Abstract
Since the discovery of leukemic stem cells (LSCs) over a decade ago, many of their critical biological properties have been elucidated, including their distinct replicative properties, cell surface phenotypes, their increased resistance to chemo-therapeutic drugs and the involvement of growth-promoting chromosomal translocations. Of particular importance is their ability to transfer malignancy to non-obese diabetic-severe combined immunodeficient (NOD-SCID) mice. Furthermore, numerous studies demonstrate that acute myeloid leukemia arises from mutations at the level of stem cell, and chronic myeloid leukemia is also a stem cell disease. In this review, we will evaluate the main characteristics of LSCs elucidated in several well-documented leukemias. In addition, we will discuss points of therapeutic intervention. Promising therapeutic approaches include the targeting of key signal transduction pathways (for example, PI3K, Rac and Wnt) with small-molecule inhibitors and specific cell surface molecules (for example, CD33, CD44 and CD123), with effective cytotoxic antibodies. Also, statins, which are already widely therapeutically used for a variety of diseases, show potential in targeting LSCs. In addition, drugs that inhibit ATP-binding cassette transporter proteins are being extensively studied, as they are important in drug resistance—a frequent characteristic of LSCs. Although the specific targeting of LSCs is a relatively new field, it is a highly promising battleground that may reveal the Holy Grail of cancer therapy.
Keywords: drug transporters, drug resistance, tumor-initiating cell, stem cells, targeted therapy
Overview of hematopoietic and leukemic stem cells
Perhaps the Holy Grail in cancer therapy today is the cancer stem cell (CSC) (also known as the cancer-initiating cell). Tumors possess a minor fraction of CSC, which maintains the propagation of the disease. In many cancer types, it is difficult to completely eliminate the CSC and reoccurrence of the cancer usually occurs. In the following review, we will discuss the leukemic stem cell (LSC) (also known as leukemia-initiating cell). Normal hematopoietic stem cells (HSCs) were first described by Till and McCulloch in 1961.1 Stem cells can be broadly defined by their potential for self-renewal and ability to proliferate and differentiate into diverse cell types. HSCs comprise a very small, but critical, sub-population of the total number of hematopoietic cells, making up less than 0.01% of cells in the bone marrow.2 An overview of the HSC and LSC is presented in Figure 1.
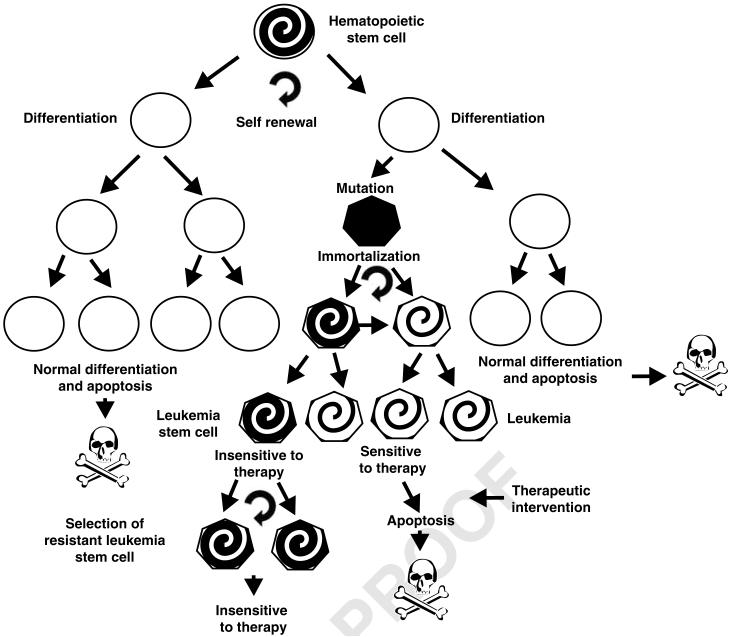
Figure 1.
Overview of normal HSCs and LSCs. LSCs originate from HSCs after various complex genetic mutations. The normal HSC differentiates into functional cells of the lymphoid and myeloid lineages, which perform their biological functions and normally die by apoptosis after an appropriate time period. In contrast, LSCs acquire mutations that allow them to grow and persist in the presence of chemotherapeutic drugs, whereas the majority of the leukemic cells that arise after mutation are unable to continue to proliferate after chemotherapy. Understanding the difference between the LSC and the majority of leukemia that are not LSC may allow the generation of more effective therapeutic strategies. The spiral shading in the HSC, LSC and leukemia cells indicates their ability to propagate indefinitely. The circular arrow indicates self-renewal.
In 1994, Dick and co-workers3 disassociated LSCs from the bulk of acute myeloid leukemia (AML) cells. The immature LSCs resided within the CD34+ CD38- sub-population and were only a small fraction of the total leukemic blasts. Importantly, LSCs were able to transmit AML to non-obese diabetic-severe combined immunodeficient (NOD-SCID) mice and repopulate the bone marrow of the irradiated recipients.4 In contrast, CD34+ CD38+ AML cells were not competent in engrafting in NOD-SCID mice. NOD-SCID mice were shown to be superior in comparison with SCID mice for the engraftment of AML and hence continue to be used extensively to investigate the differences between normal and LSC.5
The study performed in the Dick laboratory, along with similar studies by other investigators, led to the formulation of the CSC hypothesis, which postulates that tumors are maintained by a small minority of stem-like cancer cells, which possess the capacity for indefinite self-renewal. Functionally, CSCs are often defined by their capacity to transfer the malignancy to NOD-SCID mice. Since this initial discovery, CSCs have been documented in several other cancers including solid tumors.5 The studies on LSCs and CSCs have revolutionized current perceptions regarding cancer occurrence and therapeutic options.
Differences between LSCs and leukemia cells
Numerous investigations have provided evidence that AML arises from genetic mutations. It is not currently known for certain whether the mutations occur in the normal stem cells or in more differentiated cell types, which then acquire stem cell-like features. In studies by Morrison and Weissman,6 using lentiviruses, long-term (LT)-LSC and short-term (ST)-LSC cells was characterized, which have LT and ST repopulating potentials, respectively.
In other studies by Dick et al.,4,7 using clonal tracking by lentiviruses, LT-LSC and ST-LSC cells was further characterized. LT-LSCs are defined by a long-termed persistence in a xenotransplant murine model and are characterized by extensive self-renewal capacity, whereas ST-LSCs possess a reduced self-renewal and show only a transient repopulation capability. LT-LSCs can give rise to ST-LSCs. Clearly, the definition of LT-LSC and ST-LSC was derived from their repopulation capability in a murine model, but this hierarchy of LSC provides a good explanation for the emergence of a primary leukemia and its relapse in humans. Apart from their repopulation capabilities, AML LSCs in general can been identified by an aberrant CD expression pattern (see below). Conclusive discriminative parameters between LT-LSC and ST-LSC apart from of their proliferative characteristics have not been defined so far.
Some studies suggest that the original target of cellular transformation for the LSC arise from the HSC compartment.8 However, other studies by Cozzio et al.,9 using lethally irradiated recipient mice, have demonstrated that the oncogenic MLL-ELL and MLL-GAS7 chromosomal translocations can transform not only HSCs but also myeloid-restricted progenitors that lack self-renewal capacity. These MLL-ELL- and MLL-GAS7-transfected cells have LSC-like properties.9 Other oncogenic chromosomal translocations, such as MLL-ENL, are able to transform HSCs, but not differentiated progenitors into LSCs.10 This demonstrates that although the myeloid-restricted progenitor can be a transformation site in AML, it may not be a frequent site and it may depend on the particular chromosomal translocation.8 Some translocations such as MLL-ELL also result in an increase in the LSC pool. A comparison of LSC with leukemia cells is presented in Figure 2.
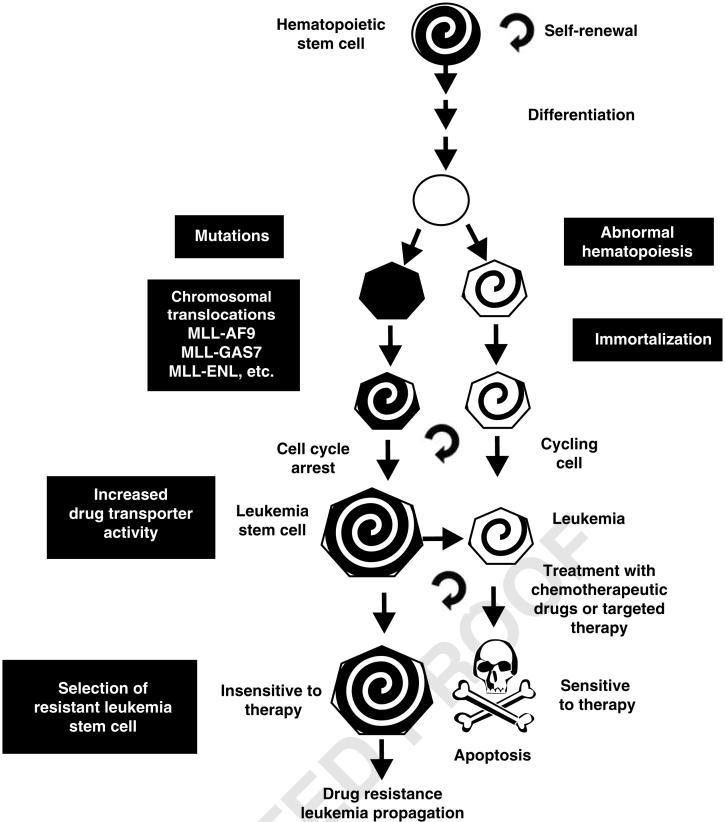
Figure 2.
Comparison of LSC and leukemic cells. Although both LSC and leukemic cells originate from HSC, they display distinct differences in terms of cell cycle status and drug transporter activity, which may be responsible for the difference in susceptibility to chemotherapeutic approaches.
Regardless of knowing the precise cell type where the original genetic mutation occurred, stem cells most likely pose both the key and barrier to discovering effective leukemia therapies. Although stem cells have the potential for self-renewal, they spend the majority of their time in the G0 phase of the cell cycle. This means that chemotherapeutic drugs, which act on cycling cell populations, such as 5-fluouracil, are less effective on LSCs than on regular leukemic cells.11 The quiescent, non-cycling state of LSCs may contribute to their resistance to conventional chemotherapeutics. Conventional chemotherapeutic drugs that target leukemic cells have been shown to be ineffective in completely eradicating LSCs. This quiescent nature of LSCs may result in low rates of long-term remission and multidrug resistance.
LSCs
Leukemias are characterized by the uncontrolled overproduction of leukocytes, which can be immature or differentiated. Acute leukemias consist of either immature progenitor cells without lineage differentiation or immature but already lineage-determined cells (for example, acute promyelocytic leukemia). Chronic leukemias are characterized by mature cells, for example, chronic myeloid leukemia (CML), which compromise mature granulocytes, and chronic lymphocytic leukemia (CLL), which comprise terminally differentiated cells. Leukemia can be further classified on the basis of the origin of malignant leukocytes. Cells of granulocyte, monocyte, erythroid or megakaryocyte lineage are considered of myeloid origin, whereas B cells, T cells and natural killer cells are considered of lymphoid origin.5
Although LSCs in AML were the earliest documented and the best characterized, stem cells exist and have also been discovered in various other leukemias including, CML, acute lymphoblastic leukemia (ALL) and CLL. Using a variety of techniques, including cytogenetics, fluorescence in situ hybridization and expression profiling by RNA analysis (reverse transcriptase-PCR), stem cells from the various leukemias have been isolated and phenotypically characterized.8 An overview of the phenotypic differences between AML stem cells and AMLs is presented in Figure 3.
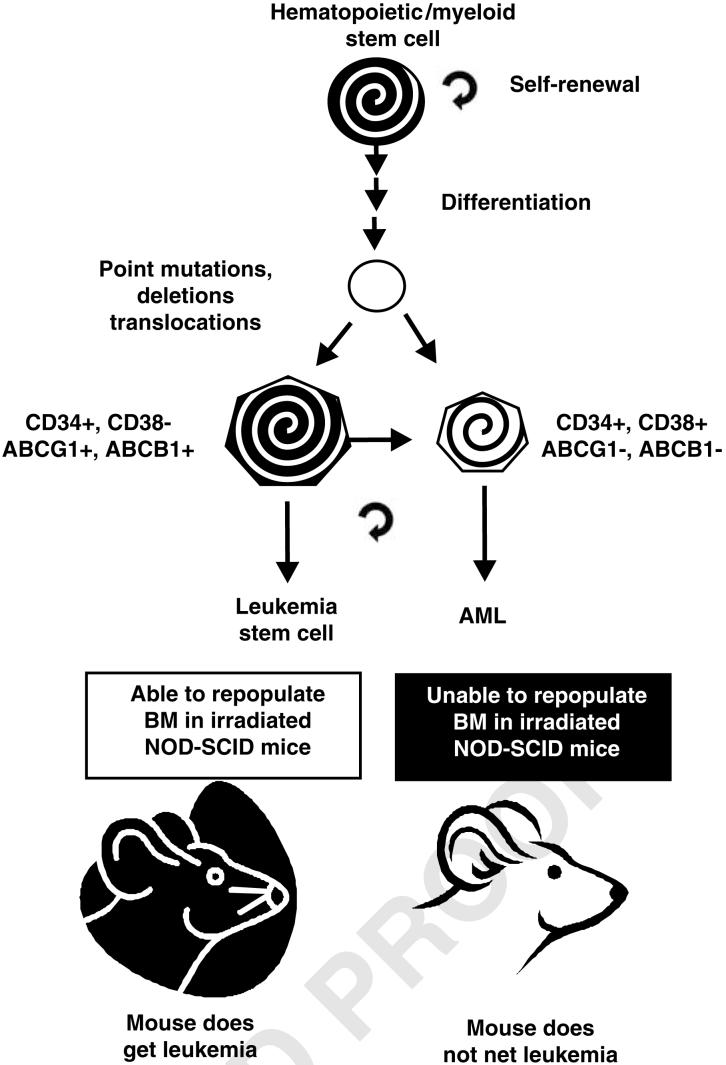
Figure 3.
Phenotypic difference between AML LSC and AMLs. The bulk of leukemic AMLs differ from AML stem cells in the expression of CD38+ and other genes. This figure is a simplification as some AML (e.g., AML FAB M3; APL) are CD34-. Functionally, these two types of cells also differ in their ability to repopulate NOD-SCID mice. NOD-SCID, non-obese diabetic-severe combined immunodeficient.
CML and CML-LSC
CML is often characterized by the overproduction of mature myeloid cells. It is further subdivided into three distinct phases, namely chronic phase (CP), acute phase (AP) and blast crisis (BC), which is phenotypically similar to AML. Whereas LSCs in AML are biologically and functionally distinct compared with HSC, LSCs in CML are often phenotypically similar to normal HSCs. CML stem cells are present in CD34+ CD38- cells and contain the Philadelphia chromosome. The Philadelphia chromosome contains the chromosomal translocation between the break point cluster region gene and the gene encoding c-Abl (BCR-ABL). The protein products of the BCR-ABL chromosomal translocation are p210BCR-ABL and p190BCR-ABL. Patients with CML have a clonal expansion of hematopoietic cells that express these BCR-ABL proteins. BCR-ABL expression has been determined to be essential for the sustained proliferation of leukemic cells in murine models.12 Current treatment of CML uses ABL kinase inhibitors, and to a much lesser extent chemotherapy, HSC transplantation and interferon-α. Problems with current therapy include acquired resistance to kinase-inhibitor therapy and a lack of sustained molecular remissions.5 An overview of the phenotypic differences between CML stem cells and CML is presented in Figure 4.
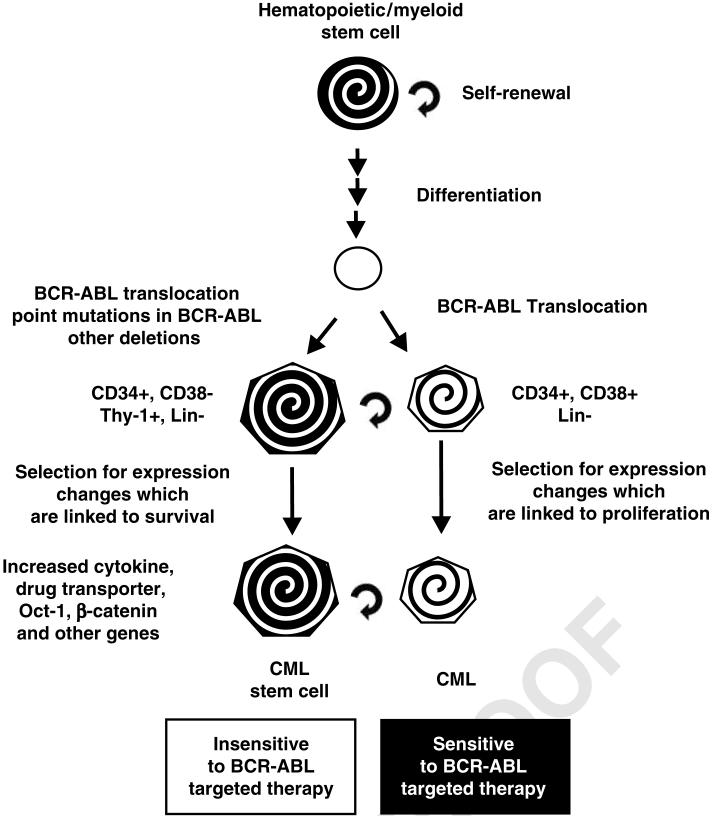
Figure 4.
Phenotypic differences between CML LSC and CMLs. Although both CML-stem cells and the CML cells contain the BCR-ABL chromosomal translocation, they differ in their proliferative capacity in the presence of certain BCR-ABL inhibitors. This may be due to the presence of certain pre-existing mutations in the BCR-ABL gene in the CML-LSCs. In addition, the CML-LSCs display differences in cytokine expression (IL-3, G-CSF), which may promote autocrine proliferation. The CML-LSC also displayed elevated transporter, Oct-1 and β-catenin pathway expression, which may result in altered proliferation in comparison with CML cells.
CML stem cells are CD90+, Thy1+ and Lin-. BC-CML patients have higher levels of the progenitor pool (CD34+ Lin- cells) than other CML patients. CML patients who are responsive to BCR-ABL-targeted therapy have fewer CD34+ Lin- cells in comparison with patients non-responsive to BCR-ABL inhibitors. Although the β-catenin pathway is equally activated in normal vs CML stem cells, this pathway was determined to be overexpressed in patients resistant to BCR-ABL inhibitors,13,14 and it will be further discussed below. The differences in response in patients resistant to BCR-ABL kinase inhibitors as compared with those not resistant imply the acquisition of self-renewal capacity and other selective genetic changes in granulocyte-macrophage progenitors present in the CML stem cells.13 These results demonstrate the complex differences between CML stem cells and the bulk of CML cells. Although both cell types display enhanced proliferative capacity in comparison with normal myeloid cells, they display different proliferative capacities in the presence of BCR-ABL inhibitors, which may be due to the presence of discrete additional mutations in the BCR-ABL gene present in the CML LSC (see below) or selection as a result of treatment.
Some of these CML LSCs display elevated BCR-ABL expression and changes in the expressions of the cytokines interleukin-3 (IL-3), granulocyte colony-stimulating factor and the drug transporters ATP-binding cassette-1 (ABC1)/multi-drug resistance-1 (MDR1), ABCG2 and the transcription factor Oct-1 upon culture with reduced levels of growth factors.14 Additional studies by Jiang et al.15 demonstrated that more than 70 different BCR-ABL mutations were present in the progeny of cultured CML LSCs. This group has hypothesized that CML patients possess LSCs, which have pre-existing BCR-ABL kinase mutations before the advent of BCR-ABL inhibitor therapy; hence, the patients already have some resistant CML stem cells. As the CML LSCs proliferate slowly, the patient may initially respond to therapy, then given time, the CML LSCs that are resistant to BCR-ABL inhibitors emerge (see mathematical models for CML presented below). This karyotype evolution of resistant cells from pre-existing CML stem cells, which already have mutations in BCR-ABL, is a lingering problem in BCR-ABL-directed therapy.15 As CML LSCs, like other LSCs, proliferate slowly, or are in a quiescent-like state, some investigators have proposed that a means to target these cells is to stimulate their proliferation and then treat with BCR-ABL inhibitors.16,17 However, this therapeutic approach will only be appropriate if the CML LSCs do not contain the T315I BCR-ABL or a similar mutation, which confers resistance to many BCR-ABL inhibitors.
Mathematical modeling provides evidence for CML LSCs
Upon careful mathematical analysis of the incidence data of CML and reappearance of the disease after the discontinuation of therapy, it has been predicted that there are CML LSC that are resistant to BCR-ABL inhibitors such as imatinib.18-21 Basically, if one examines the decrease in BCR-ABL mRNA transcripts upon and during the course of imatinib treatment, one observes a decrease in a low, constant level of BCR-ABL mRNA transcripts as long as the drug imatinib is effective; however, if the patient is taken off imatinib therapy, there is a dramatic and rapid rebound in the level of BCR-ABL mRNA transcripts, which actually may exceed the initial levels of BCR-ABL mRNA transcripts. These results suggest the existence of CML LSCs in the CML patients who are not eliminated by the imatinib treatment. In addition, this model provides a coherent explanation of the biphasic decrease in the BCR-ABL transcript level upon initial therapy with imatinib. First, imatinib rather quickly eliminates the terminally differentiated leukemic cells with an average lifespan of around 20 days, which leads to a rapid decrease in the BCR-ABL transcript levels. After elimination of these cells, the less-mature leukemic progenitors with an average lifespan of 125 days under imatinib therapy are responsible for the slower but continued decrease in the BCR-ABL transcripts. The immature CML LSCs are not sensitive to imatinib, even expand slowly over time at a slow rate and are responsible for a rapid rebound of the leukemic burden upon imatinib cessation, which can exceed the initial level. Furthermore, if there are mutations in the BCR-ABL gene that confers resistance to imatinib, the emergence of this clone(s) appears and levels of BCR-ABL mRNA transcripts increase even in the presence of imatinib, again arguing for the presence of CML LSCs in the CML leukemia patient. The models of Michor et al. also describe well the higher incidence of mutations in patients who receive therapy at a later stage of the disease. In these patients, the increased pool of CML-LSCs has a higher stochastic probability of mutations, which then lead to a rapid expansion of resistant progenitors and treatment failure.
The quiescent nature of CML LSCs may be responsible in part for the failure of imatinib to result in their elimination. CML LSC may enter a dormant, imatinib-resistant state or in some cases enter a proliferative imatinib-sensitive state. In addition, CML LSCs express higher level of the MDR p-glycoprotein (Pgp). It turns out that imatinib is a substrate for Pgp. Mathematical models, with various growth-related parameters, have been proposed to explain the asymmetrical stem cell replication; however, the biochemical mechanisms responsible for the asymmetry of LSC division are not understood. Mutations occurring in the BCR-ABL gene are commonly thought to occur before disease onset and treatment, thus they are not believed to be responsible for the quiescence nature of the CML LSC stem cells. The cells with the mutant BCR-ABL genes may emerge later, towing to the elimination of cells with the wild-type (WT) BCR-ABL gene upon imatinib treatment. This model implies that the cells with the mutant BCR-ABL genes may not proliferate as well initially as the cells with the WT BCR-ABL gene. On the other hand, conflicting data indicate that these mutations may confer a growth advantage, even without imatinib treatment.22,23 Clearly these models need further investigation.
Certain genes have been postulated to be involved in asymmetrical stem cell replication (for example, partner of inscrutable and lethal giant larvae). Further studies are necessary to confirm the mechanisms by which these and other genes and genetic processes (for example, DNA methylation) may influence asymmetrical stem cell division, quiescence and dormancy.
ALL LSC
As with CML, ALL can result from the rearrangement of BCR and ABL genes in Ph1+ cells. In CML, the translocation is thought to occur at the level of pluripotent stem cells, whereas in ALL the translocation is believed to arise in cells committed to lymphoid differentiation. ALL patients with this BCR-ABL rearrangement are referred to as Ph1-ALL. As with both CML and AML, ALL stem cells also display the cell surface phenotype CD34+CD38-. Further characterization of cell surface phenotypes is ongoing. In ALL, there are currently three morphological classifications; L1, which consists of small uniform cells; L2, which includes large varied cells; and L3, which has large, heterogeneous cells with vacuoles. ALL therapy includes treatment with chemotherapeutic drugs, such as cytarabine or daunorubicin, and bone marrow transplantation. As with both CML and AML, challenges include multidrug resistance and relapse.16
CLL LSC
Finally, the last of the true leukemias for which stem cells have been discovered is CLL. CLL is the most common leukemia in the Western world and is classified as an overproliferation of B-lymphoid cells (consisting of the majority of CLL cases) or an overproliferation of T-lymphoid cells. Treatment consists of conventional chemotherapy using drugs such as fludarabine, stem cell transplantation and immunotherapy using rituximab and alemtuzumab. As with other leukemias, disease progression often occurs which is resistant to most therapeutic approaches.5
HSCs and their microenvironmental niche
HSC quiescence, self-renewal and differentiation are regulated by both intrinsic and extrinsic mechanisms. Intrinsic mechanisms include those that affect the epigenetic state of HSCs, as it is controlled by chromatin remodelers such as (PcG) proteins. Extrinsic mechanisms include those changes in stem cell fate that are dictated by the microenvironment niche.24 The stem cell niche is an anatomical compartment located within the bone marrow cavity, and is composed by extracellular matrix molecules, osteoblasts, osteoclasts and stromal fibroblasts.
The interaction of the HSCs with specific microenvironmental elements is a key regulatory mechanism in maintenance of its self-renewal and differentiation capacities. Moreover, the stem cell niche has important functions, such as maintenance of stem cell quiescence by providing proliferative and inhibitory cues and, in some cases, it also provides stimulatory signals that induce cell growth.
The molecular events that regulate engraftment and mobilization of HSCs and progenitors are still incompletely defined. Direct physical interaction between HSCs and their niche seems to be mediated by several membrane molecules such as integrins and cadherins. Integrins such as very late antigen 4 and 5 (VLA-4 and VLA-5), for example, are efficient activators of the small GTPase family members Rac-1 and Rac-2. The deletion of both Rac1 and Rac2 murine alleles leads to a massive egress of HSCs into the blood from the marrow, demonstrating their importance in the appropriate positioning of HSCs within the bone marrow microenvironment25,26(see below).
Further support for the view that stem cells need to be physically associated with their bone marrow microenvironment arises from studies that demonstrated that in the absence of membrane-bound Kit Ligand (mKL) in the niche, HSCs are not maintained.27,28 Furthermore, it was demonstrated that matrix metalloproteinase (MMP)-9-mediated cleavage of mKit-L into soluble Kit-L resulted in the translocation of HSCs from the quiescent endosteal niche towards vascular-enriched niches favoring differentiation and HSC mobilization into the peripheral circulation.29 In MMP-9-/- mice, release of soluble Kit and HSC motility are impaired, resulting in the failure of hematopoietic recovery and increased mortality.
Once localized within the niche, locally secreted cytokines and growth factors can dictate stem cell fate by initiating specific signal transduction pathways within the HSC. Transforming growth factor-β, for example, is one of the best characterized negative regulators of HSCs. It maintains stem cells in a slow cycling or quiescent state partly by blocking the cell surface expression of cytokine receptors such as c-Kit, Fms-like tyrosine kinase-3 (Flt3), MPL (c-Mpl, receptor for thrombopoietin) and IL-6 receptor (IL-6R).30
Fibroblast growth factor-1 (FGF-1) produced by stromal cells may also have a role in stem cell self-renewal and expansion. Upon binding to its receptors, (FGFR1-4) a variety of signal transduction pathways can be activated, including the MAPK pathway, STATs and PI3K/Akt pathway.31
In addition, angiopoietin-1 produced by stromal cells enhances the ability of HSCs to become quiescent through interaction with its tyrosine kinase receptor TIE2.32 The mechanism of cell-cycle inhibition by TIE2 still remains to be elucidated, but the cyclin-dependent kinase inhibitor p21Cip1 is critical for the maintenance of HSCs quiescence.33 Interestingly, the gene encoding p21Cip1 is directly repressed by c-Myc, another key regulator in the stem cell niche.34 Resting HSCs are characterized by high p21Cip1 levels and an absence of c-Myc expression, whereas increasing levels of c-Myc activity are reduced p21Cip1 expression and a more active state of the HSC.35
Interaction of LSCs with their microenvironment
Whether LSCs also depend on the niche for self-renewal is currently unclear. Although there are clear differences between normal stem cells and LSCs, there are also striking similarities. It seems reasonable to assume that many molecular mechanisms that enable self-renewal are shared among normal stem cells and LSCs.
Indeed, both normal stem cells and leukemic HSCs depend on stromal cell-derived factor-1 (SDF-1)-mediated CXCR4 (CXC chemokine receptor-4 which is specific for SDF-1) signaling for homing and mobilization.36 Wnt-induced β-catenin signaling has been implicated in the maintenance and the expansion of murine HSCs, whereas inhibition of the β-catenin pathway severely impaired the self-renewal capacity of progenitors in CML.37-39 The adhesion of normal CD34+ stem/progenitor cells to bone marrow stroma and fibronectin is mediated by the integrins VLA-4 and VLA-5, and a similar role is fulfilled by integrins in leukemic cells.40-41 Thus, many of the molecules that mediate the interaction between stem cells and bone marrow niche are utilized by both normal stem cells and LSCs.
Whether the leukemic cell needs collaborative genetic hits to improve their interactions with the extrinsic stem cell niche remains an open question. One possibility is that LSCs have much more active migration machinery when compared with normal HSCs that allow them to escape growth inhibition or quiescence-promoting signals induced by osteoblasts and stromal cells in the niche. A number of membrane-associated ligands are normally present in the niche, and it has been shown that they can be cleaved by MMPs. MMPs and their tissue inhibitors (TIMPs) were demonstrated to have important implications in the progression and invasiveness of many malignant disorders. In contrast, the biological significance of these molecules in human leukemias is not clear. The expression levels of MMPs such as MMP9 are often elevated in AML blasts.42 It is tempting to speculate that MMPs might increase soluble concentrations of activate ligands to enable leukemic self-renewal or expansion outside the niche. Therefore, the level of marrow MMP-9 may be a useful surrogate marker for monitoring disease status in AML and it was proposed as a potential prognostic factor. Targeted inhibition of MMPs may inhibit LSCs expansion and may prove useful in leukemia therapy.
Using human AML and mouse CML model, it was recently shown that targeting CD44 with a monoclonal antibody (MoAb) could suppress both AML and CML progression and induce differentiation.43-45 The monoclonal anti-CD44 antibody may disrupt interactions between the LSCs and the bone marrow niche. Rac inhibitors may also disrupt these interactions and mobilize LSC (see below).
Importance of the Wnt/β-catenin signaling pathway in LSC
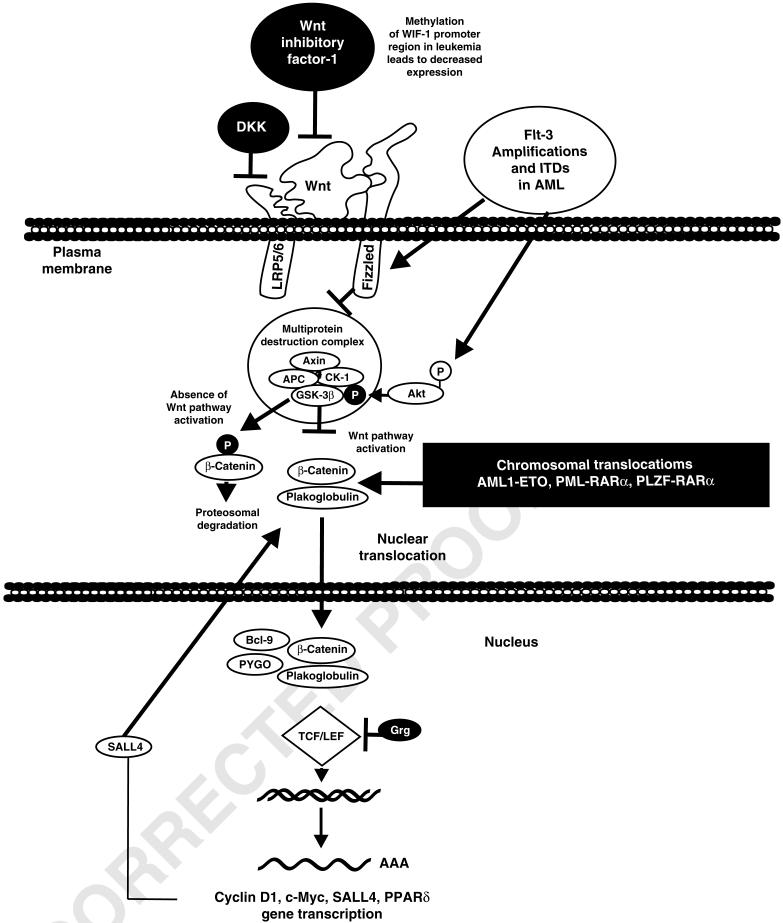
The Wnt signaling pathway plays a critical role in self-renewal of HSCs and its dysregulation may be involved in LSC. There are approximately 20 Wnt genes in the human genome and they encode lipid-modified secreted glycoproteins.46 Wnt induces cell signaling to a receptor complex consisting of a Frizzled family receptor and a co-receptor of the LDL receptor-related protein family, usually LRP5 or LRP6. This can result in the destabilization of the multiprotein destruction complex (MDC) and the subsequent stabilization of β-catenin. β-catenin normally has a very short half-life. In the absence of Wnt, β-catenin is normally phosphorylated by components of the MDC, including axin, adenomatous polyposis coli, glycogen synthase kinase 3β (GSK-3β) and Casein kinase 1 (CK1). When β-catenin is phosphorylated by these kinases, it is targeted for proteasomal degradation.
In contrast, if cytoplasmic β-catenin is stabilized by Wnt signaling (that is not phosphorylated by the MDC complex), it migrates to the nucleus and replaces the Groucho-related repressors (Grg) present on certain genes. β-catenin in the presence of the accessory proteins Bcl-9, PYGO (pygopus) and the TCF/LEF (T-cell factor/lymphocyte enhancer-binding factor) forms a complex that results in the transcription of important growth-promoting genes, such as c-Myc and cyclin D1. Furthermore, TCF/LEF can result in an increase in SALL-4 expression expression, which is an oncogene that can bind to β-catenin and enhance its activity. Plakoglobin is another important component of Wnt signaling. It is a co-activation of TCF/LEF transcription activation and stimulates the transcription of cyclin D, c-Myc and perxosome proliferator-activated receptor-delta (PPARδ). Plakoglobulin expression is enhanced by certain AML fusion proteins. The Wnt pathway is frequently dysregulated in AML.47 An overview of the Wnt signaling pathway and its dysregulation in leukemia is presented in Figure 5.
Figure 5.
Overview of Wnt/β-catenin signaling pathway in leukemia. The Wnt signaling pathway is a central pathway, which results in the transmission of growth-promoting signals that control the activity of β-catenin. Stabilization of β-catenin results in its translocation to the nucleus and promotion of gene transcription by TCF/LEF transcription complex. This can result in the transcription of genes such as Cyclin D, c-Myc, SALL-4, PPARδ and other genes. In the absence of Wnt binding of Frizzled, the multiprotein destruction complex (MDC) is activated and glycogen synthase kinase 3β (GSK-3β) phosphorylates β-catenin and it is targeted for proteasomal degradation. In the presence of Wnt binding, this complex is inactivated. Interactions with other signaling pathways such as PI3K/PTEN/Akt exist, which influence GSK-3β activity (inactivation). This pathway can be activated by Flt-3 and other receptors. Thus, there are interactions between the Wnt and PI3K/PTEN/Akt pathways. Wnt is also postulated to interact with the Notch and other signaling pathways. The Wnt pathway is frequently dysregulated in leukemia. This can occur by mutations at Flt-3, chromosomal translocations, methylation of the promoter regions inhibitory genes such as WIF-1 and many other mechanisms (e.g., mutations at either PI3K or PTEN, which result in the activation of Akt expression).
The Wnt pathway is regulated by many different inhibitors, which have been shown to have important roles in oncogenesis. These interactions are well described in the above-mentioned outstanding review by Mikesch et al.46 Some key inhibitors include members of the Dickkopf (DKK) family, which interact with LRP5/6 and prevent the transmission of signals from the Frizzled-LRP complex. The Wnt inhibitor factor 1 (Wif-1) is another inhibitor of the Wnt pathway. It is an extracellular protein that binds to Wnt, thereby inhibiting the Wnt pathways.48 The promoter regions of Wif-1 is hypermethylated in many acute promyelocytic leukemias and associated with a poorer disease-free 3-year survival rate than in patients without Wif-1 promoter methylation.49
Importantly, this pathway has been shown to have critical roles in self-renewal of HSC and LSC.37,50 Wnt expression inhibits the differentiation of HCS.51,52 Overexpression of axin, a component of the MDC, inhibits Wnt signaling and in turn this results in decreased growth of HSC.37 Constitutive overexpression of activated β-catenin increased the frequency of HSC and prevented their differentiation.37
In a recent study, which examined the role of specific deletion of β-catenin in hematopoietic lineages, it was determined that although deletion of β-catenin did not prevent the formation of HSC, the HSC were deficient in long-term growth and maintenance.50 Furthermore in these mice, the target(s) for CML LSC and BCR-ABL transformation were greatly reduced while the induction of ALL occurred fairly normally. These and other results suggest that ALL may arise from a more mature stem cell committed to the B-cell lineage. Conditional deletion of β-catenin decreased the self-renewal ability of CML LSC. The authors concluded that β-catenin is critically needed for the increased self-renewal capacity conferred by the introduced BCR-ABL oncogene on the target stem cells to permit transformation to proceed along the myeloid lineage. The authors demonstrated that BCR-ABL phosphorylation was decreased in the β-catenin-deficient mice, which also resulted in lower levels of phosphorylated STAT5a. Studies by this group and others53 have demonstrated that BCR-ABL and β-catenin form a complex and the authors have suggested that this complex may stabilize BCR-ABL. Reduction of the BCR-ABL/β-catenin complex could result in a decrease in the phosphorylation of BCR-ABL targets such as STAT5a and also decrease CML self-renewal and disease progression. These intriguing results provide further evidence of the role of β-catenin and the Wnt pathway in normal HSC as well as CML LSC and demonstrate the stem cell origin of CML but not ALL. Wnt signaling may be critical for BCR-ABL stability and the maintenance of CML LSC. The Wnt pathway may serve to differentiate CML and ALL. The successful development of Wnt inhibitors may serve to augment the ability of BCR-ABL inhibitors to eradicate CML.
Not unexpectedly, the Wnt signaling pathway interacts with other signaling pathways, including Notch, sonic hedgehog and PI3K/Akt.54-56 Increased Flt-3 signaling in AML patients with mutations/amplifications of Flt-3 may result from Akt-mediated phosphorylation and inactivation of GSK-3β, which results in higher levels of Wnt signaling and stabilized β-catenin.57 Alternatively, Frizzled-4, a Wnt receptor, is induced by certain Flt-3 mutations, which leads to increased β-catenin levels that result in augmented TCF/LEF activity and c-Myc transcription.58,59
Interactions of BCR-ABL with downstream signaling pathways leading to imatinib resistance and potential novel targets for therapy
BCR-ABL induces many different signaling pathways, that is, probably an important reason as to why it is such a ‘potent’ oncogene. BCR-ABL induces the Ras/Raf/MEK/ERK, PI3K/Akt, p38MAPK, JNK, FAK, Src and Rac signaling cascades.60 BCR-ABL can also interact with Src-family kinase Hck, which results in Stat5a activation independent of Jak kinases. This may be an important factor in the resistance of cells to imatinib therapy (see below for section on Src kinases). Recent data indicate that an important signaling family induced by BCR-ABL is the Rac family of GTPases, which has been summarized excellently in a recent review published in Leukemia.60 Rac is a multigene family, which consists of Rac-1, Rac-2 and Rac-3. Rac-1 and Rac-3 are ubiquitously expressed, whereas Rac-2 is expressed predominately in hematopoietic cells. BCR-ABL also interacts with Rho GTPases, including Rac, Rho and Cdc42. Rac-1, Rac-2 and, to a lesser extent, Rac-3 are detected at hyperactivated levels in HSCs and LSC CMLs. Rac-1 and Rac-2 cooperate with BCR-ABL and induce a myeloproliferative disease of HSC origin. By the use of Rac-1- and Rac-2-deficient mice,25,26 it has been determined that the deficiency of Rac-1 and Rac-2 reduces the severity of BCR-ABL-mediated transformation in animal models, and prolonged survival was observed. Rac-3 is believed to play an important role in the cancer development in the BCR-ABL Rac-1/Rac-2-deficient mice. These results suggest an important role for Rac GTPase in BCR-ABL-mediated CML as well as in normal HSC functions. The Rac GTPases and Ras may be important in the induction of STAT5 which may be abnormally activated by various mechanisms, including an autocrine mechanism in imatinib-resistant CML.61
Rac proteins have been shown to play key roles in the retention of murine HSC and may be an appropriate target to eliminate LSC.26,62 The effects of an experimental Rac inhibitor (NSC23766) on HSC mobilization has been recently examined.26 This inhibitor increased HSC mobilization, suppressed Rac activation and downstream p21-activated protein kinase (PAK) activation. NSC23766 also inhibited the growth of BCR-ABL-transformed cells, even those with the imatinib-resistant T315I mutation.63 This Rac inhibitor may mobilize CML LSC cells from their niche, and thus inhibit the stem cell properties of these cells. Hence, this inhibitor, unlike others such as imatinib, may effectively target the LSC.
Role of Src kinases in CML and ALL LSCs
Imatinib has proven highly effective in the treatment of CML; however, it has been shown that imatinib, by itself, will not cure CML. This is often due to the development of resistance. Src and other signaling pathways are involved in resistance to imatinib.64,65 Src family kinases are activated by BCR-ABL, and the inhibition of BCR-ABL by imatinib may not result in the complete inhibition of Src family kinases. BCR-ABL is known to activate at least three Src family kinases (LYN, HCK and FGR), which are required for the development of BCR-ABL-mediated proliferation of pre-B-ALL, but not of myeloid progenitor cells.66 However, all three of the above-mentioned Src family kinases are activated by BCR-ABL in myeloid cells. BCR-ABL may directly interact with at least two Src family kinases (LYN and HCK), which may alter their activities.67,68 By performing elegant genetic studies, it was demonstrated that LYN, HCK and FYN are required by BCR-ABL to induce CML to lymphoid blast crisis in mice injected with BCR-ABL-transduced bone marrow.64
As stated previously in this review, some BCR-ABL mutations such as T315I are resistant to imatinib, dasatinib and other BCR-ABL inhibitors. However, in the studies by Hu et al.,64 they demonstrated that although BCR-ABL kinase inhibitors prolong the survival of CML mice, they do not completely eliminate CML LSCs. In these studies, the structures of the BCR-ABL genes in CML LSC were determined and they did not have the T315I mutation. These results strongly indicate that other mutations, potentially induced by BCR-ABL, are involved in CML development and that imatinib and dasatinib are not adequate to completely eliminate the CML LSC.
Alternatively, other genetic mutations occurring in blast crisis CML may contribute to the persistent activation of Src. Mutations at other genes, including INK4a, pRb and p53 among others, are involved in CML progression.69-71 Loss of the Arf gene enhance tumorigenicity of BCR-ABL-transformed cells and limit the response to imatinib in BCR-ABL-induced B-ALL mice.72 Studies by Hu et al.,64 have demonstrated that the progression to lymphoid blast crisis requires activation of Src family kinases and the Src kinase inhibitor dasatinib was more effective than imatinib in preventing B-ALL; however, eventually the disease reoccurred. Furthermore, although dasatinib treatment prolonged the life of the CML mice, it did not eradicate CML stem cells. The authors have therefore suggested that an additional component of BCR-ABL-expressing CML LSC must be suppressed for complete eradication of the disease. Furthermore, certain BCR-ABL mutations such as T315I are resistant to imatinib, dasatinib and other BCR-ABL inhibitors. Interesting, Aurora kinase inhibitors (VX-680) have shown promise in inhibiting cells imatinib-resistant BCR-ABL, T315I-transformed cells.73-74
Src kinases may induce the Wnt/β-catenin and Raf/MEK/ERK signaling pathways, which result in LEF/TCF activation.75-76 Activation of these and other signaling pathways may contribute to the survival of B-ALL LSC.
Targeting the LSC based on differential receptor expression
For the remaining of this review, unless otherwise stated, we will focus on HSC and LSCs in the context of AML, as AML stem cells has been the most extensively studied of all the LSCs. AML, as the name describes is characterized by the overproliferation of immature myeloid cells. AML is further subdivided into eight morphological classes from M0-undifferentiated to M7-megakaryoblastic. Current treatment of AML includes FLT-3 kinase inhibitors, HSC transplantation and chemotherapeutic drugs, such as daunorubicin and others. Unfortunately, multidrug resistance during therapy and relapse often occurs.5
Although both HSC and LSC are found within the immature CD34+ CD38- fraction of normal and AML cells respectively, there are distinct markers that separate these two cell groups. The majority of studies have shown that unlike normal HSCs, AML LSCs usually lack the expressions of Thy-1 (CD90) and c-Kit (CD117), CD71 and HLA-DR.77 Furthermore, AML LSCs express IL-3R-α(CD123), CD33, the antigen C-type lectin-like molecule-1 (CLL-1) and CD96. CD33 is expressed on the cell surface of some normal HSCs, but CLL-1 is detected exclusively on malignant CD34+ CD38- cells. Surprisingly, activation of the MAPK, Akt and STAT5 pathways was not detected in response to IL-3 stimulation in some AML LSCs.78 These results may indicate AML LSCs are hyporesponsive to cytokines, which may contribute to their quiescent nature.
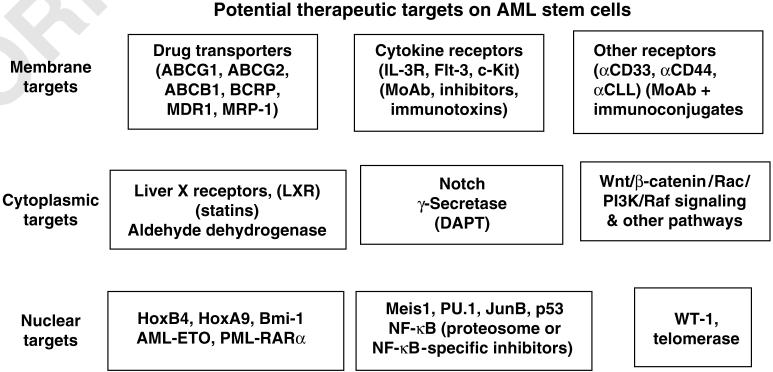
Cytotoxic antibodies can be used as a form of AML therapy. As with other therapies, the ideal treatment would target LSCs but not affect normal HSC. The CD33+ protein provides one possible target of intervention. The α-CD33 MoAb conjugated with the cytotoxic antibiotic calicheamicin induces remission in some cases of AML.79 Part of the effectiveness of this approach may be a result of the direct killing of CD33+ LSCs, as this drug has been found to be effective even in patients where the majority of the AML cells are CD33-. In the long-term, patients on this drug have developed prolonged cytopenia, which may be due to its adverse effects on normal HSCs, which are CD33+.80 Nonetheless, the calicheamicin-conjugated α-CD33 MoAb shows potential as a means of eradicating LSCs before autologous stem-cell harvest and transplantation.5 An overview of potential targets in LSC therapy based on differences in gene expression is presented in Figure 6.
Figure 6.
Targeting the AML-LSC. Potential targets on the AML-LSC are indicated. In general, these targets are not expressed or are expressed at different levels in the AML.
CD123, a receptor for IL-3, is also a potential target, as αIL-3R antibodies conjugated to ozogamicin selectively target AML LSCs in comparison with normal HSC in vitro.81 The αIL3R-ozogamicin immunoconjugate was further shown to decrease the effectiveness of AML engraftment in NOD-SCID mice and was hence indicative of its effect on LSCs.79 In addition, CLL-1 is expressed preferentially on most AML LSCs and is normally expressed on CD38+ myeloid progenitors but not on CD34+ CD38- cells. This makes CLL-1 a selective target in AML therapy.82
Another cell-surface target on LSCs is CD44. CD44 is overexpressed on both AML and CML LSCs relative to normal HSC. Using α-CD44 MoAbs, terminal differentiation and apoptosis of AML cells were observed in engrafted mice.45 The α-CD44 MoAb also reduced the leukemic burden and abolished the need for secondary transplantation of leukemia. In similar studies, CD44 has been found to be required for homing and the engraftment of CML LSCs in mice. CD44 is required for BCR-ABL-expressing cells to induce CML-like disease in mice. In contrast, CD44 was not required for the engraftment of normal HSCs or B-cell ALL in mice.45
Targeting the LSC based on altered drug transporter expression
Targeting cell surface proteins has and continues to be one approach to selectively treat and eradicate LSCs, and hence improve leukemia therapy. Another method to treat these cancers is to circumvent the problem of drug resistance, which is currently encountered after conventional chemotherapic drug treatment. Cells develop drug resistance through a variety of mechanisms that include decreased drug uptake, increased drug efflux, accelerated detoxification, defective apoptosis or altered expression of signaling pathways.83 ATP-dependent drug efflux has been linked to the increased expression of ABC transporter proteins.84
Within the ABC family of active transporters, there are currently approximately 49 separate transmembrane proteins.84 These transporters are expressed in almost all cells and play important roles in normal physiology by transporting a variety of nutrients and biologically active substances across cellular barriers. This family is divided into seven subfamilies, ABCA through ABCG. Several of these genes, including ABCG1 and ABCB1, are expressed in immature CD34+ CD38- sub-populations and are downregulated upon differentiation into the CD34+CD38+ sub-population. Studies by de Grouw et al.85 have demonstrated that 22 ABC transporters were differentially expressed in AML LSCs versus AMLs, and all were expressed lower in CD34+CD38+ cells in comparison with the CD34+ CD38- cells. Of these sub-populations, ABCB1, ABCG2 and ABCC1 are the three primary multidrug-resistant genes that have been detected to be expressed most frequently in tumor cells. Included in this subgroup are MDR1, BCRP (breast cancer resistance protein) and multidrug resistance association protein (MRP1).84 The proteins involved in drug transport have been well documented to confer drug resistance by mediating the active efflux of many anticancer drugs.5
MDR1 overexpression specifically often causes resistance to amphipathic drugs such as paclitaxel and anthracyclines.86 In addition, its expression is higher in patients with secondary leukemias as compared with those in primary disease states.87 In response, several drugs have been examined that block and compete with the Pgp (MDR1)-mediated drug efflux. Unfortunately, the treatment options with the current MDR1 inhibitors have thus far been ineffective.84-88 Second-generation MDR modulators have yielded similar results.89 This may result from MDR1 not being the unique transporter, the cell uses against chemotherapeutic drugs and suggests that additional agents that inhibit multiple transporter and other modes of drug resistance would be more effective. Upon examination of the expression of various ABC transporters, a high redundancy was observed in both normal and early AML cells.85 These results suggest that current MDR modulators may be ineffective due to the presence of multiple transporters, which can efflux the same drug, thus making targeting of a single transporter ineffective. In addition, it is possible that irrelevant transporters were inhibited or there were pharmacokinetic interactions between the chemotherapeutic agent and the ABC transporter inhibitor. Third-generation MDR modulators that are more powerful are currently being developed and examined in clinical settings.84
This ability to efflux many drugs has been practically exploited in the isolation of LSCs. Many ABC transporters expressed in stems cells efflux the fluorescent dyes Hoechst-33342 and rhodamine 123. This is in contrast to non-stem cells, which retain the dyes. After Hoechst-33342 staining and flow cytometric analysis, a large percentage of LSCs have been shown to reside in the side population cells. In addition, these side population cells are found in the bone marrow of over 80% of AML patients.84
Targeting the LSC by altered cholesterol metabolism
In addition to multidrug resistance, altered cholesterol metabolism is observed in some AML patients. This is based on research performed by several laboratories, which shows that AML cells do not exhibit efficient feedback repression of cholesterol synthesis and low-density lipoprotein-receptor expression when exposed to high sterol media in comparison with normal cells.90 This defect in cholesterol metabolism is associated with increased cell survival. This abnormality was detected by differential gene expression (for example, genes involved in cholesterol metabolism) in the CD34+ CD38- sub-population of AML cells, which protects these cells via this pathway. As ABC transporters promote cholesterol efflux, they are also important in cellular cholesterol homeostasis.84
Cholesterol efflux from cells is induced in part by the activation of liver X receptors. The activation of liver X receptors enhances the transcription of genes such as ABCA1 and ABCG1. The functions of these genes are not yet fully defined in hematopoietic cells. However, these transporters are highly expressed in immature CD34+ CD38- populations and downregulated upon differentiation. One pivotal study has shown that 58% of AMLs showed an acute cholesterol response to cytotoxic drugs. This response was not found in normal CD34+ cells and contributed to AML cell survival. Upon blocking this response, the AML cells showed an increase in chemosensitivity.91 This provides a rationale for the use of statins to inhibit such responses. As statins are already widely prescribed, their general effects are well studied and they are known to be well tolerated.92
In addition, recent reports connect central diabetes insipidus with AML. This association is believed to be linked to chromosomal abnormalities in the region 3q21q26, a commonality of the two diseases. Patients with central diabetes insipidus, and specifically the chromosomal abnormality associated with it, often have a poorer prognosis than non-diabetic AML patients.93 A common associated problem that diabetics have is increased levels of high-density lipids and elevated cholesterol levels. These patients often are treated with statins. It is not known whether the treatment of diabetic patients with statins precludes the AML development.
Furthermore, statins are of great interest because they may result in apoptosis limited to tumor cells, while not affecting the nontransformed hematopoietic cells.94 Stirewalt et al.92 have demonstrate that the co-administration of mevastatin with cytotoxic drugs resulted in reduced leukemic survival. Although the exact mechanisms regarding statin-induced apoptosis are still being elucidated, several studies point to the dysregulation of cell signaling pathways.95,96 Specifically, Wu et al.96 demonstrated that lovastatin downregulates constitutive ERK1/2 phosphorylation in AML cells. These data are interesting because in general, the tumor types that have responded well to statins are the ones associated with constitutive activation of the Raf/MEK/ERK pathway, which we discuss further in this review. It is important to note that this same group has demonstrated that lovastatin does not trigger apoptosis solely through the Raf/MEK/ERK cascade alone and the exact mechanisms continue to be studied.96 Finally, early phase I clinical trials have shown that high doses of statins are not well tolerated because of doselimiting toxicities.97 However, in vitro studies demonstrated that when statins were combined with inhibitors of the Raf/MEK/ERK pathway, such as the MEK1 inhibitor PD98059, low levels of statins achieved effective if not stronger pro-apoptotic effects.96
LSCs may also express elevated aldehyde dehydrogenase activity.98 Aldehyde dehydrogenase activity is used to define HSCs but its role in AML LSC is not well elucidated. Recent studies have indicated that some leukemic AML stem cells express elevated aldehyde dehydrogenase, which is associated with a poor prognosis.98 The targeting of aldehyde dehydrogenase is complicated as it most likely plays important functions in many different tissue types, not just in LSCs.
Targeting the LSC by signal transduction, transcription factor and apoptotic pathways
Another approach to eliminating LSCs is to determine the pathways that are aberrantly expressed and identify drugs, which selectively target LSCs while leaving normal HSCs alone, hence reducing toxicity. Although much remains to be elucidated about the regulation of HSCs and LSCs, several genes are recognized as being important in the regulation of HSCs. These include HoxB4 and HoxA9, which have both been implicated in the expansion of hematopoietic cells both in vivo and in vitro.99,100
The Bmi-1 protein, which is necessary for the self-renewal of HSCs, also has potential as a target of therapeutic intervention. Bmi-1 is a member of the Polycomb Group (PcG) gene family and its expression is limited to immature HSC and the CD34+fraction of AML patients.99,100 Studies with Bmi-1-knockout mice have shown its requirement in blood cell development by demonstrating the progressive loss of all hematopoietic lineages. When Bmi-1-/- cells were transplanted into lethally irradiated normal mice, the cells were able to partially reconstitute myeloid cells and lymphocytes. They did not persist for a prolonged time, as they were unable to self-renew.99,100
To understand the reason behind this effect, Park et al.100 compared the gene expression profiles of Bmi-1-/- HSCs with normal HSCs. Bmi-1-/- HSCs have increased levels of cell cycle inhibitors, such as p16INK4α, p19ARF and p53. p53 induced Wig1 and downregulated apoptosis inhibitors such as AI-6. The research further supports the theory that cancer is essentially a disease of stem cells. In addition, the PcG genes have long been suspected of being involved in oncogenesis and the study performed with Bmi-1 knockout mice further adds support for this hypothesis. Finally, these studies demonstrate the critical importance of Bmi-1 in stem cell self-renewal and point to a potential target of intervention that can be used to combat a variety of cancers.99
As stated earlier, the Wnt signaling pathway may be elevated in LSCs.50 This may arise from mutations in FLT-3 and chimeric transcription factors, such as promyelocytic leukemia-retinoic acid receptor-α t(15;17) (PML-RARα) and acute myeloid leukemia-1 gene (8;21) (AML-ETO). FLT-3 is mutated in over 30% of cases of AML and associated with poor prognosis and increased relapse rates.101 Internal tandem duplications of the FLT-3 gene have been detected in AML LSC.83,101 An end result of these mutations is increased expression of the Wnt signaling pathway, which may result in increased growth of the LSC. In addition, cells stimulated with the Wnt ligand are known to stabilize β-catenin. β-Catenin then translocates to the nucleus and activates specific gene transcription. Overexpression of mutant β-catenin induces the expansion of HSC.5 For these reasons, targeting of various components in these pathways (for example, FLT-3) may inhibit LSC growth. Indeed the FLT-3 inhibitor CEP-701 suppressed the engraftment of FLT-3/ITD LSC.83,101
In addition, the Notch pathway may be deregulated in LSC. Genomic analysis from AML stem cells determined that the Jagged-2 gene, a Notch ligand is overexpressed in LSC. Inhibition of γ-secretase by N-[N-(3,5-difluorophenacetyl)-L-alanyl]-S-phenylglycine t-butyl ester (DAPT) inhibits LSC growth. γ-secretase is necessary for Jagged and Notch signaling.102 Likewise, this group also determined that certain genes are detected at lower levels in LSCs, including genes involved in DNA repair, signal transduction and cell cycle progression, which is consistent with the quiescent nature of LSC.
The Wilms' tumor (WT1) gene has also been observed to be expressed at elevated levels in LSCs.103 WT1 was not expressed at high levels in normal long-term HSCs or in multipotent progenitor cells. In contrast, in AML1-ETO+TEL-PDGFRbR or BCR-ABL murine leukemias, WT1 was expressed in approximately 50% of the transplantable LSC.99 Hence, the WT1 protein may be a target for the successful treatment of LSCs.
Transcription factors have been implicated in regulating the LSC. Meis1 has recently been shown to be an essential and ratelimiting regulator of MLL LSC potential.104 Furthermore, Wong et al.,105,106 demonstrated that Meis plays a major function in establishing LSC potential by regulating self-renewal, differentiation arrest and cycling. In contrast, other transcription factors (for example, PU.1 and JunB) may display reduced expression in AML stem cells. Although targeting of transcription factors has proven problematic, it is an area with great potential.
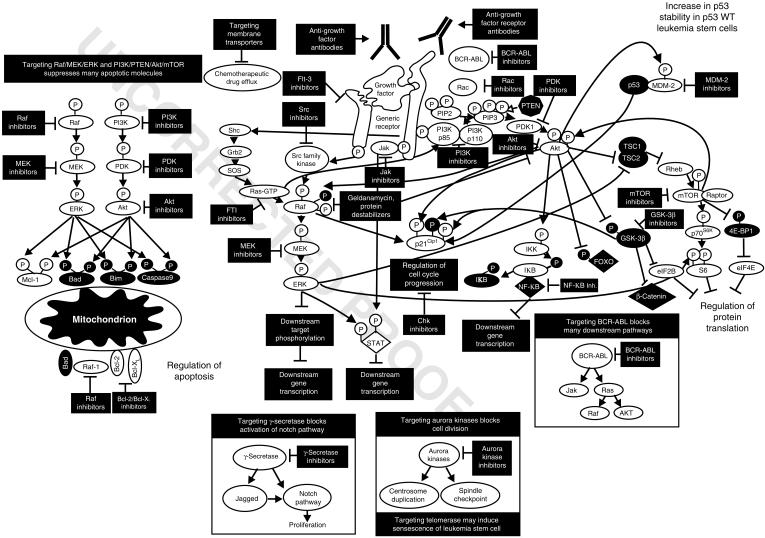
The PI3K/PTEN (phosphatase and TENsin homolog deleted on chromosome 10)/Akt/mammalian target of rapamycin (mTOR) is another cell signaling pathway of immense importance with regard to a variety of cancers. This pathway has been determined to be consistently activated in AML cell lines and strongly contributes to the proliferation, survival and drug resistance.107 In addition, its upregulation has been specifically observed in LSCs transplanted in NOD-SCID mice and shown to provide an anti-apoptotic response.108 Although this upregulation is associated with increased drug resistance and a poor prognosis, a surprising new study by Tamburini et al.109 demonstrated the upregulation of this pathway is actually a favorable factor in de novo cases of AML. This is perhaps because the upregulation of this pathway moves immature leukemic cells, including LSCs, into S phase and therefore makes them more susceptible to certain chemotherapeutic agents, especially those targeting actively replicating cells.110 An overview of the potential for targeting signal pathways in LSCs is present in Figure 7.
Figure 7.
Sites of interaction of signal transduction pathway inhibitors. Various types of inhibitors have been developed to target different molecules involved in signal transduction.
The PI3K/PTEN/Akt/mTOR pathway is of critical importance because of its effects on downstream proteins, including Bad, nuclear factor-kappa B (NF-κB) and MDM2. Inhibitors for several of these proteins, such as NF-κB and MDM2, have been developed and their efficacies in treating various leukemias and myelomas are being evaluated.111 Although its specific role remains unclear, the NF-κB transcription factor is of particular interest because it is found to be particularly active in LSCs but not in HSCs, making it a point of selective intervention. Proteasome inhibitors, such as salinosporamide A and bortezomib, are currently being examined in phase I/II trials and show potential.5
Furthermore, inhibitors of mTOR show promise in LSC therapy. These inhibitors used in conjunction with conventional therapies induce apoptosis and specifically have been shown to reduce the abundance of LSCs.5 Upstream of Akt, there also exists the tumor suppressor PTEN, which has been a further point of intervention, as it is frequently mutated or silenced in human cancer, including leukemia.112
In addition, recent evidence indicates a relationship between the PI3K/PTEN/Akt/mTOR pathway and expression of the MRP1, another ABC transporter protein that is also linked with multidrug resistance. Upregulation of this pathway was found to increase levels of the MRP1 protein.113 These data suggest a p53-dependant mechanism of MRP1, as the inhibition of MRP1 was linked with a concurrent increase in p53 levels. Inhibition of the ubiquitous ligase, MDM-2, with Nutlin-3a increases p53 stability and promotes apoptosis of leukemia, which are WT at p53.114-126 p53 has been shown to be an important target in leukemia. In addition, the connection between MRP1 and this pathway shows another possible reason for multidrug resistance in leukemias.
This connection between multidrug resistance and the PI3K/PTEN/mTOR/Akt pathway also shows the potential of targeting this cascade. Studies using inhibitors to PI3K, such as Wortmannin and LY294002 in combination with conventional chemotherapeutic drugs, demonstrated an increased sensitivity of the cells to more readily undergo apoptosis.125,126 Furthermore, as normal hematopoietic progenitors are less affected by these inhibitors, the potential to selectively target LSCs and AML cells and reduce toxicity is a possibility.110,111
Targeting leukemia stem cells by inhibition the PI3K pathway: importance of PTEN
Recent advances have highlighted extensive phenotypic and functional similarities between normal stem cells and CSCs. This raises the question of whether disease therapies can be developed to eliminate CSCs without eliminating normal stem cells.
Components of the PI3K pathway, including PTEN, Akt and mTOR, are critical regulators of both normal stem cell function and tumorigenesis. Intriguingly, inactivation of some pathway components, such as PTEN, has opposite effects on normal HSCs and leukemia-initiating cells. Therefore, mechanistic differences between normal stem cells and CSCs can thus be targeted to deplete CSCs without damaging normal stem cells.
PTEN functions as a negative regulator of the PI3K pathway, which has crucial roles in cell proliferation, survival, differentiation and migration. Pten is the most frequently mutated gene in human cancers, and is inactivated by a variety of mechanisms in some leukemias.127,128 It was well documented that conditional deletion of Pten tumor suppressor gene in bone marrow HSCs causes their short-term expansion, whereas long-term decline leads to enhanced level of HSC activation. Pten-deficient HSCs engraft normally in recipient mice, but have an impaired ability to sustain hematopoietic reconstitution, reflecting the dysregulation of their cell cycle and decreased retention in the bone marrow niche. Pten deficiency has no discernable effect on HSCs differentiation or survival; however, after 3 weeks of Pten deletion, HSCs became depleted. Thus, Pten has essential roles in restricting the activation of HSCs, in lineage fate determination and in the prevention of leukemogenesis.128,129
In contrast to this requirement for Pten in maintenance of HSCs, LSCs arose and expanded in number after Pten deletion. The LSCs were transplantable and could be enriched among cells that expressed HSC markers. Most mice died with AML and ALL within 6 weeks of Pten deletion.128,129
It was demonstrated that Pten deletion in prostate epithelium progenitor cells led to senescence response by a p53-mediated mechanism.129-131 An analogous mechanism could explain the response of HSCs after Pten deletion; moreover, mutations that occur during the progression of Pten-deficient cells to leukemia, such as p53, could bypass the senescence response in LSCs. Further investigations could elucidate whether Pten deletion in HSCs induces a senescence response and whether the p53 pathway might be involved in this mechanism.
Pten deletion leads to increased activation of Akt and mTOR. Administration of rapamycin, a potent and specific inhibitor of mTOR, to Pten-deleted mice eliminate LSCs and maintain the health of mice, but it also rescued the depletion of Pten-deficient HSCs. Pten-deficient HSCs could provide long-term multilineage reconstitution of irradiated mice as long as the mice continued to be treated with rapamycin. This demonstrates that many effect of Pten deletion were mediated by increased mTOR activation. Therefore, LSCs could be eliminated by targeting mTOR without compromising normal stem cells.
Using a murine lymphoma model, Lowe and co-workers131 demonstrated that Akt promotes tumorigenesis and drug resistance by disrupting apoptosis and that disruption of Akt signaling using the mTOR inhibitor rapamycin reverses chemoresistance in lymphomas expressing Akt, but not in those tumors in which the apoptotic machinery is compromised. Treatment of these mice with doxorubicin (a DNA-intercalating agent), together with rapamycin, resulted in a strong tumor remission, lasting more than 60 days.131 Moreover, further evidence suggests that the transcription factor NF-κB functions as an important tumor survival factor by conferring rapamycin resistance and that concomitant inhibition of NF-κB and mTOR increases cancer cells death.132 Therefore, rapamycin and its analogues may also result in synergistic interactions with other agents.
These results provide further in vivo validation for a strategy to reverse drug resistance in human cancers, such as leukemia, and highlight the potential role of translational deregulation in oncogenesis, resistance and LSCs, shedding light on the importance of cancer therapy based on tumor genotype.
Targeting leukemia stem cells by inhibition the Raf/MEK/ERK pathway
Another well-studied pathway is the Raf/MEK/ERK kinase cascade. It is overexpressed in over 70% of cases of AML.133 Drugs that target proteins in this pathway are currently being extensively studied with the belief that targeting one point of this pathway will have monumental effects on the entire pathway and its numerous downstream targets. Small-molecule weight inhibitors, which target Ras, Raf and MEK, have been developed and recently reviewed in Leukemia.111 Additional drugs such as coumermyicin, which prevents the dimerization of Raf, thus suppressing its activity, may eventually be examined in leukemia therapy.134
Although Raf/MEK/ERK and PI3K/Akt/mTOR are two seemingly separate pathways, their interaction or cross-talk is of intense interest. This cross-talk first became evident when cells treated with a PI3K inhibitor were also found to have decreased levels of ERK.135 It has been further backed by numerous studies documenting the interactions between PI3K and Akt and various isoforms of Raf.136 However, in other cell types, there is suppression of Akt, resulting in enhanced activation of ERK. In addition, inhibitors of ERK also suppressed p70S6K phosphorylation, a protein downstream of mTOR heavily implicated in its effect on protein synthesis. Indeed both the Raf/MEK/ERK and PI3K/PTEN/Akt/mTOR pathways interact to regulate p70S6K activation. The BCR-ABL oncoprotein, critical for CML, is usually effectively inhibited by the drug imatinib. However, in some cases resistance to imatinib occurs. Thus, studies combining imatinib with inhibitors targeting portions of either of these two signaling pathways are showing promise in combating this resistance.137
Conclusions
In summary, the specific therapeutic targeting of the LSC is a field in its infancy. The tumor cell microenvironment or niche may also be an important therapeutic target, and Rac inhibitors as well as various anti-integrin antibodies may be appropriate.138,139 As we learn more about the various LSCs, it undoubtedly will result in novel ways to treat leukemias. Stem cells pose challenges to classical chemotherapeutic approaches owing to their slow rate of proliferation and quiescent-like properties. In additionally, the increased expression of ABC transporter proteins and various cell-signaling pathways further adds complexity to the situation. However, their pivotal role in the overall control and propagation of a variety of cancers and some initial responses to various inhibitors and treatments shows their therapeutic potential. Ultimately, previous research has shown that we must be clever and multifaceted in our approach to design therapies, which may be effective in selectively eliminating these cancer-initiating cells.
Acknowledgements
JAM and LSS have been supported in part by a grant from the NIH (R01098195). JB was supported in part by the Deutsche Krebshilfe. MC, MM and AT have been supported in part from grants from Associazione Italiana Ricerca sul Cancro (AIRC). FN has been supported in part by the grant PRIN from Ministero dell'Istruzione, dell'Università e della Ricerca. ML has been in part supported by the grant from Lega Italiana per la Lotta contro i Tumori. AMM has been supported in part by grants from the CARISBO Foundation and the Progetti Strategici Università di Bologna EF2006.
References
- 1.Till JE, McCulloch EA. A direct measurement of the radiation sensitivity of normal mouse bone marrow cells. Radiat Res. 1961;14:213–222. [PubMed] [Google Scholar]
- 2.Rizo A, Vellenga E, de Haan G, Schuringa JJ. Signaling pathways in self-renewing hematopoietic and leukemic stem cells: do all stem cells need a niche? Hum Mol Genet. 2006;15:R210–R219. doi: 10.1093/hmg/ddl175. [DOI] [PubMed] [Google Scholar]
- 3.Lapidot T, Sirard C, Vormoor J, Murdoch B, Hoang T, Caceres-Cortes J, et al. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. 1994;367:645–648. doi: 10.1038/367645a0. [DOI] [PubMed] [Google Scholar]
- 4.Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997;3:730–737. doi: 10.1038/nm0797-730. [DOI] [PubMed] [Google Scholar]
- 5.Krause DS, Van Etten RA. Right on target: eradicating leukemic stem cells. Trends Mol Med. 2007;13:470–481. doi: 10.1016/j.molmed.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morrison SJ, Weissman IL. The long-term repopulating subset of hematopoietic stem cells is deterministic and isolatable by phenotype. Immunity. 1994;1:661–673. doi: 10.1016/1074-7613(94)90037-x. [DOI] [PubMed] [Google Scholar]
- 7.Hope KJ, Jin L, Dick JE. Acute myeloid leukemia originates from a hierarchy of leukemic stem cell classes that differ in self-renewal capacity. Nat Immunol. 2004;5:738–743. doi: 10.1038/ni1080. [DOI] [PubMed] [Google Scholar]
- 8.Satoh C, Ogata K. Hypothesis: myeloid-restricted hematopoietic stem cells with self-renewal capacity may be the transformation site in acute myeloid leukemia. Leuk Res. 2006;30:491–495. doi: 10.1016/j.leukres.2005.08.017. [DOI] [PubMed] [Google Scholar]
- 9.Cozzio A, Passegue E, Ayton PM, Karsunky H, Cleary ML, Weissman IL. Similar MLL-associated leukemias arising from self-renewing stem cells and short-lived myeloid progenitors. Genes Dev. 2003;17:3029–3035. doi: 10.1101/gad.1143403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.So CW, Karsunky H, Passegue E, Cozzio A, Weissman IL, Cleary ML. MLL-GAS7 transforms multipotent hematopoietic progenitors and induces mixed lineage leukemias in mice. Cancer Cell. 2003;3:161–171. doi: 10.1016/s1535-6108(03)00019-9. [DOI] [PubMed] [Google Scholar]
- 11.Ravandi F, Estrov Z. Eradication of leukemia stem cells as a new goal of therapy in leukemia. Clin Cancer Res. 2006;12:340–344. doi: 10.1158/1078-0432.CCR-05-1879. [DOI] [PubMed] [Google Scholar]
- 12.Jaiswal S, Traver D, Miyamoto T, Akashi K, Lagasse E, Weissman IL. Expression of BCR/ABL and BCL-2 in myeloid progenitors leads to myeloid leukemias. Proc Natl Acad Sci USA. 2003;100:10002–10007. doi: 10.1073/pnas.1633833100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jamieson CH, Ailles LE, Dylla SJ, Muijtjens M, Jones C, Zehnder JL, et al. Granulocyte-macrophage progenitors as candidate leukemic stem cells in blast-crisis CML. N Engl J Med. 2004;351:657–667. doi: 10.1056/NEJMoa040258. [DOI] [PubMed] [Google Scholar]
- 14.Jiang X, Zhao Y, Smith C, Gasparetto M, Turhan A, Eaves A, et al. Chronic myeloid leukemia stem cells possess multiple unique features of resistance to BCR-ABL targeted therapies. Leukemia. 2007;21:926–935. doi: 10.1038/sj.leu.2404609. [DOI] [PubMed] [Google Scholar]
- 15.Jiang X, Saw KM, Eaves A, Eaves C. Instability of BCR-ABL gene in primary and cultured chronic myeloid leukemia stem cells. J Natl Cancer Inst. 2007;99:680–693. doi: 10.1093/jnci/djk150. [DOI] [PubMed] [Google Scholar]
- 16.Roeder I, Horn M, Glauche I, Hochhaus A, Mueller MC, Loeffler M. Dynamic modeling of imatinib-treated chronic myeloid leukemia: functional insights and clinical implications. Nat Med. 2006;12:1181–1184. doi: 10.1038/nm1487. [DOI] [PubMed] [Google Scholar]
- 17.Cobaleda C, Gutierrez-Cianca N, Perez-Losada J, Flores T, Garcia-Sanz R, Gonzalez M, et al. A primitive hematopoietic cell is the target for the leukemic transformation in human Philadelphia-positive acute lymphoblastic leukemia. Blood. 2000;95:1007–1013. [PubMed] [Google Scholar]
- 18.Michor F, Hughes TP, Iwasa Y, Branford S, Shah NP, Sawyers CL, et al. Dynamics of chronic myeloid leukemia. Nature. 2005;435:1267–1270. doi: 10.1038/nature03669. [DOI] [PubMed] [Google Scholar]
- 19.Michor F. Mathematical models of cancer stem cells. J Clin Oncol. 2008;26:2854–2861. doi: 10.1200/JCO.2007.15.2421. [DOI] [PubMed] [Google Scholar]
- 20.Michor F. Quantitative approaches to analyzing imatinib-treated chronic myeloid leukemia. Trends Pharmacol Sci. 2007;28:197–199. doi: 10.1016/j.tips.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 21.Dingli D, Traulsen A, Michor F. (A)symmetric stem cell replication and cancer. PLOS Comput Biol. 2007;3:482–487. doi: 10.1371/journal.pcbi.0030053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gorre ME, Mohammed M, Ellwood K, Hsu N, Paquette R, Rao PN, et al. Clinical resistance to STI-571 cancer therapy caused by BCR-ABL gene mutation or amplification. Science. 2001;293:876–880. doi: 10.1126/science.1062538. [DOI] [PubMed] [Google Scholar]
- 23.Shah NP, Nicoll JM, Nagar B, Gorre ME, Paquette RL, Kuriyan J, et al. Multiple BCR-ABL kinase domain mutations confer polyclonal resistance to the tyrosine kinase inhibitor imatinib (STI571) in chronic phase and blast crisis chronic myeloid leukemia. Cancer Cell. 2002;2:117–125. doi: 10.1016/s1535-6108(02)00096-x. [DOI] [PubMed] [Google Scholar]
- 24.Shiozawa Y, Havens AM, Pienta KJ, Taichman RS. The bone marrow niche: habitat to hematopoietic and mesenchymal stem cells and unwitting host to molecular parasites. Leukemia. 2008;22:941–950. doi: 10.1038/leu.2008.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cancelas JA, Lee AW, Prabhakar R, Stringer KF, Zheng Y, Williams DA. Rac GTPases differentially integrate signals regulating hematopoietic stem cell localization. Nat Med. 2005;11:886–891. doi: 10.1038/nm1274. [DOI] [PubMed] [Google Scholar]
- 26.Gu Y, Filippi MD, Cancelas JA, Siefring JE, Williams EP, Jasti AC, et al. Hematopoietic cell regulation by Rac1 and Rac2 guanosine triphosphatases. Science. 2003;302:445–449. doi: 10.1126/science.1088485. [DOI] [PubMed] [Google Scholar]
- 27.McCulloch EA, Siminovitch L, Till JE, Russell ES, Bernstein SE. The cellular basis of the genetically determined hematopoietic defect in anemic mice of genotype Sl-Sld. Blood. 1965;26:399–410. [PubMed] [Google Scholar]
- 28.Barker JE. Early transplantation to a normal microenvironment prevents the development of Steel hematopoietic stem cell defects. Exp Hematol. 1997;25:542–547. [PubMed] [Google Scholar]
- 29.Heissig B, Hattori K, Dias S, Friedrich M, Ferris B, Hackett NR, et al. Recruitment of stem and progenitor cells from the bone marrow niche requires MMP-9 mediated release of kit-ligand. Cell. 2002;109:625–637. doi: 10.1016/s0092-8674(02)00754-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fortunel NO, Hatzfeld JA, Monier MN, Hatzfeld A. Control of hematopoietic stem/progenitor cell fate by transforming growth factor-beta. Oncol Res. 2003;13:445–453. doi: 10.3727/096504003108748483. [DOI] [PubMed] [Google Scholar]
- 31.L'Hote CG, Knowles MA. Cell responses to FGFR3 signalling: growth, differentiation and apoptosis. Exp Cell Res. 2005;304:417–431. doi: 10.1016/j.yexcr.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 32.Arai F, Hirao A, Ohmura M, Sato H, Matsuoka S, Takubo K, et al. Tie2/angiopoietin-1 signaling regulates hematopoietic stem cell quiescence in the bone marrow niche. Cell. 2004;118:149–161. doi: 10.1016/j.cell.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 33.Cheng T, Rodrigues N, Shen H, Yang Y, Dombkowski D, Sykes M, et al. Hematopoietic stem cell quiescence maintained by p21cip1/waf1. Science. 2000;287:1804–1808. doi: 10.1126/science.287.5459.1804. [DOI] [PubMed] [Google Scholar]
- 34.Wu S, Cetinkaya C, Munoz-Alonso MJ, der Lehr N, Bahram F, Beuger V, et al. Myc represses differentiation-induced p21CIP1 expression via Miz-1-dependent interaction with the p21 core promoter. Oncogene. 2003;22:351–360. doi: 10.1038/sj.onc.1206145. [DOI] [PubMed] [Google Scholar]
- 35.Murphy MJ, Wilson A, Trumpp A. More than just proliferation: Myc function in stem cells. Trends Cell Biol. 2005;15:128–137. doi: 10.1016/j.tcb.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 36.Lapidot T, Kollet O. The essential roles of the chemokine SDF-1 and its receptor CXCR4 in human stem cell homing and repopulation of transplanted immune-deficient NOD/SCID and NOD/SCID/B2 m(null) mice. Leukemia. 2002;16:1992–2003. doi: 10.1038/sj.leu.2402684. [DOI] [PubMed] [Google Scholar]
- 37.Reya T, Duncan AW, Ailles L, Domen J, Scherer DC, Willert K, et al. A role for Wnt signalling in self-renewal of haematopoietic stem cells. Nature. 2003;423:409–414. doi: 10.1038/nature01593. [DOI] [PubMed] [Google Scholar]
- 38.Willert K, Brown JD, Danenberg E, Duncan AW, Weissman IL, Reya T, et al. Wnt proteins are lipid-modified and can act as stem cell growth factors. Nature. 2003;423:448–452. doi: 10.1038/nature01611. [DOI] [PubMed] [Google Scholar]
- 39.Jamieson CH, Ailles LE, Dylla SJ, Muijtjens M, Jones C, Zehnder JL, et al. Granulocyte-macrophage progenitors as candidate leukemic stem cells in blast-crisis CML. N Engl J Med. 2004;351:657–667. doi: 10.1056/NEJMoa040258. [DOI] [PubMed] [Google Scholar]
- 40.Teixido J, Hemler ME, Greenberger JS, Anklesaria P. Role of beta 1 and beta 2 integrins in the adhesion of human CD34hi stem cells to bone marrow stroma. J Clin Invest. 1992;90:358–367. doi: 10.1172/JCI115870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hirsch E, Iglesias A, Potocnik AJ, Hartmann U, Fassler R. Impaired migration but not differentiation of haematopoietic stem cells in the absence of beta1 integrins. Nature. 1996;380:171–175. doi: 10.1038/380171a0. [DOI] [PubMed] [Google Scholar]
- 42.Lin LI, Lin DT, Chang CJ, Lee CY, Tang JL, Tien HF. Marrow matrix metalloproteinases (MMPs) and tissue inhibitors of MMP in acute leukaemia: potential role of MMP-9 as a surrogate marker to monitor leukaemic status in patients with acute myelogenous leukaemia. Br J Haematol. 2002;117:835–841. doi: 10.1046/j.1365-2141.2002.03510.x. [DOI] [PubMed] [Google Scholar]
- 43.Williams DA, Cancelas JA. Leukaemia: niche retreats for stem cells. Nature. 2006;444:827–828. doi: 10.1038/444827a. [DOI] [PubMed] [Google Scholar]
- 44.Krause DS, Lazarides K, von Adrian UH, Van Etten RA. Requirement for CD44 in homing and engraftment of BCR-ABL-expressing leukemic stem cells. Nat Med. 2006;12:1175–1180. doi: 10.1038/nm1489. [DOI] [PubMed] [Google Scholar]
- 45.Jin L, Hope KJ, Zhai Q, Smadja-Joffe F, Dick JE. Targeting of CD44 eradicates human acute myeloid leukemic stem cell. Nat Med. 2006;12:1167–1174. doi: 10.1038/nm1483. [DOI] [PubMed] [Google Scholar]
- 46.Mikesch JH, Steffen B, Berdel WE, Serve H, Muller-Tidow C. The emerging role of Wnt signaling in the pathogenesis of acute myeloid leukemia. Leukemia. 2007;21:1638–1647. doi: 10.1038/sj.leu.2404732. [DOI] [PubMed] [Google Scholar]
- 47.Jamieson CH, Ailles LE, Dylla SJ, Muijtjens M, Jones C, Zehnder JL, et al. Granulocyte-macrophage progenitors as candidate leukemic stem cells in blast-crisis CML. N Engl J Med. 2004;351:657–667. doi: 10.1056/NEJMoa040258. [DOI] [PubMed] [Google Scholar]
- 48.Kawano Y, Kypta R. Secreted antagonists of the Wnt signaling pathway. J Cell Sci. 2003;116:2627–2634. doi: 10.1242/jcs.00623. [DOI] [PubMed] [Google Scholar]
- 49.Chim CS, Chan WWL, Pang A, Kwong YL. Preferential methylation of Wnt inhibitor factor-1 in acute promyelocytic leukemia: an independent poor prognostic factor. Leukemia. 2006;20:907–909. doi: 10.1038/sj.leu.2404176. [DOI] [PubMed] [Google Scholar]
- 50.Zhao C, Blum J, Chen A, Kwon HY, Jung SH, Cook JM, et al. Loss of β-catenin impairs the renewal of normal and CML stem cells in vivo. Cancer Cell. 2007;12:528–541. doi: 10.1016/j.ccr.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Austin TW, Solar GP, Ziegler FC, Liem L, Matthews WA. A role for the Wnt gene family in hematopoiesis: expansion of multilineage progenitor cells. Blood. 1997;89:3624–3635. [PubMed] [Google Scholar]
- 52.van den Berg DJ, Sharma AK, Bruno E, Hoffman R. Role of members of the Wnt gene family in human haematopoiesis. Blood. 1998;89:3189–3202. [PubMed] [Google Scholar]
- 53.Coluccia AM, Vacca A, Dunach M, Mologni L, Redaelli S, Bustos VH, et al. Bcr-Abl stabilizes beta-catenin in chronic myeloid leukemia through its tyrosine phosphorylation. EMBO J. 2007;26:1456–1466. doi: 10.1038/sj.emboj.7601485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bhardwaj G, Murdoch B, Wu D, Baker DP, Williams KP, Chadwick K, et al. Sonic hedgehog induces the proliferation of primitive human hematopoietic cells via BMP regulation. Nat Immunol. 2001;2:172–180. doi: 10.1038/84282. [DOI] [PubMed] [Google Scholar]
- 55.Varnum-Finney B, Xu L, Brashem-Stein C, Nourigat C, Flowers D, Bakkour S, et al. Pluripotent, cytokine-dependent, hematopoietic stem cells are immortalized by constitutive Notch1 signaling. Nat Med. 2000;6:1278–1281. doi: 10.1038/81390. [DOI] [PubMed] [Google Scholar]
- 56.Hing HK, Sun X, Artavanis-Tsakonas S. Modulation of wingless signaling by Notch in Drosophilia. Mech Dev. 1994;47:261–268. doi: 10.1016/0925-4773(94)90044-2. [DOI] [PubMed] [Google Scholar]
- 57.Brandts CH, Sargin B, Rode M, Biermann C, Lindtner B, Schwable J, et al. Constitutive activation of Akt by Flt3 internal tandem duplications is necessary for increased survival, proliferation, and myeloid transformation. Cancer Res. 2005;65:9643–9650. doi: 10.1158/0008-5472.CAN-05-0422. [DOI] [PubMed] [Google Scholar]
- 58.Mizuki M, Schwable J, Steur C, Choudhary C, Agrawal S, Sargin B, et al. Suppression of myeloid transcription factors and induction of STAT response genes by AML-specific mutations. Blood. 2003;101:3164–3173. doi: 10.1182/blood-2002-06-1677. [DOI] [PubMed] [Google Scholar]
- 59.Tickenbrock L, Schwable J, Wiedehage M, Steffen B, Sargin B, Choudhary C, et al. Flt3 tandem duplication mutations cooperate with Wnt signaling in leukemic signal transduction. Blood. 2005;105:3699–3706. doi: 10.1182/blood-2004-07-2924. [DOI] [PubMed] [Google Scholar]
- 60.Thomas EK, Cancelas JA, Zheng Y, Williams DA. Rac GTPases as key regulators of p210-BCR-ABL-dependent leukemogenesis. Leukemia. 2008;22:898–904. doi: 10.1038/leu.2008.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang Y, Cai D, Brendel C, Barett C, Erben P, Manley PW, et al. Adaptive secretion of granulocyte-macrophage colony-stimulating factor (GM-CSF) mediates imatinib and nilotinib resistance in BCR-ABL+ progenitors via JAK-2/STAT-5 pathway activation. Blood. 2007;109:2147–2155. doi: 10.1182/blood-2006-08-040022. [DOI] [PubMed] [Google Scholar]
- 62.Yang FC, Atkinson SJ, Gu Y, Borneo JB, Roberts AW, Zheng Y, et al. Rac and Cdc 42 GTPases control hematopoietic stem cell shape, adhesion, migration, and mobilization. Proc Natl Acad Sci USA. 2001;98:5614–5618. doi: 10.1073/pnas.101546898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Thomas EK, Cancelas JA, Chae HD, Cox AD, Keller PJ, Perrotti D, et al. Rac guanosine triphosphates represent integrating molecular therapeutic targets for BCR-ABL-induced myeloproliferative disease. Cancer Cell. 2007;12:467–478. doi: 10.1016/j.ccr.2007.10.015. [DOI] [PubMed] [Google Scholar]
- 64.Hu Y, Swerdlow S, Duffy TM, Weinmann R, Lee FY, Li S. Targeting multiple kinase pathways in leukemic progenitors and stem cells is essential for improved treatment of Ph+ leukemia in mice. Proc Natl Acad USA. 2006;103:16870–16875. doi: 10.1073/pnas.0606509103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li S, Li D. Stem cell and kinase activity-independent pathway in resistance of leukaemia to BCR-ABL inhibitors. J Cell Mol Med. 2007;11:1251–1262. doi: 10.1111/j.1582-4934.2007.00108.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hu Y, Liu Y, Pelletier S, Buchdunger E, Warmuth M, Fabbro D, et al. Requirement of Src kinases Lyn, Hck and Fgr for BCR-ABL1-induced B-lymphoblastic leukemia but not chronic myeloid leukemia. Nat Genet. 2004;36:453–461. doi: 10.1038/ng1343. [DOI] [PubMed] [Google Scholar]
- 67.Danhauser-Riedl S, Warmuth M, Druker BJ, Emmerich B, Hellek M. Activation of Src kinases p53/p56lyn and p59hck by p210bcr/abl in myeloid cells. Cancer Res. 1996;56:3589–3596. [PubMed] [Google Scholar]
- 68.Warmuth M, Bergmann M, Priess A, Hauslmann K, Emmerich B, Hallek M. The Src family kinase Hck interacts with Bcr-Abl by a kinase-independent mechanism and phosphorylates the Grb-2-binding site of Bcr. J Biol Chem. 1997;272:33260–33270. doi: 10.1074/jbc.272.52.33260. [DOI] [PubMed] [Google Scholar]
- 69.Feinstein E, Cimino G, Gale RP, Alimena G, Berthier R, Kishi K, et al. p53 in chronic myelogenous leukemia in acute phase. Proc Natl Acad USA. 1991;88:6293–6297. doi: 10.1073/pnas.88.14.6293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sill H, Goldman JM, Cross NC. Homozygous deletions of the p16 tumor-suppressor genes are associated with lymphoid transformation of chronic myeloid leukemia. Blood. 1995;85:2013–2016. [PubMed] [Google Scholar]
- 71.Towatari M, Adachi K, Kato H, Sato H. Absence of the human retinoblastoma gene product in the megakaryoblastic crisis of chronic myelogenous leukemia. Blood. 1991;78:2178–2181. [PubMed] [Google Scholar]
- 72.Williams RT, Roussel MF, Sherr CJ. Arf gene loss enhances oncogenicity and limits imatinib response in mouse models of Bcr-Abl-induced acute lymphoblastic leukemia. Proc Natl Acad Sci USA. 2006;103:6688–6693. doi: 10.1073/pnas.0602030103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Giles FJ, Cortes J, Jones D, Bergstrom D, Kantarjian H, Freedman SJ. MK-0457, a novel kinase inhibitor, is active in patients with chronic myeloid leukemia or acute lymphocytic leukemia with the T315I BCR-ABL mutation. Blood. 2007;109:500–502. doi: 10.1182/blood-2006-05-025049. [DOI] [PubMed] [Google Scholar]
- 74.Cheetham GM, Charlton PA, Golec JM, Pollard JR. Structural basis for potential inhibition of the Aurora kinases and a T315I multi-drug resistant mutant form of Abl by VX-680. Cancer Lett. 2007;251:323–329. doi: 10.1016/j.canlet.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 75.Schroeder JA, Adriance MC, Thompson MC, Camenisch TD, Gendler SJ. MUC1 alters beta-catenin-dependent tumor formation and promotes cellular invasion. Oncogene. 2003;22:1324–1332. doi: 10.1038/sj.onc.1206291. [DOI] [PubMed] [Google Scholar]
- 76.Haraguchi K, Nishida A, Ishidate T, Akiyama T. Activation of β-catenin-TCF-mediated transcription by non-receptor tyrosine kinase v-Src. Biochem Biophys Res Commun. 2004;313:841–844. doi: 10.1016/j.bbrc.2003.12.021. [DOI] [PubMed] [Google Scholar]
- 77.van Gosliga D, Schepers H, Rizo A, van der Kolk D, Vellenga E, Schuringa JJ. Establishing long-term cultures with self-renewing acute myeloid leukemia stem/progenitor cells. Exp Hematol. 2007;35:1538–1549. doi: 10.1016/j.exphem.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 78.Jordan CT, Upchurch D, Szilvassy SJ, Guzman ML, Howard DS, Pettigrew AL, et al. The interleukin-3 receptor alpha chain is a unique marker for human acute myelogenous leukemia stem cells. Leukemia. 2000;14:1777–1784. doi: 10.1038/sj.leu.2401903. [DOI] [PubMed] [Google Scholar]
- 79.Tsimberidou AM, Giles FJ, Estey E, O'Brien S, Keating MJ, Kantarjian HM. The role of gemtuzumab ozogamicin in acute leukaemia therapy. Br J Haematol. 2006;132:398–409. doi: 10.1111/j.1365-2141.2005.05872.x. [DOI] [PubMed] [Google Scholar]
- 80.Sperr WR, Florian S, Hauswirth AW, Valent P. CD 33 as a target of therapy in acute myeloid leukemia: current status and future perspectives. Leuk Lymphoma. 2005;46:1115–1120. doi: 10.1080/10428190500126075. [DOI] [PubMed] [Google Scholar]
- 81.Hogge DE, Feuring-Buske M, Gerhard B, Frankel AE. The efficacy of diphtheria-growth factor fusion proteins is enhanced by co-administration of cytosine arabinoside in an immunodeficient mouse model of human acute myeloid leukemia. Leuk Res. 2004;28:1221–1226. doi: 10.1016/j.leukres.2004.03.015. [DOI] [PubMed] [Google Scholar]
- 82.Bakker AB, van den Oudenrijn S, Bakker AQ, Feller N, van Meijer M, Bia JA, et al. C-type lectin-like molecule-1: a novel myeloid cell surface marker associated with acute myeloid leukemia. Cancer Res. 2004;64:8443–8450. doi: 10.1158/0008-5472.CAN-04-1659. [DOI] [PubMed] [Google Scholar]
- 83.Gal H, Amariglio N, Trakhtenbrot L, Jacob-Hirsh J, Margalit O, Avigdor A, et al. Gene expression profiles of AML derived stem cells; similarity to hematopoietic stem cells. Leukemia. 2006;20:2147–2154. doi: 10.1038/sj.leu.2404401. [DOI] [PubMed] [Google Scholar]
- 84.de Jonge-Peeters SD, Kuipers F, de Vries EG, Vellenga E. ABC transporter expression in hematopoietic stem cells and the role in AML drug resistance. Crit Rev Oncol Hematol. 2007;62:214–226. doi: 10.1016/j.critrevonc.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 85.de Grouw EP, Raaijmakers MH, Boezeman JB, van der Reijden BA, van de Locht LT, de Witte TJ, et al. Preferential expression of a high number of ATP binding cassette transporters in both normal and leukemic CD34+ CD38- cells. Leukemia. 2006;20:750–754. doi: 10.1038/sj.leu.2404131. [DOI] [PubMed] [Google Scholar]
- 86.Ross DD. Novel mechanisms of drug resistance in leukemia. Leukemia. 2000;14:467–473. doi: 10.1038/sj.leu.2401694. [DOI] [PubMed] [Google Scholar]
- 87.Ross DD. Modulation of drug resistance transporters as a strategy for treating myelodysplastic syndrome. Best Pract Res Clin Haematol. 2004;17:641–651. doi: 10.1016/j.beha.2004.08.014. [DOI] [PubMed] [Google Scholar]
- 88.Fisher GA, Sikic BI. Clinical studies with modulators of multidrug resistance. Hematol Oncol Clin North Am. 1995;9:363–382. [PubMed] [Google Scholar]
- 89.Greenberg PL, Lee SJ, Advani R, Tallman MS, Sikic BI, Letendre L, et al. Mitoxantrone, etoposide, and cytarabine with or without valspodar in patients with relapsed or refractory acute myeloid leukemia and high-risk myelodysplastic syndrome: a phase III trial (E2995) J Clin Oncol. 2004;22:1078–1086. doi: 10.1200/JCO.2004.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Banker DE, Mayer SJ, Li HY, Willman CL, Appelbaum FR, Zager RA. Cholesterol synthesis and import contribute to protective cholesterol increments in acute myeloid leukemia cells. Blood. 2004;104:1816–1824. doi: 10.1182/blood-2004-01-0395. [DOI] [PubMed] [Google Scholar]
- 91.Li HY, Appelbaum FR, Willman CL, Zager RA, Banker DE. Cholesterol-modulating agents kill acute myeloid leukemia cells and sensitize them to therapeutics by blocking adaptive cholesterol responses. Blood. 2003;101:3628–3634. doi: 10.1182/blood-2002-07-2283. [DOI] [PubMed] [Google Scholar]
- 92.Stirewalt DL, Appelbaum FR, Willman CL, Zager RA, Banker DE. Mevastatin can increase toxicity in primary AMLs exposed to standard therapeutic agents, but statin efficacy is not simply associated with ras hotspot mutations or overexpression. Leuk Res. 2003;27:133–145. doi: 10.1016/s0145-2126(02)00085-1. [DOI] [PubMed] [Google Scholar]
- 93.Keung Y-K, Buss D, Powell BL, Pettenati M. Central diabetes insipidus and inv(3)(q21q26) and monosomy 7 in acute myeloid leukemia. Cancer Genet Cytogent. 2002;136:78–81. doi: 10.1016/s0165-4608(02)00521-6. [DOI] [PubMed] [Google Scholar]
- 94.Dimitroulakos J, Nohynek D, Backway KL, Hedley DW, Yeger H, Freedman MH, et al. Increased sensitivity of acute myeloid leukemias to lovastatin-induced apoptosis: a potential therapeutic approach. Blood. 1999;93:1308–1318. [PubMed] [Google Scholar]
- 95.Wong WW, Tan MM, Xia Z, Dimitroulakos J, Minden MD, Penn LZ. Cerivastatin triggers tumor-specific apoptosis with higher efficacy than lovastatin. Clin Cancer Res. 2001;7:2067–2075. [PubMed] [Google Scholar]
- 96.Wu J, Wong WW, Khosravi F, Minden MD, Penn LZ. Blocking the Raf/MEK/ERK pathway sensitizes acute myelogenous leukemia cells to lovastatin-induced apoptosis. Cancer Res. 2004;64:6461–6468. doi: 10.1158/0008-5472.CAN-04-0866. [DOI] [PubMed] [Google Scholar]
- 97.Thibault A, Samid D, Tompkins AC, Figg WD, Cooper MR, Hohl RJ, et al. Phase I study of lovastatin, an inhibitor of the mevalonate pathway, in patients with cancer. Clin Cancer Res. 1996;2:483–491. [PubMed] [Google Scholar]
- 98.Cheung AM, Wan TS, Leung JC, Chan LY, Huang H, Kwong YL, et al. Aldehyde dehydrogenase activity in leukemic blasts defines a subgroup of acute myeloid leukemia with adverse prognosis and superior NOD/SCID engrafting potential. Leukemia. 2007;21:1423–1430. doi: 10.1038/sj.leu.2404721. [DOI] [PubMed] [Google Scholar]
- 99.Raaphorst FM. Self-renewal of hematopoietic and leukemic stem cells: a central role for the Polycomb-group gene Bmi-1. Trends Immunol. 2003;24:522–524. doi: 10.1016/s1471-4906(03)00241-2. [DOI] [PubMed] [Google Scholar]
- 100.Park IK, Qian D, Kiel M, Becker MW, Pihalja M, Weissman IL, et al. Bmi-1 is required for maintenance of adult self-renewing haematopoietic stem cells. Nature. 2003;423:302–305. doi: 10.1038/nature01587. [DOI] [PubMed] [Google Scholar]
- 101.Levis M, Small D. FLT3: It does matter in leukemia. Leukemia. 2003;17:1738–1752. doi: 10.1038/sj.leu.2403099. [DOI] [PubMed] [Google Scholar]
- 102.Mumm JS, Schroeter EH, Saxena MT, Griesemer A, Tian X, Pan DJ, et al. A ligand-induced extracellular cleavage regulates gamma-secretase-like proteolytic activation of Notch 1. Mol Cell. 2000;5:197–206. doi: 10.1016/s1097-2765(00)80416-5. [DOI] [PubMed] [Google Scholar]
- 103.Hosen N, Shirakata T, Nishida S, Yanagihara M, Tsuboi A, Kawakami M, et al. The Wilms' tumor gene WT1-GFP knock-in mouse reveals the dynamic regulation of WT1 expression in normal and leukemic hematopoiesis. Leukemia. 2007;21:1783–1791. doi: 10.1038/sj.leu.2404752. [DOI] [PubMed] [Google Scholar]
- 104.Wong P, Iwasaki M, Somervaille TC, So CW, Cleary ML. Meis1 is an essential and rate-limiting regulator of MLL leukemia stem cell potential. Genes Dev. 2007;21:2762–2774. doi: 10.1101/gad.1602107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Steidl U, Rosenbauer F, Verhaak RG, Gu X, Ebralidze A, Otu HH, et al. Essential role of Jun family transcription factors in PU.1 knockdown-induced leukemic stem cells. Nat Genet. 2006;38:1269–1277. doi: 10.1038/ng1898. [DOI] [PubMed] [Google Scholar]
- 106.Somervaille TC, Cleary ML. PU.1 and Junb: suppressing the formation of acute myeloid leukemia stem cells. Cancer Cell. 2006;10:456–457. doi: 10.1016/j.ccr.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 107.Martelli AM, Nyakern M, Tabellini G, Bortul R, Tazzari PL, Evangelisti C, et al. Phosphoinositide 3-kinase/Akt signaling pathway and its therapeutical implications for human acute myeloid leukemia. Leukemia. 2006;20:911–928. doi: 10.1038/sj.leu.2404245. [DOI] [PubMed] [Google Scholar]
- 108.Xu Q, Thompson JE, Carroll M. mTOR regulates cell survival after etoposide treatment in primary AML cells. Blood. 2005;106:4261–4268. doi: 10.1182/blood-2004-11-4468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tamburini J, Elie C, Bardet V, Chapuis N, Park S, Broet P, et al. Constitutive phosphoinositide-3kinase/AKT activation represents a favourable prognostic factor in de novo AML patients. Blood. 2007;110:1025–1028. doi: 10.1182/blood-2006-12-061283. [DOI] [PubMed] [Google Scholar]
- 110.Martelli AM, Tazzari PL, Evangelisti C, Chiarini F, Blalock WL, Billi AM, et al. Targeting the phosphatidylinositol 3-kinase/Akt/mammalian target of rapamycin module for acute myelogenous leukemia therapy: from bench to bedside. Curr Med Chem. 2007;14:2009–2023. doi: 10.2174/092986707781368423. [DOI] [PubMed] [Google Scholar]
- 111.McCubrey JA, Steelman LS, Abrams SL, Bertrand FE, Ludwig DE, Basecke J, et al. Targeting survival cascades induced by activation of Ras/Raf/MEK/ERK, PI3K/PTEN/Akt/mTOR and Jak/STAT pathways for effective leukemia therapy. Leukemia. 2008;22:708–722. doi: 10.1038/leu.2008.27. [DOI] [PubMed] [Google Scholar]
- 112.Martelli AM, Tazzari PL, Evangelisti C, Chiarini F, Blalock WL, Billi AM, et al. Targeting the phosphatidylinositol 3-kinase/Akt/mammalian target of rapamycin module for acute myelogenous leukemia therapy: from bench to bedside. Curr Med Chem. 2007;14:2009–2023. doi: 10.2174/092986707781368423. [DOI] [PubMed] [Google Scholar]
- 113.Tazzari PL, Cappellini A, Ricci F, Evangelisti C, Papa V, Grafone T, et al. Multidrug resistance-associated protein 1 expression is under the control of the phosphoinositide 3 kinase/Akt signal transduction network in human acute myelogenous leukemia blasts. Leukemia. 2007;21:427–438. doi: 10.1038/sj.leu.2404523. [DOI] [PubMed] [Google Scholar]
- 114.Steelman LS, Abrams SL, Whelan J, Bertrand FE, Ludwig DE, Basecke J, et al. Contributions of the Raf/MEK/ERK, PI3K/PTEN/Akt/mTOR and Jak/STAT pathways to leukemia. Leukemia. 2008;22:686–707. doi: 10.1038/leu.2008.26. [DOI] [PubMed] [Google Scholar]
- 115.Kojima K, Knopleva M, Samudio IJ, Shikami M, Cabbreira-Hansen M, McQueen T, et al. MDM2 antagonists induce p53-dependent apoptosis in AML: implications for leukemia therapy. Blood. 2005;106:3150–3159. doi: 10.1182/blood-2005-02-0553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kojima K, Konopleva M, McQueen T, O'Brien S, Plunkett W, Andreeff M. Mdm2 inhibitor Nutlin-3a induces p53-mediated apoptosis by transcription-dependent and transcription-independent mechanism and may overcome Atm-mediated resistance to fludarabine in chronic lymphocytic leukemia. Blood. 2006;108:993–1000. doi: 10.1182/blood-2005-12-5148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kojima K, Konopleva M, Samudio IJ, Schober WD, Bornmann WG, Andreeff M. Concomitant inhibition of MDM2 and Bcl-2 protein function synergistically induce mitochondrial apoptosis in AML. Cell Cycle. 2006;5:2778–2786. doi: 10.4161/cc.5.23.3520. [DOI] [PubMed] [Google Scholar]
- 118.Kojima K, Konopleva M, Samudio IJ, Ruvolo V, Andreeff M. Mitogen-activated protein kinase inhibition enhances nuclear proapoptotic function of p53 in acute myelogenous leukemia cells. Cancer Res. 2007;67:3210–3219. doi: 10.1158/0008-5472.CAN-06-2712. [DOI] [PubMed] [Google Scholar]
- 119.Janz M, Stuhmer T, Vassilev LT, Bargou RC. Pharmacologic activation of p53-dependent and p53-independent apoptotic pathways in Hodgkin/Reed-Sternberg cells. Leukemia. 2007;21:772–779. doi: 10.1038/sj.leu.2404565. [DOI] [PubMed] [Google Scholar]
- 120.Lehman BD, McCubrey JA, Jefferson HS, Paine MS, Chappell WH, Terrian DM. A dominant role for p53-dependent cellular senescence in radiosensitization of human prostate cancer cells. Cell Cycle. 2007;6:595–605. doi: 10.4161/cc.6.5.3901. [DOI] [PubMed] [Google Scholar]
- 121.Pederson-Bjergaard J, Christiansen DH, Desta F, Andersen MK. Alternative genetic pathways and cooperating genetic abnormalities in the pathogenesis of therapy-related myelodysplasia and acute myeloid leukemia. Leukemia. 2006;20:1943–1949. doi: 10.1038/sj.leu.2404381. [DOI] [PubMed] [Google Scholar]
- 122.Pluta A, Nyman U, Joseph B, Robak T, Zhivotovsky B, Smolewski P. The role of p73 in hematological malignancies. Leukemia. 2006;20:757–766. doi: 10.1038/sj.leu.2404166. [DOI] [PubMed] [Google Scholar]
- 123.Doepfner KT, Spertini O, Arcaro A. Autocrine insulin-like growth factor-1 signaling promotes growth and survival of human acute myeloid leukemia cells via the phosphoinositide 3-kinase/Akt pathway. Leukemia. 2007;21:1921–1930. doi: 10.1038/sj.leu.2404813. [DOI] [PubMed] [Google Scholar]
- 124.Longo PG, Laurenti L, Gobessi S, Petlickovski A, Pelosi M, Chiusolo P, et al. The Akt signaling pathway determines the different proliferative capacity of chronic lymphocytic leukemia B-cells from patients with progressive and stable disease. Leukemia. 2007;21:110–120. doi: 10.1038/sj.leu.2404417. [DOI] [PubMed] [Google Scholar]
- 125.Bortul R, Tazzari PL, Cappellini A, Tabellini G, Billi AM, Bareggi R, et al. Constitutively active Akt1 protects HL60 leukemia cells from TRAIL-induced apoptosis through a mechanism involving NF-κB activation and cFLIP(L) up-regulation. Leukemia. 2003;17:379–389. doi: 10.1038/sj.leu.2402793. [DOI] [PubMed] [Google Scholar]
- 126.Xu Q, Simpson SE, Scialla TJ, Bagg A, Carroll M. Survival of acute myeloid leukemia cells requires PI3 kinase activation. Blood. 2003;102:972–980. doi: 10.1182/blood-2002-11-3429. [DOI] [PubMed] [Google Scholar]
- 127.Di Cristofano A, Pandolfi PP. The multiple roles of PTEN in tumor suppression. Cell. 2000;100:387–390. doi: 10.1016/s0092-8674(00)80674-1. [DOI] [PubMed] [Google Scholar]
- 128.Yilmaz OH, Valdez R, Theisen BK, Guo W, Ferguson DO, Wu H, et al. Pten dependence distinguishes haematopoietic stem cells from leukaemia-initiating cells. Nature. 2006;441:475–482. doi: 10.1038/nature04703. [DOI] [PubMed] [Google Scholar]
- 129.Zhang J, Grindley JC, Yin T, Jayasinghe S, He XC, Ross JT, et al. PTEN maintains haematopoietic stem cells and acts in lineage choice and leukaemia prevention. Nature. 2006;441:518–522. doi: 10.1038/nature04747. [DOI] [PubMed] [Google Scholar]
- 130.Chen BP, Fraser C, Reading C, Murray L, Uchida N, Galy A, et al. Cytokine-mobilized peripheral blood CD34+ Thy-1 +Lin-human hematopoietic stem cells as target cells for transplantation-based gene therapy. Leukemia. 1995;9:S17–S25. [PubMed] [Google Scholar]
- 131.Wendel HG, De Stanchina E, Fridman JS, Malina A, Ray S, Kogan S, et al. Survival signalling by Akt and elF4E in oncogenesis and cancer therapy. Nature. 2004;428:332–337. doi: 10.1038/nature02369. [DOI] [PubMed] [Google Scholar]
- 132.Ghosh S, Tergaonkar V, Rothlin CV, Correa RG, Bottero V, Bist P, et al. Essential role of tuberous sclerosis genes TSC1 and TSC2 in NFkappaB activation and cell survival. Cancer Cell. 2006;10:215–226. doi: 10.1016/j.ccr.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 133.McCubrey JA, Steelman LS, Chappell WH, Abrams SL, Wong EW, Chang F, et al. Roles of the Raf/MEK/ERK pathway in cell growth, malignant transformation and drug resistance. Biochim Biophys Acta. 2007;1773:1263–1284. doi: 10.1016/j.bbamcr.2006.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Blagosklonny MV. Hsp-90-associated oncoproteins: multiple targets of geldanamycin and its analogs. Leukemia. 2002;16:455–462. doi: 10.1038/sj.leu.2402415. [DOI] [PubMed] [Google Scholar]
- 135.King WG, Mattaliano MD, Chan TO, Tsichlis PN, Brugge JS. Phosphatidylinositol 3-kinase is required for integrin-stimulated AKT and Raf-1/mitogen-activated protein kinase pathway activation. Mol Cell Biol. 1997;17:4406–4418. doi: 10.1128/mcb.17.8.4406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Steelman LS, Pohnert SC, Shelton JG, Franklin RA, Bertrand FE, McCubrey JA. JAK/STAT, Raf/MEK/ERK, PI3K/Akt and BCR-ABL in cell cycle progression and leukemogenesis. Leukemia. 2004;18:189–218. doi: 10.1038/sj.leu.2403241. [DOI] [PubMed] [Google Scholar]
- 137.Daley GQ. Towards combination target-directed chemotherapy for chronic myeloid leukemia: role of farnesyl transferase inhibitors. Semin Hematol. 2003;40:11–14. doi: 10.1053/shem.2003.50035. [DOI] [PubMed] [Google Scholar]
- 138.Buzzeo MP, Scott EW, Cogie CR. The hunt for cancer-initiating cells: a history stemming from leukemia. Leukemia. 2007;21:1619–1627. doi: 10.1038/sj.leu.2404768. [DOI] [PubMed] [Google Scholar]
- 139.Ninomiya M, Abe A, Katsumi A, Xu J, Ito M, Arai F, et al. Homing, proliferation and survival sites of human leukemia cells in vivo in immunodeficient mice. Leukemia. 2007;21:136–142. doi: 10.1038/sj.leu.2404432. [DOI] [PubMed] [Google Scholar]