Abstract
Neuroimaging research has demonstrated that the posterior cingulate cortex (PCC) is functionally compromised in individuals diagnosed with amnestic Mild Cognitive Impairment (MCI), a major risk factor for the development of Alzheimer’s disease (AD). In functional magnetic resonance imaging (fMRI) studies with healthy participants, this same region is active during self-appraisal (requiring retrieval of semantic knowledge about the self) as well as episodic recognition of recently-learned information. Administering both types of tasks to people with MCI may reveal important information regarding the role of the PCC in recollection. This study investigated fMRI activation in the PCC in individuals with MCI and age, gender, and education-matched controls across two tasks. The first task was a visual episodic recognition task in which participants indicated whether pictures had or had not been presented during a study session. The second task was an autobiographical self-appraisal task in which subjects rated themselves on a set of trait adjectives. Results of a conjunction analysis revealed the PCC as the sole region commonly active during both tasks in the healthy older adults. Furthermore, additional analysis revealed an interaction in the PCC indicating a task-dependent response in the MCI group. MCI participants showed PCC activation during self-appraisal, but not during episodic retrieval. These results suggest in MCI that the PCC shows functional degradation during episodic retrieval of visual information learned in the laboratory. In contrast, the PCC’s role in retrieval and evaluation of highly-elaborated information regarding the self is more well-preserved.
INTRODUCTION
Amnestic mild cognitive impairment (MCI), defined as a marked and selective decline in memory in the context of otherwise normal cognitive and daily functioning, is a major risk factor for Alzheimer’s disease (AD) (Winblad et al., 2004). Older adults with MCI develop AD at an annual rate of approximately 12% compared to 1-2% of cognitively normal adults. Over a 6-year period, approximately 80% of individuals with MCI develop dementia (Petersen, 2004; Petersen et al., 1999).
A number of investigations have described Alzheimer-like brain changes in MCI (Wolf et al., 2003). The posterior cingulate cortex (PCC) is one of several brain regions that shows volumetric and metabolic decline in MCI (Nestor, Fryer, Ikeda, & Hodges, 2003). Furthermore, longitudinal studies indicate that PCC metabolism and regional blood flow discriminate between individuals with MCI who soon develop AD and those who do not (Chetelat, Desgranges, de la Sayette, Viader, Eustache et al., 2003; Huang, Wahlund, Svensson, Winblad, & Julin, 2002; Kogure et al., 2000). In the Chételat et al. study, glucose metabolism in the right lateral temporal cortex and PCC were most predictive of cognitive decline. In another study, Chételat et al. found a strong positive correlation between bilateral PCC metabolism and episodic memory retrieval, a primary domain of cognition affected in MCI (Chetelat, Desgranges, de la Sayette, Viader, Berkouk et al., 2003). Given evidence of compromised PCC metabolism in MCI and its putative link to clinical symptomatology of this condition, it is of interest to more fully explore the roles of the PCC in cognition and the impact of MCI on the integrity of these functions.
Before the advent of functional neuroimaging, it was difficult to assess PCC function in humans, largely due to the rarity of selective pathological lesions in this brain region. However, (Vogt, Finch, & Olson, 1992) proposed a theory of PCC function based on a review of empirical studies of PCC lesions, electrical stimulation, microelectrode recordings, and examination of anatomical connectivity. This theory implicated the PCC in the mediation of “evaluative functions” in which sensory input is associated with information in memory. The PCC’s role in memory is corroborated by a handful of human case reports describing selective PCC lesions and an amnestic syndrome (McDonald, Crosson, Valenstein, & Bowers, 2001; Valenstein et al., 1987; Yasuda, Watanabe, Tanaka, Tadashi, & Akiguchi, 1997). Growing knowledge regarding the PCC’s reciprocal anatomical connectivity with mesial temporal, thalamic, and prefrontal regions further supports the PCC’s role in mnemonic processing (Kobayashi & Amaral, 2003; Morris, Paxinos, & Petrides, 2000; Morris, Petrides, & Pandya, 1999).
Results of functional neuroimaging studies in young, healthy adults provide compelling evidence for the PCC’s involvement in memory retrieval. Studies of episodic memory implicate the PCC in successful retrieval across a multitude of stimulus types and various presentation modalities. For example, PCC activation is elicited during recognition of sounds (Shannon & Buckner, 2004), objects and pictures (Shannon & Buckner, 2004; Wiggs, Weisberg, & Martin, 1999), buildings (Maguire, Frith, & Cipolotti, 2001), dot patterns (Reber, Wong, & Buxton, 2002), and thematic narrative information (Maguire, Frith, & Morris, 1999) learned during training sessions within the laboratory. Furthermore, the PCC activation is also elicited during recognition of visual and auditory materials drawn from a person’s daily life. When individuals are presented with names of friends and family (Maddock, Garrett, & Buonocore, 2001), faces and voices of friends and family (Shah et al., 2001), famous faces (Leveroni et al., 2000), personal belongings (Leveroni et al., 2000; Sugiura, Shah, Zilles, & Fink, 2005), familiar places (Sugiura et al., 2005), and personal life events (Addis, Moscovitch, Crawley, & McAndrews, 2004; Levine et al., 2004), PCC activation is more pronounced than when viewing similarly constructed, but novel materials.
A growing body of work in functional neuroimaging also implicates the PCC in self-referential processing (see reviews by Northoff & Bermpohl, 2004; Vogeley et al., 2001). The anterior medial prefrontal cortex (AMPFC) and PCC have been found to be more active during a self-referential condition requiring subjects to reflect on their own traits than during a baseline condition in which subjects responded to statements requiring semantic knowledge (Johnson et al., 2002). Notably, this pattern of activation was consistent across all eleven participants in this study. In a similar vein, PCC and medial prefrontal activation is reliably elicited when individuals make judgments regarding their own personality traits or emotional state (Fossati et al., 2003; Kelley et al., 2002; Ochsner et al., 2004; Schmitz, Kawahara-Baccus, & Johnson, 2004).
PCC activation during both episodic retrieval and self-referential processing tasks suggests that this structure is not only involved in memory retrieval, but it is also involved in the evaluation of retrieved information (e.g., evaluation of personal-relevance, meaning, accuracy of information). This is consistent with empirical evidence suggesting that the degree of PCC activation during retrieval is directly related to depth of processing at encoding (Shannon & Buckner, 2004) as well as a subjective sense of detailed, conscious re-experiencing of items during retrieval (Wheeler & Buckner, 2004). Thus, the retrieval of self-relevant information — information that is inherently meaningful, deeply-encoded, and salient to the rememberer would be expected to yield robust PCC activation.
One main objective of the current investigation was to directly compare the functional anatomy involved in episodic recognition and self-referential appraisal in healthy older adults. This was guided by the hypothesis that both tasks would conjointly activate the PCC. A second major objective was to determine the effect of MCI on PCC activity in these types of tasks. The PCC shows structural and metabolic changes in MCI. We postulate that the PCC is part of the functional anatomy underlying retrieval processes and evaluation of the salience of retrieved information. Individuals with MCI show deficits in memory retrieval. Furthermore, MCI patients show impaired awareness of their memory deficit, a deficit that may reflect poor ability to retrieve and evaluate information pertaining to the self. Therefore, it was hypothesized that MCI patients would show attenuated PCC activation during both episodic recognition and self-appraisal tasks.
METHODS
Participants
Fourteen healthy elderly control participants and 14 individuals with MCI participated in this study. The two groups were matched on age and gender; both groups were composed of seven men and seven women. Both the MCI group, as well as their matched controls, received a battery of neuropsychological tests as part of this study to verify and document the extent of any selective memory impairment. Table 1 contains descriptive demographic and neuropsychological data for the two groups. Exclusion criteria for both groups included Hachinski score greater than four, prior neurological disease or neurosurgery, prior major psychiatric disorder, chronic major medical conditions such as insulin-dependent diabetes, poorly controlled hypertension, or cardiac disease. We obtained T2-weighted images as described below to screen for previously undetected clinically relevant brain abnormalities that were inconsistent with the MCI or control diagnosis.
Table 1.
Demographic and Neuropsychological Data.
| MCI | Controls | |||
|---|---|---|---|---|
| Mean | SD | Mean | SD | |
| Age | 73.7 | 6.9 | 72.5 | 5.7 |
| Education | 16.2 | 2.7 | 17.3 | 2.9 |
| Gender | 7m/7f | 7m/7f | ||
| MMSE | 28.6 | 1.5 | 29.4 | 0.8 |
| WRAT-III Reading (standard score) | 110 | 9.7 | 115 | 4.1 |
| Boston Naming Test (raw score) | 53.8 | 6.7 | 57.3 | 1.9 |
| RAVLT total (trials 1-5 raw score) | 31.6 | 4.6 | 48.0 | 6.8** |
| RAVLT delayed recall (raw score) | 2.1 | 1.9 | 8.9 | 2.4** |
| BVMT-R total (trials 1-3 raw score) | 12.6 | 5.7 | 25.9 | 5.4** |
| Trail Making Test A (seconds) | 38.3 | 16.5 | 32.7 | 6.9 |
| Trial Making Test B (seconds) | 100.9 | 45.1 | 65.4 | 16.5* |
p < .001
p < .05 statistical significance between MCI and control groups.
MCI = Mild Cognitive Impairment MMSE = Mini Mental State Exam; WRAT-III = Wide Range Achievement Test, 3rd Edition; RAVLT = Rey Auditory Verbal Learning Test; BVMT-R = Brief Visuospatial Memory Test, Revised
Elderly control participants were recruited from the community, predominantly by advertisement, mailings, and community outreach events. These participants exhibited normal performance across cognitive domains as assessed by a battery of neuropsychological tests.
MCI patients were referred from the several memory disorders clinics at a university-based medical center. Diagnostic criteria for MCI included: a) presence of memory complaints by patient or informant, b) relative decline in memory and learning in the context of otherwise normal functioning on a neuropsychological test battery, c) intact functional status, d) cognitive and functional status not consistent with a diagnosis of dementia. Prior to inclusion in this study, the MCI patients were presented to a diagnostic consensus panel for support of the diagnosis. Eight MCI patients were taking cholinesterase inhibitors, with dosage being stable for three months prior to taking part in this study.
Procedures
Neuropsychology
The MCI patients and elderly controls received a cognitive battery (see Table 1) using standardized administration (Spreen & Strauss, 1998).
fMRI Tasks
Episodic Recognition: Task Design
The ER task consisted of serial presentation of novel (i.e., “new”) and previously learned (i.e., “old”) line drawings obtained from a published picture set (Snodgrass & Vanderwart, 1980). The task was designed to be an exceptionally easy episodic memory task in order to ensure accurate performance of MCI participants, comparable to the controls. Old items were acquired in a training session 45 minutes prior to the task, and again during scanning setup 15 minutes prior to the task. The items were presented repeatedly in pseudorandom fashion for 15 exposures in each of the two training trials for a total of 30 exposures to each item. Participants were instructed to view the pictures and try to remember them.
In the current study, we were interested in the cerebral response during episodic recognition (i.e., response to the previously learned items versus the novel item reference condition). During the fMRI scan, old items were intermixed with new items. Old and new items were similar with respect to frequency and image complexity. The condition epochs were pseudorandomly varied in length, and ranged from single events, to five consecutive items. The variability in epoch length was implemented in order to reduce condition predictability, while preserving the comparatively greater statistical power and shorter duration of that type of paradigm (Liu, Frank, Wong, & Buxton, 2001). All stimuli in this task were presented for 2800 ms with a 200 ms interstimulus interval. Two alternate forms of the task were presented using the same old items, but different new items. The order of presentation of these alternate forms was counterbalanced among participants. The task duration for each form was four minutes and 42 seconds. Responses to old and new items were made with a two-button response device held in the right hand; the index finger was used to identify old items, the middle finger for new items.
Self-appraisal: Task Design
The self-appraisal task consisted of an experimental (self) condition and a baseline (semantic decision) condition. In the self condition, trait adjectives were presented, and each participant made quick yes/no decisions about whether each word described him/her by means of a button press. In the semantic decision condition, participants were presented with the same trait adjectives seen during the self condition (order of presentation was counterbalanced across conditions); however, they were asked to indicate whether each word was positive or not. In both self and semantic decision conditions of the self-appraisal task, adjectives were presented every 4,000 ms, remaining on screen for 3,000 ms followed by a 1,000 ms second inter-stimulus interval. An index finger button press indicated “no” and the middle finger indicated “yes.”
Two alternate forms of the task with identical timing were presented sequentially (order was counterbalanced) using a discrete 30-adjective set. Within each form, items from each of the two conditions were presented in five pseudo-randomized cycles. Adjectives were presented every four seconds in blocks of six per condition. The two different conditions each appeared in a slightly different color text, and there were prompts at the top of the screen to inform participants about the condition to which they should respond on each trial. The task duration for each form of the task was four minutes and eight seconds.
Scanning Procedures
Participants were provided with instruction on the fMRI tasks and underwent practice prior to scanning. They were then situated on the bed of a GE long bore 3.0 Tesla MRI scanner and outfitted with the MR-compatible button-box and a high-resolution goggle system, set at 800 × 600 from Resonance Technology (Northridge, CA, USA). Head motion was constrained by foam padding. The software Presentation (www.neuro-bs.com) was used to deliver visual stimuli from a personal computer via the goggle system and also record responses. A cable connecting the scanner to the presentation computer enabled the stimulus delivery software to be triggered by the start of the scan and also detect each slice acquisition for precise synchrony between scan acquisition and stimulus delivery.
Imaging Protocol
A T2* gradient-echo, echo-planar imaging (EPI) pulse sequence was used. The homogeneity of the static magnetic field (B0) in the brain was optimized using higher order shims prior to the functional trials. The EPI parameters for both tasks were as follows: echo time (TE) = 30 ms; repetition time (TR) = 2000 ms; flip angle = 90 degrees; acquisition matrix = 64 × 64 voxels; field of view (FOV) = 240 mm. Thirty sagittal slices of brain were acquired within each TR. Voxel resolution was 3.75 × 3.75 × 5 mm (4mm thick slices with a 1 mm skip). For the episodic retrieval task, 141 temporal volume images were collected. For the self-appraisal task, 124 temporal volume images were collected. The first three frames of each time-series were discarded.
Although we used higher order shimming for the EPI scans, there are typically residual magnetic field (B0) inhomogenities across the brain that cause regional image distortions in echo planar images such as near the mesial temporal lobe and in the frontal and ethmoid sinuses. Image distortions were corrected by measuring 3D field maps across the brain (co-planar with the fMRI slices). This was accomplished by measuring the phase of non-EPI gradient echo images at two echo times (7 and 10 ms). The phase difference between the two echo images is proportional to the static field inhomogeneity (Jezzard & Balaban, 1995). The warp correction was performed using custom software developed in Matlab. A 3D phase-unwrapping algorithm based on an algorithm developed by (Jenkinson, 2003) was used to estimate the continuous B0 field map. Image unwarping was performed using a nonlinear pixel shifting and B splines interpolation algorithm.
Anatomic Imaging
Following the functional scans and field mapping, a T1 weighted volume and T2 weighted anatomic images were acquired and later viewed by a neuroradiologist for abnormalities that were inconsistent with the subject diagnosis and/or requiring clinical follow-up.
Image Processing and Statistics
Following EPI image reconstruction the 4D image time-series was motion-corrected to overcome minor head movement during the scan (only individuals with <3 mm movement in the x, y, and z planes were included in this report). The field map from each subject was then applied to each image in the time series. This was followed by spatial normalization into a standard atlas space (using the T2* weighted template) resampling to 2 × 2 × 2 mm voxels, and then smoothed with an eight millimeter full-width at half-maximum Gaussian kernel.
Statistical Parametric Mapping software (SPM2) was used for statistical analysis (Friston et al., 1995). Analyses of the time series data were performed on individual participants using a boxcar model convolved with the canonical hemodynamic response function. Low-frequency components of the fMRI data were removed through use of a 128 second high-pass filter. We employed the AR1 method of estimating temporal autocorrelation in the time series. Linear contrasts of parameter estimates for each participant were taken to a second level analysis to generate statistical parametric maps of the t-statistic. The contrasts investigated in this study included: (1) response to old items relative to new items in the episodic recognition task and (2) self condition relative to the baseline semantic decision condition in the self-appraisal task. Using group random-effects procedures, a conjunction analysis was conducted to evaluate common regions of brain activation across tasks within each participant group. We employed the conjunction null (MS/CN) method. Use of the conjunction null method allowed us to evaluate brain regions that showed activation in both episodic retrieval AND self-appraisal (see Nichols, Brett, Andersson, Wager, & Poline, 2005 for a thorough discussion of valid use of conjunction analysis to test for evidence of a “logical AND”).
RESULTS
Behavioral Results
The accuracy and reaction time data for both episodic retrieval and self evaluation tasks are summarized in Table 2. On the episodic retrieval task, a 2 (group) × 2 (item type) repeated measures ANOVAs revealed no group differences in accuracy or reaction time to old or new items. Both MCI and the age and education matched control groups performed this relatively simple task with accuracy above 96%. For both participant groups, the reaction time to old items was longer than reaction time to new items (p<.05). On the self-appraisal task, a 2 × 2 repeated measures ANOVA revealed no group difference in reaction time in any condition of this task.
Table 2.
Task Behavioral Data.
| MCI | Controls | |||
|---|---|---|---|---|
| Episodic Retrieval Task | Mean | SD | Mean | SD |
| Reaction time (ms)- Old items | 924 | 12 | 918 | 20 |
| Reaction time (ms)-New items | 826 | 11 | 850 | 22* |
| Accuracy | 98% | 1.8 | 96% | 1.10 |
| Self-appraisal Task | ||||
| Reaction time (ms)- Self evaluation items | 1725 | 22 | 1714 | 22 |
| Reaction time (ms)- Emotional valence items | 1705 | 26 | 1707 | 23 |
Within groups, response times to new items were significantly faster than old items, p<.05.
fMRI Results
Elderly Controls
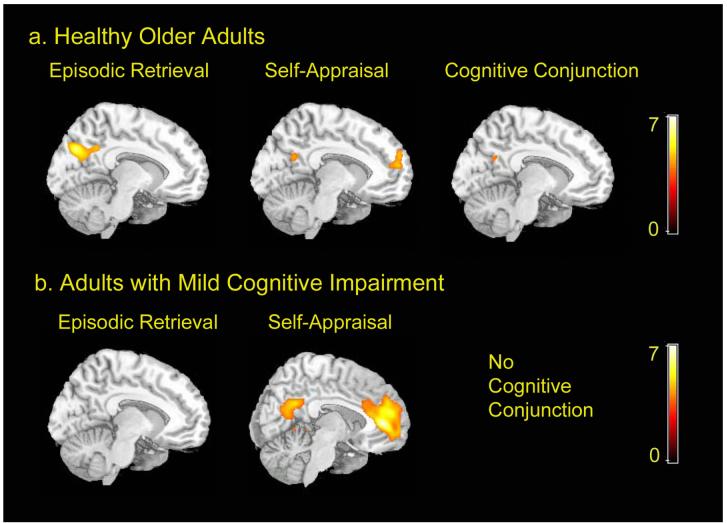
Figure 1a presents sagittal views of midline brain regions engaged in the episodic retrieval and self-appraisal tasks relative to their respective baselines in healthy older adults. At an FDR corrected threshold of 0.025, the episodic retrieval task elicited significant activity in the posterior cingulate cortex and precuneus. The self-appraisal task elicited activation in the posterior cingulate, and bilateral regions of the medial and superior prefrontal cortex.
Figure 1.
Functional MRI results a) Healthy older adults: sagittal views of activation patterns in the episodic retrieval and self-appraisal tasks (0.025 FDR-corrected) and conjunction results (0.001 uncorrected); b) Mild cognitive impairment: sagittal views of activation patterns during the two tasks (0.025 FDR-corrected) and results of conjunction analysis.
Results of the cognitive conjunction analysis are depicted in Figure 1a. This analysis revealed that in elderly controls, the PCC was the sole region of activation common to both the episodic retrieval and self-appraisal tasks at an uncorrected threshold of 0.001. The coordinates and statistics for brain regions active in each of the individual tasks and in the cognitive conjunction are presented in Table 3.
Table 3.
Statistics and voxel locations of active brain regions in elderly controls.
| Task | Brain Region | cluster size | t-value | p FDR corr | p (unc) | x,y,z (mm) |
|---|---|---|---|---|---|---|
| Episodic Retrieval | Posterior Cingulate/ Precuneus |
1356 | 6.39 | .001 | <.001 | 8, -74, 32 |
| 4.72 | .007 | <.001 | 10, -52, 32 | |||
| 3.49 | .070 | <.001 | -8, -80, 28 | |||
| L Middle Temporal Gyrus |
150 | 4.48 | .011 | <.001 | -56, -62, 16 | |
| R Middle/Superior Temporal Gyrus |
525 | 3.98 | .029 | <.001 | 48, -54, 28 | |
| 3.69 | .052 | <.001 | 58, -56, 24 | |||
| 3.62 | .058 | <.001 | 48, -58, 14 | |||
| Self-appraisal | Posterior Cingulate | 812 | 5.15 | .019 | <.001 | 8, -62, 14 |
| 4.59 | .019 | <.001 | -8, -54, 28 | |||
| 4.29 | .020 | <.001 | 8, -58, 24 | |||
| R Superior Frontal Gyrus |
1896 | 4.99 | .019 | <.001 | 10, 58, 24 | |
| 4.88 | .019 | <.001 | -12, 58, 8 | |||
| 4.87 | .019 | <.001 | -10, 48, 42 | |||
| Conjunction | Posterior Cingulate | 75 | 4.04 | ns | <.001 | 8, -60, 24 |
| 3.66 | ns | <.001 | 4, -66, 22 |
MCI Patients
Sagittal views of brain regions engaged in the episodic retrieval and self-appraisal tasks relative to their respective baselines in MCI patients are depicted in Figure 1b. In MCI patients, the episodic retrieval task did not yield any areas of significant activation at an FDR-corrected threshold of 0.025, although there were bilateral regions of activation in the lateral posterior parietal lobes at an uncorrected threshold of 0.001. The self-appraisal task elicited activation in medial prefrontal cortex, posterior cingulate, isthmus, and left parahippocampal cortex at an FDR corrected threshold of 0.025. Coordinates and statistics for brain regions active in each of the tasks in MCI patients are presented in Table 4.
Table 4.
Statistics and voxel locations of active brain regions in individuals with MCI.
| Task | Brain Region | cluster size | t-value | p FDR corr | p (unc) | x,y,z (mm) |
|---|---|---|---|---|---|---|
| Episodic Retrieval | Posterior Parietal | 106 | 4.27 | ns | <.001 | -52, -58, 42 |
| 126 | 4.06 | ns | <.001 | 6, -74, 36 | ||
| 83 | 3.86 | ns | <.001 | 46, -62, 46 | ||
| Self-appraisal | Medial Prefrontal | 4467 | 7.26 | <.001 | <.001 | -10, 44, 4 |
| 5.92 | <.001 | <.001 | -8, 50, 18 | |||
| 5.78 | <.001 | <.001 | 2, 58, 10 | |||
| Posterior Cingulate | 939 | 5.07 | .001 | <.001 | -6, -54, 28 | |
| Isthmus | 4.08 | .007 | <.001 | -10, -56, 0 | ||
| Inferior Frontal Gyrus |
115 | 3.96 | .009 | <.001 | -28, 16, -22 | |
| Parahippocampal | 3.69 | .015 | <.001 | -24, 4, -18 | ||
| Cingulate | 76 | 3.76 | .013 | <.001 | -4, -10, 34 |
The cognitive conjunction analysis in the MCI patients revealed no region of activation common to both the episodic retrieval and self-appraisal tasks.
Comparison of MCI patients to Controls
The results of the conjunction analyses indicated that although the PCC was a region of common activation during episodic retrieval and self-appraisal tasks in our control group, the PCC was not active during both tasks in the MCI group. To further characterize differences in task-dependent PCC activation between the MCI and control groups, we tested for the presence of an interaction between task and group, restricted to the region of interest (ROI) defined by the cognitive conjunction in the healthy older adults. Results of this analysis revealed a significant interaction F(1, 26)=3.83, p < .01unc. within the PCC (local max: 8, -60, 26).
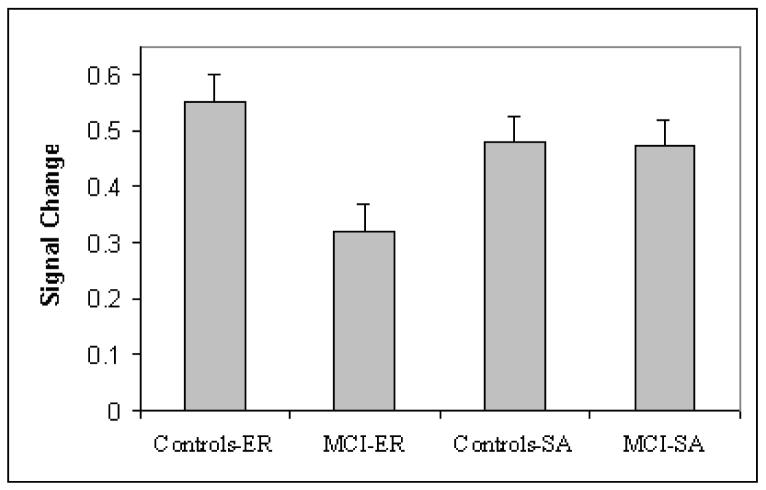
To investigate the source of this interaction, we conducted t-test comparisons of PCC activation between the two groups for each task. On the episodic retrieval task, MCI participants showed significantly attenuated PCC activation compared to controls, t (13) = 2.71, p < .01. In contrast, MCI participants and controls showed no significant difference in PCC activation during the self-appraisal task, p > .05. Figure 2 graphically depicts PCC activation during each task for the MCI and control groups.
Figure 2.
Mean PCC activation within a spherical region 2 mm in radius (7 contiguous voxels) surrounding the global maxima of the cognitive conjunction in healthy older adults. Error bars depict standard error of measurement.
DISCUSSION
The goals of the current investigation were two-fold. One goal was to provide further description of task-dependent PCC functioning in healthy older adults. To do this, we examined brain regions that showed functional overlap across two fMRI tasks hypothesized to elicit PCC activation. Our interest in investigating PCC function was motivated largely by the fact that this brain region shows structural and functional changes in individuals at risk for developing AD. Furthermore, PCC functioning also predicts development of AD in individuals with MCI. Because PCC function in individuals with MCI has received little attention in the fMRI literature, a second goal was to investigate the functional integrity of the PCC in individuals with MCI using fMRI tasks that elicit activation in this structure.
Cognitively-Healthy Elderly Adults
Consistent with the main hypothesis regarding healthy older adults, these participants showed significant PCC activation during both episodic retrieval and self-appraisal tasks. Although the literature contains reports of PCC activation in both types of tasks, the use of a cognitive conjunction analysis allowed for a more formal demonstration of the regional overlap of these two tasks in the PCC. However, the question remains: does PCC activation common to these two tasks reflect a common cognitive process that underlies both episodic retrieval and self-appraisal, or conversely, is the common PCC activation across tasks indicative of this structure’s functional heterogeneity?
It is possible that common activation across these two tasks reflects the PCC’s involvement in a common cognitive process, with memory retrieval being a likely candidate. The PCC’s reciprocal anatomical connections with other memory structures including the posterior parahippocampal cortex, presubiculum, hippocampus, entorhinal cortex, thalamic nuclei (that have reciprocal connections with the MTL), and dorsolateral prefrontal cortex suggest that the PCC has a role in mnemonic processing. Furthermore, rare reports of the cognitive sequelae of retrosplenial lesions and more abundant evidence from the functional neuroimaging literature corroborate the PCC’s role in memory retrieval. Within the current experiment, both tasks that were employed involve information retrieval. Information retrieved by participants during the episodic retrieval task had been learned quite recently, within the hour prior to the retrieval task; information retrieved by participants during the self-appraisal task was drawn from stable and long-standing schemas regarding the self.
Alternately, the cognitive conjunction in the PCC of healthy older adults could reflect this region’s involvement in somewhat disparate cognitive functions. Empirical evidence implicates PCC involvement in a wide variety of functions, including attention (Mesulam, Nobre, Kim, Parrish, & Gitelman, 2001), processing of emotional stimuli (Cato et al., 2004; Maddock, Garrett, & Buonocore, 2003), and self-monitoring during a default resting state (Greicius, Srivastava, Reiss, & Menon, 2004; Gusnard, Akbudak, Shulman, & Raichle, 2001; Raichle et al., 2001).
The healthy older adults’ data do not provide a clear answer to whether common PCC activation underlies a common construct or disparate cognitive processes. However, results from the MCI group shed some light on this, as discussed in the following section.
Individuals with MCI
Although we had hypothesized that PCC activation would be uniformly attenuated across tasks because of the structural vulnerability of this region to AD pathology, our results suggested that PCC activation was compromised in a task-dependent fashion. The MCI participants showed no significant signal change in the PCC in response to the episodic retrieval task, but normal signal change during the self-appraisal task. Similarly, in comparison to control participants, MCI participants showed less PCC activation on the episodic retrieval task. However, the magnitude of PCC activation at the maxima during the self-appraisal task was equivalent between groups (see Figure 2).
In the current investigation, our episodic retrieval task was uniquely sensitive to changes in PCC function in MCI. This finding of functional degradation in the PCC during episodic retrieval is consistent with numerous reports of functional and structural changes in the PCC. However, why does the PCC appear to be functionally intact in the self- evaluation task? This question can be addressed from the standpoint of task design and the cognitive constructs being addressed in each task.
The two tasks employed require participants to retrieve information; however, the nature of the information retrieved is qualitatively different. The self-appraisal task requires retrieval and evaluation of information relating to one’s self. Unlike the recently-learned and decontextualized information retrieved in the episodic recognition task, information regarding the self is highly-rehearsed, elaborated, and salient to the participant. Furthermore, the information retrieved during the self-appraisal task is not episodic. Instead, it falls within the rubric of semantic memory, being stored as general knowledge, not likely tied to a particular encoding episode. These qualitative differences in the nature of memory retrieval demanded by each task may underlie the dissociation in PCC activation observed in MCI participants.
Alternately, MCI participants’ demonstration of task-dependent PCC activation may relate to quantitative differences in the difficulty of memory retrieval required by each task. In other words, PCC activation during both episodic recognition and self-appraisal may relate to memory retrieval, but each task may load episodic memory to a different extent. Given the numerous differences in the design of the two tasks employed, it is not possible to accurately determine the relative difficulty of the two tasks. However, the effect of difficulty of episodic retrieval on PCC activation in MCI is an important empirical question and deserves research attention with use of an episodic retrieval task that parametrically varies retrieval effort (i.e., by varying levels of processing at encoding, by varying length of encoding-retrieval delay) while keeping other variables constant.
It is critical to note that the differential PCC activation in MCI could be due to other non-mnemonic experimental variables that varied between the tasks. First, the method of testing varied between the task: The episodic retrieval task assessed explicit memory via a direct query, whereas the self-appraisal task required each participant to make judgments about him/herself. Therefore, despite its reliance on memory, the self-appraisal task did not directly query explicit memory for a prior event. Second, the tasks also varied in the type of stimulus materials used. The episodic retrieval task used pictorial stimuli, whereas the self-appraisal task used verbal stimuli. Third, the stimulus presentation rates varied between the tasks. The relation of the other cognitive constructs that may influence PCC activation (i.e., subjectivity of responses made, type of memory assessed, method of query), also needs to be carefully investigated through development and use of paradigms in which these variables are manipulated in a systematic fashion. Empirical findings should then be extended to the study of PCC activation in MCI. This would serve to clarify this structure’s functional contribution to cognitive decline in this disorder.
Our choice of baseline conditions also affected the study results. This choice affected the results of both participant groups, and it bears reporting here because it has a strong effect on the conclusions one draws regarding both the brain regions involved in normal cognitive function and the brain regions functionally impacted by disease. Rather than use a low-level baseline condition, we employed active comparison tasks. Choice of a baseline condition is critical as the brain remains physiologically and psychologically active during “rest” (i.e., rest or attention to a fixation cross). Participants show a reliable network of activated structures (that includes the PCC) during “rest,” and it has been proposed that this activation is related to self-reflective processing when subjects are provided with a low-level baseline task that does not sufficiently engage attention (Gusnard et al., 2001; Gusnard & Raichle, 2001; Raichle et al., 2001). This point is particularly relevant to our study in which both contrasts were meant to elicit PCC activation, and one contrast of interest related significantly to self-referential processing.
With respect to our clinical participant group, one limitation of this study relates to variability in the medication regimens of MCI participants. Eight of the MCI participants were taking stable doses of cholinesterase inhibitors, and this may have affected their BOLD responses. Empirical investigation into the effect of cholinesterase inhibitors is limited, although there is some evidence that these medications may increase the BOLD response in some populations (Saykin et al., 2004; Thiel, Bentley, & Dolan, 2002). Taking cholinesterase medication would have the effect of improving cognitive function in the MCIs, and it is possible that this would be associated in a more robust task-related BOLD signal in MCI participants (i.e., mitigation of the difference in BOLD signal between controls and MCI participants). Thus, the group difference observed during episodic retrieval was above and beyond any salutary effects the cholinergic medications may have had. Clearly, a better understanding of the effects of medications on the BOLD response will lead to more precise interpretation of fMRI results in clinical populations.
Conclusion
This paper investigated PCC functioning using two fMRI tasks: an episodic retrieval and a self-appraisal task. Both tasks elicited a common region of PCC activation in healthy older adults. In contrast, MCI patients showed PCC activation during self-appraisal but not episodic retrieval, suggesting a task-dependent responsiveness in this population that may be related to the availability of prior information. One possible interpretation of our results is that the PCC shows functional degradation during episodic retrieval; however, the PCC’s role in retrieval and evaluation of highly-elaborated information regarding the self is more well-preserved.
In presenting these preliminary data, we acknowledge that our conclusions regarding the cognitive correlates of the task-dependent PCC activation in MCI are tentative. The current study compared PCC activation using two tasks that varied along a number of dimensions. More solid conclusions regarding the functional integrity of the PCC in MCI await design of studies that systematically evaluate those task conditions in which activity is preserved and conditions in which it is not. However, the current paper presents novel findings regarding PCC function that yield new, provocative questions regarding the role of the PCC in retrieval.
Acknowledgements
This study was supported by AG021155 (SCJ). The assistance of Justin Dunker, Kristine Kalmoe, Allison Wichmann, Michael Anderle, and Ronald Fisher is greatly appreciated. We extend thanks for the use of a field mapping procedure developed by Andrew Alexander.
REFERENCES
- Addis DR, Moscovitch M, Crawley AP, McAndrews MP. Recollective qualities modulate hippocampal activation during autobiographical memory retrieval. Hippocampus. 2004;14(6):752–762. doi: 10.1002/hipo.10215. [DOI] [PubMed] [Google Scholar]
- Cato MA, Crosson B, Gokcay D, Soltysik D, Wierenga C, Gopinath K, et al. Processing words with emotional connotation: an FMRI study of time course and laterality in rostral frontal and retrosplenial cortices. J Cogn Neurosci. 2004;16(2):167–177. doi: 10.1162/089892904322984481. [DOI] [PubMed] [Google Scholar]
- Chetelat G, Desgranges B, de la Sayette V, Viader F, Berkouk K, Landeau B, et al. Dissociating atrophy and hypometabolism impact on episodic memory in mild cognitive impairment. Brain. 2003;126(Pt 9):1955–1967. doi: 10.1093/brain/awg196. [DOI] [PubMed] [Google Scholar]
- Chetelat G, Desgranges B, de la Sayette V, Viader F, Eustache F, Baron JC. Mild cognitive impairment: Can FDG-PET predict who is to rapidly convert to Alzheimer’s disease? Neurology. 2003;60(8):1374–1377. doi: 10.1212/01.wnl.0000055847.17752.e6. [DOI] [PubMed] [Google Scholar]
- Fossati P, Hevenor SJ, Graham SJ, Grady C, Keightley ML, Craik F, et al. In search of the emotional self: an FMRI study using positive and negative emotional words. Am J Psychiatry. 2003;160(11):1938–1945. doi: 10.1176/appi.ajp.160.11.1938. [DOI] [PubMed] [Google Scholar]
- Friston K, Holmes AP, Worsley KJ, Poline JB, Frith C, Frackowiak RSJ. Statistical parametric maps in functional imaging: A general linear approach. Human Brain Mapping. 1995;2:189–210. [Google Scholar]
- Greicius MD, Srivastava G, Reiss AL, Menon V. Default-mode network activity distinguishes Alzheimer’s disease from healthy aging: evidence from functional MRI. Proc Natl Acad Sci USA. 2004;101(13):4637–4642. doi: 10.1073/pnas.0308627101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusnard DA, Akbudak E, Shulman GL, Raichle ME. Medial prefrontal cortex and self-referential mental activity: relation to a default mode of brain function. Proc Natl Acad Sci USA. 2001;98(7):4259–4264. doi: 10.1073/pnas.071043098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusnard DA, Raichle ME. Searching for a baseline: functional imaging and the resting human brain. Nat Rev Neurosci. 2001;2(10):685–694. doi: 10.1038/35094500. [DOI] [PubMed] [Google Scholar]
- Huang C, Wahlund LO, Svensson L, Winblad B, Julin P. Cingulate cortex hypoperfusion predicts Alzheimer’s disease in mild cognitive impairment. BMC Neurol. 2002;2(1):9. doi: 10.1186/1471-2377-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M. Fast, automated, N-dimensional phase-unwrapping algorithm. Magn Reson Med. 2003;49(1):193–197. doi: 10.1002/mrm.10354. [DOI] [PubMed] [Google Scholar]
- Jezzard P, Balaban RS. Correction for geometric distortion in echo planar images from B0 field variations. Magn Reson Med. 1995;34(1):65–73. doi: 10.1002/mrm.1910340111. [DOI] [PubMed] [Google Scholar]
- Johnson SC, Baxter LC, Wilder LS, Pipe JG, Heiserman JE, Prigatano GP. Neural correlates of self-reflection. Brain. 2002;125(Pt 8):1808–1814. doi: 10.1093/brain/awf181. [DOI] [PubMed] [Google Scholar]
- Kelley WM, Macrae CN, Wyland CL, Caglar S, Inati S, Heatherton TF. Finding the self? An event-related fMRI study. J Cogn Neurosci. 2002;14(5):785–794. doi: 10.1162/08989290260138672. [DOI] [PubMed] [Google Scholar]
- Kobayashi Y, Amaral DG. Macaque monkey retrosplenial cortex: II. Cortical afferents. J Comp Neurol. 2003;466(1):48–79. doi: 10.1002/cne.10883. [DOI] [PubMed] [Google Scholar]
- Kogure D, Matsuda H, Ohnishi T, Asada T, Uno M, Kunihiro T, et al. Longitudinal evaluation of early Alzheimer’s disease using brain perfusion SPECT. J Nucl Med. 2000;41(7):1155–1162. [PubMed] [Google Scholar]
- Leveroni CL, Seidenberg M, Mayer AR, Mead LA, Binder JR, Rao SM. Neural systems underlying the recognition of familiar and newly learned faces. J Neurosci. 2000;20(2):878–886. doi: 10.1523/JNEUROSCI.20-02-00878.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine B, Turner GR, Tisserand D, Hevenor SJ, Graham SJ, McIntosh AR. The functional neuroanatomy of episodic and semantic autobiographical remembering: a prospective functional MRI study. J Cogn Neurosci. 2004;16(9):1633–1646. doi: 10.1162/0898929042568587. [DOI] [PubMed] [Google Scholar]
- Liu TT, Frank LR, Wong EC, Buxton RB. Detection power, estimation efficiency, and predictability in event-related fMRI. Neuroimage. 2001;13(4):759–773. doi: 10.1006/nimg.2000.0728. [DOI] [PubMed] [Google Scholar]
- Maddock RJ, Garrett AS, Buonocore MH. Remembering familiar people: the posterior cingulate cortex and autobiographical memory retrieval. Neuroscience. 2001;104(3):667–676. doi: 10.1016/s0306-4522(01)00108-7. [DOI] [PubMed] [Google Scholar]
- Maddock RJ, Garrett AS, Buonocore MH. Posterior cingulate cortex activation by emotional words: fMRI evidence from a valence decision task. Hum Brain Mapp. 2003;18(1):30–41. doi: 10.1002/hbm.10075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire EA, Frith CD, Cipolotti L. Distinct neural systems for the encoding and recognition of topography and faces. Neuroimage. 2001;13(4):743–750. doi: 10.1006/nimg.2000.0712. [DOI] [PubMed] [Google Scholar]
- Maguire EA, Frith CD, Morris RG. The functional neuroanatomy of comprehension and memory: the importance of prior knowledge. Brain. 1999;122(Pt 10):1839–1850. doi: 10.1093/brain/122.10.1839. [DOI] [PubMed] [Google Scholar]
- McDonald CR, Crosson B, Valenstein E, Bowers D. Verbal encoding deficits in a patient with a left retrosplenial lesion. Neurocase. 2001;7(5):407–417. doi: 10.1076/neur.7.5.407.16250. [DOI] [PubMed] [Google Scholar]
- Mesulam MM, Nobre AC, Kim YH, Parrish TB, Gitelman DR. Heterogeneity of cingulate contributions to spatial attention. Neuroimage. 2001;13(6 Pt 1):1065–1072. doi: 10.1006/nimg.2001.0768. [DOI] [PubMed] [Google Scholar]
- Morris R, Paxinos G, Petrides M. Architectonic analysis of the human retrosplenial cortex. J Comp Neurol. 2000;421(1):14–28. doi: 10.1002/(sici)1096-9861(20000522)421:1<14::aid-cne2>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Morris R, Petrides M, Pandya DN. Architecture and connections of retrosplenial area 30 in the rhesus monkey (Macaca mulatta) Eur J Neurosci. 1999;11(7):2506–2518. doi: 10.1046/j.1460-9568.1999.00672.x. [DOI] [PubMed] [Google Scholar]
- Nestor PJ, Fryer TD, Ikeda M, Hodges JR. Retrosplenial cortex (BA 29/30) hypometabolism in mild cognitive impairment (prodromal Alzheimer’s disease) Eur J Neurosci. 2003;18(9):2663–2667. doi: 10.1046/j.1460-9568.2003.02999.x. [DOI] [PubMed] [Google Scholar]
- Nichols T, Brett M, Andersson J, Wager T, Poline JB. Valid conjunction inference with the minimum statistic. Neuroimage. 2005;25(3):653–660. doi: 10.1016/j.neuroimage.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Northoff G, Bermpohl F. Cortical midline structures and the self. Trends Cogn Sci. 2004;8(3):102–107. doi: 10.1016/j.tics.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Knierim K, Ludlow DH, Hanelin J, Ramachandran T, Glover G, et al. Reflecting upon feelings: an fMRI study of neural systems supporting the attribution of emotion to self and other. J Cogn Neurosci. 2004;16(10):1746–1772. doi: 10.1162/0898929042947829. [DOI] [PubMed] [Google Scholar]
- Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256(3):183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56(3):303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc Natl Acad Sci USA. 2001;98(2):676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reber PJ, Wong EC, Buxton RB. Comparing the brain areas supporting nondeclarative categorization and recognition memory. Brain Res Cogn Brain Res. 2002;14(2):245–257. doi: 10.1016/s0926-6410(02)00122-2. [DOI] [PubMed] [Google Scholar]
- Saykin AJ, Wishart HA, Rabin LA, Flashman LA, McHugh TL, Mamourian AC, et al. Cholinergic enhancement of frontal lobe activity in mild cognitive impairment. Brain. 2004;127(Pt 7):1574–1583. doi: 10.1093/brain/awh177. [DOI] [PubMed] [Google Scholar]
- Schmitz TW, Kawahara-Baccus TN, Johnson SC. Metacognitive evaluation, self-relevance, and the right prefrontal cortex. Neuroimage. 2004;22(2):941–947. doi: 10.1016/j.neuroimage.2004.02.018. [DOI] [PubMed] [Google Scholar]
- Shah NJ, Marshall JC, Zafiris O, Schwab A, Zilles K, Markowitsch HJ, et al. The neural correlates of person familiarity. A functional magnetic resonance imaging study with clinical implications. Brain. 2001;124(Pt 4):804–815. doi: 10.1093/brain/124.4.804. [DOI] [PubMed] [Google Scholar]
- Shannon BJ, Buckner RL. Functional-anatomic correlates of memory retrieval that suggest nontraditional processing roles for multiple distinct regions within posterior parietal cortex. J Neurosci. 2004;24(45):10084–10092. doi: 10.1523/JNEUROSCI.2625-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snodgrass J, Vanderwart M. Norms for name agreement, image agreement, familiarity, and visual complexity. Journal of Experimental Psychology: Human Learning and Memory. 1980;6:174–215. doi: 10.1037//0278-7393.6.2.174. [DOI] [PubMed] [Google Scholar]
- Spreen O, Strauss E. A compendium of neuropsychological tests: Administration, norms, and commentary. 2nd Oxford University Press; New York: 1998. [Google Scholar]
- Sugiura M, Shah NJ, Zilles K, Fink GR. Cortical representations of personally familiar objects and places: functional organization of the human posterior cingulate cortex. Journal of Cognitive Neuroscience. 2005;17(2):183–198. doi: 10.1162/0898929053124956. [DOI] [PubMed] [Google Scholar]
- Thiel CM, Bentley P, Dolan RJ. Effects of cholinergic enhancement on conditioning-related responses in human auditory cortex. Eur J Neurosci. 2002;16(11):2199–2206. doi: 10.1046/j.1460-9568.2002.02272.x. [DOI] [PubMed] [Google Scholar]
- Valenstein E, Bowers D, Verfaellie M, Heilman KM, Day A, Watson RT. Retrosplenial amnesia. Brain. 1987;110(Pt 6):1631–1646. doi: 10.1093/brain/110.6.1631. [DOI] [PubMed] [Google Scholar]
- Vogeley K, Bussfeld P, Newen A, Herrmann S, Happe F, Falkai P, et al. Mind reading: neural mechanisms of theory of mind and self-perspective. Neuroimage. 2001;14(1 Pt 1):170–181. doi: 10.1006/nimg.2001.0789. [DOI] [PubMed] [Google Scholar]
- Vogt BA, Finch DM, Olson CR. Functional heterogeneity in cingulate cortex: the anterior executive and posterior evaluative regions. Cereb Cortex. 1992;2(6):435–443. doi: 10.1093/cercor/2.6.435-a. [DOI] [PubMed] [Google Scholar]
- Wheeler ME, Buckner RL. Functional-anatomic correlates of remembering and knowing. Neuroimage. 2004;21(4):1337–1349. doi: 10.1016/j.neuroimage.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Wiggs CL, Weisberg J, Martin A. Neural correlates of semantic and episodic memory retrieval. Neuropsychologia. 1999;37(1):103–118. doi: 10.1016/s0028-3932(98)00044-x. [DOI] [PubMed] [Google Scholar]
- Winblad B, Palmer K, Kivipelto M, Jelic V, Fratiglioni L, Wahlund LO, et al. Mild cognitive impairment--beyond controversies, towards a consensus: report of the International Working Group on Mild Cognitive Impairment. J Intern Med. 2004;256(3):240–246. doi: 10.1111/j.1365-2796.2004.01380.x. [DOI] [PubMed] [Google Scholar]
- Wolf H, Jelic V, Gertz HJ, Nordberg A, Julin P, Wahlund LO. A critical discussion of the role of neuroimaging in mild cognitive impairment. Acta Neurol Scand Suppl. 2003;179:52–76. doi: 10.1034/j.1600-0404.107.s179.10.x. [DOI] [PubMed] [Google Scholar]
- Yasuda Y, Watanabe T, Tanaka H, Tadashi I, Akiguchi I. Amnesia following infarction in the right retrosplenial region. Clin Neurol Neurosurg. 1997;99(2):102–105. doi: 10.1016/s0303-8467(97)00605-7. [DOI] [PubMed] [Google Scholar]