Abstract
The complete genome sequence of Streptococcus mutans, a bacterial pathogen commonly associated with human dental caries, was published in 2002. The streamlined genome (2.03Mb) revealed an organism that was well adapted to its obligately host-associated existence in multispecies biofilms on tooth surfaces; a dynamic environment that undergoes rapid and substantial environmental fluctuations. However, S. mutans lacks many of the sensing systems and alternative sigma factors that bacteria often use to coordinate gene expression in response to stress and changes in their environment. Over the past seven years, functional genomics and proteomics have enhanced our understanding of how S. mutans has integrated the stress regulon and global transcriptional regulators to integrate responses to environmental fluctuations with modulation of virulence in a way that ensures persistence in the oral cavity and capitalizes on conditions that are favorable for the development of dental caries. Here, we highlight advances on dissection of the stress regulon of S. mutans and its intimate interrelationship with pathogenesis.
Introduction
Caries is a classic biofilm disease that develops when changes in the oral environment enhance the growth of cariogenic bacteria, which are highly efficient at converting carbohydrates to the organic acids that demineralize tooth enamel. Microbiological assessment of caries-active sites and studies with experimental animals implicated Streptococcus mutans as the primary causative agent of human dental caries (Loesche, 1986). The virulence of S. mutans resides in three core attributes; its abilities to form biofilms on the tooth surface, to produce large quantities of organic acids (acidogenicity) from a wide range of carbohydrates, and to tolerate environmental stresses, particularly low pH (aciduricity) (Lemos et al., 2005). In addition to dental caries, S. mutans is often an agent in sub-acute bacterial endocarditis, a life-threatening inflammation of heart valves.
Unlike most infectious diseases, in which classic virulence factors, such as a toxin, play a clear role in the damage elicited by the organism, the pathology of dental caries is associated almost exclusively with bacterial metabolism. Catabolism of the nutrients in saliva and the host’s diet creates stressors in the form of acids, reactive oxygen species (ROS), and other agents that damage biomolecules. Thus, stress tolerance by the bacteria is intimately intertwined with virulence. The purpose of this review is to highlight post-genomic research on genetic, biochemical and physiologic mechanisms that have evolved in S. mutans to modulate its pathogenic potential in response to nutritional, chemical and physical stresses encountered in complex biofilms.
Seven Years P.G. (Post Genome)
In 2001, the complete genome sequence of a serotype c strain of S. mutans became available (Ajdic et al., 2002). Through the application of functional genomics, transcriptomics and proteomics, researchers were able to make rapid progress in dissecting the mechanisms of stress tolerance utilized by this pathogen. One theme that emerged from these studies is that S. mutans has streamlined its genome by using pathways that cope with environmental insults to regulate a variety of virulence attributes.
A few years after the completion of the UA159 genome sequence, S. mutans microarray slides became available, with generous support from the NIDCR, through the J. Craig Venter Institute (formerly The Institute for Genomic Research, TIGR). To date, microarrays have been used to probe the responses of S. mutans to amino acid starvation (Nascimento et al., 2008), oxygen (Ahn et al., 2007), sugar transport (Ajdic & Pham, 2007), and manganese-depletion (Arirachakaran et al., 2007); to identify genes that are differentially expressed in biofilms of S. mutans compared with free-living cells (Shemesh et al., 2007); and to evaluate the consequences of gene-specific mutations (Abranches et al., 2006; Abranches et al., 2008; Lemos et al., 2008; Merritt et al., 2005; Nascimento et al., 2008; Sztajer et al., 2008; Wen et al., 2006).
Proteomic studies have been instrumental in identifying proteins and pathways that participate in acid tolerance and acid adaptation (Len et al., 2004a; Len et al., 2004b; Rathsam et al., 2005a; Rathsam et al., 2005b; Welin et al., 2003; Welin et al., 2004; Wilkins et al., 2002; Wilkins et al., 2003). Of particular interest is a report by Nick Jacques and co-workers that used continuous culture to catalogue changes in the expression of proteins involved in energy metabolism when the growth pH was lowered from 7 to 5 (Len et al., 2004b). By coupling proteomic data with measurements of end products of carbon utilization, the authors were able to propose that S. mutans tolerates growth at low pH by expending energy to extrude H+, by modulating the production of acid end products, and by using branched chain amino acid biosynthesis as a potential mechanism to reduce acid production and moderate intracellular pH (Len et al., 2004b).
Comparison of the proteome of mature biofilm and planktonic cells of S. mutans cells grown at neutral pH revealed that multiple proteins associated with carbon uptake and cell division were down-regulated in biofilms, whereas proteins required for the development of genetic competence were up-regulated (Rathsam et al., 2005a); the latter finding being consistent with the observation that the transformation efficiency of S. mutans is optimal during biofilm growth (Li et al., 2001b). This observation is thought to have significance in terms of plaque ecology. Specifically, co-ordinated production of bacteriocins from S. mutans and the development of competence have been documented in high-cell density environments, suggesting that the organism could use competence-induced cell lysis to acquire DNA from neighboring species (Kreth et al., 2005; Kreth et al., 2006; Kreth et al., 2007). Notably, a study with S. mutans implicated the presence of DNA released from competence-induced cell lysis in the extracellular matrix with proper biofilm maturation (Petersen et al., 2005). It remains to be determined whether S. mutans biofilms acquire DNA from the external environment to provide a nutrient source, to increase genetic diversity, or both (Spoering & Gilmore, 2006). In addition to the potential impact on commensal organisms in oral biofilms, a direct correlation between production of the competence stimulating peptide (CSP) and activation of autolytic pathways with biofilm formation and persistence of S. mutans has been noted. In particular, when administered at doses beyond the levels necessary to induce competence, CSP of S. mutans was found to induce cell lysis (Qi et al., 2005), suggesting the presence of an altruistic programmed cell death pathway. In this case, the “sacrifice” of a subset of cells may enable the establishment and survival of the remainder of the population.
Stress Survival Pathways
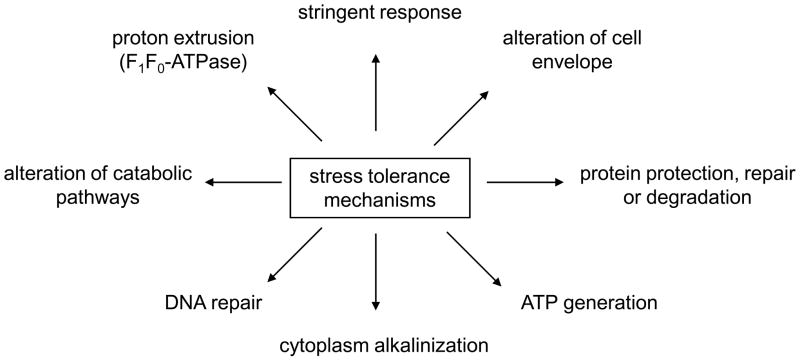
Bacteria in dental plaque experience a wide range of stresses. The intermittent ingestion of food by the host results in dramatic changes in nutrient availability and pH, but significant variability in oxygen tension and osmolality are also observed (Lemos et al., 2005). Although it has been noted that oral biofilms experience a “feast or famine” lifestyle, organisms residing in the oral cavity are really not exposed to severely-oligotrophic environments. For this reason, studies of the stress responses of S. mutans have served as an excellent model to reveal critical differences in the ways that obligately host-associated bacteria cope with environmental stresses when compared with bacteria that have both free-living and host-associated lifestyles. These studies have revealed important contrasts in the way this organism copes with stresses and uses stress regulons/enzymes to coordinate virulence and survival compared to more widely-studied bacterial paradigms (Fig. 1).
Figure 1.
ΔpH homeostasis and metabolic pathways
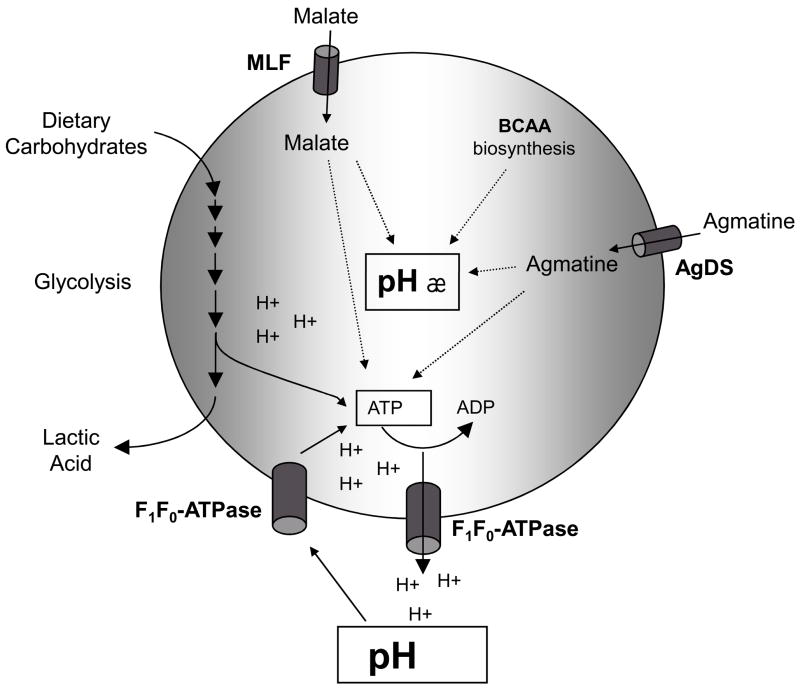
The membrane-bound F-ATPase (H+-translocating ATPase) is considered the primary determinant of acid tolerance of S. mutans because it allows the organism to maintain a cytoplasmic pH that is more alkaline than the extracellular environment (Lemos et al., 2005). Work from the laboratory of Marquis correlates acid tolerance in oral streptococci with the pH optima and absolute level of activity of the F1FO-ATPase (Bender et al., 1986). Moreover, it was recently demonstrated that the F-ATPase of S. mutans, and other oral bacteria, can function as an ATP synthase in starved cells grown at low pH (Sheng & Marquis, 2006). The authors demonstrated that in starved cells, a sudden drop in pH result in a rapid increase in ATP, followed by a rapid loss, that enhance protection against acid killing. By using specific inhibitors of F-ATPase, the authors were able to demonstrate that this increase in ATP comes from the enzyme acting as an ATP synthase. Thus, the F-ATPase may play a dual role in acid tolerance - extruding protons out of the cells and, under certain conditions, generating ATP for growth and persistence (Fig. 2).
Figure 2.
A mechanism for acid resistance used by many oral streptococci is the production of ammonia by urease enzymes or the arginine deiminase system (ADS) (Burne & Marquis, 2000). Organisms carrying these enzymes can convert urea or arginine, respectively, to produce CO2 and ammonia, which can neutralize acids and give the organisms a competitive advantage in acidified biofilms. Although S. mutans is not capable of generating significant quantities of alkali as it lacks urease and ADS pathways (Ajdic et al., 2002), an agmatine deiminase system (AgDS), analogous to the ADS, was characterized in S. mutans UA159 (Griswold et al., 2006). The AgDS converts agmatine, a decarboxylated derivative of arginine that is found in human dental plaque, to putrescine, ammonia and CO2. Whereas the ADS and urease pathways catalyze substantial environmental alkalinization and appear to be associated with caries resistance, the AgDS of S. mutans is expressed at relatively low levels and is unlikely to elicit a significant alkalinization of the environment. However, the production of ammonia from agmatine is believed to contribute to the competitive fitness of S. mutans at low pH by increasing the cytoplasmic pH and generating ATP that can be used for growth or to extrude protons (Fig. 2) (Griswold et al., 2006).
Another contributor to acid tolerance of S. mutans is malolactic fermentation (MLF), which catalyzes the conversion of dicarboxylic L-malate, a major acid in fruits such as apple, to the monocarboxylic lactic acid and CO2. It was demonstrated that although malate did not serve as a catabolite for growth of S. mutans, it did serve to protect the organism against acid killing by increasing the pH of the cytoplasm via production of CO2 (Fig. 2) (Sheng & Marquis, 2007).
Protection, Repair and Quality Control of Macromolecules
One consequence of exposure to environmental stresses is the accumulation of abnormal proteins due to increased errors in transcription and translation. Moreover, aging cells present in mature biofilms are prone to mistranslation and aggregation. In this context, molecular chaperones and proteases, which modulate the stability of proteins and prevent accumulation of misfolded proteins, are central to physiologic homeostasis. In support to this concept, proteome analysis of S. mutans grown at steady-state in continuous culture at pH 7 or 5 identified several molecular chaperones, proteases and DNA repair enzymes as up-regulated during growth at low pH (Len et al., 2004a).
The GroEL and DnaK chaperones take part in several cellular processes, including protein folding, renaturation, and presentation of proteins for degradation. In S. mutans, DnaK and GroEL appear to be indispensable, and the essential nature of these chaperones was confirmed by forced down-regulation of groEL and dnaK expression (Lemos et al., 2007b). Lowering of DnaK levels resulted in impaired capacity to form biofilms in the presence of glucose and rendered the strain more sensitive to low pH, elevated temperature and H2O2 (Lemos et al., 2007b). The acid-sensitivity of the DnaK knock-down strain was attributed, at least in part, to the DnaK chaperone participating in the biogenesis or stabilization of the F-ATPase complex (Lemos et al., 2007b). Downregulation of GroEL also resulted in high temperature sensitivity and impaired capacity to form biofilms, but did not affect growth at low pH or in the presence of H2O2(Lemos et al., 2007b). Wen and colleagues showed that the ribosome-associated peptidyl-prolyl isomerase RopA (trigger factor) is important for adherence and formation of biofilms and for tolerance to low pH and H2O2 (Wen et al., 2005). Inactivation of the surface-associated HtrA protease or the cytoplasmic ClpP peptidase generated multiple stress-sensitive phenotypes in S. mutans, and was also linked to altered biofilm formation and reduced genetic competence (Ahn et al., 2005; Biswas & Biswas, 2005; Deng et al., 2007b; Lemos & Burne, 2002). Notably, in all these cases, a unifying theme is the intimate connection between stress responses and biofilm formation, suggesting that the stress regulon of S. mutans may be responsible for controlling a broader set of biological functions when compared to organisms with more complex genomes.
Many of the stresses encountered by oral bacteria induce DNA damage, in particular acid and oxidative stresses increase the formation of abasic sites in DNA. Earlier reports have demonstrated an overlap between DNA repair systems and stress response pathways, including RecA, the endonuclease Smn and the UV repair excinuclease UvrA (Hahn et al., 1999; Hanna et al., 2001; Quivey et al., 1995). More recently, Faustoferri and colleagues characterized the Smx exonuclease in S. mutans and showed that an smx mutant strain was highly sensitive to DNA damage caused by the production of hydoxyl radicals via Fenton reaction (Faustoferri et al., 2005).
Cell Envelope Alterations
The importance of cell membrane integrity and composition in relation to changes that affect proton permeability and F-ATPase activity in S. mutans has been documented (Lemos et al., 2005). Fozo and Quivey showed that, in response to the acidification of its environment, S. mutans increases the proportion of monounsaturated membrane fatty acids (Fozo & Quivey, 2004b), which is thereby predicted to decrease proton permeability. Inactivation of the gene responsible for biosynthesis of monounsaturated fatty acids, fabM, resulted in a strain that was extremely sensitive to low pH and unable to maintain ΔpH (Fozo & Quivey, 2004a). Rats infected with the fabM mutant exhibited substantially reduced caries, as compared to the parent strain (Fozo et al., 2007).
The significance of membrane protein biogenesis to stress tolerance was demonstrated in a study with mutated strains lacking the signal recognition particle-translocation (SRP) pathway or the membrane-localized chaperone YidC, both involved in the translocation and assembly of membrane proteins. Once considered essential for the viability of all organisms, the SRP pathway was found to be dispensable in S. mutans (Hasona et al., 2005), although mutants lacking proteins of the SRP pathway or YidC were impaired in growth under a variety of stress conditions (Hasona et al., 2005). The authors observed that YidC and a functional SRP pathway is necessary for optimal insertion of membrane proteins, including the F-ATPase, providing a partial explanation for the diminished acid tolerance of strains lacking YidC or components of the SRP pathway (Hasona et al., 2005; Hasona et al., 2007). Notably, mutations in SRP-related genes were also associated with decreases in biofilm formation, providing another example of the overlap between pathways that govern stress tolerance and biofilm formation (Hasona et al., 2007).
Finally, the surface-associated protein BrpA was found to play a role in biofilm development, autolysis, cell division and stress tolerance (Wen et al., 2006). A comparison of the transcriptomes of the brpA mutant and the parent reveal significant alterations in the expression of genes involved in cell wall biogenesis, stress tolerance and adherence (Wen et al., 2006). Although the function of BrpA has not been defined, increased autolysis in the ΔbrpA strain indicates that this protein may play a role in modulating cell wall integrity through modulation of autolytic activities, which could mechanistically link BrpA to the increases in susceptibility to acid and oxidative stresses observed in the BrpA-deficient strain (Wen et al., 2006).
Two-component signal transduction systems
S. mutans lives almost exclusively in densely-populated biofilms that form on the tooth surface. The structure and composition of these biofilms are influenced by the capacity of its constituents to adapt to environmental changes. As is typical of bacteria with specialized niches, there are very few alternative sigma factors in the UA159 genome (Ajdic et al., 2002). Thus, regulatory systems, such as two-component systems (TCS), which integrate various chemical and physical signals to coordinate appropriate gene expression patterns, play a central role in stress tolerance and are viewed as desirable targets for the development of new antimicrobial therapies.
TCS are composed of a transmembrane sensor kinase that detects environmental changes and a cytosolic response regulator, which is a DNA binding protein that modulates expression of target genes when phosphorylated by the kinase. In streptococcal species, the number of TCS is small compared to organisms with a free living life-style, ranging from as few as 10 in S. thermophilus, to more than 20 in S. agalactiae. Sequence analysis initially revealed the presence of 13 TCS in S. mutans UA159 (Ajdic et al., 2002), but the Biswas lab identified an additional pair in this same strain (Biswas et al., 2008).
Over the past few years, studies that evaluated the role of TCS in S. mutans have shown that they regulate virulence gene expression, induction of competence, biofilm development, bacteriocin production and stress tolerance (Biswas et al., 2008; Chen et al., 2007; Deng et al., 2007a; Levesque et al., 2007; Li et al., 2001a; Li et al., 2002a; Li et al., 2002b; Qi et al., 2004; Senadheera et al., 2005; Zeng et al., 2006). In particular, two studies from independent laboratories systematically inactivated the genes encoding sensor kinases of all TCS and evaluated their role in stress tolerance by S. mutans (Biswas et al., 2008; Levesque et al., 2007). In the study by Lévesque and colleagues, smu1814c (scnK) and smu1965c (levS) mutants displayed significantly slower growth at pH 5.5, whereas the smu1128c (ciaH) mutant grew better than the parental strain in the presence of NaCl or H2O2 (Levesque et al., 2007). Biswas and co-workers found that inactivation of three sensor kinases, smu486 (liaS), smu1128c (ciaH) and smu1516c (vicK), affected stress tolerance of strain UA159 (Biswas et al., 2008). However, the vicK mutant showed an increased tolerance to puromycin, which causes premature chain termination during protein synthesis (Biswas et al., 2008). The liaS and ciaH mutants showed reduced growth when incubated in aerobic conditions or on agar medium supplemented with H2O2 (Biswas et al., 2008). The liaS and ciaH mutants also showed increased sensitivity to puromycin, while the ciaH mutant showed significant reduction of growth at pH 5 and displayed increased sensitivity to DNA damage caused by mitomycin C (Biswas et al., 2008). Notably, previous reports have also shown that inactivation of ciaH resulted in an acid sensitive phenotype in strains UA159 and UA140 (Ahn et al., 2006; Qi et al., 2004). The S. mutans VicRK system was shown to respond to, and protect against, oxidative stress in one particular study (Deng et al., 2007a). A role in oxidative stress response was also assigned to ScnRK, as scnRK mutants were more sensitive to H2O2 and more susceptible to phagocytic killing in non-activated macrophages (Chen et al., 2007). Studies from the Cvitkovitch lab have shown that inactivation of LiaS or ComDE conferred an acid-sensitive phenotype to strains NG8 and BM71, respectively (Li et al., 2001a; Li et al., 2002a), although comD (smu1916c) or comE (smu1917c) do not appear to affect acid tolerance in strain UA159 (Ahn et al., 2006). Finally, the smu927-smu928 TCS, designated relRS, that is co-transcribed with a newly described (p)ppGpp-synthetase (relP) has been implicated in survival and persistence as it may help regulate (p)ppGpp metabolism (Lemos et al., 2007a).
In S. pyogenes, the TCS CovRS regulates expression of approximately 15% of the genome, including key virulence genes (Graham et al., 2002). In S. mutans UA159, CovR is an orphan response regulator that controls expression of genes related to biofilm formation and virulence (Biswas et al., 2007; Biswas & Biswas, 2006). Similar to what has been observed for the S. pyogenes covRS, expression of the S. mutans covR is autoregulated, optimal during exponential-growth and induced by addition of Mg2+ in a dose-dependent manner (Chong et al., 2008). The extent of the genes controlled by CovR in S. mutans is not known but based on the findings obtained in other streptococci, it is expected that CovR participate in the stress responses.
Collectively, these data support the idea that there may be substantial heterogeneity among strains in the role of specific TCS, not only in the genes they regulate, but also in the external stimuli to which they respond. Nevertherless, CiaRH have been consistently found to play a role in the stress responses by S. mutans. Moreover, CiaRH have also been implicated in competence development, bacteriocin production and biofilm formation (Ahn et al., 2006; Levesque et al., 2007; Qi et al., 2004). More recently, it was demonstrated that the ciaRH operon of S. mutans consists of three genes with the first gene, ciaX, encoding a small, double-glycine signaling peptide that allows CiaRH to modulate its own expression in response to calcium (He et al., 2008). Inactivation of ciaX, or point mutations in its calcium-binding domain resulted in diminished biofilm formation that was rescued by addition of calcium. Human saliva is saturated in calcium (Agha-Hosseini et al., 2006) and calcium is the principal cation in tooth enamel, so calcium signaling may be an important regulator, through CiaRH, of stress responses and virulence in S. mutans.
Other regulators
Metal ions, including iron and manganese, have been implicated in the regulation of virulence expression by S. mutans. In particular, the SloR metalloregulator was shown to modulate S. mutans biofilm formation, genetic competence and oxidative stress tolerance in response to manganese availability (Rolerson et al., 2006). Work from the Spatafora lab has linked SloR repression of the transcriptional regulator gcrR with acid stress tolerance (Dunning et al., 2008). More specifically, a gcrR mutant was more sensitive to low pH and this phenotype was linked to the inability of the mutant to maintain ΔpH homeostasis.
As mentioned above, the AgDS has been proposed to enhance acid resistance through alkalinization of the cytoplasm (Griswold et al., 2006). The AgDS of S. mutans is subject to complex regulation by substrate, catabolite control, and relevant environmental stresses. A LuxR-like transcriptional regulator, named aguR, was identified upstream the aguBDAC operon. Inactivation of aguR decreased AgD activity and eliminated agmatine induction, indicating that AguR is a major regulator of AgDS (Griswold et al., 2006).
Nutritional Regulators and alteration of catabolic pathways
In order to thrive in dental plaque where there is considerable fluctuation in the nutrient pools, S. mutans must be able to adjust its metabolism and gene expression patterns to maximize the use of available substrates (Lemos et al., 2005). Despite the need to endure periods of nutrient limitation, abrupt exposure to an excess amount of carbohydrate in the diet can result in the rapid accumulation of toxic glycolytic intermediates, acidification of the environment, and osmotic stress. To survive nutrient starvation, to cope with the detrimental effects of glycolytic intermediates, and to maintain proper NAD/NADH+ balances, S. mutans has developed a sophisticated regulatory nextwork that combines transcriptional regulation with allosteric modulation of enzymatic activities to coordinate optimal flow of carbohydrates.
Carbohydrate source and availability are key factors affecting the pathogenic potential of oral biofilms. The sugar phosphotransferase system (PTS) is the major carbohydrate transport system in oral streptococci, especially under carbohydrate-limiting conditions. In addition to participating in sugar uptake, PTS components influence many other cellular processes. Mutations in the ManL PTS permease influenced biofilm development, regulation of acid tolerance and global control of gene expression, in particular carbon catabolite repression (Abranches et al., 2006; Abranches et al., 2008). Two global regulators of central metabolism genes, CcpA and CodY, have been shown to impact acid tolerance and the expression of other virulence traits of S. mutans (Abranches et al., 2008; Lemos et al., 2008). CcpA, a regulator of carbon-metabolism in Gram-positive bacteria, has been shown to globally regulate transcription in response to carbohydrate availability, and a CcpA-deficient strain was substantially more acid resistant than its parent (Abranches et al., 2008). The enhanced acid tolerance of the CcpA mutant has been associated with increases in the expression of the PTS that result in higher rates of ATP generation through glycolysis. Microarrays revealed that CodY, a regulator that helps cells to adapt to poor nutritional conditions, is indeed a global regulator of gene expression in S. mutans (Lemos et al., 2008). Phenotypic studies revealed that the codY mutant had reduced capacities to form biofilms and was more sensitive to growth at low pH (Lemos et al., 2008).
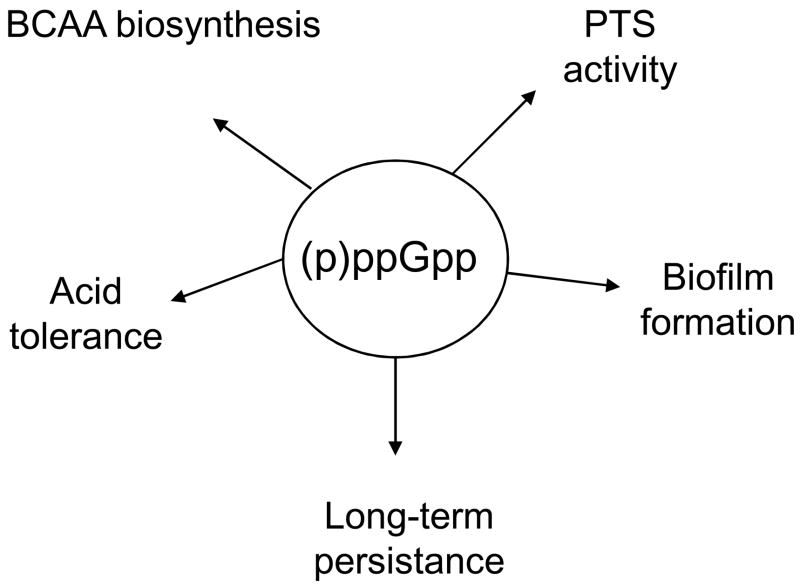
The nutritional alarmone (p)ppGpp also appears to play an important role in orchestrating an appropriate response to multiple environmental and physiologic inputs that S. mutans encounters in the oral cavity (Fig. 3). When limited for essential amino acids, bacteria accumulate (p)ppGpp by enzymatic phosphorylation of GDP and GTP, resulting in down-regulation of genes for macromolecular biosynthesis and up-regulation of genes for amino acid biosynthesis and stress tolerance. In Gram-positive bacteria (GPB), RelA is a bi-functional enzyme with potent (p)ppGpp-synthetic and -degradative activities. In S. mutans, RelA was shown to play major roles in the regulation of phenotypic traits that are required for establishment, persistence and survival (Lemos et al., 2004; Nascimento et al., 2008), further supporting an overlap between circuits that govern nutrient starvation, general stress tolerance and biofilm fomation. Until recently, RelA was considered the sole enzyme responsible for synthesis and degradation of (p)ppGpp in Gram-positive bacteria. However, our group recently identified two novel enzymes, designated RelP and RelQ, with (p)ppGpp-synthase activities in S. mutans that could be found in a number of related GPB (Lemos et al., 2007a). A relAPQ triple mutant was auxotrophic for the branched-chain amino acids (BCAA) leucine and valine, but not isoleucine, a phenotype that was directly related to CodY-dependent repression of genes involved in the synthesis of BCAA (Lemos et al., 2008). As mentioned above, RelP is co-transcribed with, and apparently regulated by, the RelRS TCS (Lemos et al., 2007a) suggesting that S. mutans may use environmental signals to optimize cell growth and survival in a manner that allows the organism to balance growth during dietary intake by the host with the capacity to rapidly mount an adaptive response during fasting periods. Consistent with the role of (p)ppGpp in bacteria, homologues of RelRS in S. pyogenes, designated SptRS, were shown to be critical for this bacterium to survive in saliva (Shelburne et al., 2005).
Figure 3.
Concluding Remarks
Genomic and proteomic studies have enabled researchers to make rapid progress on the identification of genes, proteins and pathways that are associated with stress tolerance in S. mutans. Because there is a strong overlap between stress tolerance and biofilm development pathways, some of these gene products are attractive targets for the development of new anti-caries therapies (Matsushita & Janda, 2002). In particular, strategies that short-circuit regulatory pathways used by S. mutans to sense and respond to environmental signals may have a potent capacity to disrupt cariogenic biofilms.
Acknowledgments
We would like to thank Jacqueline Abranches and Jessica Kajfasz for critical reading of this manuscript. The authors’ research in this area was supported by grants RO1 DE13239, DE10362 and DE12236 from the NIDCR.
References
- Abranches J, Candella MM, Wen ZT, Baker HV, Burne RA. Different roles of EIIABMan and EIIGlc in regulation of energy metabolism, biofilm development, and competence in Streptococcus mutans. Journal of bacteriology. 2006;188:3748–3756. doi: 10.1128/JB.00169-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abranches J, Nascimento MM, Zeng L, Browngardt CM, Wen ZT, Rivera MF, Burne RA. CcpA regulates central metabolism and virulence gene expression in Streptococcus mutans. Journal of bacteriology. 2008;190:2340–2349. doi: 10.1128/JB.01237-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agha-Hosseini F, Dizgah IM, Amirkhani S. The composition of unstimulated whole saliva of healthy dental students. J Contemp Dent Pract. 2006;7:104–111. [PubMed] [Google Scholar]
- Ahn SJ, Lemos JA, Burne RA. Role of HtrA in growth and competence of Streptococcus mutans UA159. Journal of bacteriology. 2005;187:3028–3038. doi: 10.1128/JB.187.9.3028-3038.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn SJ, Wen ZT, Burne RA. Multilevel control of competence development and stress tolerance in Streptococcus mutans UA159. Infect Immun. 2006;74:1631–1642. doi: 10.1128/IAI.74.3.1631-1642.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn SJ, Wen ZT, Burne RA. Effects of oxygen on virulence traits of Streptococcus mutans. Journal of bacteriology. 2007;189:8519–8527. doi: 10.1128/JB.01180-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ajdic D, McShan WM, McLaughlin RE, et al. Genome sequence of Streptococcus mutans UA159, a cariogenic dental pathogen. Proc Natl Acad Sci U S A. 2002;99:14434–14439. doi: 10.1073/pnas.172501299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ajdic D, Pham VT. Global transcriptional analysis of Streptococcus mutans sugar transporters using microarrays. Journal of bacteriology. 2007;189:5049–5059. doi: 10.1128/JB.00338-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arirachakaran P, Benjavongkulchai E, Luengpailin S, Ajdic D, Banas JA. Manganese affects Streptococcus mutans virulence gene expression. Caries Res. 2007;41:503–511. doi: 10.1159/000110883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender GR, Sutton SV, Marquis RE. Acid tolerance, proton permeabilities, and membrane ATPases of oral streptococci. Infect Immun. 1986;53:331–338. doi: 10.1128/iai.53.2.331-338.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas I, Drake L, Biswas S. Regulation of gbpC expression in Streptococcus mutans. Journal of bacteriology. 2007;189:6521–6531. doi: 10.1128/JB.00825-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas I, Drake L, Erkina D, Biswas S. Involvement of sensor kinases in the stress tolerance response of Streptococcus mutans. Journal of bacteriology. 2008;190:68–77. doi: 10.1128/JB.00990-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas S, Biswas I. Role of HtrA in surface protein expression and biofilm formation by Streptococcus mutans. Infect Immun. 2005;73:6923–6934. doi: 10.1128/IAI.73.10.6923-6934.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas S, Biswas I. Regulation of the glucosyltransferase (gtfBC) operon by CovR in Streptococcus mutans. Journal of bacteriology. 2006;188:988–998. doi: 10.1128/JB.188.3.988-998.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burne RA, Marquis RE. Alkali production by oral bacteria and protection against dental caries. FEMS microbiology letters. 2000;193:1–6. doi: 10.1111/j.1574-6968.2000.tb09393.x. [DOI] [PubMed] [Google Scholar]
- Chen PM, Chen HC, Ho CT, Jung CJ, Lien HT, Chen JY, Chia JS. The two-component system ScnRK of Streptococcus mutans affects hydrogen peroxide resistance and murine macrophage killing. Microbes Infect. 2007 doi: 10.1016/j.micinf.2007.12.006. [DOI] [PubMed] [Google Scholar]
- Chong P, Drake L, Biswas I. Modulation of covR expression in Streptococcus mutans UA159. Journal of bacteriology. 2008;190:4478–4488. doi: 10.1128/JB.01961-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng DM, Liu MJ, ten Cate JM, Crielaard W. The VicRK system of Streptococcus mutans responds to oxidative stress. J Dent Res. 2007a;86:606–610. doi: 10.1177/154405910708600705. [DOI] [PubMed] [Google Scholar]
- Deng DM, ten Cate JM, Crielaard W. The adaptive response of Streptococcus mutans towards oral care products: involvement of the ClpP serine protease. Eur J Oral Sci. 2007b;115:363–370. doi: 10.1111/j.1600-0722.2007.00477.x. [DOI] [PubMed] [Google Scholar]
- Dunning DW, McCall LW, Powell WF, Jr, Arscott WT, McConocha EM, McClurg CJ, Goodman SD, Spatafora GA. SloR modulation of the Streptococcus mutans acid tolerance response involves the GcrR response regulator as an essential intermediary. Microbiology. 2008;154:1132–1143. doi: 10.1099/mic.0.2007/012492-0. [DOI] [PubMed] [Google Scholar]
- Faustoferri RC, Hahn K, Weiss K, Quivey RG., Jr Smx nuclease is the major, low-pH-inducible apurinic/apyrimidinic endonuclease in Streptococcus mutans. Journal of bacteriology. 2005;187:2705–2714. doi: 10.1128/JB.187.8.2705-2714.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fozo EM, Quivey RG., Jr The fabM gene product of Streptococcus mutans is responsible for the synthesis of monounsaturated fatty acids and is necessary for survival at low pH. Journal of bacteriology. 2004a;186:4152–4158. doi: 10.1128/JB.186.13.4152-4158.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fozo EM, Quivey RG., Jr Shifts in the membrane fatty acid profile of Streptococcus mutans enhance survival in acidic environments. Appl Environ Microbiol. 2004b;70:929–936. doi: 10.1128/AEM.70.2.929-936.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fozo EM, Scott-Anne K, Koo H, Quivey RG., Jr Role of unsaturated fatty acid biosynthesis in virulence of Streptococcus mutans. Infect Immun. 2007;75:1537–1539. doi: 10.1128/IAI.01938-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham MR, Smoot LM, Migliaccio CA, et al. Virulence control in group A Streptococcus by a two-component gene regulatory system: global expression profiling and in vivo infection modeling. Proc Natl Acad Sci U S A. 2002;99:13855–13860. doi: 10.1073/pnas.202353699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griswold AR, Jameson-Lee M, Burne RA. Regulation and physiologic significance of the agmatine deiminase system of Streptococcus mutans UA159. Journal of bacteriology. 2006;188:834–841. doi: 10.1128/JB.188.3.834-841.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn K, Faustoferri RC, Quivey RG., Jr Induction of an AP endonuclease activity in Streptococcus mutans during growth at low pH. Molecular microbiology. 1999;31:1489–1498. doi: 10.1046/j.1365-2958.1999.01292.x. [DOI] [PubMed] [Google Scholar]
- Hanna MN, Ferguson RJ, Li YH, Cvitkovitch DG. uvrA is an acid-inducible gene involved in the adaptive response to low pH in Streptococcus mutans. Journal of bacteriology. 2001;183:5964–5973. doi: 10.1128/JB.183.20.5964-5973.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasona A, Crowley PJ, Levesque CM, Mair RW, Cvitkovitch DG, Bleiweis AS, Brady LJ. Streptococcal viability and diminished stress tolerance in mutants lacking the signal recognition particle pathway or YidC2. Proc Natl Acad Sci U S A. 2005;102:17466–17471. doi: 10.1073/pnas.0508778102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasona A, Zuobi-Hasona K, Crowley PJ, Abranches J, Ruelf MA, Bleiweis AS, Brady LJ. Membrane composition changes and physiological adaptation by Streptococcus mutans signal recognition particle pathway mutants. Journal of bacteriology. 2007;189:1219–1230. doi: 10.1128/JB.01146-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X, Wu C, Yarbrough D, Sim L, Niu G, Merritt J, Shi W, Qi F. The cia operon of Streptococcus mutans encodes a unique component required for calcium-mediated autoregulation. Molecular microbiology. 2008 doi: 10.1111/j.1365-2958.2008.06390.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreth J, Merritt J, Shi W, Qi F. Co-ordinated bacteriocin production and competence development: a possible mechanism for taking up DNA from neighbouring species. Molecular microbiology. 2005;57:392–404. doi: 10.1111/j.1365-2958.2005.04695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreth J, Merritt J, Zhu L, Shi W, Qi F. Cell density- and ComE-dependent expression of a group of mutacin and mutacin-like genes in Streptococcus mutans. FEMS microbiology letters. 2006;265:11–17. doi: 10.1111/j.1574-6968.2006.00459.x. [DOI] [PubMed] [Google Scholar]
- Kreth J, Hung DC, Merritt J, Perry J, Zhu L, Goodman SD, Cvitkovitch DG, Shi W, Qi F. The response regulator ComE in Streptococcus mutans functions both as a transcription activator of mutacin production and repressor of CSP biosynthesis. Microbiology. 2007;153:1799–1807. doi: 10.1099/mic.0.2007/005975-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemos JA, Burne RA. Regulation and Physiological Significance of ClpC and ClpP in Streptococcus mutans. Journal of bacteriology. 2002;184:6357–6366. doi: 10.1128/JB.184.22.6357-6366.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemos JA, Brown TA, Jr, Burne RA. Effects of RelA on key virulence properties of planktonic and biofilm populations of Streptococcus mutans. Infect Immun. 2004;72:1431–1440. doi: 10.1128/IAI.72.3.1431-1440.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemos JA, Abranches J, Burne RA. Responses of cariogenic streptococci to environmental stresses. Curr Issues Mol Biol. 2005;7:95–107. [PubMed] [Google Scholar]
- Lemos JA, Lin VK, Nascimento MM, Abranches J, Burne RA. Three gene products govern (p)ppGpp production by Streptococcus mutans. Molecular microbiology. 2007a;65:1568–1581. doi: 10.1111/j.1365-2958.2007.05897.x. [DOI] [PubMed] [Google Scholar]
- Lemos JA, Luzardo Y, Burne RA. Physiologic effects of forced down-regulation of dnaK and groEL expression in Streptococcus mutans. Journal of bacteriology. 2007b;189:1582–1588. doi: 10.1128/JB.01655-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemos JA, Nascimento MM, Lin VK, Abranches J, Burne RA. Global Regulation by (p)ppGpp and CodY in Streptococcus mutans. Journal of bacteriology. 2008 doi: 10.1128/JB.00288-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Len AC, Harty DW, Jacques NA. Stress-responsive proteins are upregulated in Streptococcus mutans during acid tolerance. Microbiology. 2004a;150:1339–1351. doi: 10.1099/mic.0.27008-0. [DOI] [PubMed] [Google Scholar]
- Len AC, Harty DW, Jacques NA. Proteome analysis of Streptococcus mutans metabolic phenotype during acid tolerance. Microbiology. 2004b;150:1353–1366. doi: 10.1099/mic.0.26888-0. [DOI] [PubMed] [Google Scholar]
- Levesque CM, Mair RW, Perry JA, Lau PC, Li YH, Cvitkovitch DG. Systemic inactivation and phenotypic characterization of two-component systems in expression of Streptococcus mutans virulence properties. Lett Appl Microbiol. 2007;45:398–404. doi: 10.1111/j.1472-765X.2007.02203.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YH, Hanna MN, Svensater G, Ellen RP, Cvitkovitch DG. Cell density modulates acid adaptation in Streptococcus mutans: implications for survival in biofilms. Journal of bacteriology. 2001a;183:6875–6884. doi: 10.1128/JB.183.23.6875-6884.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YH, Lau PC, Lee JH, Ellen RP, Cvitkovitch DG. Natural genetic transformation of Streptococcus mutans growing in biofilms. Journal of bacteriology. 2001b;183:897–908. doi: 10.1128/JB.183.3.897-908.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YH, Lau PC, Tang N, Svensater G, Ellen RP, Cvitkovitch DG. Novel two-component regulatory system involved in biofilm formation and acid resistance in Streptococcus mutans. Journal of bacteriology. 2002a;184:6333–6342. doi: 10.1128/JB.184.22.6333-6342.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YH, Tang N, Aspiras MB, Lau PC, Lee JH, Ellen RP, Cvitkovitch DG. A quorum-sensing signaling system essential for genetic competence in Streptococcus mutans is involved in biofilm formation. Journal of bacteriology. 2002b;184:2699–2708. doi: 10.1128/JB.184.10.2699-2708.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loesche WJ. Role of Streptococcus mutans in human dental decay. Microbiological reviews. 1986;50:353–380. doi: 10.1128/mr.50.4.353-380.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushita M, Janda KD. Histidine kinases as targets for new antimicrobial agents. Bioorg Med Chem. 2002;10:855–867. doi: 10.1016/s0968-0896(01)00355-8. [DOI] [PubMed] [Google Scholar]
- Merritt J, Kreth J, Shi W, Qi F. LuxS controls bacteriocin production in Streptococcus mutans through a novel regulatory component. Molecular microbiology. 2005;57:960–969. doi: 10.1111/j.1365-2958.2005.04733.x. [DOI] [PubMed] [Google Scholar]
- Nascimento MM, Lemos JA, Abranches J, Lin VK, Burne RA. Role of RelA of Streptococcus mutans in global control of gene expression. Journal of bacteriology. 2008;190:28–36. doi: 10.1128/JB.01395-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen FC, Tao L, Scheie AA. DNA binding-uptake system: a link between cell-to-cell communication and biofilm formation. Journal of bacteriology. 2005;187:4392–4400. doi: 10.1128/JB.187.13.4392-4400.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi F, Merritt J, Lux R, Shi W. Inactivation of the ciaH Gene in Streptococcus mutans diminishes mutacin production and competence development, alters sucrose-dependent biofilm formation, and reduces stress tolerance. Infect Immun. 2004;72:4895–4899. doi: 10.1128/IAI.72.8.4895-4899.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi F, Kreth J, Levesque CM, Kay O, Mair RW, Shi W, Cvitkovitch DG, Goodman SD. Peptide pheromone induced cell death of Streptococcus mutans. FEMS microbiology letters. 2005;251:321–326. doi: 10.1016/j.femsle.2005.08.018. [DOI] [PubMed] [Google Scholar]
- Quivey RG, Jr, Faustoferri RC, Clancy KA, Marquis RE. Acid adaptation in Streptococcus mutans UA159 alleviates sensitization to environmental stress due to RecA deficiency. FEMS microbiology letters. 1995;126:257–261. doi: 10.1111/j.1574-6968.1995.tb07427.x. [DOI] [PubMed] [Google Scholar]
- Rathsam C, Eaton RE, Simpson CL, Browne GV, Berg T, Harty DW, Jacques NA. Up-regulation of competence- but not stress-responsive proteins accompanies an altered metabolic phenotype in Streptococcus mutans biofilms. Microbiology. 2005a;151:1823–1837. doi: 10.1099/mic.0.27830-0. [DOI] [PubMed] [Google Scholar]
- Rathsam C, Eaton RE, Simpson CL, Browne GV, Valova VA, Harty DW, Jacques NA. Two-dimensional fluorescence difference gel electrophoretic analysis of Streptococcus mutans biofilms. J Proteome Res. 2005b;4:2161–2173. doi: 10.1021/pr0502471. [DOI] [PubMed] [Google Scholar]
- Rolerson E, Swick A, Newlon L, Palmer C, Pan Y, Keeshan B, Spatafora G. The SloR/Dlg metalloregulator modulates Streptococcus mutans virulence gene expression. Journal of bacteriology. 2006;188:5033–5044. doi: 10.1128/JB.00155-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senadheera MD, Guggenheim B, Spatafora GA, et al. A VicRK signal transduction system in Streptococcus mutans affects gtfBCD, gbpB, and ftf expression, biofilm formation, and genetic competence development. Journal of bacteriology. 2005;187:4064–4076. doi: 10.1128/JB.187.12.4064-4076.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelburne SA, 3rd, Sumby P, Sitkiewicz I, Granville C, DeLeo FR, Musser JM. Central role of a bacterial two-component gene regulatory system of previously unknown function in pathogen persistence in human saliva. Proc Natl Acad Sci U S A. 2005;102:16037–16042. doi: 10.1073/pnas.0505839102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shemesh M, Tam A, Steinberg D. Differential gene expression profiling of Streptococcus mutans cultured under biofilm and planktonic conditions. Microbiology. 2007;153:1307–1317. doi: 10.1099/mic.0.2006/002030-0. [DOI] [PubMed] [Google Scholar]
- Sheng J, Marquis RE. Enhanced acid resistance of oral streptococci at lethal pH values associated with acid-tolerant catabolism and with ATP synthase activity. FEMS microbiology letters. 2006;262:93–98. doi: 10.1111/j.1574-6968.2006.00374.x. [DOI] [PubMed] [Google Scholar]
- Sheng J, Marquis RE. Malolactic fermentation by Streptococcus mutans. FEMS microbiology letters. 2007;272:196–201. doi: 10.1111/j.1574-6968.2007.00744.x. [DOI] [PubMed] [Google Scholar]
- Spoering AL, Gilmore MS. Quorum sensing and DNA release in bacterial biofilms. Curr Opin Microbiol. 2006;9:133–137. doi: 10.1016/j.mib.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Sztajer H, Lemme A, Vilchez R, Schulz S, Geffers R, Yip CY, Levesque CM, Cvitkovitch DG, Wagner-Dobler I. Autoinducer-2-regulated genes in Streptococcus mutans UA159 and global metabolic effect of the luxS mutation. Journal of bacteriology. 2008;190:401–415. doi: 10.1128/JB.01086-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welin J, Wilkins JC, Beighton D, Wrzesinski K, Fey SJ, Mose-Larsen P, Hamilton IR, Svensater G. Effect of acid shock on protein expression by biofilm cells of Streptococcus mutans. FEMS microbiology letters. 2003;227:287–293. doi: 10.1016/S0378-1097(03)00693-1. [DOI] [PubMed] [Google Scholar]
- Welin J, Wilkins JC, Beighton D, Svensater G. Protein expression by Streptococcus mutans during initial stage of biofilm formation. Appl Environ Microbiol. 2004;70:3736–3741. doi: 10.1128/AEM.70.6.3736-3741.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen ZT, Suntharaligham P, Cvitkovitch DG, Burne RA. Trigger factor in Streptococcus mutans is involved in stress tolerance, competence development, and biofilm formation. Infect Immun. 2005;73:219–225. doi: 10.1128/IAI.73.1.219-225.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen ZT, Baker HV, Burne RA. Influence of BrpA on critical virulence attributes of Streptococcus mutans. Journal of bacteriology. 2006;188:2983–2992. doi: 10.1128/JB.188.8.2983-2992.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkins JC, Homer KA, Beighton D. Analysis of Streptococcus mutans proteins modulated by culture under acidic conditions. Appl Environ Microbiol. 2002;68:2382–2390. doi: 10.1128/AEM.68.5.2382-2390.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng L, Wen ZT, Burne RA. A novel signal transduction system and feedback loop regulate fructan hydrolase gene expression in Streptococcus mutans. Molecular microbiology. 2006;62:187–200. doi: 10.1111/j.1365-2958.2006.05359.x. [DOI] [PubMed] [Google Scholar]