Staining for nitrotyrosine is shown in representative brain sections (top) from an untreated SIV-infected animal (B) and an SIV-infected minocycline-treated animal (C). Representative staining present in subcortical white matter, as quantitated in (A), is also shown at increased magnification (bottom). Original magnifications 100x and 200x.
Minocycline Suppresses NO-Induced Activation of p38 and JNK In Vitro
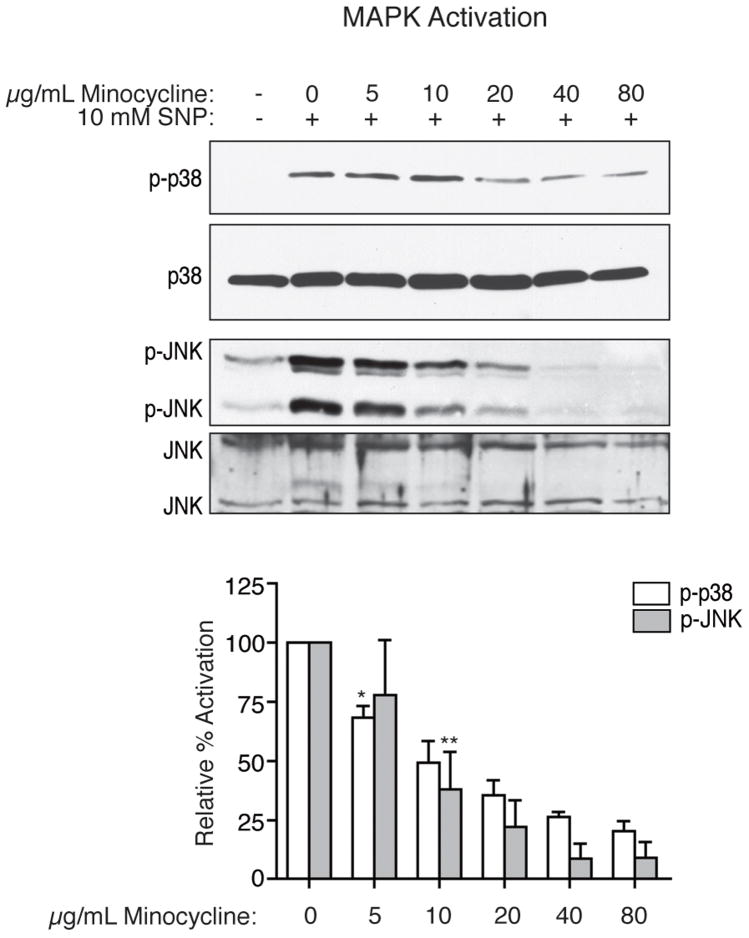
To explore how minocycline suppresses the activation of both p38 and JNK, we first examined the mechanism by which minocycline modulates the activities of these MAPKs in vitro, following exposure of differentiated U937 cells to the NO donor sodium nitroprusside (SNP). Because of their similarity to macrophages, which are key players in the inflammatory neuro-environment in our SIV/macaque model, these cells provided a homogeneous and well-characterized culture model. As expected, SNP treatment (10 mM) induced activation of both p38 and JNK above basal levels in these cells. When pretreated with minocycline, the activation of p38 in response to the NO donor was inhibited, as determined by western blot analysis using anti-active phospo-p38 antibody. This inhibition was dose-dependent for concentrations of minocycline from 5 to 80 μg/mL, with statistically significant decreases observed with doses of 5 μg/mL of minocycline (p = 0.008; one-sample t-test) or higher (Figure 4). As with p38, NO-induced activation of JNK was inhibited dose-dependently by minocycline, as determined by western blot analysis using phospho-specific antibody to active JNK (p = 0.018 for 10 μg/mL, all higher doses also resulted in significant decreases; one-sample t-test; Figure 4). While activation of both p38 and JNK was suppressed by minocycline, expression of these MAPKs remained stable with treatment.
Figure 4.
Inhibition by minocycline of activation of p38 and JNK MAPKs with SNP treatment in differentiated U937 cells. After four hours of treatment with 10 mM SNP, both p38 and JNK were activated, as indicated by phospho-specific western blot analysis (representative blots shown). Pretreatment for four hours with minocycline led to a dose-dependent reduction in the activation of p38 and JNK by SNP. Western blot analyses for total p38 and JNK expression also are shown. Results are the means of four independent experiments, with error bars illustrating the SEM. Densitometric analysis was performed with Kodak MI software. Values are presented as percent activation relative to activated samples (SNP treated, no minocycline). A one-sample t-test was performed to compare the mean activation level of each treatment group to 100%. Asterisks represent the lowest dose at which a statistically significant decrease in activation was observed compared to SNP stimulation (*p = 0.008, **p = 0.018); all higher doses also resulted in significant decreases.
Dose-Dependent Reduction in Activation of Upstream MAPK Kinases by Minocycline
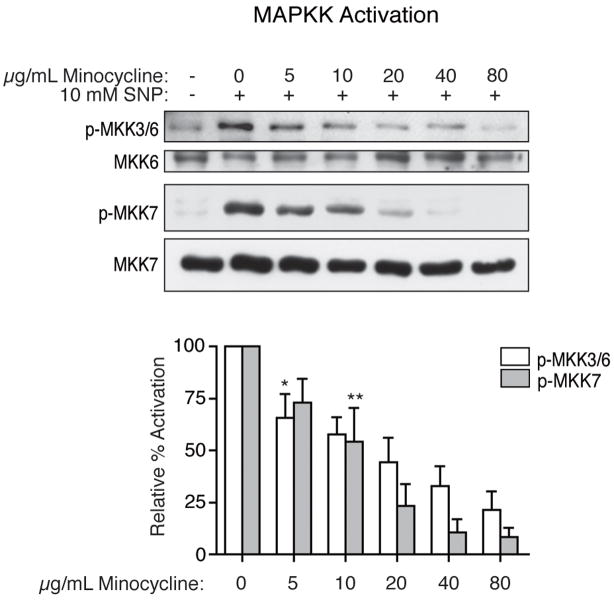
We next examined activation of the MAPK kinases (MAPKKs) required for activation of p38 and JNK in the classical hierarchical cascade of MAPK signaling pathways. Western blot analysis using a phospho-specific antibody against MKK3 and MKK6, upstream activators of p38, demonstrated that NO-induced activation of these MAPKKs was inhibited by minocycline. NO-induced activation of MKK7, the upstream activator of JNK, also was inhibited by minocycline. Expression of the MAPKKs remained stable throughout treatment. (Figure 5)
Figure 5.
Inhibition by minocycline of the activation of MKK3/6 and MKK7 in vitro. After four hours of treatment with 10 mM SNP, MKK3/6 and MKK7 were activated, as indicated by phospho-specific western blot analysis (representative blots shown). Pretreatment for four hours with minocycline prior to the addition of SNP led to a decrease in the activation of these MAPKKs. Western blot analyses for total MKK6 and MKK7 expression also are shown. Results are the means of four independent experiments, with error bars representing the SEM. Values illustrated are percent activation relative to activated samples (SNP treated, no minocycline). A one-sample t-test was performed to compare the mean activation level of each treatment group to 100%. Asterisks represent the lowest dose at which a statistically significant decrease in activation was seen compared to SNP stimulation (*p = 0.040, **p = 0.006); all higher doses also resulted in significant decreases.
Minocycline Treatment Reduces Activated ASK1 In Vitro
The above observations suggested that minocycline might modulate the activity of a MAPK kinase kinase (MAPKKK) upstream of all three MAPKKs examined, prompting us to examine the effect of minocycline on the MAPKKK ASK1, a selective activator of both p38 and JNK pathways. ASK1 activity was evaluated using an in vitro kinase assay that quantitates phosphorylation of myelin basic protein (MBP), an exogenous substrate for ASK1 (Figure 6A) (Dorion et al, 2002; Saitoh et al, 1998; Zhang and Zhang, 2002). Following three hours of treatment with 10 mM SNP, ASK1 activity was elevated well above basal levels. In contrast, ASK1 immunoprecipitates prepared from minocycline-treated cells exhibited reduced phosphorylation of MBP (p = 0.033 for 5 μg/mL, all higher doses also resulted in significant decreases; one-sample t-test; Figure 6A).
Figure 6.
Effects of minocycline on activation and expression of ASK1 in vitro. (A) ASK1 was activated after three hours of SNP treatment, as shown by an increase in phosphorylation of MBP in in vitro kinase assay. With minocycline pretreatment, decreased phosphorylation of MBP by immunoprecipitated ASK1 was observed (*p = 0.033 for 5 μg/mL, all higher doses also resulted in significant decreases; one sample t-test). A corresponding dose-dependent decrease in the amount of ASK1 immunoprecipitated from minocycline-treated cells was quantitated in metabolic labeling experiments (*p = 0.035 for 5 μg/mL, all higher doses also resulted in significant decreases; one sample t-test) (B) Western blot analysis for ASK1 illustrates a dose-dependent decrease in total ASK1 present in whole cell lysates, normalized to actin expression (*p = 0.050 for 5 μg/mL, all higher doses also resulted in significant decreases; one-sample t-test). (C) RT-PCR for ASK1 normalized to GAPDH showed no decrease in levels of ASK1 transcript produced with minocycline treatment (p > 0.05 for all comparisons; one sample t-test). All results are representative of three or four independent experiments, with error bars representing the SEM. Values shown are percent activation or expression relative to activated samples (SNP treated, no minocycline).
Unlike the downstream MAPKs and MAPKKs examined, the reduction of ASK1 activity in minocycline-treated cells correlated with a reduction in the expression of ASK1. Immunoprecipitation of ASK1 from cells that were labeled with Tran35S-Label and treated with minocycline revealed a dose-dependent reduction in the amount of ASK1 present, paralleling the reduction in activity observed in the kinase assays (p = 0.035 for 5 μg/mL, all higher doses also resulted in significant decreases; one-sample t-test; Figure 6A). A similar decrease in ASK1 levels was also observed in western blot analysis of whole cell lysates when normalized to a loading control (p = 0.050 for 5 μg/mL, all higher doses also resulted in significant decreases; one-sample t-test; Figure 6 B). Since NO-induced activation of ASK1 is necessary for activation of p38 (Han et al, 2001; Jibiki et al, 2003), it is reasonable to conclude that reduced levels of ASK1 in the presence of minocycline, and hence reduced NO-induced ASK1 activity, results in decreased NO-induced activity not only of MAPKK3, MAPKK6 and p38, but also of MAPKK7 and JNK.
RT-PCR analysis of ASK1 mRNA levels in cells treated with minocycline did not reveal a downward trend paralleling the observed decrease in ASK1 protein expression; levels remained essentially constant (p > 0.05 for all comparisons; one-sample t-test; Figure 6C). These data suggest that minocycline affects processes that regulate ASK1 expression post-transcriptionally.
Reduced ASK1 Activation in the Brains of Minocycline-Treated SIV-Infected Macaques
The results of our in vitro analyses strongly suggested that the ability of minocycline to inhibit NO-induced activation of p38 and JNK was linked to its ability to suppress levels of activated ASK1. We therefore examined ASK1 activation in our SIV/macaque model in vivo. Activated phospho-Thr845 ASK1 (autophosphorylation at threonine residue 845 in the activation loop of ASK1 renders the kinase functionally active, Tobiume et al, 2002) was detected in brain homogenates made from fresh frozen subcortical white matter tissue samples. SIV-infected animals displayed a strong trend toward elevated levels of activated ASK1 compared to uninfected control animals, based on comparisons of the ratios of phospho-ASK1 to total ASK1 for each animal (p = 0.093; Mann-Whitney test; Figure 7A). But for one outlying control animal with significant activation, this difference would have been significant (p = 0.009). Regardless, this demonstrated that ASK1 is activated during terminal infection in the brain. SIV-infected minocycline-treated animals had significantly lower levels of activated ASK1 versus SIV-infected untreated animals (p = 0.004; Mann-Whitney test), demonstrating the ability of minocycline to suppress ASK1 activation in the brain (Figure 7A).
Figure 7.
Activation and expression of ASK1 in the macaque brain. (A) Active phospho-Thr845 ASK1 in macaque brain homogenates as observed by western blot analysis after immunoprecipitation of ASK1. The total levels of ASK1 immunoprecipitated for each animal also are shown. Determination of a ratio of phospho-ASK1 to total ASK1 for each animal from densitometric analysis of bands allowed for the comparison of relative levels of ASK1 activation between animals. Activation of ASK1 was observed in SIV-infected untreated animals, and this was significantly decreased by minocycline treatment (p = 0.004; Mann-Whitney test; results are the means of values obtained in duplicate blots). (B) ASK1 expression in white matter brain homogenate. By western blot analysis (performed in duplicate), there was no significant difference in relative ASK1 expression between any of the treatment groups (p > 0.05; Mann-Whitney tests). Using immunohistochemistry, total ASK1 expression was observed clearly in axons (C), as well as in astrocytes (as shown by colocalization with GFAP, D) in the subcortical white matter of all macaques, regardless of group. ASK1 expression also was observed in activated microglia and macrophages when they were detected in sites of inflammation (as shown by colocalization with CD68, E). Original magnification 200x.
To evaluate the relative expression levels of ASK1 in the animals, western blot analysis was performed on equal amounts of the macaque brain homogenate samples, and ASK1 levels were quantitated and normalized to an actin loading control (Figure 7B). Unlike the in vitro observations, we did not find that decreased expression of ASK1 could account for the decreased ASK1 activation observed. ASK1 expression in SIV-infected minocycline-treated macaques was not different from that in SIV-infected untreated macaques (p = 0.931; Mann-Whitney test; Figure 7B). This result was upheld in quantitative immunohistochemistry for total ASK1 in subcortical white matter sections (p = 0.329; Mann-Whitney test; data not shown). ASK1 expression consistently was detected strongly in axons and astrocytes in subcortical white matter, accounting for a majority of the total staining, with no readily perceptible differences in distribution between animal groups (Figure 7C, D). ASK1 also colocalized with activated microglia/macrophages in animals where inflammation was observed (Figure 7E). Thus, although minocycline suppressed ASK1 activation in vitro and in vivo, the processes involved in the complex, multi-cellular, inflammatory environment of the SIV-infected CNS lacked the simplicity of those observed in a single cell type in culture.
Discussion
The ability of minocycline to inhibit p38 activation has been demonstrated in many disease models and is thought to be an important part of its neuroprotective properties (Stirling et al, 2005). Importantly, recent literature continues to reveal pathological activation of JNK, as well, in many of the neurodegenerative diseases in which p38 activation has been observed (Borsello and Forloni, 2007; Morishima et al, 2001). This study demonstrated that minocycline is able to inhibit not only p38 activation, but also activation of JNK. Relative to p38, effects of minocycline on JNK have rarely been studied (Nikodemova et al, 2006; Wilkins et al, 2004). In addition to the present study, however, two other reports have recently demonstrated inhibition of JNK activation by minocycline in models of CNS viral infection (Michaelis et al, 2007; Mishra and Basu, 2008), suggesting that inhibition of JNK activation may also contribute to minocycline’s neuroprotective effects. Changes in JNK activation in the brain are characteristic of the progression of CNS disease in our SIV model, and the ability of minocycline to prohibit rebounding JNK activation in the cells of the CNS at terminal infection underscores the importance of this MAPK to neuropathogenic mechanisms leading to SIV encephalitis and likely HIV CNS disease.
To examine potential mechanisms by which minocycline inhibits both p38 and JNK in vivo, we studied NO, a known activator of both pathways in vitro, and demonstrated not only elevated levels of reactive nitrogen species during SIV encephalitis by the detection of increased nitrotyrosine, but also a reduction in nitrotyrosine in the brain by minocycline. The ability of minocycline to inhibit NO-induced activation in vitro of the MAPKKs that activate both p38 and JNK prompted us to examine ASK1, a selective activating kinase for the p38 and JNK pathways that is activated by diverse stress stimuli including endoplasmic reticulum stress, reactive oxygen species resulting from TNFα or LPS stimulation, and of relevance here, NO and nitrosative stress (Hayakawa et al, 2006; Nagai et al, 2007; Sekine et al, 2006).
Numerous reports have illustrated that NO induces ASK1 activation, and have confirmed that ASK1 is required for NO-induced activation of p38 in multiple cell types through the use of dominant negative ASK1 constructs (Han et al, 2001; Jibiki et al, 2003; Sarker et al, 2003; Sumbayev, 2003). We found that minocycline treatment reduced the expression of, and hence, activation of ASK1 in vitro. Moreover, we demonstrated for the first time that ASK1 is activated in the brain during SIV infection, and that ASK1 activation in vivo is decreased with minocycline treatment. Thus, suppressed ASK1 activation is common to both our in vitro and in vivo studies.
Interestingly, the processes leading to this suppression in vitro appear at first glance to differ from those occurring in the more complex environment in vivo; however recent studies have revealed that many of the protein interactions affecting ASK1 activation are also involved in regulating its expression levels in the cell (He et al, 2006; Hwang et al, 2005; Kutuzov et al, 2007; Liu and Min, 2002). Therefore, it is conceivable that effects of minocycline in vitro and in vivo share a common mechanism that simply manifests differently in short-term cell culture as compared to several weeks in the brain in vivo. At least 31 different proteins have been shown to have regulatory effects on ASK1 activation, many of which may be found complexed with ASK1 in what has been coined the “ASK1 signalosome” (Takeda et al, 2008). Minocycline likely modulates one or more of these interactions. Many of these associations have been identified as dependent upon redox status, and in this regard an interesting observation of minocycline is its potential to act as an anti-oxidant in the presence of multiple different reactive oxygen and nitrogen species (Borderie et al, 2001; Kraus et al, 2005; Morimoto et al, 2005; Whiteman and Halliwell, 1997). While the specific mechanism remains to be defined, the previously unexamined suppression of ASK1 activation nevertheless represents an intriguing potential therapeutic target, and places SIV CNS disease with the growing number of neurological diseases in which ASK1 is suggested to play a pathological role (Akterin et al, 2006; Kadowaki et al, 2005; Ouyang and Shen, 2006; Sekine et al, 2006).
In the context of our SIV model, the ability to suppress ASK1 activation enables minocycline to interrupt the vicious cycle of inflammation and virus replication that culminates in encephalitis since the downstream effectors of this kinase, p38 and JNK, are not only involved in cellular responses to inflammatory mediators, but also in the production of these inflammatory molecules. The decrease in nitrotyrosine observed in minocycline-treated animals suggests that this could be the case for NO; not only does minocycline suppress NO-induced p38 and JNK activation, but it also leads to a decreased level of NO production when examined in vivo. Extending this concept to another example, because ASK1 is required for sustained p38 and JNK activation in response to tumor necrosis factor alpha (TNF-α) and oxidative stress (Tobiume et al, 2001), the ability of minocycline to suppress ASK1 activation likely decreases the activation of p38 and JNK by this stimulus as well. In turn, since p38 MAPK activation is necessary for the production of TNF-α (Brook et al, 2000; Guo et al, 2003; Lee et al, 1994; Wang et al, 1999), minocycline would suppress TNF-α production and further amplification of the cycle.
While reducing ASK1 activation in the brain during late stage infection is not the only mode of minocycline’s neuroprotective action, we believe it is an important part of the suppression of CNS disease. We have previously demonstrated effects of minocycline earlier in infection, such as a significant decrease in monocyte chemoattractant protein-1 (MCP-1) in the CSF, starting at just 28 days p.i. (Zink et al, 2005). This is a time at which elevated CCL2 levels are highly predictive of the influx of inflammatory cells to the brain and the development of encephalitis terminally (Wright et al, 2006). Thus, minocycline provides a first line of defense for the brain by inhibiting some of the immune cell infiltration leading to encephalitis. Unfortunately, such upstream effects of minocycline are not sufficient to totally preempt the processes leading to the development of CNS disease in this SIV model. Indeed, before minocycline treatment is even initiated at 21 days, it is evident that changes in the neuro-signaling environment have already been set in motion, such as the changing levels of p38 and JNK activation. Even with minocycline treatment, there are still activated CD68+ immune cells in the brain terminally, and while the levels are significantly decreased with treatment, virus replication is still observed in the brain terminally and is readily detected in the CSF throughout infection (Zink et al, 2005). How does minocycline prevent these processes, once started, from amplifying unchecked in the brain? We propose that through the suppression of ASK1 activation, minocycline provides an additional level of defense for the brain by interrupting ongoing inflammatory processes, even within the environment of established infection.
While many results of animal studies with minocycline have been positive, there also have been contradictory reports, and reports of minocycline exacerbating some disease conditions (Diguet et al, 2004; Gordon et al, 2007; Jackson et al, 2007; Mievis et al, 2007; Tsuji et al, 2004). Further understanding of minocycline’s mechanisms of action, the cellular mechanisms of disease pathology, and host immune components of disease management will be necessary to predict clinical applications. Access to a macaque model that closely recapitulates HIV CNS disease has provided us a unique opportunity to investigate minocycline in vivo, lending support to its evaluation in HIV-infected individuals. Perhaps ongoing clinical trials of minocycline for HIV-associated CNS disease will provide much needed insight to advance new approaches to treatment.
Materials and Methods
Animals
Eleven juvenile pigtailed macaques (Macaca nemestrina) were intravenously inoculated with SIV/DeltaB670 (50 AID50) and SIV/17E-Fr (10 000 AID50) as previously described (Zink et al, 1999). Minocycline at a dose of 4 mg/kg per day (Yen and Shaw, 1975) divided over two doses was administered orally to five of the macaques starting 21 days after inoculation. No adverse effects of minocycline treatment were identified in these animals. All infected macaques were euthanized during terminal infection (approximately 84 days after inoculation) in accordance with federal guidelines and institutional policies. Six age- and gender-matched uninfected macaques also were euthanized to provide control tissues (at the time of the first experiment in which levels of activated JNK in the brain are examined, tissue was available from just four control macaques, whereas all later experiments in this study include these same four animals plus two additional control macaques for a total n = 6). Macaques were perfused with sterile saline while under deep anesthetic to remove blood from the vasculature, and then tissues were frozen or fixed. Multiple brain sections from each animal were examined microscopically and scored in a blinded fashion to determine an encephalitis status of none, mild, moderate, or severe, according to previously described criteria (Zink et al, 1999). These animal studies were approved by the Johns Hopkins Animal Care and Use Committee. All animals were humanely treated in accordance with federal guidelines and institutional policies.
Immunohistochemistry
Fixed, paraffin embedded, subcortical white matter tissue from macaque brains was cut in 5 μm sections and immunohistochemically stained with an automated immunostainer (XMatrx, Biogenex, San Ramon, CA). Tissue sections were first deparaffinized and rehydrated, then heated in a microwave in sodium citrate buffer (0.01 M, pH 6.0) for eight minutes for antigen retrieval. Endogenous peroxidase activity was quenched by incubation in 3% H2O2 for 20 minutes. The sections were then blocked for 10 minutes (Power Block, Biogenex), followed by incubation in primary antibody (phopsho-JNK 1:100, Promega, Madison, WI; nitrotyrosine 1:6000, Upstate, Charlottesville, VA; ASK1 H300 1:200, Santa Cruz Biotechnology, Inc.) for one hour at room temperature. Wash steps were performed using PBS with 0.05% tween, followed by incubation in biotinylated secondary antibody (Multi-link, Biogenex) for 20 minutes. Colorimetric detection was performed with the application of streptavidin conjugated horseradish peroxidase (HRP) followed by liquid diaminobenzidine tetrahydrochloride (DAB) substrate (Biogenex). The stained sections were washed, dehydrated and mounted.
Digital image analysis was used for quantification of staining. Stained slides were blinded and examined at 200x magnification. Twenty adjacent fields of white matter constituting approximately 3 mm2 were imaged for each animal and images were analyzed using IP Lab imaging software (Scanalytics, BD Biosciences, Rockville, MD). Images were binarized (each pixel converted to a value of 1 for positive or 0 for negative) and the total area occupied by positive pixels was calculated. This provides a quantitative measure of the total area occupied by positively stained cells or portions of cells in the area evaluated. Specificity of the antibodies used for phosho-JNK and ASK1 was confirmed by performing the immunohistochemical staining procedure as above except for the omission of the primary antibodies, and by western blotting. For the anti-nitrotyrosine antibody, specific staining was eliminated by omitting the primary antibody, or by pre-absorption of the antibody with 10 mM 3-nitro-L-tyrosine (Sigma). To identify cell types stained for ASK1, double labeling was performed using anti-glial fibrillary acidic protein (GFAP, DAKO, 1:4000) and anti-CD68 (DAKO, 1:2000) primary antibodies, in conjunction with Vector SG staining reagents (Vector Laboratories, Inc., Burlingame, CA).
Cell Culture and Treatments
The U937 promonocytic cell line was obtained from American Type Culture Collection (Manassas, VA). Cell cultures were maintained under 5% CO2 in RPMI 1640 media (Gibco, Carlsbad, CA), supplemented with 10% fetal bovine serum (FBS, Atlanta Biologicals, Lawrenceville, Georgia), 2 mM L-glutamine (Gibco), 10 mM HEPES (Gibco), and 0.5 mg/mL gentamycin (Gibco). Prior to use in assays, cells were seeded at a density of 106/mL and cultured overnight in the RPMI based media with a reduction in the supplemented FBS to 2% and the addition of 16 nM phorbol 12-myristate 13-acetate (PMA, Sigma, St. Louis, MO) to induce differentiation toward a macrophage phenotype. Cells were then treated or not with minocycline (doses from 0 to 80 μg/mL, Sigma) for four hours prior to the addition of the nitric oxide donor sodium nitroprusside (SNP, Sigma) at 10 mM.
Western Blotting of Cell Lysates
Whole cell lysates were prepared from treated U937 cell cultures using radioimmunoprecipitation assay (RIPA) lysis buffer containing protease and phosphatase inhibitors (20 mM Tris-HCl pH 7.4, 150 mM NaCl, 0.1 mM EDTA, 1% NP-40, 1% sodium deoxycholate, 0.1% sodium dodecyl sulfate [SDS], 50 mM sodium fluoride [NaF], 1 mM sodium orthovanadate [NaOV], 1 mM phenylmethylsulphonyl fluoride [PMSF], 2 μg/mL aprotinin and 2 μg/mL leupeptin). Lysates (40 μg) were electrophoretically separated on polyacrilamide gels (10% or 4 to 15% gradient) and transferred to polyvinylidene difluoride (PVDF) membranes (Millipore, Bedford, MA) for western detection using both phospho-specific and non-phospho-specific antibodies to MAPK signaling pathway proteins. Antibodies to p38 MAP Kinase, phospho-p38 MAPK (Thr180/Tyr182), phospho-SAPK/JNK (Thr185/Tyr185), phospho-MKK3/MKK6 (Ser189/207), MKK7 and phospho-MKK7 (Ser271/Thr275) were from Cell Signaling Technology (Beverly, MA); antibodies to JNK, MEK-6 (MKK6), and ASK1 were from Santa Cruz Biotechnology, Inc (Santa Cruz, CA). Secondary HRP conjugated anti-rabbit, anti-mouse and anti-goat antibodies (DAKO, Carpinteria, CA) were used, and blots were visualized by enhanced chemiluminescence using DuraSignal substrate (Pierce, Rockford, IL). Blots were quantitated by relative densitometry using Kodak MI software.
Immunoprecipitation and In Vitro Kinase Assay
Cells were washed twice in ice cold PBS and lysed (20 mM Tris-HCl pH 7.4, 12 mM β-glycerophosphate, 150 mM NaCl, 5 mM EGTA, 10 mM NaF, 1% triton X-100, 1% sodium desoxycholate, 1 mM NaOV, 1 mM PMSF, 1 mM dithiothreitol (DTT), 2 μg/mL aprotinin and 2 μg/mL leupeptin). Immunoprecipitation was carried out overnight from 1 mg of cell lysate using 4 μg of anti-ASK1 (H300, Santa Cruz Biotechnology, Inc.). Activity of the immunoprecipitated kinase was assayed in vitro with the addition of 10 μg of the exogenous substrate myelin basic protein (MBP, Upstate) and 30 μCi [32P]-γATP (Perkin Elmer, Waltham, MA) in kinase buffer (20mM Tris pH 7.4, 20 mM MgCl2), followed by a 30-minute incubation with shaking at 37°C. Phosphorylated substrate was resolved by SDS-PAGE and imaged with a Typhoon 9210 phosphorimager (Amersham, Piscataway, NJ). ImageQuant software (version 5.5, Molecular Dynamics) was used for densitometric analysis of autoradiograph images.
Metabolic Labeling
U937 cells plated at 106 cells/mL and PMA-treated overnight were washed once in HBSS to remove media, and then starved for one hour in RPMI 1640 without methionine or cysteine (Invitrogen, Carlsbad, CA), supplemented with 2% FBS, 2 mM L-glutamine, 10 mM HEPES, and 0.5 mg/mL gentamycin. Tran35S-Label (MP Biomedicals, Inc., Irvine, CA) was added to each flask at 0.2 miC/mL for one hour followed by the addition of minocycline and SNP as described above. Following treatment, cells were washed in ice cold PBS, and lysed in the same buffer used for kinase assays. 300 μg samples of lysate were pre-cleared over night by rotation at 4°C with 30 μL of protein A/G agarose beads. ASK1 was then immunoprecipitated with anti-ASK1 (H300, Santa Cruz Biotechnology) and resolved by SDS-PAGE on a 4 to 15% gradient gel. Dried gels were imaged with a Typhoon 9210, and band densities analyzed with ImageQuant software.
RT-PCR
Total RNA was extracted from cells using the RNEasy kit (Qiagen, Valencia, CA). Superscript II (Invitrogen) was used to make cDNA from 1 μg of each RNA sample, using random hexamer priming. RT-PCR was performed on equal amounts of the cDNA samples (2 μL of the 30 μL reaction) using primers for ASK1 (Hershko et al, 2006) (5′-ACAGCAGATACTCTCAGCC and 5′-CATTGTCACCCTTTATGTCCC) and GAPDH (5′-TGCCATCAATGACCCCTTCATTGACCTC and 5′-CCCAGCCTTCTCCATGGTGGTGAAGAC). Products were resolved on agarose gels and stained with ethidium bromide. The relative densities of the resulting bands were determined using the Typhoon 9210 (excitation 532 nm, emission filter 610BP30) and ImageQuant software.
Phospho-Thr845 ASK1 Detection in Macaque Brain Homogenates
Fresh frozen tissue samples of subcortical white matter were homogenized in buffer (30 mM Tris pH 8.5, 2 M thiourea, 7 M urea, 4% CHAPS) containing protease inhibitor (Calbiochem, San Diego, CA) and phosphatase inhibitor cocktails (I and II, Sigma). To enable detection of active phoshpo-ASK1 (Thr845), ASK1 was first immunoprecipitated from 200 μg of homogenate with 1 μg of anti-ASK1 (F9, Santa Cruz Biotechnology). Immunoprecipitated protein was run on a 4 to 15% gradient gel, followed by western blotting for phospho-ASK1 (Thr845, Cell Signaling) using secondary anti-rabbit HRP (DAKO) and enhanced chemiluminescence detection (DuraSignal, Pierce). After detection of phospho-ASK1, blots were stripped and re-probed for detection of total ASK1. Kodak MI software was used to determine band densities, and ratios of phospho-ASK1:ASK1 were determined for each animal. Ratios were normalized to a standard sample on each blot.
Fluorescent Western Blotting on Macaque Brain Homogenates
To most accurately quantify any ASK1 expression differences in macaque brain homogenates, fluorescent western blotting was employed. Brain homogenate samples (20 μg) were run on 4 to 15% gradient gels followed by transfer to PVDF membrane. Blots were probed with primary antibody to ASK1 (F9, Santa Cruz), followed by secondary anti-mouse AP (GE Healthcare; Piscataway, NJ). ECF reagent (GE Healthcare) was used for detection and blots were imaged using the Typhoon 9210 scanner with fluorescence emission filter 526SP (excitation 532 nm). The same blots were then probed with primary anti-actin (Sigma) followed by secondary anti-mouse cy3 (GE Healthcare), which was directly detected using the CY3 580 BP30 emission filter (excitation 532 nm). Serial dilutions of a standard sample on each blot were analyzed for both proteins (ASK1 and actin) to insure that detection occurred within a linear range, and to determine relative concentrations of detected protein based on the linear curve equation produced. The resulting values for ASK1 were normalized to actin for each sample as a loading control. Inter-blot normalization was performed based on a standard sample run on all blots.
Acknowledgments
This research was supported by NIH grants MH069116, MH070306, NS44815, and NS55648.
The authors would like to thank Dr. Patrick Tarwater for discussion of statistical analysis, Dr. Robert Adams for assistance with the macaques, and John Anderson and Christopher Bartizal for excellent technical assistance. Additionally, we thank the members of the Retrovirus laboratory for helpful discussion.
Footnotes
Drs. Zink and Barber are named as inventors on a patent pending for minocycline to treat HIV infection. The patent will be held by the Johns Hopkins University.
References
- Akterin S, Cowburn RF, Miranda-Vizuete A, Jimenez A, Bogdanovic N, Winblad B, Cedazo-Minguez A. Involvement of glutaredoxin-1 and thioredoxin-1 in beta-amyloid toxicity and Alzheimer’s disease. Cell Death Differ. 2006;13:1454–65. doi: 10.1038/sj.cdd.4401818. [DOI] [PubMed] [Google Scholar]
- Ances BM, Ellis RJ. Dementia and neurocognitive disorders due to HIV-1 infection. Semin Neurol. 2007;27:86–92. doi: 10.1055/s-2006-956759. [DOI] [PubMed] [Google Scholar]
- Barber SA, Uhrlaub JL, DeWitt JB, Tarwater PM, Zink MC. Dysregulation of mitogen-activated protein kinase signaling pathways in simian immunodeficiency virus encephalitis. Am J Pathol. 2004;164:355–62. doi: 10.1016/S0002-9440(10)63125-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borderie D, Hernvann A, Hilliquin P, Lemarchal H, Kahan A, Ekindjian OG. Tetracyclines inhibit nitrosothiol production by cytokine-stimulated osteoarthritic synovial cells. Inflamm Res. 2001;50:409–14. doi: 10.1007/PL00000263. [DOI] [PubMed] [Google Scholar]
- Borsello T, Forloni G. JNK signalling: a possible target to prevent neurodegeneration. Curr Pharm Des. 2007;13:1875–86. doi: 10.2174/138161207780858384. [DOI] [PubMed] [Google Scholar]
- Boven LA, Gomes L, Hery C, Gray F, Verhoef J, Portegies P, Tardieu M, Nottet HS. Increased peroxynitrite activity in AIDS dementia complex: implications for the neuropathogenesis of HIV-1 infection. J Immunol. 1999;162:4319–27. [PubMed] [Google Scholar]
- Brigham and Women’s Hospital. ClinicalTrials.gov [Internet] Bethesda (MD): National Library of Medicine (US); 2000. A Study of the Effects of Minocycline on Cognitive Function After Carotid Endarterectomy. [cited 2008 Feb 1]. Available from: http://clinicaltrials.gov/ct/show/NCT00401921 NLM Identifier: NCT00401921. [Google Scholar]
- Brook M, Sully G, Clark AR, Saklatvala J. Regulation of tumour necrosis factor alpha mRNA stability by the mitogen-activated protein kinase p38 signalling cascade. FEBS Lett. 2000;483:57–61. doi: 10.1016/s0014-5793(00)02084-6. [DOI] [PubMed] [Google Scholar]
- Bukrinsky MI, Nottet HS, Schmidtmayerova H, Dubrovsky L, Flanagan CR, Mullins ME, Lipton SA, Gendelman HE. Regulation of nitric oxide synthase activity in human immunodeficiency virus type 1 (HIV-1)-infected monocytes: implications for HIV-associated neurological disease. J Exp Med. 1995;181:735–45. doi: 10.1084/jem.181.2.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diguet E, Fernagut PO, Wei X, Du Y, Rouland R, Gross C, Bezard E, Tison F. Deleterious effects of minocycline in animal models of Parkinson’s disease and Huntington’s disease. Eur J Neurosci. 2004;19:3266–76. doi: 10.1111/j.0953-816X.2004.03372.x. [DOI] [PubMed] [Google Scholar]
- Dore GJ, McDonald A, Li Y, Kaldor JM, Brew BJ. Marked improvement in survival following AIDS dementia complex in the era of highly active antiretroviral therapy. Aids. 2003;17:1539–45. doi: 10.1097/00002030-200307040-00015. [DOI] [PubMed] [Google Scholar]
- Dorion S, Lambert H, Landry J. Activation of the p38 signaling pathway by heat shock involves the dissociation of glutathione S-transferase Mu from Ask1. J Biol Chem. 2002;277:30792–7. doi: 10.1074/jbc.M203642200. [DOI] [PubMed] [Google Scholar]
- Duncan AJ, Heales SJ. Nitric oxide and neurological disorders. Mol Aspects Med. 2005;26:67–96. doi: 10.1016/j.mam.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Elewa HF, Hilali H, Hess DC, Machado LS, Fagan SC. Minocycline for short-term neuroprotection. Pharmacotherapy. 2006;26:515–21. doi: 10.1592/phco.26.4.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EMD Serono. ClinicalTrials.gov [Internet] Bethesda (MD): National Library of Medicine (US); 2000. Minocycline as Add-on to Interferon-Beta-1a (Rebif®) in RRMS (Recycline) [cited 2008 Feb 1]. Available from: http://clinicaltrials.gov/ct/show/NCT00381459 NLM Identifier: NCT00381459. [Google Scholar]
- Gordon PH, Moore DH, Miller RG, Florence JM, Verheijde JL, Doorish C, Hilton JF, Spitalny GM, MacArthur RB, Mitsumoto H, Neville HE, Boylan K, Mozaffar T, Belsh JM, Ravits J, Bedlack RS, Graves MC, McCluskey LF, Barohn RJ, Tandan R. Efficacy of minocycline in patients with amyotrophic lateral sclerosis: a phase III randomised trial. Lancet Neurol. 2007;6:1045–53. doi: 10.1016/S1474-4422(07)70270-3. [DOI] [PubMed] [Google Scholar]
- Guo X, Gerl RE, Schrader JW. Defining the involvement of p38alpha MAPK in the production of anti- and proinflammatory cytokines using an SB 203580-resistant form of the kinase. J Biol Chem. 2003;278:22237–42. doi: 10.1074/jbc.M300847200. [DOI] [PubMed] [Google Scholar]
- Halliwell B. What nitrates tyrosine? Is nitrotyrosine specific as a biomarker of peroxynitrite formation in vivo? FEBS Lett. 1997;411:157–60. doi: 10.1016/s0014-5793(97)00469-9. [DOI] [PubMed] [Google Scholar]
- Han OJ, Joe KH, Kim SW, Lee HS, Kwon NS, Baek KJ, Yun HY. Involvement of p38 mitogen-activated protein kinase and apoptosis signal-regulating kinase-1 in nitric oxide-induced cell death in PC12 cells. Neurochem Res. 2001;26:525–32. doi: 10.1023/a:1010917129951. [DOI] [PubMed] [Google Scholar]
- Harper SJ, LoGrasso P. Signalling for survival and death in neurones: the role of stress-activated kinases, JNK and p38. Cell Signal. 2001;13:299–310. doi: 10.1016/s0898-6568(01)00148-6. [DOI] [PubMed] [Google Scholar]
- Hayakawa T, Matsuzawa A, Noguchi T, Takeda K, Ichijo H. The ASK1-MAP kinase pathways in immune and stress responses. Microbes Infect. 2006;8:1098–107. doi: 10.1016/j.micinf.2005.12.001. [DOI] [PubMed] [Google Scholar]
- He Y, Zhang W, Zhang R, Zhang H, Min W. SOCS1 inhibits tumor necrosis factor-induced activation of ASK1-JNK inflammatory signaling by mediating ASK1 degradation. J Biol Chem. 2006;281:5559–66. doi: 10.1074/jbc.M512338200. [DOI] [PubMed] [Google Scholar]
- Hershko T, Korotayev K, Polager S, Ginsberg D. E2F1 modulates p38 MAPK phosphorylation via transcriptional regulation of ASK1 and Wip1. J Biol Chem. 2006;281:31309–16. doi: 10.1074/jbc.M601758200. [DOI] [PubMed] [Google Scholar]
- Huntington Study Group, FDA Office of Orphan Products Development. ClinicalTrials.gov [Internet] Bethesda (MD): National Library of Medicine (US); 2000. Pilot Study of Minocycline in Huntington’s Disease. [cited 2008 Feb 1]. Available from: http://clinicaltrials.gov/ct/show/NCT00277355 NLM Identifier: NCT00277355. [Google Scholar]
- Hwang JR, Zhang C, Patterson C. C-terminus of heat shock protein 70-interacting protein facilitates degradation of apoptosis signal-regulating kinase 1 and inhibits apoptosis signal-regulating kinase 1-dependent apoptosis. Cell Stress Chaperones. 2005;10:147–56. doi: 10.1379/CSC-90R.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson AC, Scott CA, Owen J, Weli SC, Rossiter JP. Therapy with minocycline aggravates experimental rabies in mice. J Virol. 2007;81:6248–53. doi: 10.1128/JVI.00323-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jibiki I, Hashimoto S, Maruoka S, Gon Y, Matsuzawa A, Nishitoh H, Ichijo H, Horie T. Apoptosis signal-regulating kinase 1-mediated signaling pathway regulates nitric oxide-induced activator protein-1 activation in human bronchial epithelial cells. Am J Respir Crit Care Med. 2003;167:856–61. doi: 10.1164/rccm.2204042. [DOI] [PubMed] [Google Scholar]
- Kadowaki H, Nishitoh H, Urano F, Sadamitsu C, Matsuzawa A, Takeda K, Masutani H, Yodoi J, Urano Y, Nagano T, Ichijo H. Amyloid beta induces neuronal cell death through ROS-mediated ASK1 activation. Cell Death Differ. 2005;12:19–24. doi: 10.1038/sj.cdd.4401528. [DOI] [PubMed] [Google Scholar]
- Kraus RL, Pasieczny R, Lariosa-Willingham K, Turner MS, Jiang A, Trauger JW. Antioxidant properties of minocycline: neuroprotection in an oxidative stress assay and direct radical-scavenging activity. J Neurochem. 2005;94:819–27. doi: 10.1111/j.1471-4159.2005.03219.x. [DOI] [PubMed] [Google Scholar]
- Kutuzov MA, Andreeva AV, Voyno-Yasenetskaya TA. Regulation of apoptosis signal-regulating kinase 1 degradation by G{alpha}13. FASEB J. 2007;21:3727–3726. doi: 10.1096/fj.06-8029com. [DOI] [PubMed] [Google Scholar]
- Lee JC, Laydon JT, McDonnell PC, Gallagher TF, Kumar S, Green D, McNulty D, Blumenthal MJ, Heys JR, Landvatter SW, et al. A protein kinase involved in the regulation of inflammatory cytokine biosynthesis. Nature. 1994;372:739–46. doi: 10.1038/372739a0. [DOI] [PubMed] [Google Scholar]
- Liu Y, Min W. Thioredoxin promotes ASK1 ubiquitination and degradation to inhibit ASK1-mediated apoptosis in a redox activity-independent manner. Circ Res. 2002;90:1259–66. doi: 10.1161/01.res.0000022160.64355.62. [DOI] [PubMed] [Google Scholar]
- Michaelis M, Kleinschmidt MC, Doerr HW, Cinatl J., Jr Minocycline inhibits West Nile virus replication and apoptosis in human neuronal cells. J Antimicrob Chemother. 2007;60:981–986. doi: 10.1093/jac/dkm307. [DOI] [PubMed] [Google Scholar]
- Mielke K, Herdegen T. JNK and p38 stresskinases--degenerative effectors of signal-transduction-cascades in the nervous system. Prog Neurobiol. 2000;61:45–60. doi: 10.1016/s0301-0082(99)00042-8. [DOI] [PubMed] [Google Scholar]
- Mievis S, Levivier M, Communi D, Vassart G, Brotchi J, Ledent C, Blum D. Lack of Minocycline Efficiency in Genetic Models of Huntington’s Disease. Neuromolecular Med. 2007;9:47–54. doi: 10.1385/nmm:9:1:47. [DOI] [PubMed] [Google Scholar]
- Mishra MK, Basu A. Minocycline neuroprotects, reduces microglial activation, inhibits caspase-3 induction, and viral replication following Japanese Encephalitis. J Neurochem. 2008;105:1582–1595. doi: 10.1111/j.1471-4159.2008.05238.x. [DOI] [PubMed] [Google Scholar]
- Morimoto N, Shimazawa M, Yamashima T, Nagai H, Hara H. Minocycline inhibits oxidative stress and decreases in vitro and in vivo ischemic neuronal damage. Brain Res. 2005;1044:8–15. doi: 10.1016/j.brainres.2005.02.062. [DOI] [PubMed] [Google Scholar]
- Morishima Y, Gotoh Y, Zieg J, Barrett T, Takano H, Flavell R, Davis RJ, Shirasaki Y, Greenberg ME. Beta-amyloid induces neuronal apoptosis via a mechanism that involves the c-Jun N-terminal kinase pathway and the induction of Fas ligand. J Neurosci. 2001;21:7551–60. doi: 10.1523/JNEUROSCI.21-19-07551.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai H, Noguchi T, Takeda K, Ichijo H. Pathophysiological roles of ASK1-MAP kinase signaling pathways. J Biochem Mol Biol. 2007;40:1–6. doi: 10.5483/bmbrep.2007.40.1.001. [DOI] [PubMed] [Google Scholar]
- National Institute of Allergy and Infectious Diseases, Neuologic AIDS Research Consortium. Minocycline for the Treatment of Decreased Mental Function in HIV Infected Adults. ClinicalTrials.gov. [Internet]. Bethesda (MD): National Library of Medicine (US). 2000 – [cited 2008 Feb 1]. Available from: http://clinicaltrials.gov/ct/show/NCT00361257 NLM Identifier: NCT00361257.
- National Institute of Mental Health. ClinicalTrials.gov [Internet] Bethesda (MD): National Library of Medicine (US); 2000. Minocycline to Treat Childhood Regressive Autism. [cited 2008 Feb 1]. Available from: http://clinicaltrials.gov/ct/show/NCT00409747 NLM Identifier: NCT00409747. [Google Scholar]
- Nikodemova M, Duncan ID, Watters JJ. Minocycline exerts inhibitory effects on multiple mitogen-activated protein kinases and IkappaBalpha degradation in a stimulus-specific manner in microglia. J Neurochem. 2006;96:314–23. doi: 10.1111/j.1471-4159.2005.03520.x. [DOI] [PubMed] [Google Scholar]
- Ouyang M, Shen X. Critical role of ASK1 in the 6-hydroxydopamine-induced apoptosis in human neuroblastoma SH-SY5Y cells. J Neurochem. 2006;97:234–44. doi: 10.1111/j.1471-4159.2006.03730.x. [DOI] [PubMed] [Google Scholar]
- Pacher P, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev. 2007;87:315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson KR, Smurzynski M, Parsons TD, Wu K, Bosch RJ, Wu J, McArthur JC, Collier AC, Evans SR, Ellis RJ. The prevalence and incidence of neurocognitive impairment in the HAART era. Aids. 2007;21:1915–21. doi: 10.1097/QAD.0b013e32828e4e27. [DOI] [PubMed] [Google Scholar]
- Sacktor N, McDermott MP, Marder K, Schifitto G, Selnes OA, McArthur JC, Stern Y, Albert S, Palumbo D, Kieburtz K, De Marcaida JA, Cohen B, Epstein L. HIV-associated cognitive impairment before and after the advent of combination therapy. J Neurovirol. 2002;8:136–42. doi: 10.1080/13550280290049615. [DOI] [PubMed] [Google Scholar]
- Saitoh M, Nishitoh H, Fujii M, Takeda K, Tobiume K, Sawada Y, Kawabata M, Miyazono K, Ichijo H. Mammalian thioredoxin is a direct inhibitor of apoptosis signal-regulating kinase (ASK) 1. Embo J. 1998;17:2596–606. doi: 10.1093/emboj/17.9.2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapadin AN, Fleischmajer R. Tetracyclines: nonantibiotic properties and their clinical implications. J Am Acad Dermatol. 2006;54:258–65. doi: 10.1016/j.jaad.2005.10.004. [DOI] [PubMed] [Google Scholar]
- Sarchielli P, Galli F, Floridi A, Floridi A, Gallai V. Relevance of protein nitration in brain injury: a key pathophysiological mechanism in neurodegenerative, autoimmune, or inflammatory CNS diseases and stroke. Amino Acids. 2003;25:427–36. doi: 10.1007/s00726-003-0028-6. [DOI] [PubMed] [Google Scholar]
- Sarker KP, Biswas KK, Rosales JL, Yamaji K, Hashiguchi T, Lee KY, Maruyama I. Ebselen inhibits NO-induced apoptosis of differentiated PC12 cells via inhibition of ASK1-p38 MAPK-p53 and JNK signaling and activation of p44/42 MAPK and Bcl-2. J Neurochem. 2003;87:1345–53. doi: 10.1046/j.1471-4159.2003.02096.x. [DOI] [PubMed] [Google Scholar]
- Sekine Y, Takeda K, Ichijo H. The ASK1-MAP kinase signaling in ER stress and neurodegenerative diseases. Curr Mol Med. 2006;6:87–97. doi: 10.2174/156652406775574541. [DOI] [PubMed] [Google Scholar]
- Stirling DP, Koochesfahani KM, Steeves JD, Tetzlaff W. Minocycline as a neuroprotective agent. Neuroscientist. 2005;11:308–22. doi: 10.1177/1073858405275175. [DOI] [PubMed] [Google Scholar]
- Sumbayev VV. S-nitrosylation of thioredoxin mediates activation of apoptosis signal-regulating kinase 1. Arch Biochem Biophys. 2003;415:133–6. doi: 10.1016/s0003-9861(03)00199-1. [DOI] [PubMed] [Google Scholar]
- Takeda K, Noguchi T, Naguro I, Ichijo H. Apoptosis signal-regulating kinase 1 in stress and immune response. Annu Rev Pharmacol Toxicol. 2008;48:199–225. doi: 10.1146/annurev.pharmtox.48.113006.094606. [DOI] [PubMed] [Google Scholar]
- Tobiume K, Matsuzawa A, Takahashi T, Nishitoh H, Morita K, Takeda K, Minowa O, Miyazono K, Noda T, Ichijo H. ASK1 is required for sustained activations of JNK/p38 MAP kinases and apoptosis. EMBO Rep. 2001;2:222–8. doi: 10.1093/embo-reports/kve046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobiume K, Saitoh M, Ichijo H. Activation of apoptosis signal-regulating kinase 1 by the stress-induced activating phosphorylation of pre-formed oligomer. J Cell Physiol. 2002;191:95–104. doi: 10.1002/jcp.10080. [DOI] [PubMed] [Google Scholar]
- Tozzi V, Balestra P, Bellagamba R, Corpolongo A, Salvatori MF, Visco-Comandini U, Vlassi C, Giulianelli M, Galgani S, Antinori A, Narciso P. Persistence of neuropsychologic deficits despite long-term highly active antiretroviral therapy in patients with HIV-related neurocognitive impairment: prevalence and risk factors. J Acquir Immune Defic Syndr. 2007;45:174–82. doi: 10.1097/QAI.0b013e318042e1ee. [DOI] [PubMed] [Google Scholar]
- Tsuji M, Wilson MA, Lange MS, Johnston MV. Minocycline worsens hypoxic-ischemic brain injury in a neonatal mouse model. Exp Neurol. 2004;189:58–65. doi: 10.1016/j.expneurol.2004.01.011. [DOI] [PubMed] [Google Scholar]
- Vincent VA, De Groot CJ, Lucassen PJ, Portegies P, Troost D, Tilders FJ, Van Dam AM. Nitric oxide synthase expression and apoptotic cell death in brains of AIDS and AIDS dementia patients. Aids. 1999;13:317–26. doi: 10.1097/00002030-199902250-00003. [DOI] [PubMed] [Google Scholar]
- Wang SW, Pawlowski J, Wathen ST, Kinney SD, Lichenstein HS, Manthey CL. Cytokine mRNA decay is accelerated by an inhibitor of p38-mitogen-activated protein kinase. Inflamm Res. 1999;48:533–8. doi: 10.1007/s000110050499. [DOI] [PubMed] [Google Scholar]
- Whiteman M, Halliwell B. Prevention of peroxynitrite-dependent tyrosine nitration and inactivation of alpha1-antiproteinase by antibiotics. Free Radic Res. 1997;26:49–56. doi: 10.3109/10715769709097783. [DOI] [PubMed] [Google Scholar]
- Wilkins A, Nikodemova M, Compston A, Duncan I. Minocycline attenuates nitric oxide-mediated neuronal and axonal destruction in vitro. Neuron Glia Biology. 2004;1:297–305. doi: 10.1017/S1740925X05000104. [DOI] [PubMed] [Google Scholar]
- Wright EK, Jr, Clements JE, Barber SA. Sequence variation in the CC-chemokine ligand 2 promoter of pigtailed macaques is not associated with the incidence or severity of neuropathology in a simian immunodeficiency virus model of human immunodeficiency virus central nervous system disease. J Neurovirol. 2006;12:411–9. doi: 10.1080/13550280601009538. [DOI] [PubMed] [Google Scholar]
- Yen PK, Shaw JH. Minocycline and its influence on calcifying tissues of young monkeys. J Dent Res. 1975;54:423. [PubMed] [Google Scholar]
- Zhang Q, Zhang G. Activation and autophosphorylation of apoptosis signal-regulating kinase 1 (ASK1) following cerebral ischemia in rat hippocampus. Neurosci Lett. 2002;329:232–6. doi: 10.1016/s0304-3940(02)00650-x. [DOI] [PubMed] [Google Scholar]
- Zink MC, Suryanarayana K, Mankowski JL, Shen A, Piatak M, Jr, Spelman JP, Carter DL, Adams RJ, Lifson JD, Clements JE. High viral load in the cerebrospinal fluid and brain correlates with severity of simian immunodeficiency virus encephalitis. J Virol. 1999;73:10480–8. doi: 10.1128/jvi.73.12.10480-10488.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zink MC, Uhrlaub J, DeWitt J, Voelker T, Bullock B, Mankowski J, Tarwater P, Clements J, Barber S. Neuroprotective and anti-human immunodeficiency virus activity of minocycline. Jama. 2005;293:2003–11. doi: 10.1001/jama.293.16.2003. [DOI] [PubMed] [Google Scholar]