Abstract
The anterior medial prefrontal cortex (aMPFC) is consistently active during personally salient decisions, yet the differential contributory processes of this region along the dorsal—ventral axis are less understood. Using a self-appraisal decision-making task and functional magnetic resonance imaging, we demonstrated task-dependent connectivity of ventral aMPFC with amygdala, insula, and nucleus accumbens, and dorsal aMPFC connectivity with dorsolateral PFC and bilateral hippocampus. These aMPFC networks appear to subserve distinct contributory processes inherent to self-appraisal decisions, specifically a dorsally mediated cognitive and a ventrally mediated affective/self-relevance network.
Introduction
The anterior medial prefrontal cortex (aMPFC) comprises large-scale networks that are selectively engaged by appraisal of and decisions about self-relevant stimuli (Gusnard, Akbudak, Shulman, & Raichle, 2001; Macrae, Moran, Heatherton, Banfield, & Kelley, 2004; Northoff & Bermpohl, 2004; Zysset, Huber, Samson, Ferstl, & von Cramon, 2003). Specifically, functional magnetic resonance imaging (fMRI) paradigms requiring cognitively based appraisals of one’s personal characteristics (Fossati et al., 2003; Johnson et al., 2002; Kelley et al., 2002; Schmitz, Kawahara, & Johnson, 2004; Schmitz, Rowley, Kawahara, & Johnson, In press) as well as one’s subjective preference (Johnson et al., in press; Seger, Stone, & Keenan, 2004; Zysset, Huber, Ferstl, & von Cramon, 2002; Zysset et al., 2003) evoke widespread activation extending along the midline dorsal—ventral axis of the aMPFC. Bounded by the right and left superior frontal sulci, dorsal aMPFC encompasses Brodmann Areas (BA) 9m, superior sections of BA 10m and rostral sections of BA 32m, whereas the ventral aMPFC includes inferior sections of BAs 10m and 32m (Petrides & Pandya, 1994). Yet given the extensive coverage of aMPFC activation evoked by the above appraisal paradigms, as well as the multimodal nature inherent to midline frontal cortices, these data provide little information as to whether self-appraisal decisions instantiate potentially separable contributory aMPFC pathways.
A recent meta-analytic study has approached the question of process specificity within the aMPFC through stereotactic comparison of activation evoked by emotive and cognitively-based neuroimaging tasks, demonstrating aggregate patterns of orbitofrontal/vMPFC activation for the prior and dorsomedial PFC/anterior cingulate (ACC) for the latter task classification (Steele & Lawrie, 2004). In a critical review of animal, lesion, and functional neuroimaging literature, Phillips and colleagues (2003) posit a more systemic distinction between dorsal and ventral aMPFC function, wherein appraisal of stimuli for emotional significance and immediate response biases evokes a ventral aMPFC mediated network inclusive of the amygdala, ventral striatum, insula, and ventral ACC; followed by regulatory processing of this initial appraisal to guide contextually appropriate goal-directed behavior, mediated by a dorsal aMPFC network inclusive of the hippocampus (HF), dorsolateral PFC, and dorsal ACC. Recent functional neuroimaging studies have provided compelling support for the ventral axis of this model through a diverse array of appraisal paradigms, demonstrating ventral MPFC interactions with the amygdala during affective face presentation (Das et al., 2005) and as well as pain (Phelps, Delgado, Nearing, & LeDoux, 2004), and with the amygdala and ventral striatum during reward response (Elliott, Newman, Longe, & Deakin, 2003; Knutson, Taylor, Kaufman, Peterson, & Glover, 2005). In contrast, there are few neuroimaging paradigms that directly evidence the dorsal axis of this model, with respect to observations of dorsal aMPFC interaction with the dorsolateral PFC, HF, and ACC. Ridderinkhof and colleagues (2004) have highlighted a functional association between the posterior MFC (rostral ACC) and dorsolateral PFC in a meta-analysis of tasks requiring cognitive control processes, such as decision-making and self-monitoring of performance (e.g. the Stroop task). Although this research, as a whole, has implicated potentially distinct cognitive and affective aMPFC pathways underlying appraisal of salient stimuli, a clear differentiation of dorsal—ventral aMPFC contributions to explicitly self-relevant appraisal decisions has yet to be demonstrated in the same experimental paradigm.
We addressed the question of process specificity within the aMPFC using functional MRI (fMRI) and psycho-physiological interaction analysis (Friston et al., 1997) to localize spatially distinct brain regions demonstrating condition dependent connectivity with either the ventral or dorsal aMPFC during self-appraisal decisions. In contrast to functional connectivity analysis, which provides only a correlative index of task-independent covariation between regions, psycho-physiological interaction analysis (PPI) allows inference as to whether inter-regional coactivation changes significantly as a function of task manipulation. For this study, our objective to delineate aMPFC process specificity was accomplished first by implementing an established appraisal task requiring self-referential decisions about positive and negative trait adjectives, known to evoke robust activation in both the dorsal and ventral aMPFC (Johnson et al., 2002; Kelley et al., 2002; Schmitz et al., 2004). Second, PPI connectivity analyses were performed to identify brain regions selectively influenced by either the dorsal or ventral aMPFC as a function of self-appraisal. Based on the above reviews of animal, lesion, and neuroimaging literature, as well as the extant fMRI appraisal studies demonstrating ventral cortico-limbic interactions, we hypothesized that the dorsal and ventral aMPFC would engage distinct cortico-limbic networks reflective of cognitive and affective processes inherent to self-appraisal, specifically a dorsally mediated cognitive and a ventrally meditated affective network.
Methods
Participants
Fifteen right-handed, physically and cognitively healthy participants (mean age: 21.2 ± 1.9; sex: 10f/5m; mean education: 15 ± 1; all right-handed) were recruited from the University of Wisconsin—Madison campus via advertisement. Prior to study procedures, participants were screened for MRI safety/compatibility and given a standardized questionnaire covering general health history. The exclusion criteria consisted of any chronic medical condition (e.g. neurological, cardiovascular, cancer), prior invasive surgical procedures, incidence of head trauma, diagnosis of a major psychiatric disorder, and history of alcohol/substance abuse, learning disability, or vision/hearing impairment. Participants who met criteria provided written informed consent prior to engaging in study procedures. The participants were compensated with $40.00 for taking part in this Institutional Review Board approved study, and were treated in accordance with U.S. federal regulations and ethical standards of the American Psychological Association.
fMRI Task
The fMRI paradigm consisted of a task that required patients to make yes/no decisions based on individually presented trait adjectives across two conditions: referential self-appraisal (SA) and non-referential affective valence appraisal (AVA). The same set of 30 adjectives was presented in the SA and AVA conditions. This set consisted of a roughly even distribution of positive and negative valence trait adjectives (based on ratings gathered in prior studies implementing the same paradigm) and was equated based on normative data for familiarity and frequency (Francis & Kucera, 1982) using the MRC Psycholinguistics Database (www.psy.uwa.edu.au/mrcdatabase/uwa_mrc.htm). The adjective set contained a broad spectrum of personality traits, including general disposition (e.g. “daring”), interactive social characteristics (e.g. “shy”), as well as cognitive (e.g. “intelligent”) and physical traits (e.g. “weak”).
The SA condition required patients to decide whether or not adjectives described their own personal traits and abilities whereas the non-referential AVA condition required patients to decide whether or not adjectives in the set were of positive valence. The AVA condition was implemented to control for activation resulting from non-referential appraisal decisions of emotionally valenced stimuli, as well as for language demands, attention, and motoric response. That the adjective set and response paradigm (yes/no decision-making) were identical across the SA and AVA conditions, the prior was intended to isolate emotional and cognitive processes unique to self-appraisal. Measures were also taken to facilitate easy differentiation between the conditions by varying the font hue slightly for each condition and by including a brief instructional reminder above each adjective (SA: “I am … ”; AVA: “Is the word positive?”). A response reminder was also continuously displayed below the adjective, with the words ‘yes’ right of center and ‘no’ left of center corresponding to the configuration of a two-button box (held in patient’s right hand), on which middle-finger (right) button presses conveyed ‘yes’ and index-finger (left) button presses conveyed ‘no.’ Participants reported no difficulty understanding task instructions or viewing the stimuli.
Two discrete but equivalent runs of the task were presented sequentially, each using a separate 30-adjective set. First presentation of each adjective was counterbalanced across conditions such that novelty was not confounded with condition order. Furthermore, the order of task run administration was counterbalanced across subjects. Within both task runs, each of the 2 conditions was presented in 5 pseudo-randomized cycles. For each cycle, adjectives were presented every 4 s in blocks of 6 per condition. Adjectives remained on screen for 3 s followed by a one second inter-stimulus interval.
Procedures
Participants were first situated on the bed of a GE 3.0 Tesla scanner and outfitted with the MR-compatible button-box and a high-resolution goggle system, set at 800 × 600 from Resonance Technology (Northridge, CA, USA). Head motion was constrained by foam padding around the head-coil. The software package Presentation ® (Albany, CA, USA; NeuroBehavioral Systems) was used to deliver visual stimuli and record responses via the goggle system and button-box, respectively. A cable connecting the scanner to the stimulus-delivery computer enabled Presentation to monitor TTL pulses from the scanner, thereby enabling precise synchrony between slice acquisition and stimulus delivery.
Imaging protocol
Echo-planar imaging
For each subject, a T2* weighted gradient-echo echoplanar image (EPI) pulse sequence was prescribed and higher-order shimmed for the functional trials. The EPI parameters were as follows: echo time (TE) = 30 ms; repetition time (TR) = 2000 ms; flip angle = 90°; acquisition matrix = 64 × 64 voxels; field of view (FOV) = 240 mm. Thirty sagittal slices of the brain were acquired within the TR at each time point, with a voxel resolution of 3.75 × 3.75 × 4 mm, with a 1 mm skip between slices. In both 4 min 8 s scanning trials, 124 time points were collected, of which 3 images acquired during the first 6 s were discarded (for a total of 242 reconstructed time points).
Field Mapping
After higher-order shimming, residual magnetic field (Bo) inhomogenieties across the brain often continue to cause regional image distortions in echo planar images, especially within inferior prefrontal regions near the frontal and ethmoid sinuses. Given the importance of ventral prefrontal cortical and limbic loci to the current study hypotheses, these regional image distortions were corrected by measuring 3D field maps across the brain (co-planar with the fMRI slices). This was accomplished by measuring the phase of non-EPI gradient echo images at 2 echo times (7 and 10 ms). The phase difference between the two echo images is proportional to the static field inhomogeniety (Jezzard & Balaban, 1995). The warp correction was performed using custom software developed in Matlab6.5. A 3D phase-unwrapping algorithm (Jenkinson, 2003) was used to estimate the continuous field map. Image unwarping was performed using a nonlinear pixel shifting and B splines interpolation algorithm.
Data Analysis
Functional activation was determined from the blood oxygenation level dependent (BOLD) signal using the software Statistical Parametric Mapping (SPM2, University College London, UK; http://www.fil.ion.ucl.ac.uk). Following image reconstruction, the files were then converted to Analyze file format and reoriented to the axial plane. The image time-series was motion-corrected, field map corrected as described above, normalized into the MNI standard space using the T2* weighted template provided through SPM2 (written out with 2×2×2 mm voxel resolution), and then smoothed with an 8 mm full-width at half-maximum Gaussian kernel.
Single participant analyses
The time-series data for individual patients were analyzed using a boxcar model convolved with the canonical hemodynamic response function (HRF). The statistical model included high frequency signal filtering (high pass filter = 128 seconds) and the AR1 method of estimating temporal autocorrelation. The time series data were statistically analyzed using the general linear model (Friston, 1995). Two subtractive contrasts were specified per individual analysis: SA versus AVA (contrast coding: 1 1; referred to as SA forthwith) and the inverse, AVA versus SA (contrast coding: –1 –1 ).
Group analysis of task
The individual subject SA contrast images were entered into a random effects analysis. Inference of statistical significance in the SA and AVA group contrasts used an FDR corrected p-value < 0.01 (Genovese, Lazar, & Nichols, 2002). However, the task group results are also presented at a threshold with an uncorrected p-value < 0.001 for purposes of uniform statistical comparison with the results of the PPI group analyses.
Psycho-physiological interaction analysis
The coordinates of the ventral aMPFC (x, y, z, = -10, 64, 8) and dorsal aMPFC (x, y, z, = -14, 50, 42) maxima identified in the second level analysis of task were subsequently used as seed points for the two primary PPI analyses of connectivity, enabling data-led observations of potential condition-dependent covariance with voxels activated by the main effects of task. As additional subsidiary tests for the process specificity of cortico-limbic dorsal—ventral aMPFC networks to SA, the remaining maxima identified in the second level task analysis (ACC: x, y, z, = -10, 44, 8; and RSC; x, y, z, = -12, -50, 34) were also seeded for PPI analyses.
First, the design matrices of the two task runs were concatenated into one linear series, such that VOI extractions at each seed locus incorporated time series for the entire task duration. The single participant analyses were then repeated in accordance with the previously described method. In each participant, mean-corrected activity (adjusted against persistent average activation to main effects of task) was extracted from volumes of interest (first eigenvariate of a spherical VOI; 3 mm radius) centered on the pre-specified seed points. For each resultant VOI, a PPI regressor was calculated (using the PPI function in SPM2) as the point-by-point product of the deconvolved VOI time series and a binary vector coding for sequential blocks of the task design; 1 for the SA condition, –1 for the AVA condition (Friston et al., 1997; Gitelman, Penny, Ashburner, & Friston, 2003). The single participant PPIs (dorsal and ventral aMPFC, ACC, and RSC) were then separately reconvolved with the canonical HRF and modeled with the same specifications as the concatenated single participant analysis. In this way, a significant effect for PPI (referred to as connectivity estimates forthwith) indicated that covariance between seed origin and a coupled region (referred to as CR forthwith) during SA differs significantly than that during AVA (see Figure 1 for example). Two contrasts were specified per PPI seed origin (1 0 0, and –1 0 0) reflecting activations either positively or negatively related to the PPI interaction term, respectively.
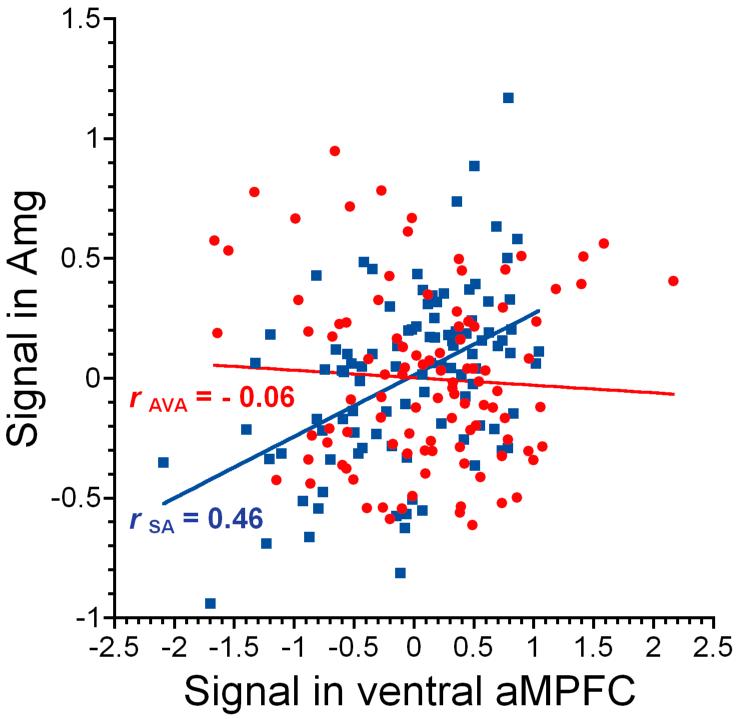
Figure 1.
Example of a psycho-physiological interaction (PPI) analysis for a single participant. The two regression slopes represent average time-course activity in the ventral aMPFC for the SA (blue squares) and AVA (red circles) conditions. Mean corrected activity in the amygdala is selectively coupled with mean corrected activity in the ventral aMPFC (extracted from a 3 mm radius volume of interest; x, y, z: -10, 64, 8) during the SA condition. The PPI, or condition-dependent coupling between regions, is calculated as the difference between regression slopes (p corrected < 0.001, t = 3.42).
As the current study was based on model driven (Phillips et al., 2003) a priori hypotheses implicating differential dorsal—ventral aMPFC networks in the mediation of SA, connectivity estimates for the SA condition were evaluated in second level analyses. Statistical significance in the connectivity group analyses was determined by: (a.) calculating the SPMt based on an uncorrected voxel threshold p < 0.001; and (b.) applying a small volume voxel-wise p-value correction (6 mm radius spherical VOI; FDR corrected p-value = 0.05) to the hypothesized regions of interest.
aMPFC network specificity analysis
The second level aMPFC PPI analyses described above identified distal CRs that significantly interact with the respective seed origins (i.e. regions where the average interaction is greater than zero, indicating condition-dependent connectivity). However, because these separate group PPI analyses are univariate statistical parametric maps (SPMs) that test voxels against the null (zero), inferences cannot be drawn as to whether connectivity estimates of the ventral and dorsal aMPFC seed origins were indeed selective to the CRs observed in either respective analysis. In order to determine whether CRs were uniquely networked with their corresponding aMPFC seed origin during SA, a multivariate approach was taken in the form of two separate repeated measures ANOVAs: (1) compared the connectivity estimates of aMPFC seed origins to CRs originally identified in the SA contrast of the ventral aMPFC PPI group analysis; (2) compared the connectivity estimates of aMPFC seed origins to CRs originally identified in the SA contrast of the dorsal aMPFC PPI group analysis. For each ANOVA, mean-corrected activity was extracted from 3 mm radius spherical VOIs centered on the CRs of interest in both the dorsal and ventral aMPFC PPI group analyses. Post hoc comparisons were then conducted.
Results
Behavioral Analyses
Repeated measures ANOVAs were conducted to examine potential differences in response times (RTs) between the SA and AVA conditions, and in yes versus no responses across conditions. There were no significant differences in response times between conditions F(1, 14) = 1.16, p = 0.291, or in yes versus no responses F(1, 14) = 3.10, p = 0.089, indicating that cognitive demand was roughly equivalent between conditions. In terms of response patterns between conditions, the proportion of yes responses was 53% in the SA condition and 49% in the AVA condition; this difference, although only four percentage points, was significant in a paired t-test at p < 0.05. Furthermore, there was a significant interaction between condition and adjective valence F(1, 14) = 22.52, p < 0.001, indicating a differential proportion of yes responses between positive and negative trait adjectives during the SA condition and an equivalent proportion during the AVA condition (consistent with the intended structuring of the adjective set). The interaction occurred as a function of participants’ willingness to endorse negative traits (∼25%), suggesting that self-appraisals were performed subjectively and honestly.
Imaging Results
Group Analysis of Task
Consistent with previous findings, robust cortical midline activation was detected during the SA condition, specifically at loci in the ventral and dorsal aMPFC, anterior cingulated, and retrosplenial cortex. The AVA condition evoked activation in the precuneus, superior parietal lobule, bilateral inferior frontal gyri, medial frontal gyrus, superior temporal gyrus, and occipital cortex, indicating processes subserving semantic and non self-referential appraisal of affective word stimuli (e.g. attention, language). Table 1 provides a summary of the findings for the group analysis of task.
Table 1.
Main effect of task group analysis: Coordinates (MNI space) a and peak activation statistics for the self-appraisal (SA) and affective valence appraisal (AVA) conditions
|
Talairach coordinates |
|||||||
|---|---|---|---|---|---|---|---|
| Group main effect | Region | x | y | z | t-value | uncorrected p < 0.001 |
FDR corr p < 0.01 |
| SA | dorsal aMPFC | -14 | 50 | 42 | 10.96 | 0.000000015 | 0.001 |
| ventral aMPFC | -10 | 64 | 8 | 6.78 | 0.0000045 | 0.003 | |
| ACC b | -10 | 44 | 8 | 9.73 | 0.000000065 | 0.001 | |
| RSC c | -12 | -50 | -34 | 6.24 | 0.000011 | 0.004 | |
| AVA | Precuneus | 22 | -70 | 28 | 9.54 | 0.000000084 | 0.002 |
| SPL d | -26 | -60 | 40 | 8.66 | 0.00000027 | 0.002 | |
| r IFG e | 36 | 36 | 10 | 8.87 | 0.00000020 | 0.002 | |
| l IFG | -32 | 38 | 4 | 5.96 | 0.000017 | 0.003 | |
| r MFG f | 24 | 4 | 62 | 4.72 | 0.00016 | 0.009 | |
| STG g | 40 | 2 | -14 | 5.25 | 0.000061 | 0.006 | |
| Lingual gyrus | 6 | -86 | 2 | 7.36 | 0.0000018 | 0.002 | |
MNI = Montreal Neurological Institute
ACC = anterior cingulate cortex
RSC = retrosplenial cortex
SPL = superior parietal lobule
IFG = inferior frontal gyrus
MFG = middle frontal gyrus
STG = superior temporal gyrus
PPI Connectivity Analyses
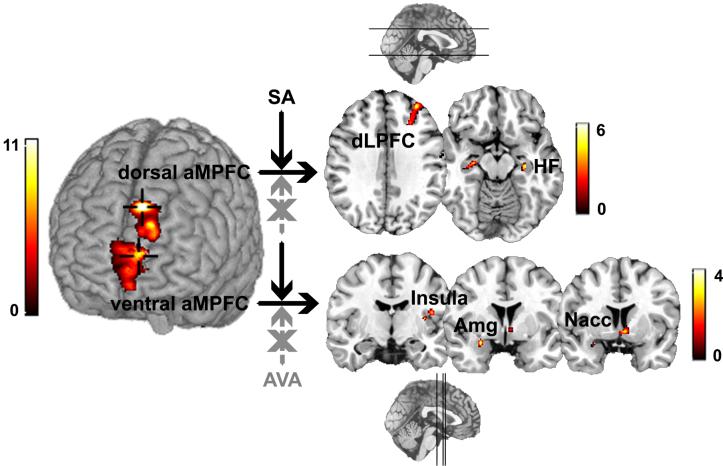
Of the four seed origins (dorsal and ventral aMPFC, ACC, and RSC) examined in separate group connectivity analyses, only the aMPFC seeds demonstrated condition-dependent coupling patterns as a function of self-appraisal, and this coupling was observed only in the positive interaction contrast (seed and coupled region exhibited co-active increases in signal). Furthermore, the dorsal and ventral aMPFC instantiated dissociated networks (see Figure 2), specifically a ventrally mediated coupling with the left amygdala (Amg), nucleus accumbens (Nacc), and insula, and dorsally mediated coupling with the right dorsolateral prefrontal cortex (dLPFC) and bilateral anterior hippocampus (HF). Subsequent interrogation of these coupled regions with small volume correction revealed statistical significance well beyond an FDR corrected p threshold < 0.05. Table 2 provides a summary of the findings for the PPI group analyses. These results indicate that appraisal of one’s self across a set of positive and negative trait/ability adjectives evoke discrete changes in dorsal and ventral aMPFC coupling with distal brain locations, above and beyond any main effects of task as well as condition-independent activation coupling.
Figure 2.
Connectivity analyses were conducted at the ventral and dorsal aMPFC maxima (shown at crosshairs on the 3 dimensional anatomical rendering; left hemisphere is on viewer’s right side) in order to identify condition-dependent activation coupling with distal regions during self-appraisal decisions (SA condition). The ventral aMPFC demonstrated coupling with nucleus accumbens, left amygdala, and insula, whereas the dorsal aMPFC was selectively coupled with the right dorsolateral PFC and anterior hippocampus bilaterally (shown on 2 dimensional coronal and axial slices, respectively, with sagittal references; left hemisphere is on viewer’s left side).
Table 2.
PPI a group analyses for the SA contrast: MNI coordinates and peak activation statistics for connectivity estimates of ventral and dorsal aMPFC seed origin to CRs b
|
Talairach coordinates |
|||||||
|---|---|---|---|---|---|---|---|
| PPI seed origin | → CR | x | y | z | t-value | uncorrected p < 0.001 |
FDR corr S.V.C. c p < 0.05 |
| ventral aMPFC | Amg d | -28 | 2 | -16 | 4.19 | 0.00050 | 0.012 |
| Nacc e | 4 | 6 | -4 | 3.99 | 0.00068 | 0.008 | |
| Insula | 42 | -8 | 16 | 3.85 | 0.00090 | 0.017 | |
| dorsal aMPFC | dLPFC f | 30 | 46 | 34 | 5.59 | 0.000034 | < 0.001 |
| HF g | 26 | -22 | -8 | 6.16 | 0.000012 | 0.001 | |
PPI = psycho-physiological interaction
CR = coupled region
S.V.C. = small volume correction
Amg = amygdala
Nacc = Nucleus accumbens
dLPFC = dorsolateral prefrontal cortex
HF = Hippocampal formation
aMPFC network specificity analysis
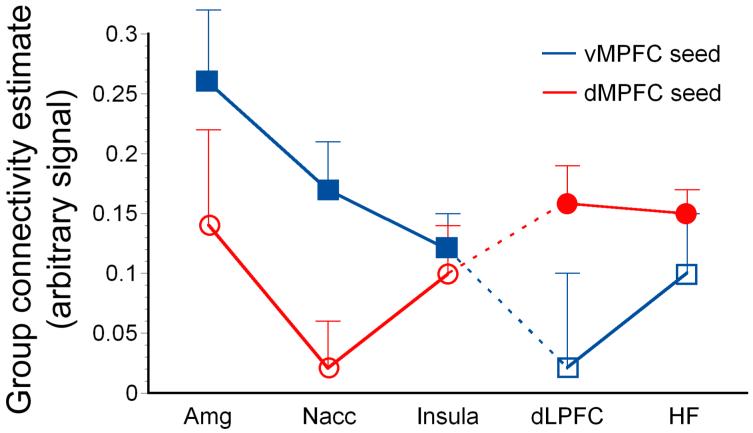
Two separate analyses of variance compared the connectivity estimates of the ventral and dorsal aMPFC seed origins to coupled regions (CRs) identified in either respective aMPFC PPI group analysis: (1) the Amg, Nacc, and insula; (2.) the dLPFC and HF. When comparing the connectivity estimates of seed origins to ventral axis CRs, there was a significant effect of seed origin F(1, 14) = 7.73, p = 0.015, wherein collectively the Nacc, Amg, and insula demonstrated significant connectivity with the ventral aMPFC and not with the dorsal aMPFC, indicating a network dissociation. When comparing the connectivity estimates of seed origins to dorsal axis CRs, the effect of seed origin was near significance F(1, 14) = 4.31, p = 0.057, indicating a weaker dissociation (see Figure 3 for plots of the two ANOVAs). Post hoc comparisons were then conducted to further interrogate significant differences in the connectivity estimates of seed origin to each CR identified above. The ventral and dorsal aMPFC seed origins demonstrated significantly different connectivity estimates for the Nacc and dLPFC regions, wherein the Nacc was preferentially coupled with the ventral aMPFC and dLPFC with the dorsal aMPFC (see Table 3).
Figure 3.
These plots display findings from two separate analyses of variance (demarcated by dashed lines) that compared group connectivity estimates of the ventral and dorsal aMPFC seed origins to coupled regions (CRs) identified in the SA contrast of either respective aMPFC PPI group analysis: (1.) the Amg, Nacc, and Insula; (2.) the dLPFC and HF. Y axis—seed origins: ventral aMPFC data-series = blue square symbols; dorsal aMPFC data-series = red circle symbols. X axis—coupled regions: Amg = amygdala, Nacc = nucleus accumbens, Insula; dLPFC = dorsal lateral prefrontal cortex; HF = hippocampal formation). Solid symbols indicate significant coupling, whereas hollow symbols indicate non-significant coupling with the respective aMPFC seed origins during SA (uncorrected of p threshold < 0.001; see Table 2 for post hoc comparisons). Standard error bars are included.
Table 3.
Post-hoc comparisons a for 2 ANOVAs differentiating group connectivity estimates of the aMPFC seed origins to CRs during SA
| CR | → Difference in seed origin connectivity |
t-value | uncorrected p < 0.05 |
|---|---|---|---|
| Amg | ventral aMPFC vs. dorsal aMPFC |
1.43 | 0.088 |
| Nacc | 3.72 | 0.001 | |
| Insula | 0.58 | 0.285 | |
| dLPFC | 2.04 | 0.031 | |
| HF | 0.96 | 0.178 |
Post-hoc comparisons = paired 1-tailed t-tests of dorsal and ventral aMPFC connectivity estimates to CRs identified in either aMPFC PPI group analysis
Discussion
Ventral aMPFC network
The current study dissociated two networks along the dorsal—ventral axis of the aMPFC that are selectively activated in the context of self-appraisal decisions (i.e., the SA condition). First, we identified a ventral aMPFC pathway involving condition dependent co-activation in the nucleus accumbens (Nacc), insula, and left amygdala (Amg). In addition to surviving the FDR corrected p < 0.05 small volume correction (see Table 2), a repeated measures ANOVA revealed significantly greater connectivity estimates for Nacc, insular, and Amg coupling with the ventral compared to the dorsal aMPFC seed origin (see Figure 3). Post hoc comparisons of Nacc, insular, and Amg coupling between aMPFC seed origins yielded a consistent pattern, with the Nacc exhibiting significantly stronger, and the Amg near significant estimates of preferential connectivity with the ventral aMPFC (see Table 3). Taken together, these multivariate comparisons provide evidence that process specificity is indeed conferrable to a ventral aMPFC—Nacc—insula—Amg network in the context of self-appraisal decisions.
Separate evidence for a ventral aMPFC—Amg pathway subserving personally salient appraisal decisions originate from lesion studies of reward and emotion, in which damage selective to either the Amg or ventral aMPFC has been shown to cause impairments in the development of such processes (Bechara, Damasio, & Damasio, 2003; Phillips et al., 2003). Based on these and other lesion findings (Eslinger & Damasio, 1985), Stuss and Levine (2002) have put forth that the ventral aMPFC plays a role in maintaining an accurate mental representation of the self “on-line” in order to guide emotion, reward, and inhibition processing to an appropriate behavioral response. Similarly, Phillips et al. (2003) have proposed a systems level model based on extensive review of lesion as well as animal and functional neuroimaging literature, wherein appraisal of stimulus-induced emotional significance, the generation of an initial affective state, and immediate response biases engage a ventral aMPFC mediated network inclusive of the Amg, insula, ventral striatum and anterior cingulate (ACC). Taken together, the lesion literature and derivational model posed by Phillips and colleagues are highly consistent with the ventral axis network identified in the present study. However, that the present finding demonstrates condition-dependent connectivity, wherein instantiation of the ventral axis network occurred explicitly as a function of self-appraisal and not affective valence appraisal, we propose a more general function of this ventromedial cortico-limbic appraisal system, inclusive of rather than specific to appraisal of emotional cues. In line with Stuss and Levine (2002), the findings presented in this study suggest that the ventral aMPFC is involved in the representation of external stimuli as implicitly self-relevant. Furthermore, the observed ventromedial corticolimbic connectivity implicates that the network is involved, as a whole, in the identification and appraisal of personal salience, or immediate relevance to one’s self, conveyed by a stimulus.
Recent functional neuroimaging research spanning an array of appraisal-based paradigms has provided convergent in vivo evidence of vental PFC—limbic interactivity, similar to that identified in this study. Specifically, ventral MPFC—Amg connectivity has been observed with PPI analysis during passive viewing of affective as opposed to neutral face stimuli (Das et al., 2005), whereas other studies have demonstrated right ventrolateral PFC—Amg connectivity during presentations of negative face stimuli in a simple gender discrimination task (Iidaka et al., 2001), during affective valence appraisals of face stimuli that were subliminally primed with an angry face (Nomura et al., 2004), and during categorical decisions of threatening pictorial stimuli (Hariri, Mattay, Tessitore, Fera, & Weinberger, 2003). Of note, these latter studies all required veridical cognitively-based appraisals of affectively laden stimuli (similar to the AVA condition of this paradigm), yet these occurred in the absence of an explicitly self-relevant context, and therefore did not evoke cortical midline structures. Phan (2004) addressed the question of potentially dissociable neural substrates underlying appraisal of emotion and personal relatedness, comparing separate ratings of these criteria to activation evoked during presentation of affective pictorial stimuli. That study identified differential but overlapping neural substrates associated with ratings along either dimension, specifically ventral MPFC activation (as well as dorsal MPFC and anterior insula) was associated with appraisal of personal relatedness, the Amg with emotional intensity, and the Nacc with both appraisal types. The identification and interpretation of rewarding (i.e. financially gainful) stimuli has also been shown to differentially engage neural response within this ventral cortico-limbic network, with Amg and Nacc activity demonstrating an all-or-nothing response to the absolute presence of reward, and ventral MPFC activity differentially responding to specific values of reward (Elliott et al., 2003; ventromedial cortico-striatal coactivation was also observed in the context of expected value, see Knutson et al., 2005). Still further support for the present ventromedial cortico-limbic finding comes from a computational modeling investigation, in which a representative network of the ventral MPFC, Amg, and Nacc successfully generated decisions requiring cognitive-affective integration, yet failed to do so when the ventral MPFC component was removed (Wagar & Thagard, 2003). Taken together, these findings fit well with the model of emotion appraisal proposed by Phillips et al. (2003) and with the ventral aMPFC—Nacc—insula—Amg network identified in this study. Additionally, the general overlap of the above in vivo findings, acquired across an array of paradigms including appraisal of affective salience, personal relatedness, and reward, suggests a cross-modality of function for this network. In light of ventral axis connectivity findings from the present study, we interpret this cross-modality as further indicative that this system subserves appraisal of the self-relevance conveyed by endogenous phenomena (i.e. how a given stimulus will affect one’s self), rather than a specific type of emotional cue (i.e. happy, sad, rewarding).
Dorsal aMPFC network
In contrast to the ventromedial cortico-limbic network, we found that the dorsal aMPFC was significantly (FDR p < 0.05 S.V.C.) coupled with the right dLPFC and bilateral anterior hippocampi during self-appraisal decisions (see Figure 2 and Table 2). A repeated measures ANOVA revealed near significant differences in connectivity estimates between aMPFC seed origins to the dLPFC and HF (see plots in Figure 3). Upon subsequent interrogation of these plots, the absence of differential connectivity between seed origins was most likely driven by the HF locus, whereat aMPFC connectivity estimates differed only slightly (although less variability was observed in the dorsal aMPFC estimate). Post-hoc comparisons of dLPFC and HF coupling between aMPFC seed origins identified significantly stronger estimates of dLPFC connectivity with the dorsal aMPFC, whereas expectedly, the HF exhibited no significant differences between seed origins (see Table 3). These multivariate comparisons lend support to a process specific dorsal aMPFC—dLPFC network in the context of self-appraisal decisions.
The dorsal aMPFC—dLPFC coupling pattern implicates a cognitive control mechanism, extending upon evidence that the dorsal aMPFC is selective, or process specific, to monitoring and evaluating decisions about self-relevant stimuli (Northoff & Bermpohl, 2004; Zysset et al., 2003). Furthermore, the observed dorsal axis network overlaps quite well with the appraisal model proposed by Phillips et al. (2003), wherein initial appraisal and response biases induced by a stimulus (subserved by the ventral axis) are subsequently processed through a series of regulatory and inhibitive checks to guide contextually appropriate goal-directed behavior, mediated by the dorsal aMPFC, ACC, and LPFC, as well as the HF. Based on this model and a recent meta-analysis of the MFC contributions to goal-directed behavior (Ridderinkhof et al., 2004), the present findings suggest interactive roles for the dorsal aMPFC in self-monitoring decisions, and the right dLPFC in mediating performance adjustments to maintain the veracity of such decisions relative to one’s core self-schema. Consistent with this finding, robust dorsal aMPFC activation has been extensively demonstrated in studies requiring explicitly self-referential decision-making (Fossati et al., 2003; Johnson et al., 2002; Kelley et al., 2002; Schmitz et al., 2004; Schmitz et al., In press; Seger et al., 2004; Zysset et al., 2002; Zysset et al., 2003), yet direct comparisons of activation evoked by self-referential relative to other-referential decisions (which do not require appraisal of one’s internal self-schema) have shown a preferential right-hemisphere dorsal prefrontal neural response (Fossati et al., 2003; Schmitz et al., 2004). Among a group of traumatic brain injury patients, the magnitude of activation in this right dorsal PFC region was also recently shown to relate with self-evaluative accuracy about one’s post-injury traits and abilities (Schmitz et al., In press). Thus, as a function of more stringently controlled conditions, these latter paradigms support the presently held notion that the right dLPFC facilitates the dorsal aMPFC in generating accurate decisions, in this case, about the self.
With respect to the significant, albeit network-independent, coupling observed in the bilateral anterior HF, less fMRI data is available on interactions between the medial temporal lobe and dorsal aMPFC in the context of self-appraisal decisions. However, recent neuroimaging studies investigating cognitive control of episodic memory retrieval (Bunge, Burrows, & Wagner, 2004) and regularity learning across trial episodes (Doeller, Opitz, Krick, Mecklinger, & Reith, 2004) have implicated a modulative relationship between the dLPFC and HF. Taken together, these studies implicate cognitively mediated top-down influence of the dLPFC on memory processes subserved by the hippocampus. Given the dorsal aMPFC—dLPFC—HF network presented here, it is possible that these dorsal prefrontal cortical regions engage the HF in encoding personally salient decisions across episodes of the self-appraisal condition. Phillips et al. (2003) include the HF in the dorsal axis of their emotion appraisal model based largely on Gray and McNaughton (2000), who theorize that the structure plays a role in affective state regulation, specifically as a comparator between different and conflicting potential goal-directed behaviors, biasing more exploratory patterns of behavior to effect conflict resolution. The observed dorsal aMPFC—HF coupling in the context of self-appraisal decisions substantiates this theory, as the process of self-ascribing a positive and especially a negative trait adjective would potentially generate competing decision outcomes, a biasing of the more self-congruent outcome, and an ultimate rejection of the (self-incongruent) alternative to enable a response. Further study is required to disambiguate the connectional influences of cognitive and memory processes within this network.
Neuroanatomic boundaries
The process dissociation between dorsal and ventral aMPFC networks, as demonstrated in this study, closely parallels underlying neuroanatomical divisions and interconnectivity. At the most basic level, the ventral PFC and dLPFC have been shown to segregate cytoarchitectonically in accordance with models of cortical evolution (Pandya & Yeterian, 1996). Within the ventral division, dense axonal projections extend between the ventral PFC and limbic regions inclusive of the Amg and Nacc, which together also share robust interconnectivity (de Olmos & Heimer, 1999). The dLPFC comprises part of the archicortical trend emerging from the hippocampus, along which axonal interconnections extend in a reciprocal fashion (Goldman-Rakic, Selemon, & Schwartz, 1984). Furthermore, anatomical research in monkeys has implicated that the dLPFC and dMPFC also share reciprocal axonal connectivity (Petrides & Pandya, 1999). These neuroantomical data support the gross division of dorsal and ventral aMPFC in terms of cognitive and affective functions, respectively, and furthermore the separate extended networks regulated by each of these aMPFC sub-regions.
Conclusion
Though the current finding of dissociated dorsal—ventral MPFC networks represents a correlative interaction, and therefore does not invoke causal inter-regional relationships, we present evidence that the observed neural coupling patterns behave in a condition-dependent fashion, specifically as a function of self-appraisal decisions. Furthermore, in general accord with the appraisal model posed by Phillips et al (2003), we argue that these dorsal—ventral networks subserve differential global processing demands in the context of such decisions, with the ventral aMPFC—Nacc—insula—Amg mediating identification and appraisal of stimulus-induced self-relevance, and the dorsal aMPFC—dLPFC—HF mediating cognitive control in the generation of explicitly self-referential decisions. Future research of brain networks subserving self-appraisal decisions may benefit from the usage of different stimulus types as well as task manipulations designed to evaluate the temporal coordination within and across these networks.
Acknowledgments
This study was supported by R01 MH65723 (SCJ).
References
- Bechara A, Damasio H, Damasio AR. Role of the amygdala in decision-making. Ann N Y Acad Sci. 2003;985:356–369. doi: 10.1111/j.1749-6632.2003.tb07094.x. [DOI] [PubMed] [Google Scholar]
- Bunge SA, Burrows B, Wagner AD. Prefrontal and hippocampal contributions to visual associative recognition: interactions between cognitive control and episodic retrieval. Brain Cogn. 2004;56(2):141–152. doi: 10.1016/j.bandc.2003.08.001. [DOI] [PubMed] [Google Scholar]
- Das P, Kemp AH, Liddell BJ, Brown KJ, Olivieri G, Peduto A, et al. Pathways for fear perception: modulation of amygdala activity by thalamo-cortical systems. Neuroimage. 2005;26(1):141–148. doi: 10.1016/j.neuroimage.2005.01.049. [DOI] [PubMed] [Google Scholar]
- de Olmos JS, Heimer L. The concepts of the ventral striatopallidal system and extended amygdala. Ann N Y Acad Sci. 1999;877:1–32. doi: 10.1111/j.1749-6632.1999.tb09258.x. [DOI] [PubMed] [Google Scholar]
- Doeller CF, Opitz B, Krick CM, Mecklinger A, Reith W. Prefrontal-hippocampal dynamics involved in learning regularities across episodes. Cereb Cortex. 2004 doi: 10.1093/cercor/bhh211. [DOI] [PubMed] [Google Scholar]
- Elliott R, Newman JL, Longe OA, Deakin JF. Differential response patterns in the striatum and orbitofrontal cortex to financial reward in humans: a parametric functional magnetic resonance imaging study. J Neurosci. 2003;23(1):303–307. doi: 10.1523/JNEUROSCI.23-01-00303.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eslinger PJ, Damasio AR. Severe disturbance of higher cognition after bilateral frontal lobe ablation: patient EVR. Neurology. 1985;35(12):1731–1741. doi: 10.1212/wnl.35.12.1731. [DOI] [PubMed] [Google Scholar]
- Fossati P, Hevenor SJ, Graham SJ, Grady C, Keightley ML, Craik F, et al. In search of the emotional self: an FMRI study using positive and negative emotional words. Am J Psychiatry. 2003;160(11):1938–1945. doi: 10.1176/appi.ajp.160.11.1938. [DOI] [PubMed] [Google Scholar]
- Francis WN, Kucera H. Frequency Analysis of English Usage: Lexicon and Grammar. Houghton Mifflin Company; Boston: 1982. [Google Scholar]
- Friston KJ. Commentary and opinion: II. Statistical parametric mapping: ontology and current issues. Journal of Cerebral Blood Flow & Metabolism. 1995;15(3):361–370. doi: 10.1038/jcbfm.1995.45. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Buechel C, Fink GR, Morris J, Rolls E, Dolan RJ. Psychophysiological and modulatory interactions in neuroimaging. Neuroimage. 1997;6(3):218–229. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- Genovese CR, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage. 2002;15(4):870–878. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- Gitelman DR, Penny WD, Ashburner J, Friston KJ. Modeling regional and psychophysiologic interactions in fMRI: the importance of hemodynamic deconvolution. Neuroimage. 2003;19(1):200–207. doi: 10.1016/s1053-8119(03)00058-2. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS, Selemon LD, Schwartz ML. Dual pathways connecting the dorsolateral prefrontal cortex with the hippocampal formation and parahippocampal cortex in the rhesus monkey. Neuroscience. 1984;12(3):719–743. doi: 10.1016/0306-4522(84)90166-0. [DOI] [PubMed] [Google Scholar]
- Gray J, McNaughton N. The Neuropsychology of Anxiety: An Enquiry into the Functions of the Septohippocampal System. 2nd Oxford University Press; Oxford, UK: 2000. [Google Scholar]
- Gusnard DA, Akbudak E, Shulman GL, Raichle ME. Medial prefrontal cortex and self-referential mental activity: Relation to a default mode of brain function. Proc Natl Acad Sci U S A. 2001;20:20. doi: 10.1073/pnas.071043098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri AR, Mattay VS, Tessitore A, Fera F, Weinberger DR. Neocortical modulation of the amygdala response to fearful stimuli. Biol Psychiatry. 2003;53(6):494–501. doi: 10.1016/s0006-3223(02)01786-9. [DOI] [PubMed] [Google Scholar]
- Iidaka T, Omori M, Murata T, Kosaka H, Yonekura Y, Okada T, et al. Neural interaction of the amygdala with the prefrontal and temporal cortices in the processing of facial expressions as revealed by fMRI. J Cogn Neurosci. 2001;13(8):1035–1047. doi: 10.1162/089892901753294338. [DOI] [PubMed] [Google Scholar]
- Jenkinson M. Fast, automated, N-dimensional phase-unwrapping algorithm. Magn Reson Med. 2003;49(1):193–197. doi: 10.1002/mrm.10354. [DOI] [PubMed] [Google Scholar]
- Jezzard P, Balaban RS. Correction for geometric distortion in echo planar images from B0 field variations. Magn Reson Med. 1995;34(1):65–73. doi: 10.1002/mrm.1910340111. [DOI] [PubMed] [Google Scholar]
- Johnson S, Baxter L, Wilder L, Pipe J, Heiserman J, Prigatano G. Neural correlates of self-reflection. Brain. 2002;125(8):1808–1814. doi: 10.1093/brain/awf181. [DOI] [PubMed] [Google Scholar]
- Johnson S, Schmitz T, Kawahara-Baccus T, Rowley H, Alexander A, Lee J, et al. (in press). The Cerebral Response During Subjective Choice With and Without Self-Reference Journal of Cognitive Neuroscience [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley WM, Macrae CN, Wyland CL, Caglar S, Inati S, Heatherton TF. Finding the self? An event-related fMRI study. J Cogn Neurosci. 2002;14(5):785–794. doi: 10.1162/08989290260138672. [DOI] [PubMed] [Google Scholar]
- Knutson B, Taylor J, Kaufman M, Peterson R, Glover G. Distributed neural representation of expected value. J Neurosci. 2005;25(19):4806–4812. doi: 10.1523/JNEUROSCI.0642-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macrae CN, Moran JM, Heatherton TF, Banfield JF, Kelley WM. Medial prefrontal activity predicts memory for self. Cereb Cortex. 2004;14(6):647–654. doi: 10.1093/cercor/bhh025. [DOI] [PubMed] [Google Scholar]
- Nomura M, Ohira H, Haneda K, Iidaka T, Sadato N, Okada T, et al. Functional association of the amygdala and ventral prefrontal cortex during cognitive evaluation of facial expressions primed by masked angry faces: an event-related fMRI study. Neuroimage. 2004;21(1):352–363. doi: 10.1016/j.neuroimage.2003.09.021. [DOI] [PubMed] [Google Scholar]
- Northoff G, Bermpohl F. Cortical midline structures and the self. Trends Cogn Sci. 2004;8(3):102–107. doi: 10.1016/j.tics.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Pandya DN, Yeterian EH. Comparison of prefrontal architecture and connections. Philos Trans R Soc Lond B Biol Sci. 1996;351(1346):1423–1432. doi: 10.1098/rstb.1996.0127. [DOI] [PubMed] [Google Scholar]
- Petrides M, Pandya DN. Comparative architectonic analysis of the human and the macaque frontal cortex. In: Boller F, Spinnler H, editors. Handbook of Neuropsychology. Vol. 9. Elsevier Science; Amsterdam: 1994. pp. 17–58. [Google Scholar]
- Petrides M, Pandya DN. Dorsolateral prefrontal cortex: comparative cytoarchitectonic analysis in the human and the macaque brain and corticocortical connection patterns. Eur J Neurosci. 1999;11(3):1011–1036. doi: 10.1046/j.1460-9568.1999.00518.x. [DOI] [PubMed] [Google Scholar]
- Phan KL, Taylor SF, Welsh RC, Ho SH, Britton JC, Liberzon I. Neural correlates of individual ratings of emotional salience: a trial-related fMRI study. Neuroimage. 2004;21(2):768–780. doi: 10.1016/j.neuroimage.2003.09.072. [DOI] [PubMed] [Google Scholar]
- Phelps EA, Delgado MR, Nearing KI, LeDoux JE. Extinction learning in humans: role of the amygdala and vmPFC. Neuron. 2004;43(6):897–905. doi: 10.1016/j.neuron.2004.08.042. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception I: The neural basis of normal emotion perception. Biol Psychiatry. 2003;54(5):504–514. doi: 10.1016/s0006-3223(03)00168-9. [DOI] [PubMed] [Google Scholar]
- Ridderinkhof KR, Ullsperger M, Crone EA, Nieuwenhuis S. The role of the medial frontal cortex in cognitive control. Science. 2004;306(5695):443–447. doi: 10.1126/science.1100301. [DOI] [PubMed] [Google Scholar]
- Schmitz TW, Kawahara TN, Johnson SC. Metacognitive evaluation, self-relevance, and the right prefrontal cortex. Neuroimage. 2004;22(2):941–947. doi: 10.1016/j.neuroimage.2004.02.018. [DOI] [PubMed] [Google Scholar]
- Schmitz TW, Rowley HA, Kawahara TN, Johnson SC.(in press). Neural correlates of self-evaluative accuracy after traumatic brain injury Neuropsychologia [DOI] [PubMed] [Google Scholar]
- Seger CA, Stone M, Keenan JP. Cortical Activations during judgments about the self and an other person. Neuropsychologia. 2004;42(9):1168–1177. doi: 10.1016/j.neuropsychologia.2004.02.003. [DOI] [PubMed] [Google Scholar]
- Steele JD, Lawrie SM. Segregation of cognitive and emotional function in the prefrontal cortex: a stereotactic meta-analysis. Neuroimage. 2004;21(3):868–875. doi: 10.1016/j.neuroimage.2003.09.066. [DOI] [PubMed] [Google Scholar]
- Stuss D, Levine B. Adult clinical neuropsychology: lessons from studies of the frontal lobes. Annu Rev Psychol. 2002;53:401–433. doi: 10.1146/annurev.psych.53.100901.135220. [DOI] [PubMed] [Google Scholar]
- Wagar BM, Thagard P. Using computational neuroscience to investigate the neural correlates of cognitive-affective integration during covert decision making. Brain Cogn. 2003;53(2):398–402. doi: 10.1016/s0278-2626(03)00153-2. [DOI] [PubMed] [Google Scholar]
- Zysset S, Huber O, Ferstl E, von Cramon DY. The Anterior Frontomedian Cortex and Evaluative Judgment: An fMRI Study. Neuroimage. 2002;15(4):983–991. doi: 10.1006/nimg.2001.1008. [DOI] [PubMed] [Google Scholar]
- Zysset S, Huber O, Samson A, Ferstl EC, von Cramon DY. Functional specialization within the anterior medial prefrontal cortex: a functional magnetic resonance imaging study with human subjects. Neurosci Lett. 2003;335(3):183–186. doi: 10.1016/s0304-3940(02)01196-5. [DOI] [PubMed] [Google Scholar]