Abstract
This study examined the functionality of the mesial temporal lobe (MTL) and posterior cingulate (PC) in Mild Cognitive Impairment amnestic type (MCI), a syndrome that puts patients at greater risk for developing Alzheimer Disease (AD). Functional MRI (fMRI) was used to identify regions normally active during encoding of novel items and recognition of previously learned items in a reference group of 77 healthy young and middle-aged adults. The pattern of activation in this group guided further comparisons between 14 MCI subjects and 14 age-matched controls. The MCI patients exhibited less activity in the PC during recognition of previously learned items, and in the right hippocampus during encoding of novel items, despite comparable task performance to the controls. Reduced fMRI signal change in the MTL supports prior studies implicating the hippocampus for encoding new information. Reduced signal change in the PC converges with recent research on its role in recognition in normal adults as well as metabolic decline in people with genetic or cognitive risk for AD. Our results suggest that a change in function in the PC may account, in part, for memory recollection failure in AD.
1. Introduction
Amnestic mild cognitive impairment (MCI) is a condition in which patients display relative memory impairment beyond typical age-related declines, and unaccounted for by other medical conditions. Memory impairment is among the earliest symptoms of typical Alzheimer Disease (AD), and amnestic MCI is a major risk factor for development of AD [44]. Brain volumetric studies using MRI in MCI have shown reductions in the medial temporal lobe (MTL) [e.g.4, 23] as well as the Posterior Cingulate (PC)[55]. Furthermore, F-18 fluoro-deoxy-glucose (FDG) positron emission tomography (PET) studies show reduced cerebral metabolic rate of glucose (CMRgl) in many of the areas affected by AD including the posterior cingulate (PC) and parietal lobes [40], and this is related to neuropsychological status [10]. Two studies have shown that reduced CMRgl in the PC of MCI patients predicts subsequent decline to AD [11, 16]. An in-vivo PET study of amyloid burden in AD has shown disproportionate aggregation of amyloid in the PC, among other areas [28], indicating that this region may be a primary site for AD neuropathology [29]. This same region of the brain has been found to be active during retrieval or recognition in functional MRI (fMRI) studies with healthy adults [9, 32, 57].
While CMRgl and structural MRI studies have shown some sensitivity to MCI, the use of fMRI has not been widely applied to this disorder. FMRI offers a method of examining memory-associated brain regions while those regions are functionally engaged in memory tasks. A small body of functional imaging studies suggest that the hippocampus is responsive to new information in controls more than MCI or early AD [26, 36, 59, 61]. One study showed that the extent of MTL activity in MCI was correlated with successful encoding, but paradoxically was also correlated with clinical impairment [15]. Some fMRI studies of mild AD and persons at genetic risk for AD have found greater activation associated with disease presence or risk, perhaps reflecting a compensatory response [5, 6, 53]. Further study is needed to resolve the discordant findings. The current study sought to mitigate this discrepancy by utilizing larger samples, a validated episodic memory task, regionally focused statistical analyses to protect against Type 1 error, and higher MRI field strength to increase signal sensitivity.
In the present study, we identify changes in fMRI activation in MCI patients during encoding and recognition. FMRI data were first acquired in a large reference group of healthy adult volunteers. This reference group was used to determine the areas of the brain normally active on the task, and guide the subsequent patient-control comparisons. The large sample size in the reference group provided sufficient statistical power and protection from spurious outliers influencing the functionally defined search regions [13]. The task was a variant of a well-known fMRI paradigm [14, 54, 64] in which participants distinguished between novel and previously learned items. The stimuli were line drawings of common nameable objects. The task was intentionally designed to be very simple, so that persons with memory impairment would be able to perform it at the same level as cognitively intact participants. We expected that the mesial temporal lobe would be more active to novel information [21, 67] and that this would be attenuated in persons with MCI. Given the accumulating findings of PC involvement in recollection in healthy adults [57], we hypothesized that the posterior cingulate would be responsive during recognition, but less so in MCI.
2. Methods
2.1 Participants
Three groups were studied under a protocol approved by the local Institutional Review Board. Written informed consent was obtained from all participants after the procedures had been fully explained.
2.1.1 MCI group
Fourteen subjects (seven men, seven women) with MCI were studied and compared to 14 matched controls (see Table 1). Criteria for MCI included: a) memory complaints, b) relative decrement in the domain of memory and learning, but otherwise normal functioning on a battery of neuropsychological tests, c) intact functional status, d) not demented [45]. Prior to inclusion in this study, the MCI patients were presented to a diagnostic consensus panel for support of the diagnosis. Exclusion criteria consisted of an abnormal anatomic scan, Hachinski score greater than four, prior neurological disease or neurosurgery, prior major psychiatric disorder, chronic major medical conditions such as diabetes, poorly controlled hypertension, or cardiac disease. Eight of the MCI patients were taking cholinesterase inhibitors, but were stable for three months prior to participating in this study. The patients were referred from the several memory disorders clinics at a university-based medical center.
Table 1.
Demographic and Neuropsychological Data.
| MCI | Matched Controls | Reference Group | ||||
|---|---|---|---|---|---|---|
| Avg | SD | Avg | SD | Avg | SD | |
| Age | 73.7 | 6.9 | 72.5 | 5.7 | 43.7 | 14.3 |
| Education | 16.2 | 2.7 | 17.3 | 2.9 | 15.9 | 2.1 |
| Gender | 7m/7f | -- | 7f/7m | -- | 47f / 30m | -- |
| MMSE | 28.6 | 1.5 | 29.4 | 0.84 | -- | -- |
| WRAT-III reading (std score) | 110 | 9.7 | 115 | 4.1 | 108.4 | 6.7 |
| Boston Naming Test (raw) | 53.8 | 6.7 | 57.3 | 1.9 | 58.9 | 1.1 |
| RAVLT raw total (trials 1-5) | 31.6 | 4.6** | 48.0 | 6.8 | -- | -- |
| RAVLT delayed recall (raw) | 2.1 | 1.9 ** | 8.9 | 2.4 | -- | -- |
| BVMT-R raw total (trials 1-3) | 12.6 | 5.7 ** | 25.9 | 5.4 | -- | -- |
| BVMT-R delayed recall (raw) | 5.2 | 2.8 * | 9.4 | 3.6 | -- | -- |
| Trail Making A (seconds) | 38.3 | 16.5 | 32.7 | 6.9 | 27 | 8.2 |
| Trail Making B (seconds) | 100.9 | 45.1 * | 65.4 | 16.5 | 58.8 | 21.3 |
p<.05
p<.001 statistical significance between MCI and matched controls.
MCI = Mild Cognitive Impairment; MMSE=Mini Mental State Exam; WRAT-III = Wide Range Achievement Test-3rdEdition; RAVLT= Rey Auditory Verbal Learning Test; BVMT-R = Brief Visuospatial Memory Test-Revised
2.1.2 Matched Elderly Control Group (EC)
The exclusion criteria for MCI patients were also applied to the EC group. They exhibited neuropsychological evidence of normal cognitive function at the time of the study. The demographic characteristics and neuropsychological data are shown in Table 1. The EC group was recruited from the community, predominantly by advertisement, mailings, and community outreach events.
2.1.3 Reference group
In order to determine the areas of the brain normally active during this task we studied 77 cognitively normal, physically healthy adults recruited from the University of Wisconsin and from the community via advertisement and public outreach events, and through existing registries of healthy adults who had previously expressed willingness to participate in aging research.
2.2 Neuropsychology
The MCI and EC groups received a cognitive battery (see Table 1) using standardized administration [62]. The younger reference group received a subsample of these tests sufficient to verify normal cognitive functioning. These tests and the results are shown in Table 1.
2.3 fMRI task
The task consisted of serial presentation of novel (NV) and previously learned (PL) line drawings obtained from a published set [60]. PL items were acquired in two training sessions 45 minutes and 15 minutes prior to the task. The training set consisted of five items with similar frequency and image complexity as the novel items. The training items were presented repeatedly in pseudorandom fashion for 15 exposures in each of the two training trials for a total of 30 exposures to each item. Participants were instructed to view the training pictures and try to remember them.
During the fMRI scan PL items were intermixed with NV items. A picture was presented every three seconds for the duration of the scanning run. The task was to decide if the picture was previously learned or novel. Items within each condition occurred as trains of events and ranged from a single event to five consecutive items. The variability in epoch length was implemented in order to reduce condition predictability, while preserving the comparatively greater statistical power and shorter duration of boxcar style paradigms [34]. All stimuli in this task were presented for 2800 ms with a 200 ms interstimulus interval. There were no fixation epochs or null events. The subject maintained the same cognitive set throughout the experiment, which was to decide if the item had been studied earlier or whether it was new. Responses to PL and NV items were made with a two-button response device held in the right hand; the first finger was used to identify PL items, the middle finger for NV items. Two iterations of the task were sequentially presented (order was counterbalanced across subjects) using the same PL items, but different NV items. The total task duration was 9 minutes and 24 seconds.
2.4 Scanning procedures
Participants were provided with instruction and practice prior to scanning. They were then situated on the bed of a GE 3.0 Tesla MRI scanner and outfitted with the MR-compatible button-box and a high-resolution goggle system, set at 800 × 600 from Resonance Technology (Northridge, CA, USA). The head was constrained by foam padding. The software Presentation (v .70) was used to deliver visual stimuli, via the goggle system, and record responses.
A T2* gradient-echo, echo-planar imaging (EPI) pulse sequence was used. Higher order shimming was applied to the static magnetic field (B0). The EPI parameters were as follows: echo time = 30 ms; repetition time (TR) = 2000 ms; flip angle = 90 degrees; acquisition matrix = 64 × 64 voxels; field of view = 240 mm. Thirty sagittal slices of brain were acquired within each TR. Voxel resolution was 3.75 × 3.75 × 5 mm (4mm thick slices with a 1 mm skip). A time course of 141 temporal volume images was collected, of which the initial 3 image volumes of each scan were discarded.
Three dimensional field maps across the brain (co-planar with the fMRI slices) were acquired on each subject by measuring the phase of non-EPI gradient echo images at two echo times (7 and 10 ms). The phase difference between the two echo images is proportional to the static field inhomogeneity [25]. The warp correction was performed using custom software developed in Matlab. A 3D phase-unwrapping algorithm [based on an algorithm developed by Jenkinson, 24] was used, and the corrected images were written out using a nonlinear pixel shifting and B-splines interpolation algorithm.
Following the functional scans and field mapping, a T1 weighted inversion recovery prepared volume and T2 weighted anatomic images were acquired and later viewed by a neuroradiologist for abnormalities that were inconsistent with the diagnosis and/or requiring clinical follow-up.
2.5 Image processing and statistics
The 4D time-series was motion-corrected (using Statistical Parametric Mapping software; SPM2). The field map from each subject was then applied to the time series. This was followed by spatial normalization into a standard atlas space (using the T2* weighted template in Montreal standard space provided with SPM2), and spatial smoothing to 8 mm. To estimate single subject activations, the analysis employed the canonical hemodynamic response function, high frequency signal filtering (high pass filter = 128 seconds) and the AR1 method of estimating temporal autocorrelation. The contrasts NV>PL and PL>NV were computed for each subject and subsequently entered into second-level random effects analyses. One-sample t-tests were computed on the reference group in order to determine regions normally active. MCI patients were compared to age-matched controls using a two-sample t-test in a manner that was constrained to only include those regions active in the reference group for the relevant contrast. This was accomplished by using the reference group activation (False Discovery Rate, FDR thresholding p<.05) as an explicit mask in the two-sample t-test models comparing MCIs to their controls (at an uncorrected threshold of p<.01 within the small volume of the mask).
3. Results
3.1 Behavioral results
Accuracy and reaction times are presented in Table 2. Analysis of variance and post-hoc comparisons revealed that all groups performed this task with accuracy above 96%. Importantly, the MCI and EC group did not differ on overall accuracy or reaction time to NV and PL items. However, compared to both the MCI and EC groups, the reference group performed at significantly higher accuracy for both the NV and PL conditions, and exhibited significantly faster response times for the NV condition (p<.05). All groups had equivalent response times for the PL condition. For all three groups the reaction time to PL items was longer than reaction time to NV items (p<.05).
Table 2.
Task Behavioral Data.
| MCI | Matched Controls |
Reference Group |
||||
|---|---|---|---|---|---|---|
| Avg | SD | Avg | SD | Avg | SD | |
| Reaction Time- Learned items (seconds) | 0.915 | 0.13 | 0.924 | 0.20 | 0.921 | 0.19 |
| Reaction time-Novel items** (seconds) | 0.834* | 0.11 | 0.848 * | 0.21 | 0.758 | 0.12 |
| Accuracy | 97% | 1.8 | 96%* | 1.10 | 99% | 0.5 |
Significantly different from reference group, p<.05.
Within all groups, response times to NV were significantly faster than PL items, p<.05.
3.2 Imaging results: reference group
Figure 1 depicts the cerebral response during encoding of NV items relative to PL items, and Figure 2 the response to PL items versus NV items in the reference group. Significant signal change (activation) to NV items was present in the ventral temporal lobe and anteriorly in the MTL including the hippocampus. The relative response during recognition of PL items was seen in the PC, precuneus, lateral parietal lobe (right more than left), right lateral temporal lobe and right prefrontal cortex. Table 3 contains the coordinates and statistics for these regions.
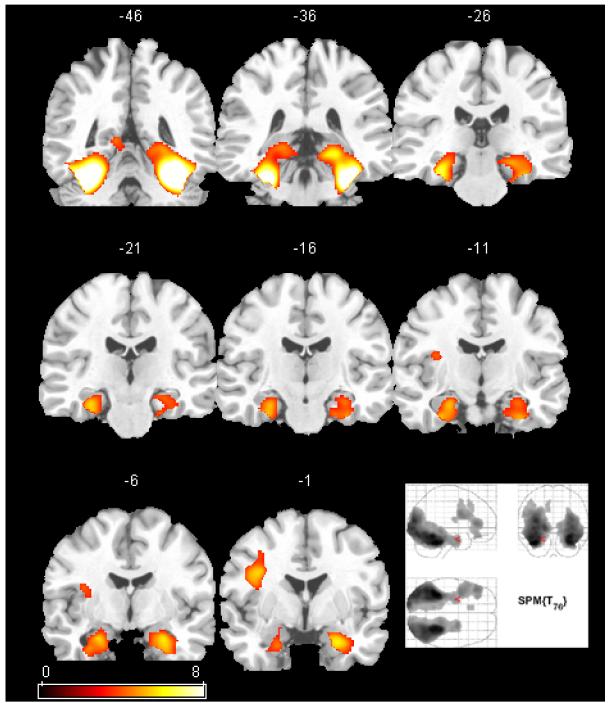
Figure 1.
The SPM(t) map of the cerebral response to novel line drawings versus previously learned drawings in 77 healthy adults (reference group). The map is thresholded using FDR correction (p<0.01). A selection of coronal views of an atlas brain are shown with y-values of the coordinate system shown above each slice (left is on left). The maximum intensity projection of the same map is shown in the bottom right. Activation is strongest in the ventral temporal lobes extending into the mesial temporal lobes bilaterally. The left lateral frontal lobe was also active.
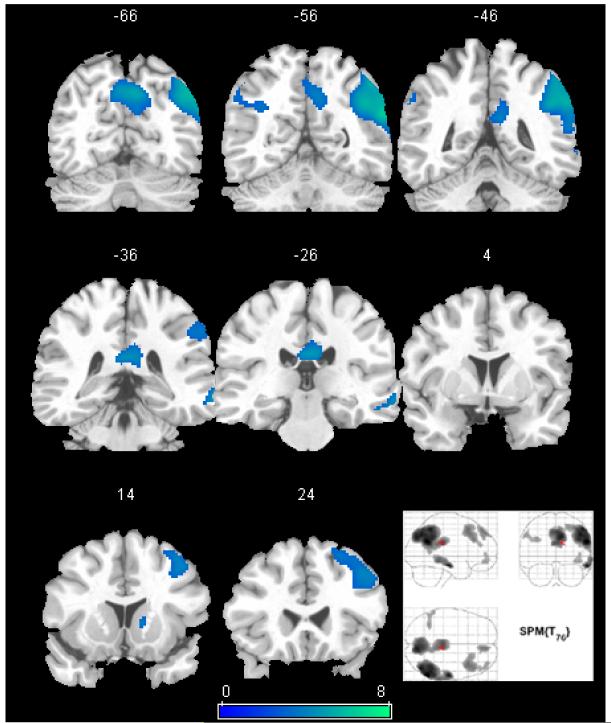
Figure 2.
The SPM(t) map of the cerebral response to recognition of previously learned line drawings versus novel items in the reference group. The map is thresholded using FDR correction (p<0.05). A selection of coronal views of the MNI standard atlas brain are shown with y-values of the coordinate system shown above each slice (left is on left). The maximum intensity projection of the same map is shown in the bottom right. Activations are visualized in the posterior cingulate and precuneus, as well as the right lateral parietal, frontal, and temporal lobes.
Table 3.
Statistics and voxel locations of activated areas in the normative group.
| t-value | p(FDR) | x,y,z (mm) | Description |
|---|---|---|---|
| Novel items | |||
| 13.2 | <.001 | -34 -44 -28 | Left ventral temporal lobe |
| 12.4 | <.001 | 30 -44 -26 | Right ventral temporal lobe |
| 6.95 | <.001 | -44 34 8 | Left lateral frontal lobe |
| 6.43 | <.001 | 24 -4 -24 | Right anterior hippocampus |
| 5.77 | <.001 | -25 -8 -22 | Left anterior hippocampus |
| 3.98 | .001 | -12 22 46 | Left anterior cingulate |
| Familiar Items | |||
| 6.58 | <.001 | 58 -58 28 | Right lateral parietal lobe |
| 5.99 | <.001 | 58 -18 -18 | Right lateral temporal lobe |
| 5.90 | <.001 | 8 -76 36 | Precuneus |
| 5.00 | <.001 | 2 -28 26 | Posterior cingulate |
| 4.43 | .001 | 36 18 52 | Right frontal lobe |
| 4.29 | .002 | 8 52 54 | Right medial prefrontal |
| 3.64 | .01 | -36 -54 32 | Left lateral parietal lobe |
Because the reference group recognition findings were lateralized to the right, a secondary analysis was performed to determine if the right hemisphere activity was statistically greater than the left. This was accomplished by extracting a spherical region of interest (ROI) with a 2 mm radius at the maxima of right side clusters (see Table 3 for locations) in the right parietal lobe, right lateral temporal lobe, right posterior cingulate and right lateral frontal lobe; homologous ROIs were also extracted from the left side. Paired t-tests were performed between right and left ROIs, each indicating right lateralized activity (p values ranged from .046 for the right PC to .00001 for the right parietal lobe).
3.3 Imaging results: elderly controls versus MCI
In the EC > MCI comparison of the cerebral response to NV versus PL items, EC demonstrated significantly greater signal change than MCI in the right hippocampal head and body (Figure 3). Other significant areas included the left lateral frontal lobe, and the right inferior temporal lobe (see Table 4). In the group comparison to PL items, EC demonstrated significantly more signal change than MCI in the right PC/precuneus. There were no regions where MCI activated more than EC in either contrast.
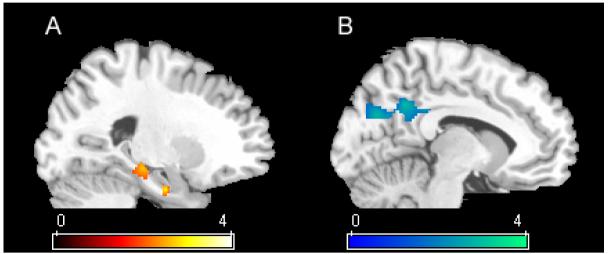
Figure 3.
Areas of significance in two sample t-tests where age-matched controls are significantly more active than patients with MCI on the two comparisons. A) Novel items: The hippocampus is significantly more active in controls than MCI. B) Previously learned items: the posterior cingulate and precuneus are more active in controls during recognition. For each of these comparisons, the reference group was used to constrain the analysis to only those regions significantly active in the reference group (FDR p<.05).
Table 4.
Statistics and voxel locations of areas more active in elderly controls versus MCI.
| t-value | p (unc) | x,y,z (mm) | Description |
|---|---|---|---|
| Novel items | |||
| 3.95 | <.001 | -54 22 30 | Left lateral frontal lobe |
| 2.64 | .007 | 40 -68 -24 | Right ventral temporal lobe |
| 2.61 | .007 | 22 -8 -26 | Right anterior hippocampus |
| 2.51 | .009 | 24 -26 -10 | Right hippocampus |
| Previously Learned Items | |||
| 3.57 | .001 | 16 -72 28 | Precuneus |
| 3.17 | .002 | 12 -52 16 | Posterior cingulate |
4. Discussion
In a healthy sample of young and middle aged adults, the task presented here evoked a cerebral response in many of the same areas that are involved in AD, including the hippocampus during encoding of NV items; and posterior cingulate as well as medial and lateral parietal lobes during recognition of PL items. There was significant rightward laterality in the normal reference group during recognition of PL items, likely reflecting the visuospatial and feature perception demands of the task. The EC group exhibited statistically greater relative signal change in the right hippocampus than MCI to NV items, and greater relative signal change in the PC during recognition of PL items. Importantly, the behavioral data was equivalent, indicating comparable task difficulty in the elderly groups. However, the younger reference group had faster reaction times to novel items; this may have been an aging effect of processing speed [52].
4.1 Greater activation in controls
Many functional imaging studies of the cerebral response to encoding novel information have found MTL activity [30, 54, 69] and at least three studies have found reduced activity in the MTL in MCI during encoding of new information using conceptually similar paradigms [26, 36, 59]. A fourth study [61] found reduced activity in the MTL for elderly controls compared to young adult controls, and reduced activity in mild AD patients versus elderly controls. However, two studies comparing carriers to non-carriers of the APOE e4 allele (a gene that increases susceptibility to AD) have shown greater activity in persons with greater AD risk [5, 6]. One longitudinal study [15] consisting only of mild MCI patients examined signal change to novel scenes versus repeating scenes. MCI subjects who exhibited more activated voxels in the MTL to novel items also scored the highest on a measure of encoding success. Paradoxically, that study also found a relationship between the number of activated MTL voxels and level of overall impairment. Further, they found that subsequent conversion to AD over a 2.5 year follow up was also significantly related to the number of voxels activated in the MTL. The findings from that correlational study [15] are not directly comparable to the present report and prior studies because it did not contain a control group and thus it is not known whether the MCI group as a whole had more or less MTL activity than controls.
There are several fundamental differences in design between the current report and the aforementioned studies. First, the stimuli were less predictable than the aforementioned reports. Novel and previously learned items were intermixed pseudorandomly in trains of events ranging from a single item up to five consecutive items of the same condition, and there was no warning/indication of what item type the subject would see next. The cognitive set was thus constant throughout the scan (deciding if each object was old or new). Other reports [e.g. 5, 15] presented novel and repeating/familiar items in discrete blocks such that item type was quite predictable. Second, the task design of the present study allowed us to assess reaction time of the old-new decisions. There were no differences in reaction time or accuracy between the EC and MCI groups, indicating that the task was equated on difficulty level. Other reports with at—risk groups have not always been designed to ensure that cognitive effort and other task processes were equated between patients and controls. This is a vital experimental component. A group by region effect is difficult to interpret in the presence of differences in cognitive effort/task difficulty. Third, we constrained our patient control comparisons to only those regions found to be typically active in a large younger cohort. This method reduced the number of voxel-wise comparisons and limited the search region to task-related structures. The method prevented us from examining age-related or disease-related neural compensatory effects outside of the typically activated structures. However, given the number of voxel-wise comparisons in this and other fMRI studies and the limited number of subjects in the patient and control groups, conducting whole brain comparisons would have further raised the risk of false positive (Type I) statistical error. While studies of compensatory neural processes (e.g. neurotrophic change) in disease groups are sorely needed, conclusions regarding compensatory processes will require greater numbers of subjects in patient and control groups to effectively address the multiple comparison problem, and will need to use tasks that are cognitively equivalent between groups as discussed above. Studies of cognitive compensation (including parametrically manipulating mnemonic and attentional strategies) are also needed to fully understand aging and disease effects on fMRI signal.
Like the MTL, the right ventral temporal lobe was also differentially more active to NV items in the controls. This region is consistently implicated in object identification [27]. The reference group demonstrated robust signal change in this region to novel relative to previously learned objects, indicating that efficient semantic and perceptual representations of the previously learned objects were formed. Event-related potentials in the inferior temporal lobes of nonhuman primates show reduced amplitude when the same stimulus is viewed again [51]. This stimulus specific adaptation has generally been interpreted as a learning effect [3, 8, 12, 22, 50, 70]. The finding of differentially less signal change in this region in the MCI group (but generally intact naming ability as measured by the Boston Naming Test) may suggest decreased efficiency of the ventral temporal system in learning or maintaining perceptual representations of the previously learned items.
The left lateral frontal lobe near Broca’s area was also differentially responsive to novel items in the EC group. The involvement of this region in the controls is likely due to semantic processes associated with identifying and silently naming the novel objects more so than the previously identified and learned objects. It may be that the MCIs employed object naming processes to both novel and previously learned items. In light of the above discussion regarding task factors, we attribute this effect to differential cognitive processes rather than specific disease related effects in Broca’s area.
4.2 The role of the PC in memory
While the MTL has long been known to support formation of new memories, the role of the PC in memory [46, 57] and related self referential processes [43] are more recent. As part of the limbic system, the PC has reciprocal connections with other memory areas including the dorsomedial and dorsolateral prefrontal cortex, the posterior parahippocampal cortex, presubiculum, hippocampus, entorhinal cortex, and thalamus [17, 41]. Its role in recollection is supported not only by its functional anatomy and connectivity with other memory structures, but also by lesion studies [e.g. 68]. Studies of episodic memory implicate the PC in successful retrieval across a multitude of stimulus types and various presentation modalities. For example, PC activation is elicited during recognition of sounds [57], objects and pictures [57, 71], buildings [38], dot patterns [47], and thematic narrative information [39] learned during training sessions within the laboratory. Furthermore, the PC activation is also elicited during recognition of visual and auditory materials drawn from a person’s daily life. When individuals are presented with names of friends and family [37], faces and voices of friends and family [56], famous faces [31], personal belongings [31, 65], familiar places [65], and personal life events [1, 33], PC activation is more pronounced than when viewing similarly constructed, but novel materials.
PC hypometabolism and blood flow are frequent findings in mild AD [42] and MCI [66], and in people with genetic risk for AD [48, 49, 58]. FMRI and resting PET studies in normal volunteers find that the PC is active during low level baselines relative to goal directed tasks [19, 20], but less so in early AD [35]. The findings that the PC is adversely affected very early on in AD, and also that this region is relatively more active during retrieval or recognition in the healthy brain, lend support to the idea that dysfunction in this region primarily contributes to memory retrieval difficulty in persons with MCI and mild AD. This may be due to loss of afferent connections from the mesial temporal lobe, as well as amyloid burden in the PC region [7, 28]. At least one study [49] suggests that PC dysfunction may begin as much as four decades before typical AD onset. The time course and mechanism of cognitive decline and neural activity in the PC and MTL requires further study.
4.3 Design issues and limitations
There were some limitations to this study that need to be recognized. Eight of the MCIs were taking stable doses of cholinesterase inhibitors at the time of the study and this may have affected their fMRI response. This difference would have the potential effect of improving cognitive function in the MCIs. Thus, the group differences observed were above and beyond any salutary effects the medications may have had. Both elderly groups were highly educated and this may limit generalizability to the general population. Also, although we collected response times and accuracy of decisions in the scanner, we did not employ recognition testing after the scan to test how well the novel items were encoded and subsequently recognized. This might have provided a more accurate marker of encoding success and would have undoubtedly enhanced the design and the conclusions drawn.
We note that this experiment did not have a third baseline condition to which the NV and PL conditions could be independently compared. We chose to use only two conditions in order to keep the task simple and the scan length short for use with cognitively impaired older patients. The choice of baseline is critical for interpreting fMRI experiments as illustrated by Stark and Squire [63]. Low-level baselines such as rest or visual fixation have the unintentional effect of reduced experimenter control over the cognitive state of the participant, and may actually evoke PC activation due to increased task independent selfreferential cognition [18, 20]. For these reasons, a low-level baseline was avoided.
The analysis strategy of this study was to restrict the patient-control comparisons only to regions that are normally responsive on the task in a reference group of young and middle-aged adults. This approach focused the analysis to task-specific memory relevant areas of the brain, and helped avoid the possibility of invalid inferences regarding false-positive errors. The disadvantage of restricting the analysis in this manner is that it does not allow inferences regarding compensatory neural processes that might be occurring elsewhere in the brain outside the empirically defined system of interest. In view of the small sample size of the patients and use of uncorrected p-values in the patient-control comparison, it seemed prudent to minimize the possibility of Type 1 error.
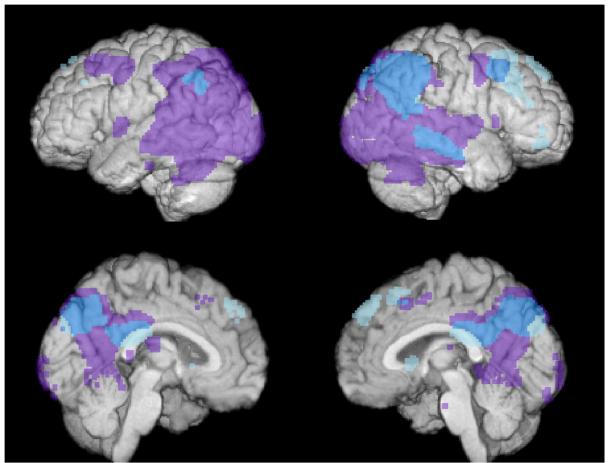
Figure 4.
Surface render of brain activation in the reference group during recognition (cyan and light blue). The result is superimposed over data from Alexander et al. 2002 showing regions that are hypometabolic in 14 AD patients compared to 34 age-matched controls (purple). See reference [2] for details on the AD findings and PET methodology. This figure illustrates areas of overlap (shown in cyan blue) between recognition in healthy adults and hypometabolism in AD, particularly in the medial and lateral parietal lobes and posterior cingulate.
Acknowledgement
This study was supported by R01 AG21155 (SCJ). The helpful expertise of the faculty and staff at the Waisman Center for Brain Imaging and Behavior at the University of Wisconsin—Madison is greatly appreciated.
References
- 1.Addis DR, Moscovitch M, Crawley AP, McAndrews MP. Recollective qualities modulate hippocampal activation during autobiographical memory retrieval. Hippocampus. 2004;14(6):752–62. doi: 10.1002/hipo.10215. [DOI] [PubMed] [Google Scholar]
- 2.Alexander GE, Chen K, Pietrini P, Rapoport SI, Reiman EM. Longitudinal PET Evaluation of Cerebral Metabolic Decline in Dementia: A Potential Outcome Measure in Alzheimer’s Disease Treatment Studies. Am J Psychiatry. 2002;159(5):738–45. doi: 10.1176/appi.ajp.159.5.738. [DOI] [PubMed] [Google Scholar]
- 3.Baylis GC, Rolls ET. Responses of neurons in the inferior temporal cortex in short term and serial recognition memory tasks. Exp Brain Res. 1987;65(3):614–22. doi: 10.1007/BF00235984. [DOI] [PubMed] [Google Scholar]
- 4.Bigler ED, Tate DF, Miller MJ, Rice SA, Hessel CD, Earl HD, Tschanz JT, Plassman B, Welsh-Bohmer KA. Dementia, asymmetry of temporal lobe structures, and apolipoprotein E genotype: relationships to cerebral atrophy and neuropsychological impairment. J Int Neuropsychol Soc. 2002;8(7):925–33. doi: 10.1017/s1355617702870072. [DOI] [PubMed] [Google Scholar]
- 5.Bondi MW, Houston WS, Eyler LT, Brown GG. fMRI evidence of compensatory mechanisms in older adults at genetic risk for Alzheimer disease. Neurology. 2005;64(3):501–8. doi: 10.1212/01.WNL.0000150885.00929.7E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bookheimer SY, Strojwas MH, Cohen MS, Saunders AM, Pericak-Vance MA, Mazziotta JC, Small GW. Patterns of brain activation in people at risk for Alzheimer’s disease. N Engl J Med. 2000;343(7):450–6. doi: 10.1056/NEJM200008173430701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bradley KM, O’Sullivan VT, Soper ND, Nagy Z, King EM, Smith AD, Shepstone BJ. Cerebral perfusion SPET correlated with Braak pathological stage in Alzheimer’s disease. Brain. 2002;125(Pt 8):1772–81. doi: 10.1093/brain/awf185. [DOI] [PubMed] [Google Scholar]
- 8.Brown MW, Wilson FA, Riches IP. Neuronal evidence that inferomedial temporal cortex is more important than hippocampus in certain processes underlying recognition memory. Brain Res. 1987;409(1):158–62. doi: 10.1016/0006-8993(87)90753-0. [DOI] [PubMed] [Google Scholar]
- 9.Buckner RL. Memory and executive function in aging and AD: multiple factors that cause decline and reserve factors that compensate. Neuron. 2004;44(1):195–208. doi: 10.1016/j.neuron.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 10.Chetelat G, Desgranges B, de la Sayette V, Viader F, Berkouk K, Landeau B, Lalevee C, Le Doze F, Dupuy B, Hannequin D, Baron JC, Eustache F. Dissociating atrophy and hypometabolism impact on episodic memory in mild cognitive impairment. Brain. 2003;126(Pt 9):1955–67. doi: 10.1093/brain/awg196. [DOI] [PubMed] [Google Scholar]
- 11.Chetelat G, Desgranges B, De La Sayette V, Viader F, Eustache F, Baron JC. Mild cognitive impairment: Can FDG-PET predict who is to rapidly convert to Alzheimer’s disease? Neurology. 2003;60(8):1374–7. doi: 10.1212/01.wnl.0000055847.17752.e6. [DOI] [PubMed] [Google Scholar]
- 12.Desimone R. Neural mechanisms for visual memory and their role in attention. Proc Natl Acad Sci U S A. 1996;93(24):13494–9. doi: 10.1073/pnas.93.24.13494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Desmond JE, Glover GH. Estimating sample size in functional MRI (fMRI) neuroimaging studies: statistical power analyses. J Neurosci Methods. 2002;118(2):115–28. doi: 10.1016/s0165-0270(02)00121-8. [DOI] [PubMed] [Google Scholar]
- 14.Detre JA, Maccotta L, King D, Alsop DC, Glosser G, D’Esposito M, Zarahn E, Aguirre GK, French JA. Functional MRI lateralization of memory in temporal lobe epilepsy. Neurology. 1998;50(4):926–32. doi: 10.1212/wnl.50.4.926. [DOI] [PubMed] [Google Scholar]
- 15.Dickerson BC, Salat DH, Bates JF, Atiya M, Killiany RJ, Greve DN, Dale AM, Stern CE, Blacker D, Albert MS, Sperling RA. Medial temporal lobe function and structure in mild cognitive impairment. Ann Neurol. 2004;56(1):27–35. doi: 10.1002/ana.20163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Drzezga A, Lautenschlager N, Siebner H, Riemenschneider M, Willoch F, Minoshima S, Schwaiger M, Kurz A. Cerebral metabolic changes accompanying conversion of mild cognitive impairment into Alzheimer’s disease: a PET follow-up study. Eur J Nucl Med Mol Imaging. 2003;30(8):1104–13. doi: 10.1007/s00259-003-1194-1. [DOI] [PubMed] [Google Scholar]
- 17.Duvernoy H. The Human Hippocampus. 2nd Springer-Verlag; Berlin: 1998. [Google Scholar]
- 18.Greicius MD, Menon V. Default-Mode Activity during a Passive Sensory Task: Uncoupled from Deactivation but Impacting Activation. J Cogn Neurosci. 2004;16(9):1484–92. doi: 10.1162/0898929042568532. [DOI] [PubMed] [Google Scholar]
- 19.Greicius MD, Srivastava G, Reiss AL, Menon V. Default-mode network activity distinguishes Alzheimer’s disease from healthy aging: evidence from functional MRI. Proc Natl Acad Sci U S A. 2004;101(13):4637–42. doi: 10.1073/pnas.0308627101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gusnard DA, Raichle ME. Searching for a baseline: functional imaging and the resting human brain. Nat Rev Neurosci. 2001;2(10):685–94. doi: 10.1038/35094500. [DOI] [PubMed] [Google Scholar]
- 21.Habib R, McIntosh AR, Wheeler MA, Tulving E. Memory encoding and hippocampally-based novelty/familiarity discrimination networks. Neuropsychologia. 2003;41(3):271–9. doi: 10.1016/s0028-3932(02)00160-4. [DOI] [PubMed] [Google Scholar]
- 22.Henson RN, Rugg MD. Neural response suppression, haemodynamic repetition effects, and behavioural priming. Neuropsychologia. 2003;41(3):263–70. doi: 10.1016/s0028-3932(02)00159-8. [DOI] [PubMed] [Google Scholar]
- 23.Jack CR, Petersen RC, Xu YC, O’Brien PC, Smith GE, Ivnik RJ, Boeve BF, Waring SC, Tangalos EG, Kokmen E. Prediction of AD with MRI-based hippocampal volume in mild cognitive impairment. Neurology. 1999;52(7):1397–403. doi: 10.1212/wnl.52.7.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jenkinson M. Fast, automated, N-dimensional phase-unwrapping algorithm. Magn Reson Med. 2003;49(1):193–7. doi: 10.1002/mrm.10354. [DOI] [PubMed] [Google Scholar]
- 25.Jezzard P, Balaban RS. Correction for geometric distortion in echo planar images from B0 field variations. Magn Reson Med. 1995;34(1):65–73. doi: 10.1002/mrm.1910340111. [DOI] [PubMed] [Google Scholar]
- 26.Johnson S, Baxter L, Susskind-Wilder L, Connor DJ, Sabbagh MN, Caselli RJ. Hippocampal adaptation to face repetition in healthy elderly and mild cognitive impairment. Neuropsychologia. 2004;42(7):980–9. doi: 10.1016/j.neuropsychologia.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 27.Joseph JE. Functional neuroimaging studies of category specificity in object recognition: a critical review and meta-analysis. Cogn Affect Behav Neurosci. 2001;1(2):119–36. doi: 10.3758/cabn.1.2.119. [DOI] [PubMed] [Google Scholar]
- 28.Klunk WE, Engler H, Nordberg A, Wang Y, Blomqvist G, Holt DP, Bergstrom M, Savitcheva I, Huang GF, Estrada S, Ausen B, Debnath ML, Barletta J, Price JC, Sandell J, Lopresti BJ, Wall A, Koivisto P, Antoni G, Mathis CA, Langstrom B. Imaging brain amyloid in Alzheimer’s disease with Pittsburgh Compound-B. Ann Neurol. 2004;55(3):306–19. doi: 10.1002/ana.20009. [DOI] [PubMed] [Google Scholar]
- 29.Kobayashi K, Hayashi M, Nakano H, Shimazaki M, Sugimori K, Koshino Y. Correlation between astrocyte apoptosis and Alzheimer changes in gray matter lesions in Alzheimer’s disease. J Alzheimers Dis. 2004;6(6):623–32. doi: 10.3233/jad-2004-6606. [DOI] [PubMed] [Google Scholar]
- 30.LePage M, Habib R, Tulving E. Hippocampal PET activations of memory encoding and retrieval: The HIPER model. Hippocampus. 1998;8:313–322. doi: 10.1002/(SICI)1098-1063(1998)8:4<313::AID-HIPO1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 31.Leveroni CL, Seidenberg M, Mayer AR, Mead LA, Binder JR, Rao SM. Neural systems underlying the recognition of familiar and newly learned faces. J Neurosci. 2000;20(2):878–86. doi: 10.1523/JNEUROSCI.20-02-00878.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Levine B, Turner G, Tisserand D, Hevenor S, Graham S, McIntosh A. The functional neuroanatomy of episodic and semantic autobiographical remembering: A prospective functional MRI study. Journal of Cognitive Neuroscience. 2004;16(9):1633–1646. doi: 10.1162/0898929042568587. [DOI] [PubMed] [Google Scholar]
- 33.Levine B, Turner GR, Tisserand D, Hevenor SJ, Graham SJ, McIntosh AR. The functional neuroanatomy of episodic and semantic autobiographical remembering: a prospective functional MRI study. J Cogn Neurosci. 2004;16(9):1633–46. doi: 10.1162/0898929042568587. [DOI] [PubMed] [Google Scholar]
- 34.Liu TT, Frank LR, Wong EC, Buxton RB. Detection power, estimation efficiency, and predictability in event-related fMRI. Neuroimage. 2001;13(4):759–73. doi: 10.1006/nimg.2000.0728. [DOI] [PubMed] [Google Scholar]
- 35.Lustig C, Snyder AZ, Bhakta M, O’Brien KC, McAvoy M, Raichle ME, Morris JC, Buckner RL. Functional deactivations: change with age and dementia of the Alzheimer type. Proc Natl Acad Sci U S A. 2003;100(24):14504–9. doi: 10.1073/pnas.2235925100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Machulda MM, Ward HA, Borowski B, Gunter JL, Cha RH, O’Brien PC, Petersen RC, Boeve BF, Knopman D, Tang-Wai DF, Ivnik RJ, Smith GE, Tangalos EG, Jack CR., Jr. Comparison of memory fMRI response among normal, MCI, and Alzheimer’s patients. Neurology. 2003;61(4):500–506. doi: 10.1212/01.wnl.0000079052.01016.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maddock RJ, Garrett AS, Buonocore MH. Remembering familiar people: the posterior cingulate cortex and autobiographical memory retrieval. Neuroscience. 2001;104(3):667–76. doi: 10.1016/s0306-4522(01)00108-7. [DOI] [PubMed] [Google Scholar]
- 38.Maguire EA, Frith CD, Cipolotti L. Distinct neural systems for the encoding and recognition of topography and faces. Neuroimage. 2001;13(4):743–50. doi: 10.1006/nimg.2000.0712. [DOI] [PubMed] [Google Scholar]
- 39.Maguire EA, Frith CD, Morris RG. The functional neuroanatomy of comprehension and memory: the importance of prior knowledge. Brain. 1999;122(Pt 10):1839–50. doi: 10.1093/brain/122.10.1839. [DOI] [PubMed] [Google Scholar]
- 40.Matsuda H. Cerebral blood flow and metabolic abnormalities in Alzheimer’s disease. Ann Nucl Med. 2001;15(2):85–92. doi: 10.1007/BF02988596. [DOI] [PubMed] [Google Scholar]
- 41.Mesulam M-M. Principles of Behavioral and Cognitive Neurology. Oxford; New York: 2000. Behavioral neuroanatomy: Large scale networks, association cortex, frontal syndromes, the limbic system, and hemispheric specializations; pp. 1–173. [Google Scholar]
- 42.Minoshima S, Foster NL, Kuhl DE. Posterior cingulate cortex in Alzheimer’s disease. Lancet. 1994;344(8926):895. doi: 10.1016/s0140-6736(94)92871-1. [DOI] [PubMed] [Google Scholar]
- 43.Northoff G, Bermpohl F. Cortical midline structures and the self. Trends Cogn Sci. 2004;8(3):102–107. doi: 10.1016/j.tics.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 44.Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256(3):183–94. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- 45.Petersen RC, Doody R, Kurz A, Mohs RC, Morris JC, Rabins PV, Ritchie K, Rossor M, Thal L, Winblad B. Current concepts in mild cognitive impairment. Arch Neurol. 2001;58(12):1985–92. doi: 10.1001/archneur.58.12.1985. [DOI] [PubMed] [Google Scholar]
- 46.Piefke M, Weiss PH, Zilles K, Markowitsch HJ, Fink GR. Differential remoteness and emotional tone modulate the neural correlates of autobiographical memory. Brain. 2003;126(Pt 3):650–68. doi: 10.1093/brain/awg064. [DOI] [PubMed] [Google Scholar]
- 47.Reber PJ, Wong EC, Buxton RB. Comparing the brain areas supporting nondeclarative categorization and recognition memory. Brain Res Cogn Brain Res. 2002;14(2):245–57. doi: 10.1016/s0926-6410(02)00122-2. [DOI] [PubMed] [Google Scholar]
- 48.Reiman EM, Caselli RJ, Yun LS, Chen K, Bandy D, Minoshima S, Thibodeau SN, Osborne D. Preclinical evidence of Alzheimer’s disease in persons homozygous for the epsilon 4 allele for apolipoprotein E. N Engl J Med. 1996;334(12):752–8. doi: 10.1056/NEJM199603213341202. [DOI] [PubMed] [Google Scholar]
- 49.Reiman EM, Chen K, Alexander GE, Caselli RJ, Bandy D, Osborne D, Saunders AM, Hardy J. Functional brain abnormalities in young adults at genetic risk for late-onset Alzheimer’s dementia. Proc Natl Acad Sci U S A. 2004;101(1):284–9. doi: 10.1073/pnas.2635903100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Riches IP, Wilson FA, Brown MW. The effects of visual stimulation and memory on neurons of the hippocampal formation and the neighboring parahippocampal gyrus and inferior temporal cortex of the primate. J Neurosci. 1991;11(6):1763–79. doi: 10.1523/JNEUROSCI.11-06-01763.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ringo JL. Stimulus specific adaptation in inferior temporal and medial temporal cortex of the monkey. Behav Brain Res. 1996;76(12):191–7. doi: 10.1016/0166-4328(95)00197-2. [DOI] [PubMed] [Google Scholar]
- 52.Salthouse TA. General and specific speed mediation of adult age differences in memory. J Gerontol B Psychol Sci Soc Sci. 1996;51(1):30–42. doi: 10.1093/geronb/51b.1.p30. [DOI] [PubMed] [Google Scholar]
- 53.Saykin AJ, Flashman LA, Frutiger S, Johnson SC, Mamourian AC, Moritz CH, O’Jile JR, Riordan HJ, Santulli RB, Smith CA, Weaver JB. Neuroanatomic substrates of semantic memory impairment in Alzheimer’s disease: Patterns of functional MRI activation. Journal of the International Neuropsychological Society. 1999;5:377–392. doi: 10.1017/s135561779955501x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Saykin AJ, Johnson SC, Flashman LA, McAllister TW, Sparling MB, Darcey TM, Moritz CH, Guerin SJ, Weaver JB, Mamourian A. Functional differentiation of medial temporal and frontal regions involved in processing novel and familiar words: An fMRI study. Brain. 1999;122:1963–1971. doi: 10.1093/brain/122.10.1963. [DOI] [PubMed] [Google Scholar]
- 55.Scahill RI, Schott JM, Stevens JM, Rossor MN, Fox NC. Mapping the evolution of regional atrophy in Alzheimer’s disease: unbiased analysis of fluidregistered serial MRI. Proc Natl Acad Sci U S A. 2002;99(7):4703–7. doi: 10.1073/pnas.052587399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shah NJ, Marshall JC, Zafiris O, Schwab A, Zilles K, Markowitsch HJ, Fink GR. The neural correlates of person familiarity. A functional magnetic resonance imaging study with clinical implications. Brain. 2001;124(Pt 4):804–15. doi: 10.1093/brain/124.4.804. [DOI] [PubMed] [Google Scholar]
- 57.Shannon BJ, Buckner RL. Functional-anatomic correlates of memory retrieval that suggest nontraditional processing roles for multiple distinct regions within posterior parietal cortex. J Neurosci. 2004;24(45):10084–92. doi: 10.1523/JNEUROSCI.2625-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Small G, Ercoli LM, Silverman DH, Huang SC, Komo S, Bookheimer SY, Lavretsky H, Miller K, Siddarth P, Rasgon NL, Mazziotta JC, Saxena S, Wu HM, Mega MS, Cummings JL, Saunders AM, Pericak-Vance MA, Roses AD, Barrio JR, Phelps ME. Cerebral metabolic and cognitive decline in persons at genetic risk for Alzheimer’s disease. Proc Natl Acad Sci U S A. 2000;97(11):6037–42. doi: 10.1073/pnas.090106797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Small S, Perera GM, DeLaPaz R, Mayeux R, Stern Y. Differential regional dysfunction of the hippocampal formation among elderly with memory decline and Alzheimer’s disease. Ann Neurol. 1999;45(4):466–72. doi: 10.1002/1531-8249(199904)45:4<466::aid-ana8>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 60.Snodgrass J, Vanderwart M. A standardized set of 260 pictures: Norms for name agreement, image agreement, familiarity, and visual complexity. Journal of Experimental Psychology: Human Learning and Memory. 1980;6(2):174–215. doi: 10.1037//0278-7393.6.2.174. [DOI] [PubMed] [Google Scholar]
- 61.Sperling RA, Bates JF, Chua EF, Cocchiarella AJ, Rentz DM, Rosen BR, Schacter DL, Albert MS. fMRI studies of associative encoding in young and elderly controls and mild Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 2003;74(1):44–50. doi: 10.1136/jnnp.74.1.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Spreen O, Strauss E. A Compendium of Neuropsychological Tests. Oxford; New York: 1998. [Google Scholar]
- 63.Stark CE, Squire LR. When zero is not zero: the problem of ambiguous baseline conditions in fMRI. Proc Natl Acad Sci U S A. 2001;98(22):12760–6. doi: 10.1073/pnas.221462998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stern CE, Corkin S, Gonzalez RG, Guimaraes AR, Baker JR, Jennings PJ, Carr CA, Sugiura RM, Vedantham V, Rosen BR. The hippocampal formation participates in novel picture encoding: evidence from functional magnetic resonance imaging. Proc Natl Acad Sci U S A. 1996;93(16):8660–5. doi: 10.1073/pnas.93.16.8660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sugiura M, Shah NJ, Zilles K, Fink GR. Cortical representations of personally familiar objects and places: functional organization of the human posterior cingulate cortex. Journal of Cognitive Neuroscience. 2005;17(2):183–98. doi: 10.1162/0898929053124956. [DOI] [PubMed] [Google Scholar]
- 66.Tanaka M, Fukuyama H, Yamauchi H, Narita M, Nabatame H, Yokode M, Fujimoto N, Kita T, Murakami M. Regional cerebral blood flow abnormalities in nondemented patients with memory impairment. J Neuroimaging. 2002;12(2):112–8. doi: 10.1111/j.1552-6569.2002.tb00106.x. [DOI] [PubMed] [Google Scholar]
- 67.Tulving E, Markowitsch HJ, Craik FE, Habib R, Houle S. Novelty and familiarity activations in PET studies of memory encoding and retrieval. Cereb Cortex. 1996;6(1):71–9. doi: 10.1093/cercor/6.1.71. [DOI] [PubMed] [Google Scholar]
- 68.Valenstein E, Bowers D, Verfaellie M, Heilman KM, Day A, Watson RT. Retrosplenial amnesia. Brain. 1987;110(Pt 6):1631–46. doi: 10.1093/brain/110.6.1631. [DOI] [PubMed] [Google Scholar]
- 69.Wagner AD, Schacter DL, Rotte M, Koutstaal W, Maril A, Dale AM, Rosen BR, Buckner RL. Building memories: remembering and forgetting of verbal experiences as predicted by brain activity. Science. 1998;281(5380):1188–91. doi: 10.1126/science.281.5380.1188. [DOI] [PubMed] [Google Scholar]
- 70.Wiggs CL, Martin A. Properties and mechanisms of perceptual priming. Current Opinion in Neurobiology. 1998;8(2):227–33. doi: 10.1016/s0959-4388(98)80144-x. [DOI] [PubMed] [Google Scholar]
- 71.Wiggs CL, Weisberg J, Martin A. Neural correlates of semantic and episodic memory retrieval. Neuropsychologia. 1999;37(1):103–18. doi: 10.1016/s0028-3932(98)00044-x. [DOI] [PubMed] [Google Scholar]