Abstract
Objectives
Non-invasive, sensitive and specific tools for early identification of chronic inflammatory bowel diseases (IBD) are needed for clinical practice. The aim was to identify new non-invasive test combinations for characterization of IBD in children and adolescents by comparing serological responses to microbial antigens and fecal calprotectin, a new promising marker for intestinal inflammation.
Patients and methods
Our study included 73 children who underwent endoscopies because of suspicion of IBD. Their sera were tested for antibodies to the Pseudomonas fluorescens-associated sequence I2, a Bacteroides caccae TonB-linked outer membrane protein, OmpW and anti-Saccharomyces cerevisiae (ASCA). Simultaneously, samples for fecal calprotectin measurements were obtained from 55 subjects.
Results
IBD was diagnosed in 60 patients (CD in 18 patients, UC in 36 and IC in six). Thirteen children had a non-IBD disease. Fecal calprotectin levels were elevated (≥ 100ug/g) more frequently in IBD patients (89%, 39/44) compared to non-IBD cases (9%, 1/11, p<0.001). ASCA antibodies in sera were detected in 67% (12/18) of patients with CD, in 14% (5/36) of the children with UC and in 50% (3/6) of patients with IC. Seroreactivity for I2 was observed in 42% of the IBD patients, this frequency being higher than in non-IBD cases (7,7% seropositive; p=0.025). Serum anti-I2 IgA levels (median absorbances) were higher in those with IBD compared to those without gut inflammation (p=0.039). The combination of the measurements of fecal calprotectin and serological responses to microbial antigens (ASCA, I2 and OmpW) identified 100% of CD patients (sensitivity 100%, specificity 36%, PPV 66%, NPV 100%) and 89% of UC patients (sensitivity 89%, specificity 36%, PPV 77%, NPV 57%).
Conclusions
Increased levels of serological responses to microbial antigens (ASCA, I2 and OmpW) and fecal calprotectin are evident in both CD and UC patients. The combination of these markers provides valuable, non-invasive tools for the diagnostics of IBD.
Keywords: Crohn’s disease, ulcerative colitis, microbial antigens, I2, OmpW, calprotectin
INTRODUCTION
Crohn’s disease (CD) and ulcerative colitis (UC) are chronic inflammatory bowel diseases (IBD) characterized as a group of immune-mediated disorders of the intestine. To increase the diagnostic accuracy, non-invasive, sensitive and specific tools for early identification of IBD are needed. Fecal calprotectin, a cytoplasmatic protein released by activated neutrophilic polymorphonuclear cells and/or monocytes-macrophages, is a marker of intraluminal intestinal inflammation. 1,2 This protein has been demonstrated to be useful in detecting active IBD and clinical relapse, and in monitoring the response to treatment both in adults and children. 1–7
Serological markers such as anti-Saccharomyces cerevisiae antibodies (ASCA) and perinuclear anti-neutrophilic antibodies, (pANCAs) have proved to be valuable in the diagnosis and differentiation of CD and UC. 8–16 These markers may also predict the development of IBD years before the disease is clinically diagnosed. 17 Familial clustering of ASCA and pANCA has also been demonstrated suggesting that these antibodies might be familial disease markers. 18–19 The variety of serologic markers for IBD has expanded and an increasing amount of experimental data are nowadays available on new antibodies directed against various microbial antigens such as the Pseudomonas fluorescens associated sequence I2, a Bacteroides caccae TonB-linked outer membrane protein, OmpW, and E. Coli outer membrane porin C, OmpC. 20–26 The appearance of these antibodies reflects a loss of tolerance to different intestinal bacteria. Varying responses to selected microbial and autoantigens have been described in subsets of CD patients and also in UC patients. 23, 26–27
The aim of the present study was to examine the association of fecal calprotectin with serological markers in children and adolescents with IBD. Furthermore, we wanted to identify new possible non-invasive test combinations for detecting the IBD patients.
MATERIALS AND METHODS
Serum and fecal samples were collected in 73 children and adolescents examined for suspicion of IBD (CD, IC, UC) at the Hospital for Children and Adolescents in Helsinki, Finland during May 2005-November 2006. At the time of primary diagnostics samples of 64 patients with IBD suspicion were available for analyses (9 cases excluded, see table). All 73 subjects underwent upper and lower endoscopies and their sera were collected for further analysis. The diagnosis of IBD was based on histopathological criteria. 28 Subjects were grouped for the final analysis into IBD patients (n=60), including patients with CD (n= 18), UC (n= 36), and IC (n=6), and non-IBD control subjects (n=13). The presence and degree of inflammation were determined in the upper gastrointestinal biopsies using the modified Sydney system. 29
Measurement of fecal calprotectin
Fecal calprotectin was measured by enzyme immunoassay in fecal samples available for analysis in 55 patients (median age 12.8 years, range 5.8–19.9). Calprotectin levels were determined from feces as previously described and fecal calprotectin level higher than 100μg/g was considered as elevated. 30
Serological tests
Sera for the determination of anti-I2 and anti-OmpW IgA were drawn at the time of endoscopy from all 73 children and adolescents and stored at −20 ºC until testing. The majority of the samples (64/73) were collected at the time of primary diagnostics. In our laboratory, E. Coli XL-1-blue and E. Coli BL-21 (Stratagene, La Jolla, California, USA) strains were used for all cloning and recombinant expression experiments. I2-GST and OmpW were produced by using previously reported antigen purification techniques. 20,22 Sera were analysed by IgA enzyme-linked immunosorbent assays (ELISA) for the determination of the Pseudomonas fluorescens-associated sequence I2 and the Bacteroides caccae TonB-linked outer membrane protein OmpW as previously described. 26 An enzyme immunoassay kit (QUANTA Lite ASCA, INOVA Diagnostics Inc, San Diego, CA, USA) was used to determine ASCA of both IgG and IgA isotypes. Quantitative results in arbitrary enzyme immunoassay units were obtained from standard curves defined by the manufacturer, but the results were statistically handled as qualitative. Equivocal/borderline results were interpreted as negative. Results exceeding 25 U for IgG and/or IgA ASCA were regarded as positive.
Statistical analysis
Numerical analysis of laboratory measurements was made by Mann-Whitney U test. Other parameters between study groups were compared by Pearson’s [chi]2 and Fisher exact tests. Statistical calculations were carried out with SPSS (version 11.0, SPSS Inc, Chicago I11). Analyses for specificity, sensitivity, positive and negative predictive value were performed.
Ethical Considerations
The study protocol was approved by the Ethics Committee of Helsinki University Hospital, and informed consent was obtained from the parents and from the children when appropriate.
RESULTS
IBD was detected in 60 patients (CD in 18 patients, UC in 36 and IC in six), and 13 children presented a non-IBD disease including celiac disease (n=1), allergy (n=1), lymphatic adenopathy (n=1), extrahepatic portal obstruction (n=1), juvenile polyp (n=1), hematochezia (n=1), hemorrhagia intestinalis NAS (n=1), operated intestinal invagination (n=1), lumbosacralic meningomyelocele (n=1) and normal (n=4). Gender and age of study population are shown in Table 1.
Table 1.
Gender and age (median, range) in the study population
| IBD | CD | UC | IC | Non-IBD | |
|---|---|---|---|---|---|
| N | 60 | 18 | 36 | 6 | 13 |
| Sex (F/M) | 26/34 | 7/11 | 14/23 | 6/0 | 7/6 |
| Age median (range) | 13.8 yrs (2.7–19.9) | 15.5 yrs (9.0–19.6) | 13.7 yrs (2.7–19.9) | 7.2 yrs (6.1–16.3) | 8.1 yrs (5.8–14.9) |
Seropositivity rates
At the time of diagnosis fecal calprotectin levels were increased (≥ 100ug/g) in 89% (39/44) of IBD patients. This frequency of positivity was significantly higher compared to non-IBD patients (9%, 1/11, p<0.001). Frequencies of fecal calprotectin positivity in all IBD subgroups (CD, UC and IC) are shown in Table 2. Calprotectin levels in feces were significantly elevated in patients with IBD when compared to patients who were excluded from the disease-group (p<0.001). (Figure 1). The sensitivity, specificity, PPV and NPV are presented in Table 2.
Table 2.
Sensitivity, Specificity, Positive and Negative Predictive Values of Serum ASCA, I2 and OmpW and fecal calprotectin Tests in IBD Patients (n=60, including CD, UC and IC) and in CD (n=17) and UC (n=38) Subgroups. Results are presented as IBD (CD, UC)
| ASCA | I2 | OmpW | Calprotectin | |
|---|---|---|---|---|
| Sensitivity | 33% (67%, 14%) | 42% (44%, 42%) | 32% (28%, 33%) | 89% (100%, 82%) |
| Specificity | 77% (77%, 77%) | 92% (92%, 92%) | 77% (77%, 77%) | 90% (90%, 91%) |
| PPV | 87% (80%, 63%) | 96% (89%, 94%) | 86% (63%, 80%) | 97% (93%, 96%) |
| NPV | 20% (63%, 25%) | 26% (55%, 36%) | 20% (44%, 29%) | 67% (100%, 67%) |
FIGURE 1.
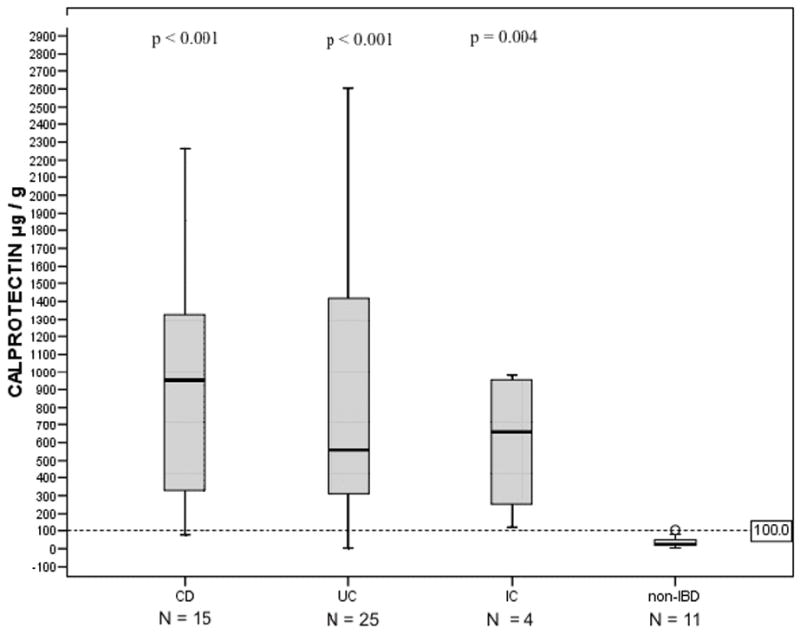
Fecal calprotectin levels in children and adolescents according to diagnosis into inflammatory bowel disease (IBD) subgroups (Crohn’s disease; CD, ulcerative colitis; UC and indeterminate colitis; IC) and non-IBD group. Horizontal line denotes the cut-off value for positivity (see text). P values compare absorbances between the IBD subgroups and non-IBD group, using the Mann-Whitney U-test.
In our laboratory, the mean optical density in the IgA-class I2-GST antibody ELISA test for all 60 children with untreated IBD was 0.72 (95% confidence interval, CI 0.34–1.1), and the respective value for all non-IBD patients was 0.30 (95% CI 0.01–0.59). The mean optical density in the IgA-class OmpW antibody test with untreated IBD patients was 0.70 (95% CI 0.33–1.08) and in children with non-IBD disease 0.36 (95% CI 0.16–0.56). The cut-off levels were set as previously described. 26 Sensitivity, specificity, PPV and NPV of I2 and OmpW tests are shown in Table 2. The frequencies of seropositivity towards different antigens in children with IBD and children with non-IBD disease are presented in Table 3.
Table 3.
The Frequency of Positive Seroreactivity to Anti-Saccharomyces Cerevisiae (ASCA), I2 and OmpW and Frequency of Fecal Calprotectin positivity among 60 Patients with IBD and 13 non-IBD disease patients
| ASCA IgA and/or IgG | I2 IgA | OmpW IgA | Fecal calprotectin | |
|---|---|---|---|---|
| All IBD | 20/60 (33.3%) | 25/60 (41.7%) | 19/60 (31.7%) | 39/44 (88.6%) |
| N=60 | p1=0.743 | p1=0.025 | p1=0.742 | p1<0.001 |
| N=51* | 18/51 (35.3%) | 22/51 (44.0%) | 17/51 (33.3%) | 32/37 (86.5%) |
| p2=0.518 | p2=0.022 | p2=0.739 | p2<0.001 | |
|
| ||||
| CD | 12/18 (66.7%) | 8/18 (44.4%) | 5/18 (27.8%) | 14/15 (93.3%) |
| p1=0.024 | p1=0.092 | p1=1.000 | p1<0.001 | |
| 10/16 (62.5%) | 8/16 (50%) | 5/16 (31.3%) | 12/13 (92.3%) | |
| p2=0.049 | p2=0.038 | p2=1.0 | p2<0.001 | |
|
| ||||
| UC | 5/36 (13.9%) | 15/36 (41.7%) | 12/36 (33.3%) | 21/25 (84%) |
| p1=0.701 | p1=0.038 | p1=0.730 | p1<0.001 | |
| 5/30 (16.7%) | 12/30 (40%) | 10/30 (37%) | 17/21 (81%) | |
| p2=1.000 | p2=0.038 | p2=0.724 | p2<0.001 | |
|
| ||||
| IC | 3/6 (50.0%) | 2/6 (33.3%) | 2/6 (33.3%) | 4/4 (100%) |
| p1=0.583 | p1=0.172 | p1=0.583 | p1=0.011 | |
| 3/5 (60.0%) | 2/5 (40.0%) | 2/5(40.0%) | 3/3 (100%) | |
| p2=0.538 | p2=0.121 | p2=0.538 | p2=0.038 | |
|
| ||||
| Non-IBD | 3/13 (23.1%) | 1/13 (7.7%) | 3/13 (23.1%) | 1/11 (9.1%) |
IBD, inflammatory bowel disease; CD, Crohn’s disease; UC, ulcerative colitis; IC, indeterminate colitis; ASCA, anti- Saccharomyces cerevisiae antibodies; I2, anti-I2; antibodies to P. fluorescens associated sequence I2; OmpW, a Bacteroides caccae TonB-linked outer membrane protein. For p values, patient groups were compared to the non-IBD group using Pearson’s χ2 and Fisher’s exact tests.
9 patients diagnosed 5.3 years (2.0–12.8 years) before first available serum or feces sample are excluded. p2; the subgroups of patients with all measurements determined at the time of primary diagnostics.
Seromarker levels
Anti-I2 levels in the sera were significantly higher in IBD patients when compared to those without IBD (p=0.039; median of absorbances, Mann-Whitney U-test) (Figure 2A). At the time of diagnosis children and adolescents with CD and UC expressed higher serum anti-I2 IgA levels in comparison to those with non-IBD disease (p=0.032 and p=0.042, respectively), (Figure 3). In contrast, serum anti-OmpW levels were not markedly elevated in IBD patients; in children and adolescents with CD and UC, anti-OmpW IgA levels did not differ significantly from patients with non-IBD disease in this material (p=0.207, p=0.217, respectively), (Figure 2B).
FIGURE 2.
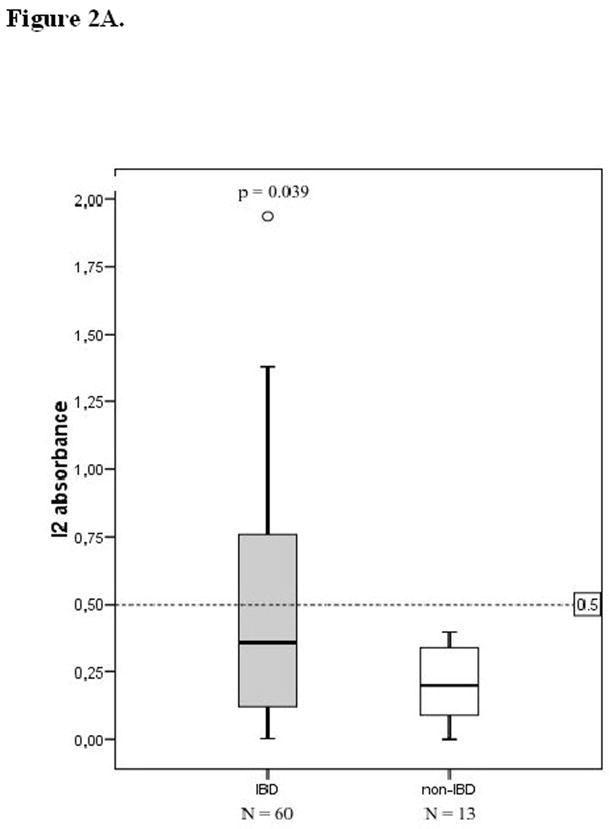
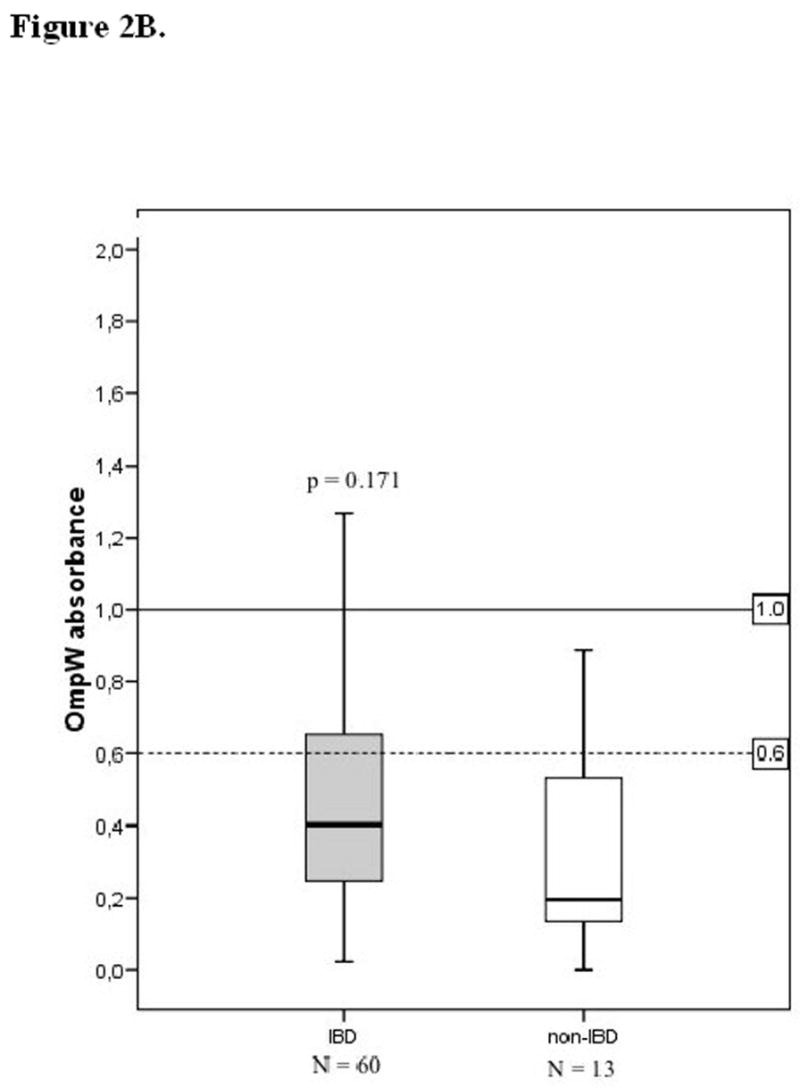
Serological responses to the Pseudomonas fluorescens-associated sequence I2 (A) and Bacteroides caccae TonB-linked outer membrane protein OmpW (B) in children and adolescents according to their diagnosis into inflammatory bowel disease (IBD) and non-IBD group. Horizontal lines denote the cut-off values for seropositivity (see text). P values compare absorbances between the IBD and non-IBD groups, using the Mann-Whitney U-test.
FIGURE 3.
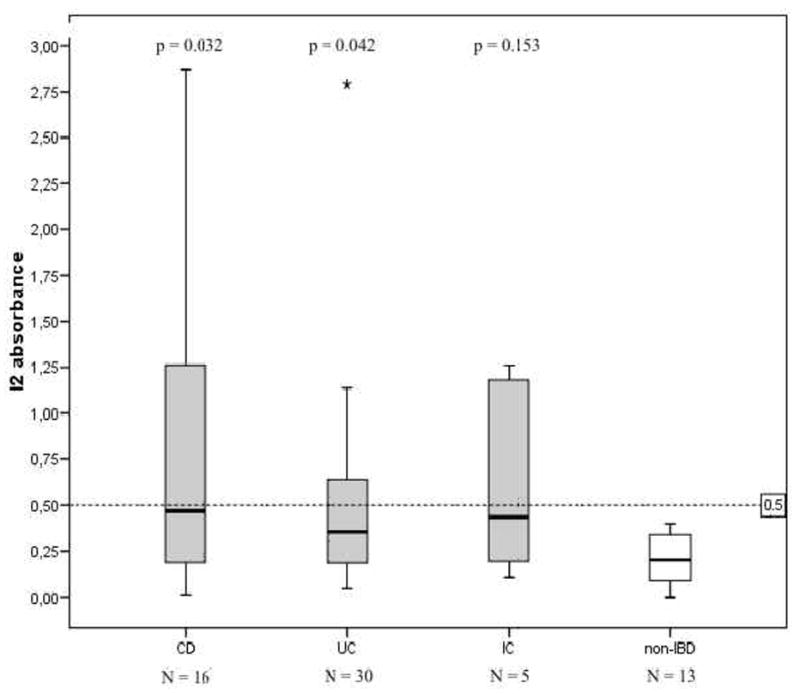
Serological responses to the Pseudomonas fluorescens-associated sequence I2 in 51 children and adolescents with inflammatory bowel disease (IBD) at the time of diagnosis. Patients are grouped according to their final diagnosis into Crohn’s disease (CD), ulcerative colitis (UC), indeterminate colitis (IC) and non-IBD patients. Horizontal lines denote the cut-off value for seropositivity (see text). P values compare absorbances between the IBD and non-IBD groups, using the Mann-Whitney U-test.
ASCA antibodies were detected in 67% (12/18) of patients with CD. Serum titres of IgA and IgG ASCA were significantly elevated in children and adolescents with CD as compared to the patients with non-IBD disease (p=0.001 and p=0.007, respectively). Three out of six CD patients negative for ASCA showed seropositivity to I2 and/or OmpW. The combination of ASCA, OmpW and I2 tests was able to detect 83% of all CD patients and 61% of all UC patients and 46% of patients with non-IBD disease. Among UC patients 14% (5/36) of the children and adolescents and 50% (3/6) with IC were ASCA-positive (Table 2). Serum IgA and IgG ASCA titres were not significantly higher in UC patients than in patients with non-IBD disease however (p=0.226 and p=0.461, respectively). IgA ASCA titres were significantly increased in IC patients compared to patients excluded for IBD (p=0.041). Fifty-six per cent (20/36) of UC patients were positive for I2 and/or OmpW antibodies. Fourteen UC cases were negative for all serological tests (ASCA, I2, OmpW) used in this study. Samples of seven out of those 14 UC patients were available for fecal calprotectin measurement. Fifty-seven per cent (4/7) of those turned out to have positive (>100ug/g) levels. Four UC patients were positive only for OmpW antibodies in serological testing, Figure 4. Altogether, the combination of the measurements identified 100% (15/15) of CD patients and 88% (22/25) of UC patients who were available for all four tests.
FIGURE 4.
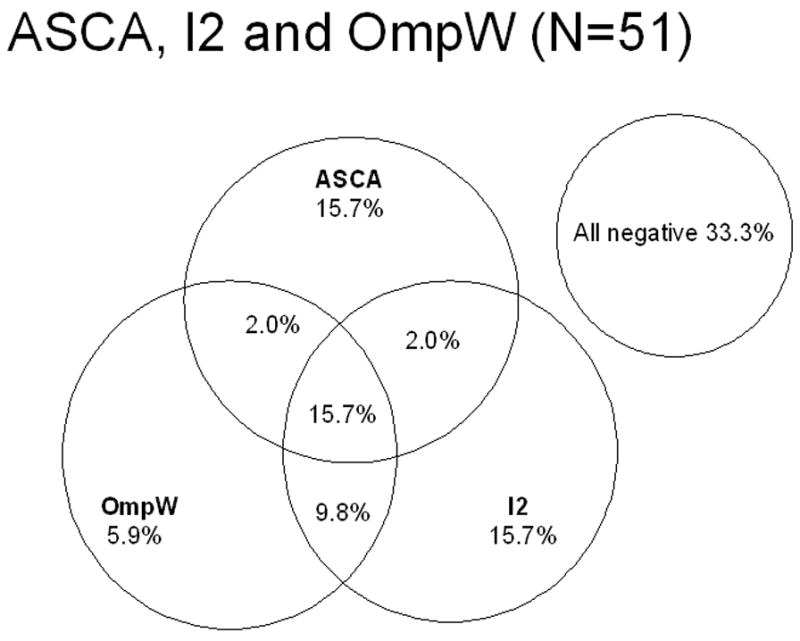
Relationship between serum antibodies to Saccharomyces cerevisiae, the Pseudomonas fluorescens-associated sequence I2 and to a Bacteroides caccae TonB-linked outer membrane protein, OmpW and 51 inflammatory bowel disease (IBD) patients at the time of primary diagnosis by presence vs. absence. The percentage of patients positive for any of each marker and of two or all three markers is presented.
When analyzing the whole IBD group 66% of patients were seropositive toward at least one of the microbial antigens (ASCA, I2, OmpW) at the time of primary diagnosis (Figure 4). The best three test combinations concerning relationship between serum antibodies to microbial antigens and fecal calprotectin in IBD patients by presence vs. absence were examined. Samples of 37 IBD patients were available for testing of all four tests (ASCA, I2, OmpW and fecal calprotectin) at the time of primary diagnosis. By testing serology (ASCA, I2, OmpW), 76% of patients were positive at least in one of the tests (Figure 5). Fecal calprotectin test alone was positive in 87% of IBD patients at the time of diagnosis. When selecting fecal calprotectin and any two out of three serological tests, 89–92% of IBD patients showed positivity at least in one of the tests. The combination of these four non-invasive tests was highly sensitive at the time of diagnosis for IBD (sensitivity 92%, specificity 36%, PPV 83% and NPV 57%) and also for subgroups; CD (sensitivity 100%, specificity 36%, PPV 63% and NPV 100%), UC (sensitivity 87%, specificity 36%, PPV 74% and NPV 57%) and IC (sensitivity 100%, specificity 36%, PPV 22% and NPV 100%).
DISCUSSION
There is a need, especially in children, for non-invasive, sensitive and specific tools to improve the early diagnosis of IBD. Fecal calprotectin is remarkably stable and easy to detect in stool. Calprotectin levels have been shown to correlate well with the results of invasive measures of colonic and small bowel inflammation in pediatric IBD. 3 We confirm the results of previous reports by showing that the frequency of positive (>100ug/g) fecal calprotectin levels is significantly increased in children and adolescents with IBD compared with those with no intestinal inflammation. 5–7, 30–32 The highest levels of calprotectin were found in patients with CD, which may refer to different histopathology and pathogenesis of CD and UC 31
Serological markers are promising in the diagnosis of IBD and in the differentiation of CD and UC. ASCA is thought to be a specific marker of CD as several studies have provided evidence of seroreactivity to ASCA both in adult and pediatric CD patients 7–13, 27–28, 33–34 Our study agrees with the previous ones as ASCA positivity was found in 65% of patients with CD. Interestingly, ASCA positivity was also observed in a small proportion of UC patients (14% and in three children with non-IBD disease. These patients, especially those with non-IBD disease, are of major interest for follow-up because it has been suggested that ASCA is an early marker preceding the onset of clinical disease. 17
Recently, the variety of serologic markers for IBD has expanded and antibodies directed to various bacterial antigens are under continuous investigation. 21–26, 34 High prevalence of antibodies to Pseudomonas fluorescens-associated sequence I2 in the sera has been pointed out in patients with CD. 23, 26, 36 In the present study, the frequency of anti-I2 antibodies was significantly increased in children and adolescents with IBD. Concordant with our previous findings, positive seroreactivity to a Bacteroides caccae TonB-linked outer membrane protein OmpW was frequent in children with CD and with UC (61%, 42%, respectively). 26 Alone the prevalence of anti-OmpW antibodies was not significantly elevated in IBD subgroups. However, one possibility is that serum OmpW-antibodies could be useful to identify those few cases who are negative for all other serological tests.
To support this idea, a CD patient subset has been reported in which the IgA response to the E. Coli outer membrane porin C (OmpC) was suggested to have clinical utility in diagnosing IBD, specifically in ASCA negative patients. 23–25, 36 We have previously pointed out that most ASCA negative children with CD and pANCA-negative patients with UC express positive seroreactivity to OmpW and/or I2. 26 In the present study, 50% of ASCA-negative CD patients had elevated levels of serum anti- I2 and/or anti-OmpW antibodies. In addition, unlike adult IBD patients (in whom bacterial seromarkers are uncommon) 53% of pediatric UC patients were positive for serum anti- I2 and/or anti-OmpW antibodies. Recently, reactivity to bacterial flagellins (CBir) has also been found to be characteristic for CD patients. 36–39 Thus, inclusion of these and other emerging bacterial seromarkers of IBD may further improve the specificity and sensitivity of early diagnosis of both pediatric UC and CD.
The key conclusion of this study is that the combination of fecal calprotectin levels with bacterial seromarkers increases the sensitivity of noninvasive identification of children and adolescents with IBD. Fecal calprotectin measurement together with serological tests (ASCA, I2, OmpW) showed high sensitivity in detecting IBD patients (92%), both CD and UC (100%, 87%, respectively) in this patient cohort. The fecal calprotectin test showed the best sensitivity and specificity for IBD than any other test alone.
As shown here, marked serological responses to microbial antigens are evident among children and adolescents with IBD suggesting that immune responses to commensal enteric bacteria have an important role in the induction of mucosal damage in the pathogenesis of chronic bowel inflammation. In clinical practice, non-invasive, sensitive and specific tools for early detection of IBD are needed and serological specificities together with fecal calprotectin measurement provide valuable help in the diagnostics of the disease. Noninvasive testing may decrease unnecessary invasive procedures and further, facilitate clinical decision-making when the work up of IBD in children is initially uncertain. Early identification of the subgroups of IBD patients is important also because it has been shown that seroreactivity to microbial components in Crohn’s disease is associated with disease severity, progression and the frequency of complications. 40–41 Additionally, it has also been suggested that a proportion of IBD patients may benefit from a relatively aggressive therapeutic approach and this could even decrease the necessity for surgery in the future. 42 The positivity of any of the present tests (ASCA, I2, OmW, fecal calprotectin) should encourage investigators for further examinations in aiming to the final diagnosis and addressing the treatment.
Supplementary Material
Acknowledgments
This study was supported by grants from the Paediatric Research Foundation, The Competitive Research Funding of the Pirkanmaa Hospital District, and NIH PO1-DK 46763 (J.B.). The authors would like to acknowledge Ms Sari Honkanen for excellent assistance in recruitment of the patients and Ms Marja-Leena Koskinen for technical support.
References
- 1.Roseth AG, Schmidt PN, Fagerhol MK. Correlation between faecal excretion of indium-111-labelled granulocytes and calprotectin, a granulocyte marker protein, in patients with inflammatory bowel disease. Scand J Gastroenterol. 1999;34:50–4. doi: 10.1080/00365529950172835. [DOI] [PubMed] [Google Scholar]
- 2.Tibble JA, Sighthorsson G, Bridger S, et al. Surrogate markers of intestinal inflammation are predictive of relapse in patients with inflammatory bowel disease. Gastroenterology. 2000;119:15–22. doi: 10.1053/gast.2000.8523. [DOI] [PubMed] [Google Scholar]
- 3.Bunn SK, Bisset WM, Main MJ, et al. Fecal calprotectin: validation as a non-invasive measure of bowel inflammation in childhood inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2001;33:14–22. doi: 10.1097/00005176-200107000-00003. [DOI] [PubMed] [Google Scholar]
- 4.Aadland E, Fagerhol MK. Faecal calprotectin: a marker of inflammation throughout the intestinal tract. Eur J Gastroenterol Hepatol. 2002;14:823–5. doi: 10.1097/00042737-200208000-00002. [DOI] [PubMed] [Google Scholar]
- 5.Olafsdottir E, Aksnes L, Fluge G, et al. Faecal calprotectin levels in infants with infantile colic, healthy infants, children with inflammatory bowel disease, children with recurrent abdominal pain and healthy children. Acta Paediatr. 2002;91:45–50. doi: 10.1080/080352502753457932. [DOI] [PubMed] [Google Scholar]
- 6.Wassell J, Dolwani S, Metzner M, et al. Faecal calprotectin: a new marker for Crohn’s disease? Ann Clin Biochem. 2004;41:230–2. doi: 10.1258/000456304323019613. [DOI] [PubMed] [Google Scholar]
- 7.Angriman I, Scarpa M, D’Inca R, et al. Enzymes in feces: useful markers of chronic inflammatory bowel disease. Clin Chim Acta. 2007;381:63–8. doi: 10.1016/j.cca.2007.02.025. [DOI] [PubMed] [Google Scholar]
- 8.McKenzie H, Main J, Pennington CR, et al. Antibody to selected strains of Saccharomyces cerevisiae (baker’s and brewer’s yeast) and Candida albicans in Crohn’s disease. Gut. 1990;31:536–538. doi: 10.1136/gut.31.5.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vasiliauskas EA, Kam LY, Karp LC, et al. Marker antibody expression stratifies Crohn’s disease into immunologically homogeneous subgroups with distinct clinical characteristics. Gut. 2000;47:487–496. doi: 10.1136/gut.47.4.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dubinsky MC, Ofman JJ, Urman M, et al. Clinical utility of serodiagnostic testing in suspected pediatric inflammatory bowel disease. Am J Gastroenterol. 2001;96:758–765. doi: 10.1111/j.1572-0241.2001.03618.x. [DOI] [PubMed] [Google Scholar]
- 11.Bartunkova J, Kolarova I, Sediva A, et al. Antineutrophil cytoplasmic antibodies, anti-Saccharomyces cervisie antibodies, and specific IgE to food allergens in children with inflammatory bowel disease. Clinical Immunology. 2002;102:162–168. doi: 10.1006/clim.2001.5145. [DOI] [PubMed] [Google Scholar]
- 12.Reese GE, Constantinides VA, Simillis C, et al. Diagnostic precision of anti-Saccharomyces cerevisiae antibodies and perinuclear antineutrophil cytoplasmic antibodies in inflammatory bowel disease. Am J Gastroenterol. 2006;101:2410–22. doi: 10.1111/j.1572-0241.2006.00840.x. [DOI] [PubMed] [Google Scholar]
- 13.Desplat-Jego S, Johanet C, Escande A, et al. Update on Anti-Saccharomyces cerevisiae antibodies, anti-nuclear associated anti-neutrophil antibodies and antibodies to exocrine pancreas detected by indirect immunofluorescence as biomarkers in chronic inflammatory bowel diseases: Results of a multicenter study. World J Gastroenterol. 2007;13:2312–2318. doi: 10.3748/wjg.v13.i16.2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saxon A, Shanahan F, Landers CJ, et al. A distinct subset of antineutrophil cytoplasmic antibodies is associated with inflammatory bowel disease. J Allergy Clin Immunol. 1990;86:202–210. doi: 10.1016/s0091-6749(05)80067-3. [DOI] [PubMed] [Google Scholar]
- 15.Cohavy O, Harth G, Horwitz M, et al. Identification of a novel mycobacterial histone H1 homologue (HupB) as an antigenic target of pANCA monoclonal antibody and serum immunoglobulin A from patients with Crohn’s disease. Infect Immun. 1999;67:6510–6517. doi: 10.1128/iai.67.12.6510-6517.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cohavy O, Bruckner D, Gordon LK, et al. Colonic bacteria express an ulcerative colitis pANCA-related protein epitope. Infect Immun. 2000;68:1542–1548. doi: 10.1128/iai.68.3.1542-1548.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Israeli E, Grotto I, Gilburd B, et al. Anti-Saccharomyces cerevisiae and antineutrophil cytoplasmic antibodies as predictors of inflammatory bowel disease. Gut. 2005;54:1232–36. doi: 10.1136/gut.2004.060228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sendid B, Quinton JF, Charrier G, et al. Anti-Saccharomyces cerevisiae mannan antibodies in familial Crohn’s disease. Am J Gastroenterol. 1998;93:1306–10. doi: 10.1111/j.1572-0241.1998.00415.x. [DOI] [PubMed] [Google Scholar]
- 19.Vermeire S, Peeters M, Vlietinck R, et al. Anti-Saccharomyces cerevisiae antibodies (ASCA), phenotypes of IBD, and intestinal permeability: A study in IBD families. Inflamm Bowel Dis. 2001;7:8–15. doi: 10.1097/00054725-200102000-00002. [DOI] [PubMed] [Google Scholar]
- 20.Sutton CL, Kim J, Yamane A, et al. Identification of a novel bacterial sequence associated with Crohn’s disease. Gastroenterology. 2000;119:23–31. doi: 10.1053/gast.2000.8519. [DOI] [PubMed] [Google Scholar]
- 21.Wei B, Huang T, Dalwadi H, et al. Pseudomonas fluorescens encodes the Crohn’s disease-associated I2 sequence and T-cell superantigen. Infect Immunol. 2002;70:6567–6575. doi: 10.1128/IAI.70.12.6567-6575.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wei B, Dalwadi H, Gordon LK, et al. Molecular cloning of a Bacteroides caccae TonB-linked outer membrane protein identified by an inflammatory bowel disease marker antibody. Infect Immun. 2001;69:6044–6054. doi: 10.1128/IAI.69.10.6044-6054.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Landers CJ, Cohavy O, Misra R, et al. Selected loss of tolerance evidenced by Crohn’s disease-associated immune responses to auto- and microbial antigens. Gastroenterology. 2002;123:689–699. doi: 10.1053/gast.2002.35379. [DOI] [PubMed] [Google Scholar]
- 24.Zholudev A, Zurakowski D, Young W, et al. Serologic testing with ANCA, ASCA, and anti-OmpC in children and young adults with Crohn’s disease and ulcerative colitis: diagnostic value and correlation with disease phenotype. Am J Gastroenterol. 2004;99:2235–2241. doi: 10.1111/j.1572-0241.2004.40369.x. [DOI] [PubMed] [Google Scholar]
- 25.Jaskowski TD, Litwin CM, Hill HR. Analysis of serum antibodies in patients suspected of having inflammatory bowel disease. Clin Vaccine Immunol. 2006 Jun;13(6):655–60. doi: 10.1128/CVI.00034-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Iltanen S, Tervo L, Halttunen T, Wei B, Braun J, Rantala I, Honkanen T, Kronenberg M, Cheroutre H, Turovskaya O, Autio V, Ashorn M. Elevated serum anti-I2 and anti-OmpW antibody levels in children with IBD. Inflamm Bowel Dis. 2006;12:389–94. doi: 10.1097/01.MIB.0000218765.84087.42. [DOI] [PubMed] [Google Scholar]
- 27.Arnott ID, Landers CJ, Nimmo EJ, et al. Sero-reactivity to microbial components in Crohn’s disease is associated with disease severity and progression, but not NOD2/CARD15 genotype. Am J Gastroenterol. 2004;99:2376–2384. doi: 10.1111/j.1572-0241.2004.40417.x. [DOI] [PubMed] [Google Scholar]
- 28.Evans CM, Beattie RM, Walker-Smith JA. Inflammatory bowel disease in childhood. In: Allan RN, Rhodes JM, Hanauer SB, editors. Inflammatory bowel diseases. 3. New York: Churchill Livingstone; 1997. pp. 647–670. [Google Scholar]
- 29.Dixon FM, Genta RM, Yardley JH, et al. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol. 1996;20:1161–1181. doi: 10.1097/00000478-199610000-00001. [DOI] [PubMed] [Google Scholar]
- 30.Kolho KL, Raivio T, Lindahl H, et al. Fecal calprotectin remains high during glucocorticoid therapy in children with inflammatory bowel disease. Scand J Gastroenterol. 2006;41:720–5. doi: 10.1080/00365520500419623. [DOI] [PubMed] [Google Scholar]
- 31.Amati L, Passeri ME, Selicato F, et al. New insights into the biological and clinical significance of fecal calprotectin in inflammatory bowel disease. Immunopharmacol Immunotoxicol. 2006;28:665–81. doi: 10.1080/08923970601067326. [DOI] [PubMed] [Google Scholar]
- 32.von Roon AC, Karamountzos L, Purkayastha S, et al. Diagnostic precision of fecal calprotectin for inflammatory bowel disease and colorectal malignancy. Am J Gastroenterol. 2007;102:803–13. doi: 10.1111/j.1572-0241.2007.01126.x. [DOI] [PubMed] [Google Scholar]
- 33.Riis L, Vind I, Vermeire S, et al. The prevalence of genetic and serologic markers in an unselected European population-based cohort of IBD patients. Inflamm Bowel Dis. 2007;13:24–32. doi: 10.1002/ibd.20047. [DOI] [PubMed] [Google Scholar]
- 34.Papp M, Norman GL, Altorjay I, et al. Utility of serological markers in inflammatory bowel diseases: Gadget or magic? World J Gastroenterol. 2007;13:2028–36. doi: 10.3748/wjg.v13.i14.2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Melmed GY, Elashoff R, Chen GC, et al. Predicting a change in diagnosis from ulcerative colitis to Crohn’s disease: a nested, case-control study. Clin Gastroenterol Hepatol. 2007;5:602–8. doi: 10.1016/j.cgh.2007.02.015. [DOI] [PubMed] [Google Scholar]
- 36.Dubinsky MC, Lin YC, Dutridge D, et al. Serum immune responses predict rapid disease progression among children with Crohn’s disease: immune responses predict disease progression. Am J Gastroenterol. 2006;101:360–7. doi: 10.1111/j.1572-0241.2006.00456.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lodes MJ, Cong Y, Elson CO, et al. Bacterial flagellin is a dominant antigen in Crohn disease. J Clin Invest. 2004;113:1296–1306. doi: 10.1172/JCI20295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Targan SR, Landers CJ, Yang H, et al. Antibodies to Cbir1 flagellin define a unique response that is associated independently with complicated Crohn’s disease. Gastroenterology. 2005;128:2020–2028. doi: 10.1053/j.gastro.2005.03.046. [DOI] [PubMed] [Google Scholar]
- 39.Bossuyt X. Serologic markers in inflammatory bowel disease. Clin Chem. 2006;52:171–81. doi: 10.1373/clinchem.2005.058560. [DOI] [PubMed] [Google Scholar]
- 40.Mow WS, Vasiliauskas EA, Lin YC, et al. Association of antibody responses to microbial antigens and complications of small bowel Crohn’s disease. Gastroenterology. 2004;126:414–424. doi: 10.1053/j.gastro.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 41.Ferrante M, Henckaerts L, Joossens M, et al. New serological markers in inflammatory bowel disease are associated with complicated disease behaviour. Gut. 2007;56:1394–403. doi: 10.1136/gut.2006.108043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Forcione DG, Rosen MJ, Kisiel JB, et al. Anti-Saccaharomyces cerevisiae antibody (ASCA) positivity is associated with increased risk for surgery. Gut. 2004;53:1117–22. doi: 10.1136/gut.2003.030734. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


