Abstract
The G protein alpha subunit type-2 (Gαi2)-deficient mouse develops inflammatory bowel disease (IBD) with increased severity in mice on a 129SvEv (129) background compared to the C57BL/6 (B6) background. By breeding these strains to homozygosity for the first time, it became possible to study innate immunity, in this animal model, with more precision than ever before. We learned that dendritic cell distribution and function are significantly different in the two strains of mice.
In contrast to Gαi2-/- B6, Gαi2-/- 129 mice display accelerated onset and increased severity of colitis, abnormal mucosal DC distribution, accompanied by preponderance for Th1 and Th17-associated gut cytokine expression. TLR9 activation of bone marrow-derived dendritic cells (BMDCs) induces sustained p38 MAPK activation and greater Th1- and Th17-type cytokine secretion in both strains of Gαi2-deficient compared to wild-type BMDCs. However, only B6 Gαi2-/- BMDCs concomitantly produce IL-10 while Gαi2-/- 129 BMDCs do not. A relative IL-10 insufficiency in Gαi2-/- 129 BMDCs may account for the striking difference in disease.
Keywords: dendritic cell, Gi alpha, inflammatory bowel disease, cytokine
Introduction
How tolerance is maintained by the gut-associated lymphoid tissue (GALT) against the enormous diversity of antigens from commensal microflora and food remains a fundamental question in mucosal immunology. Preservation of immune equilibrium, while simultaneously surveying the vast pool of antigens for pathogens, requires an intricate and self-correcting immune response. A sophisticated mucosal immune system includes sentinel cells including T cells, B cells, macrophages, and dendritic cells (DCs), which serve to maintain homeostasis. Most of these cells lie in a hypo-responsive state within the GALT, poised for action against pathogens.1, 2
Inflammatory bowel diseases (IBD) are chronic relapsing disorders exemplified by inappropriate and exaggerated mucosal immune responses, involving Toll-like receptors (TLRs) and the activation of other pattern-recognition receptors (PRRs) against the indigenous microflora.3, 4 The exact immunopathogenesis of IBD remains unknown, although a combination of environmental and genetic factors are presumed to affect one or more of the cell types within the GALT.5
Animal models have provided numerous insights into mucosal immunity relevant to the pathogenesis and treatment of IBD. A mouse model that closely resembles ulcerative colitis, including colon adenocarcinoma, is the inhibitory G protein alpha subunit type 2 (Gαi2)-deficient mouse.6 The Gαi2-deficient mouse spontaneously develops ulcerative colitis by 16-21 weeks, and 30-40% of the animals develop non-polypoid adenocarcinoma, typical of human ulcerative colitis.6
Genetic differences between mouse strains affect the susceptibility to colitis in the Gαi2-deficient mouse. In multigenic diseases, as IBD, hybrid mice can limit our understanding of the effect of this gene deletion in mucosal inflammation due to possible confounding effects of background strain modifiers.
Gαi2-deficient C57BL/6 (B6) mice are relatively resistant to colitis, whereas Gαi2-deficient 129 mice develop inflammatory bowel disease earlier and with greater frequency and severity.7 Linkage analysis has identified a locus on mouse chromosome 3 termed Gpdc1, which confers colitogenic susceptibility in the Gαi2-deficient mouse.8 An overlapping locus, called CdcS1, is linked with disease susceptibility in another IBD mouse model, due to IL-10 deficiency.9, 10 The Cdcs1/Gpdc1 locus appears to be the major independent genetic determinant for strain susceptibility in these two IBD models.
Although Gαi2 protein is expressed in many cell types, it is interesting that only colonic inflammation is observed in Gai2-deficient mice. Progress has been made in characterizing the cellular and molecular deficiencies in the Gαi2-deficient mouse, but the relevance of immune cell types and signaling pathways in colitogenesis remains controversial.11, 12
The dendritic cell links and regulates the adaptive immune system response to innate immune system stimulation. DCs play a vital role in the induction and maintenance of immunity because of their role as immune sentinels, chief antigen presenting cells (APCs), and directors of helper T cell function. However, DCs can also contribute to an inappropriate immune response in IBD.13-17 Human patients with active IBD have reduced counts of peripheral plasmacytoid and myeloid DCs, possibly due to migration of these cells into the gut.18 Notably, IL-23 is elaborated by intestinal lamina propria DCs,19 which drives inflammation in several models of T cell colitis.20-22
IL-23 is needed for the clonal survival and expansion23 of the Th17 cell, a recently described class of CD4+ T cells,24 that has been associated with human inflammatory diseases such as autoimmune arthritis, systemic sclerosis, psoriasis and obstructive airway diseases.25 Differentiation of naïve CD4+ cells into Th17 cells requires TGF-β and IL-6.23, 26 Th17 cells produce the cytokine, IL-17, which up-regulates production of IL-8, IL-1, IL-6, GM-CSF and prostaglandin E2.27 The Th17 cell also produces IL-6 and TNF-α.24
Early descriptions of the Gαi2-/- mouse reported significant increases in CD4+ T cells producing Th1 cytokines in the colon of Gαi2-deficient mouse with active colitis,28, 29 an increase in hyper-responsive single-positive thymocytes,30, 31 and a deficiency in marginal zone B cells.12 Clearly, cells involved in adaptive immunity are altered in this model. Induction of the Th1 phenotype in the Gαi2-/- mouse has been attributed to IL-12 p40 expression, following LPS-mediated TLR stimulation, in splenic dendritic cells from Gαi2-/- mice compared to wild-type counterparts.32 However, the innate response of the Gαi2-/- mouse to commensal bacterial antigens remains to be fully explored.
Understanding molecular pathways where Gαi2 protein participates in the development of colitis may contribute to newer, more effective treatments for IBD. Here, we investigate the possibility that DCs, lacking Gαi2, lose the regulatory balance of the innate immune response to enteric antigens.
Materials & Methods
Animals and genotyping
Mice were raised and bred in an Association for Assessment and Accreditation of Laboratory Animal Care-approved facility at Baylor College of Medicine (BCM), Houston, TX, under specific pathogen-free (SPF) conditions in micro-isolator cages. Animal husbandry and experiments were carried out in accordance to protocols approved by the BCM Institutional Animal Care and Use Committee. Incipient congenic Gαi2-/- mice were reared by backcrossing 129×B6 Gαi2-/- hybrids onto 129S6/SvEvTac (129) or C57BL/6NTac (B6) (Taconic Farms, Germantown, NY) to the 8th generation (N8) or beyond.
Allelic discrimination between 129 and B6 mice were carried out by PCR amplification of the cdcS1 locus. Genomic DNA were extracted from tail clippings (Wizard® SV Genomic DNA Purification System, Promega, Madison, Wisconsin) and primers for D3Mit348 (ResGen-Invitrogen, Carlsbad, California) used in microsatellite mapping reactions. Gαi2-deletion was confirmed in progeny by PCR using primers targeted against the neomycin cassette used to disrupt the Gαi2 gene.6
Colon histologic analysis and explant cultures
Colons were resected from mice, cut longitudinally to expose the lumen then rinsed in DC media (see BMDC cell culture section) for 10 min at 37 °C. Colons were cut into ~1mm × 1mm pieces, weighed then cultured in 24 well plates with 2 ml DC media. After 18-24 h the supernatants were collected and debris removed by centrifugation. Aliquots were frozen at -80 °C and analyzed by cytometric bead capture (see Cytokine and MAPK analyses section).
Representative fragments of each colon section were sections were also fixed in 10% neutral-buffered formalin and processed for routine hematoxylin and eosin (H&E) staining. Pathology was scored in a blinded fashion using a previously described scoring system.33 Criteria were as follows: Grade 0: no changes compared to normal tissue; Grade 1: occasional mononuclear infiltrates in the mucosa lacking any injury to epithelial cells or loss of goblet cells; Grade 2: multi-focal, mild infiltrates of mononuclear cells and occasional neutrophils with rare involvement of submucosa. Mild epithelial cell damage with occasional mucus depletion; Grade 3: more frequent and severe lesions compared to grade 2 with more acute inflammation (i.e. polymorphonuclear cells), moderate involvement of submucosa with rare transmural infiltration, mucin depletion evident and moderate epithelial hyperplasia; Grade 4: the entire region of colon involved with severe lesions including mononuclear cells and neutrophils with transmural infiltration. Epithelial cells hyperplastic with diffusely elongated crypts and severe depletion of goblet cells.
Additional samples were used for immunofluorescence and immunohistochemical staining. Segments of normal and Gαi2-/- bowel were formed into Swiss rolls and frozen in Tissue Tek OCT media, sectioned on a Leica CM1850 cryostat (Leica Microsystems, Bannockburn, Illinois) at 5 μm intervals onto Superfrost/plus slides (Fisher Scientific, Pittsburgh, Pennsylvania), post-fixed with 2% paraformaldehyde prior to H&E or fluorescent staining. For double labeling, the tissue sections were incubated with hamster anti-mouse CD11c (a pan-DC marker; BD Pharmingen, San Jose, California) and rat anti-mouse CD11b (a macrophage/DC marker; BD Pharmingen) and visualized sequentially using HRP-conjugated goat anti-rat IgG (Jackson ImmunoResearch, West Grove, Pennsylvania) followed by tyramide based TSA fluorescent labeling reagent Alexa FL 568, (Molecular Probes-Invitrogen, Eugene, Oregon) which was then blocked with hydrogen peroxide and stained with HRP-conjugated anti-hamster IgG (Jackson ImmunoResearch) then visualized with Alexa FL 488 (Molecular Probes). Isotype control antibodies were used to test the effectiveness of the hydrogen peroxide block after the Alexa FL 568 step. The fluorescently labeled sections were counterstained with ProLong Gold antifade reagent with DAPI (Molecular Probes), and examined with a Zeiss Axiovert 200 fluorescence microscope (Carl Zeiss Imaging, Thronwood, New York) and analyzed by ImagePro plus software (MediaCybernetics, Bethesda, Maryland). Routine immunohistochemistry was performed using anti-B220/CD45R, -CD3 or -CD11c monoclonal antibodies (BD Pharmingen) followed by a biotin-based diaminobenzidine chromogen detection system (Link-label Detection kit, BioGenex, San Ramon, California) and counterstained with hematoxylin.
Bone marrow-derived dendritic cell culture
Tibia and femurs were collected from female mice following CO2 asphyxiation. Long bones were gently crushed, flushed with PBS and cells recovered by straining through a sterile nylon mesh. Mononuclear cells were concentrated by gradient centrifugation (Lympholyte M®, Cedarlane Laboratories, Burlington, North Carolina). Cells were washed three times with 1% heat-inactivated FBS, 100 U/ml penicillin, 10 mg/ml streptomycin and 1 mM HEPES in Hank's balanced salt solution. Cells were resuspended to 106 cells/ml in DC media, which is 10% heat inactivated fetal bovine serum (Hyclone, Logan, Utah), 2 mM L-glutamine, 100 U/ml penicillin, 10 mg/ml streptomycin, 1 mM HEPES (Invitrogen, Carlsbad, California) in RPMI 1640 (BioWhittaker, Walkersville, Maryland), and cultured in the presence of recombinant GM-CSF (R&D Systems, Minneapolis, Minnesota) at 100 ng/ml for seven days in 100 mm non-treated tissue culture dishes. Media was replenished every other day by removing ½ of the media and replacing with ½ of fresh media.
Cell sorting and bioassays
Bone marrow-derived dendritic cells (BMDCs) were cultured for seven days, CD11c-enriched by magnetic bead sorting (StemCell Technologies, Canada) and stained with fluorescent labeled antibodies CD11b-FITC and CD11c-PE (BD Pharmingen). CD11c-enriched BMDCs were treated with an Fc receptor blocking agent (Fc blockTM, BD Pharmingen) prior to staining with anti-CD11c/PE and anti-CD11b/FITC antibodies (BD Pharmingen). Enriched BMDCs were then sorted according to CD11b and CD11c expression on a MoFlo® Flow cytometer (DAKO Cytomations, Fort Collins, Colorado). CD11c-enriched and FACS-sorted cells were washed once, resuspended to 106 cells/ml and plated at 100 ml volumes in 96-well flat bottom clusters in the presence of media, phosphorothiorate-modified oligodeoxynucleotide (CpG-ODN or CpG), designated “1826” (5'-TCC ATG ACG TTC CTG ACG TT-3') (5 μg/ml) or E. coli O127:B8 lipopolysaccharide (LPS) (Sigma) (100 pg/ml). Culture supernatants were collected at 6 and 24 h later, stored at -80 °C and assayed for cytokine production.
Cytokine and MAPK analyses
Production of cytokines was analyzed by cytometric bead capture (Luminex®, Austin, Texas). Levels of IL-2, IFN-γ, IL-6, IL-10, IL-12p40, IL-17, IP-10, KC and TNF-α were measured using antibody beads (Biosource, Camarillo, California). IL-23 p19/p40 levels were measured by ELISA (eBioscience, San Diego, California). We assessed mitogen-activated protein kinase (MAPK) pathway activation by stimulation of CD11c-enriched BMDCs with CpG-ODN 1826 (5 μg/ml) or LPS (100 pg/ml) for 30 and 60 min at 37 °C. Total and phosphorylated (active) ERK, JNK and p38 MAP kinases were assayed (Biosource). Whole cell lysates were prepared from DCs by treatment of cell pellets with cell lysis buffer (50 mM Tris pH 7.4, 250 mM NaCl, 5 mM EDTA, 50 mM NaF, 1 mM Na3VO4, 1% Nonidet P40 supplemented with 4 mM PMSF, AEBSF HCl, Aprotinin, Bestatin, E-64, Leupeptin hemisulfate and Pepstatin A) (Sigma, St Louis, Missouri). Pellets were incubated with cell lysis buffer for 30 min on ice with vortexing every 10 min. Lysates were clarified by centrifugation at maximum speed and stored at -80 °C.
Statistical analyses
Statistics were performed using SPSS for Windows version 15.0 (SPSS Inc., Chicago, Illinois). Cytokine and histologic data were analyzed using Kruskal-Wallis tests followed by Mann-Whitney U test for independent samples. IL-23 data were compared by ANOVA followed by t-test for independent samples. Correlations between cytokine production and disease severity were determined using Spearman's method and reported as rho (ρ). P values of ≤ 0.05 were considered significant.
Results
Gαi2-/- 129 mice are more susceptible to IBD compared to Gαi2-/- B6 mice
To clarify the strain background effects on dendritic cell function in the Gαi2-/- mouse, we reared incipient congenic Gαi2-/- mice by backcrossing 129×B6 Gαi2-/- hybrids onto 129 or B6. Using microsatellite mapping, we screened N8 (or later) mice for homozygosity of 129 or B6 Cdcs1 alleles, shown to be important in both Gαi2-/- and IL-10-/- mouse models of IBD.8, 10 We found that after eight backcrosses, we were able to achieve the C57BL/6 Cdcs1 locus homozygosity in our Gαi2-/- B6 mouse line and the 129SvEv Cdcs1 locus homozygosity in both lines.
In our SPF animal facility, Gαi2-/- 129 mice consistently succumb to IBD by 4-6 months of age with a median inflammation score of 2 (out of 4) and a 100% penetrance, whereas our Gαi2-/- B6 mice show no disease at 4-6 m and manifest inflammation only after nine to 12 m of age (Figure 1) (Table I).
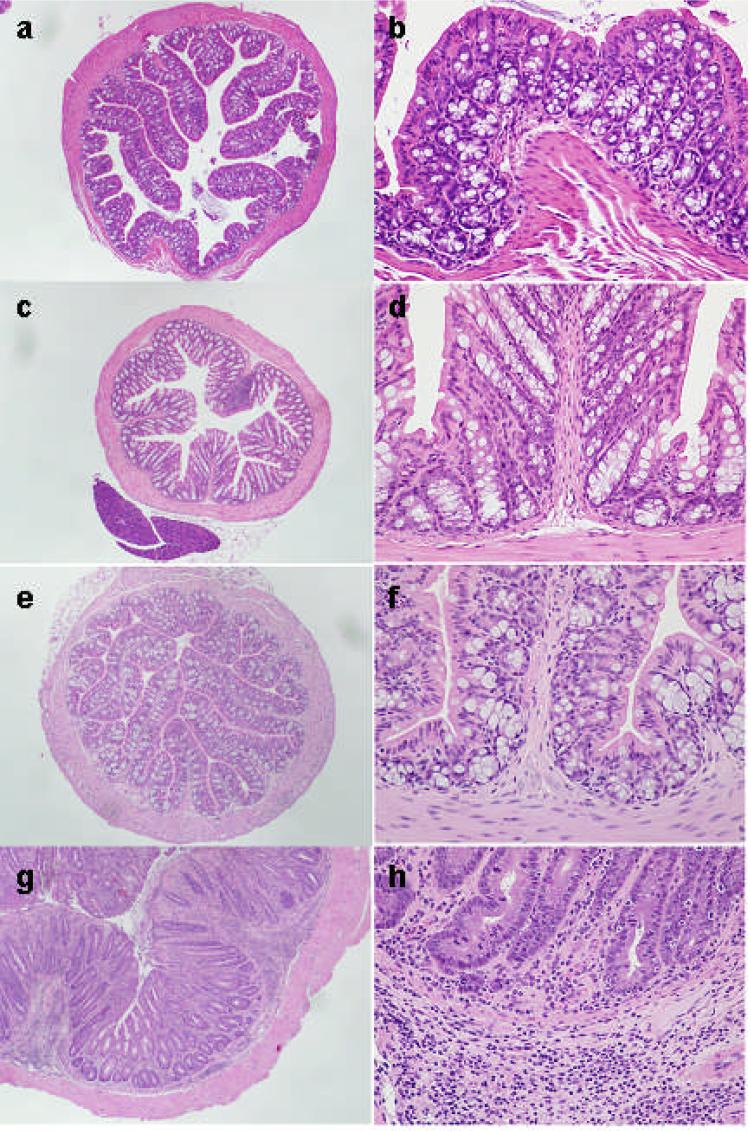
Figure 1.
H&E stained sections of colons from 4 month old mice. (a,b) wild-type C57BL/6 (B6) mouse, (c,d) Gαi2-deficient B6 mouse, (e,f) wild-type 129SvEv (129) mouse and (g,h) Gαi2-deficient 129 mouse. (a) through (f) show show overall preserved mucosal architecture, cytology and mucin production. A few lymphocytes are present in the lamina propria. Epithelial cells do not show increased mitotic activity or reactive changes. The Gαi2-deficient 129 mouse colon displays architectural distortion with occasional bifurcation of glands, mucosal mucin depletion and predominantly plasma lymphocytic infiltrate, involving the lamina propria, muscularis mucosa and submucosa. Inflammation also contains rare eosinophils and occasional neutrophils. There is focally prominent mitotic activity in the glandular epithelium. Epithelial cells show reactive changes, with hyperchromasia and enlargement of nuclei, irregularity of the nuclear contours and occasionally prominent nucleoli. No atypical mitoses are seen. Magnification 40x (a,c,e,g) and 200x (b,d,f,h)
Table 1.
Histologic scores in the colon
| Group | Age (m) | N | Histologic Score Median (Interquartile range) |
|---|---|---|---|
| WT B6 | 4-6 | 3 | 0 (0-0.5) |
| Gαi2 B6 | 4-6 | 3 | 0 (0-0.5)* |
| WT B6 | 9-10 | 2 | 0.5 (0-1) |
| Gαi2 B6 | 9-10 | 2 | 2 (1-3) |
| WT129 | 4-6 | 6 | 0 (0) |
| Gαi2 129 | 4-6 | 6 | 2 (2-3)* |
Histologic scores were significantly worse in Gαi2 129 mice than B6 counterparts at 4-6 m of age (P=0.024) but not at 9-10 m age (P>0.05).
Th1- and Th17-type cytokines are produced by inflamed colons in Gαi2-/- mice
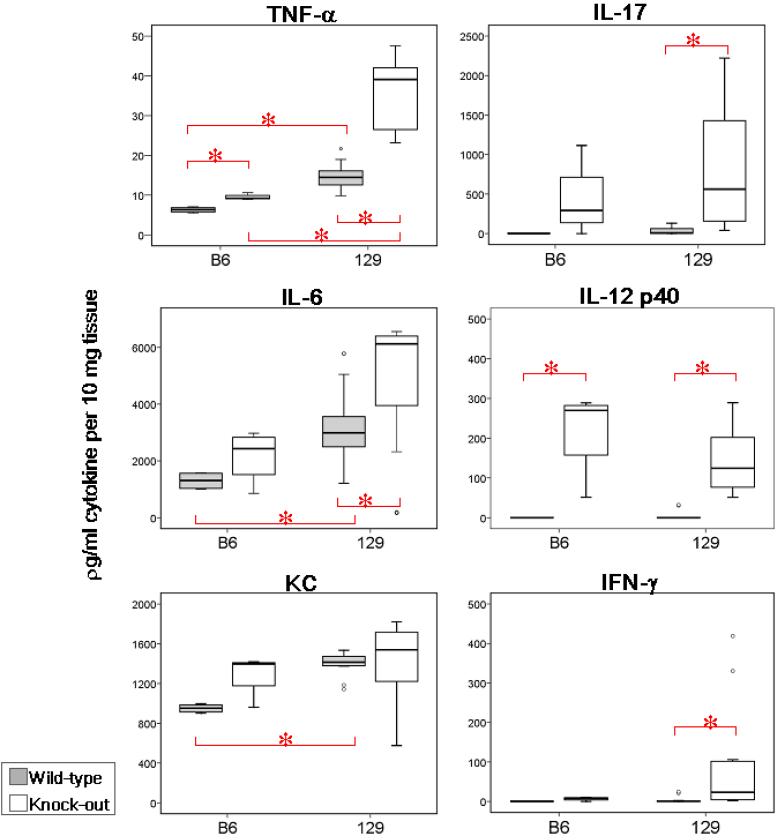
In order to correlate cytokine production with the degree of inflammation, adjacent segments of colon were examined histologically and for cytokine production by ex vivo colon explant culture. To enable us to compare mucosal cytokine expression in the setting of colitis, it was essential to use four to six week old 129 mice and nine to ten month-old B6 mice because the age of IBD onset in these two background strains differs so dramatically. Colon cultures from mice on 129 background, aged four to six months, lacking the Gαi2 gene, produced significantly greater levels of IFN-γ, IL-6, IL-12 p40, IL-17, and TNF-α over their WT counterparts (all P<0.05) (Figure 2). As inflammation tends to develop at nine months or older in the B6 mice, we compared cytokine expression from mice at this age. Only TNF-α and IL-12 p40 were significantly increased in Gαi2-/- B6 colons compared to WT B6 mice (all P<0.05). IL-17, IL-12 p40 and IFN-γ appear to be produced in colons of both the 4-6 m old Gαi2-/- 129 mice and the 9 m old Gαi2-/- B6 mice. However inflammatory cytokines, including IFN-γ, TNF-α and IL-6 are more prominent in the Gαi2-/- 129 colons compared to the Gαi2-/-B6 mice despite similar histologic scores (Table I).
Figure 2.
Ex vivo cytokine production by mouse colons. Colons were cultured for 24h without added TLR ligand and supernatants were assayed for cytokines. Experiments represent at least 4 animals per strain and genotype. Data in box-plots with median (heavy line inside box), interquartile range (box), range (error bars) and outliers (circles beyond error bars). Intergroup comparisons yielding significantly different levels of cytokines are represented (*red bars, P<0.05)
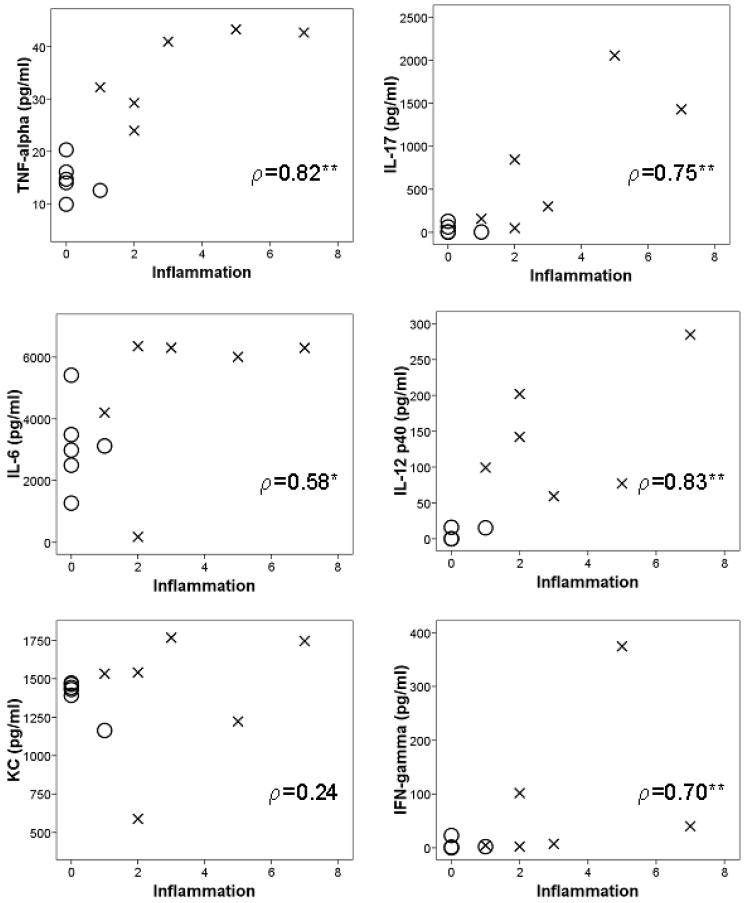
To determine which cytokines correlate with the severity of inflammation, we directly compared cytokine production with degree of colonic inflammation (Figure 3). When comparing colitis-susceptible 4-6 m old Gαi2-/- 129 mice to age-matched WT 129 mice, a strong correlation was detected between inflammation and the increased levels of IL-12 p40 (p=0.834), TNF-α (p=0.822), IL-17 (p=0.746), and IFN-γ (p=0.792) (all correlations P<0.01) in colon supernatants. This is in contrast to the lack of correlation (all P>0.05) between cytokine secretion and disease severity in mice on a B6 background alone (data not shown).
Figure 3.
Correlations between cytokine production and histologic disease severity in colons of Gi2-/- 129 mice. Spearman's correlation coefficient is indicated, ρ, with significant correlations indicated by ** (P<0.01) and * (P<0.05) Degree of inflammation is positively correlated with increased levels of TNF-α, IL-6, IL-17, IL-12 p40 and IFN-γ. Note that expression of KC is not related to inflammation in these mice.
DCs in the intestinal lamina propria of Gαi2-/- mice have abnormal distribution
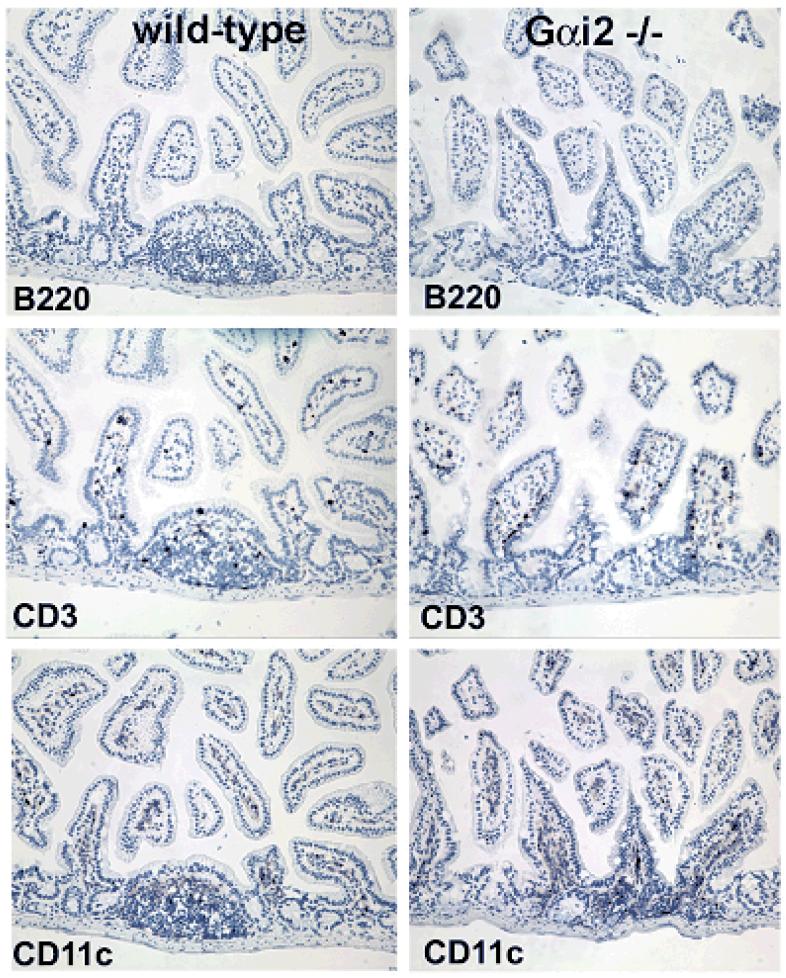
Activation of the immune response is thought to precede intestinal damage in the Gαi2-deficient mouse.34 Lymphoid follicles in ileal GALT display abnormal distributions of DCs in Gαi2-/- mice as young as eight weeks old, prior to visible inflammation in the colon, which appears at 4-6 m of age. Unlike the localized sub-epithelial dome distribution of the DCs in WT 129 mice, the Gαi2-/-129 mice at 2-6 m of age consistently show a random distribution of CD11c+ cells within the gut associated lymphoid follicles in the intestines (Figure 4a). Colons from Gαi2-/- 129 mouse also show increased numbers of CD11c+ cells in the crypts and lamina propria long before the development of intestinal inflammation (Figure 4b).
Figure 4a.
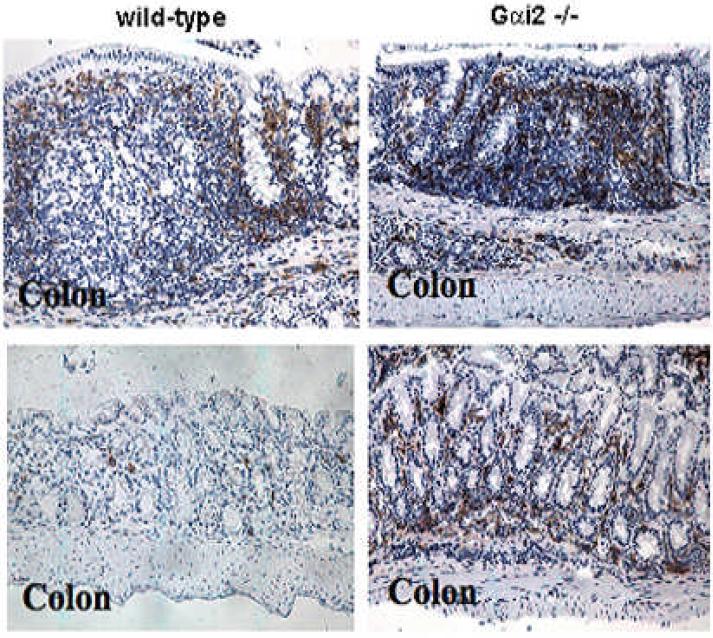
Serial immunohistochemical staining of ileum from 129SvEv mice for B220/CD45R, CD3 and CD11c. Gαi2-deficient mice have increased numbers of CD11c+ cells in the lamina propria as well as the absence of well-formed Peyer's patches compared to WT mice. Note the random distribution of CD11c-staining cells in Gαi2-/- mice compared with the ordered subepithelial dome distribution in WT mucosa. No difference in T cell (CD3) and B cell (B220) staining was observed between the two genotypes of mice. Magnification 40x.
Figure 4b.
Immunohistochemical staining of colon from 129SvEv mice for CD11c. Gαi2-deficient mice have increased CD11c staining in the lamina propria as well as the absence of well-formed lymphoid follicles compared to WT mice. Note the random distribution of CD11c-staining cells in Gαi2-/- mice compared with the orderly localized subepithelial dome distribution in WT mucosa. Magnification 40x (top); 100x (bottom).
By double-labeled immunofluorescence microscopy, the largest differences that we observed in gastrointestinal DCs is the increased number of DCs in the colon of the Gαi2-/- 129 SvEv mice compared to their B6 counterparts, as early as 8 weeks of age (Figure 5). In contrast, Gαi2-/- B6 mice do not have an increase in DC numbers in their colons. This suggests that the abnormal distribution of mucosal DCs in the Gαi2-/- 129 mice could affect T cell and NK cell activation and cytokine production, perhaps leading to the observed inflammation commencing at about 4 m of age.
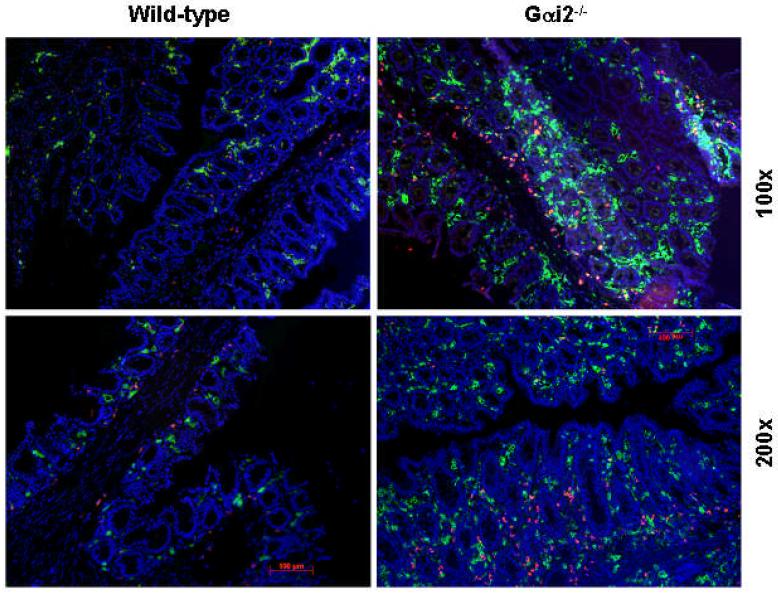
Figure 5.
CD11b and CD11c immunofluorescence microscopy of cecum of 129SvEv mice. There is increased CD11c staining (green) as well as CD11b (red) in the lamina propria of Gαi2-/- mice compared to WT counterparts. Double-staining cells (yellow) are seen only in inflamed tissue from Gαi2-/- mice and not WT 129, representing CD11c+b+ (double-positive) cells, likely DCs.
Differences in cytokine expression by BMDC subsets exist in the Gαi2-/- mouse
Studies of DC function in the gut are complicated by environmental influences. Dendritic cell function is partially determined by signals encountered through maturation, development and location.35-37 Isolation of DCs from the mucosal tissue can alter their function and decrease viability, and posing problems with functional analyses. We therefore chose to study immature DCs derived from bone marrow cultures (BMDCs), from the two strains, to determine whether Gαi2 deficiency alters the intrinsic response of naïve BMDCs to TLR signals.
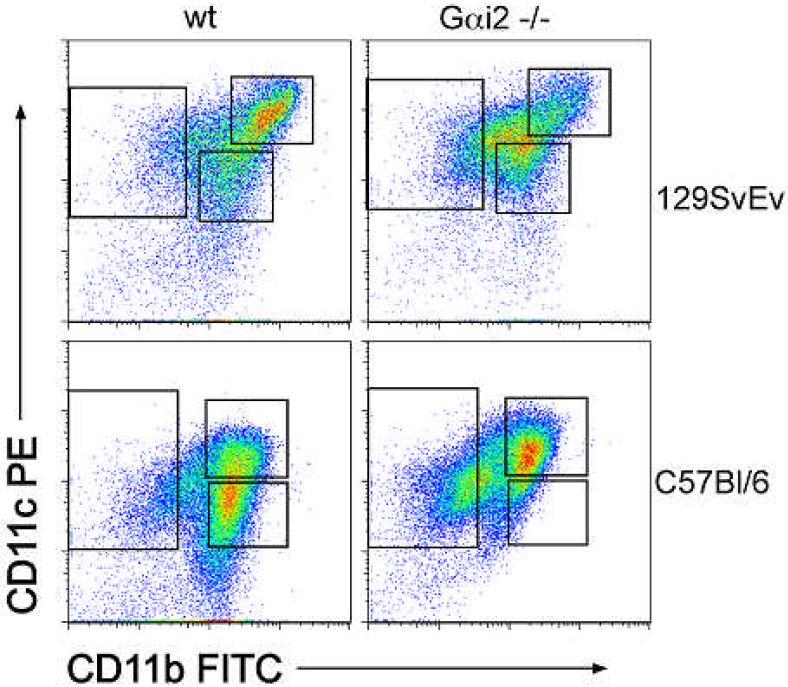
Sorted cultures of DCs from bone marrow non-lymphoid mononuclear cell precursors yielded three distinct populations of immature BMDCs (all CD11c+ with varying CD11b expression). The following subsets of dendritic cells were isolated from BMDC cultures by flow cytometry: CD11c+b- (single-positive), CD11c+hi CD11b+hi (double-hi) and CD11c+lo CD11b+lo (double-lo) (Figure 6). To compare cytokine production in response to TLR stimulation the DC subsets were cultured overnight with LPS or CpG. TLR9 stimulation, by CpG-ODN, activates a major pathway in naïve DCs 38 and has been implicated as a major source of stimulation from commensal Lactobacillus sp.39 IL-17 and IFN-g were undetectable in BMDC supernatants, while IL-6, IL-10, IL-12 p40, IP-10, KC and TNF-α were all produced in DC culture supernatants. Cytokine production differences depended on time, stimulus and DC subtype. Unstimulated BMDCs of all three subtypes from WT 129 animals secreted relatively lower levels of cytokines at 24 h when compared to those stimulated with LPS or CpG for 24h. Immature BMDCs produced little or no detectable cytokines in response to LPS alone (data not shown), indicating a low expression of TLR4 in these cells.
Figure 6.
Flow cytometric analysis of CD11b and CD11c expression by BMDCs. Three populations of BMDCs were isolated following seven-day culture with GM-CSF: CD11c+b- (single-positive), CD11c+hib+hi (double-hi) and CD11c+lob+lo (double-lo). There is slightly increased expression of CD11b expression in double-hi cells from 129 mice compared to B6 mice.
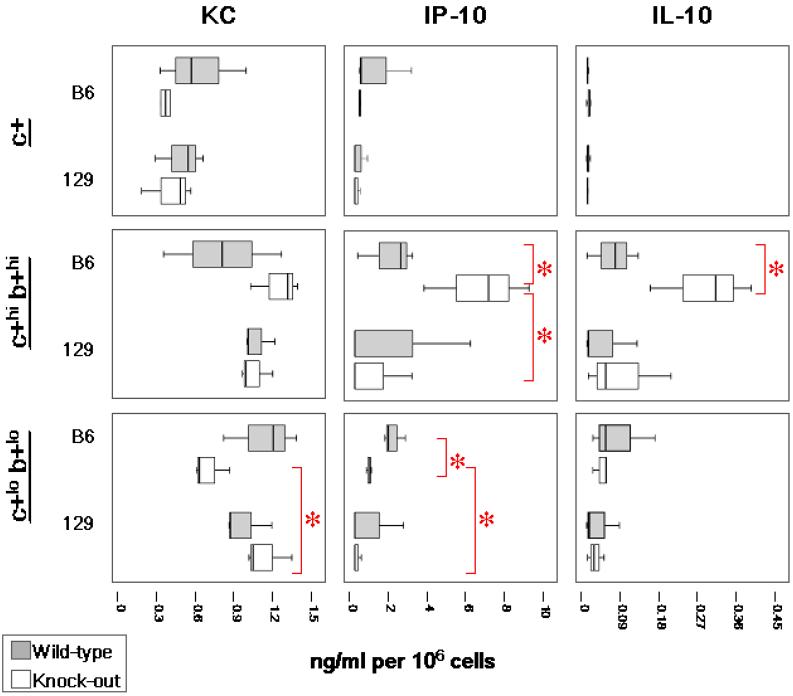
Single-positive BMDCs from Gαi2-/- 129 or B6 mice did not produce significantly different levels of TNF-α, IL-6, IL-12 p40 or the chemokines, KC (CXCL1) and IP-10 (CXCL10), in response to CpG stimulation compared to their WT counterpart cells (all P>0.05) (data not shown). However, these single-positive BMDCs, from either mouse strain, respond to CpG with a generally lower cytokine output compared to the double-hi and double-lo cells with the exception of IL-12 p40 (Figure 7). Apparently, immature single-positive DCs are primed to respond immediately to CpG stimulation by IL-12 p40 production in both strains of mice.
The most significant difference we observe in the DC subtypes isolated from the BMDC is the response of either the double-hi or double-lo BMDCs to TLR9 stimulation by CpG-ODN 1826. Figure 7 shows the in vitro responses of BMDC subsets to CpG. At 24 h post-stimulation with CpG, no significant differences in amounts inflammatory cytokines were produced by Gαi2-/- B6 double-hi BMDCs compared to the same DC subset from B6 WT mice, with the exception of greater IP-10 expression (P=0.05). Interestingly, this subset of Gαi2-/- B6 BMDCs simultaneously produces significantly higher levels of the anti-inflammatory cytokine, IL-10 (P=0.05), while the BMDCs from 129 mice produce a minimal IL-10 response to CpG (Figure 7). In addition, 24h after CpG stimulation, the Gαi2-/- 129 double-lo cells produce significantly more TNF-α, IL-6 and KC than the Gαi2-/- B6 double-lo cells. These findings suggest that production of anti-inflammatory cytokines by the DCs from Gαi2-/- B6 mice, compared with increased pro-inflammatory cytokine production by Gαi2-/- 129 DCs, could account for the different pathologies in these mouse strains.
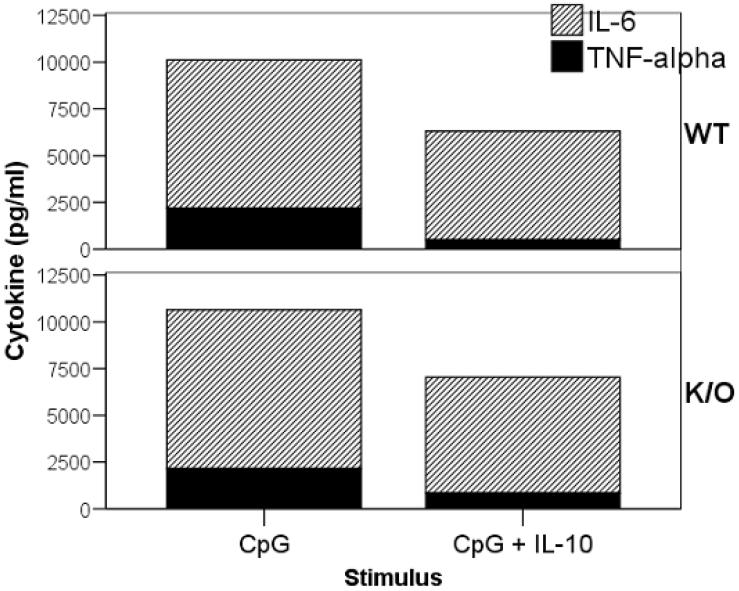
As production of IL-10 was decreased in TLR-mediated responses of 129 BMDCs (relative to B6), we also determined if the increased inflammatory phenotype of 129 mice was simply due to a decreased IL-10 production or whether IL-10 receptor signaling was also impaired. We tested whether CD11c-enriched BMDCs from the Gαi2-/- or WT 129 mice could respond to exogenous IL-10. BMDCs from the 129 mice responded to the anti-inflammatory signals of IL-10 during CpG stimulation as evidenced by decreasing TNF-α and IL-6 production compared to cells not receiving exogenous IL-10 (Figure 8). This indicates that 129 mice have the necessary machinery for IL-10 regulation of TLR-activated DCs, but may lack the appropriate pathways related to TLR9 activation to induce IL-10 secretion.
Figure 8.
TNF-α and IL-6 suppression by exogenous IL-10. BMDCs from 129 mice were stimulated by CpG or CpG with exogenous IL-10 (5 ng/ml) and assayed for IL-6 and TNF-α. Expression of inflammatory cytokines in both WT and Gαi2-deficient (K/O) mice were decreased in the presence of exogenous IL-10.
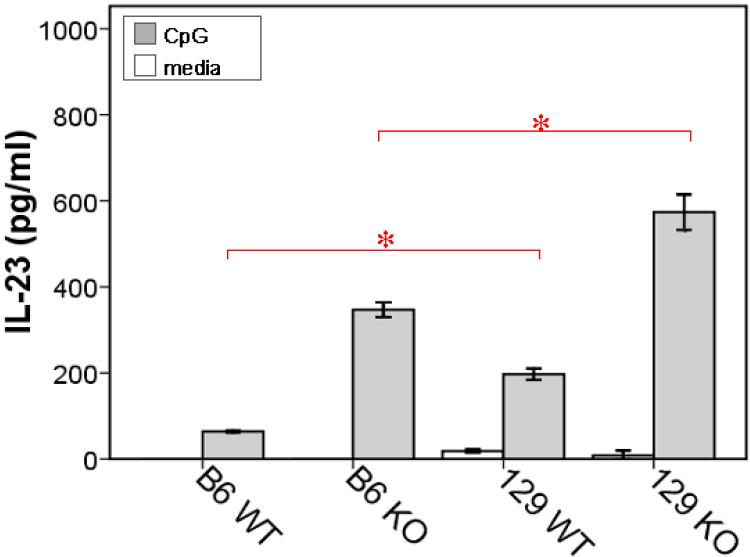
Since we had observed an increase in IL-12 p40 in the colons of Gαi2-/- mice from both strains, but no significant differences in CpG-induced IL-12 p40 expression in the immature DC subsets, we sought to determine if there could be any differences in IL-23 expression. The heterodimeric cytokines IL-12 and IL-23 share the p40 subunit, but each contains unique subunits, p35 and p19, respectively. The cytometric bead assay for the p40 subunit may represent IL-12 or IL-23. We found that CpG-stimulated Gαi2-/- 129 CD11c-enriched BMDCs compared to Gαi2-/- B6 DCs by IL-23-specific ELISA (Figure 9).
Figure 9.
IL-23 production by CD11c-enriched BMDCs following stimulation with CpG-ODN. Data shown is peak response at 18-24 h. Data shown are means (bars) and SD (error bars). Experiments represent at least 3 animals per strain and genotype. Intergroup comparisons yielding significantly different levels of IL-23 are represented (*red bars, P<0.05)
Our cytokine data indicates that Gαi2-deficiency promotes an increased inflammatory cytokine response to TLR9 activation in immature BMDCs. When considering background strain differences, DCs from B6 mice simultaneously express IL-10, which may counteract these inflammatory mediators, suggesting a possible mechanism by which Gαi2-/- B6 mice develop resistance to inflammation. In the Gαi2-/- 129 mouse however, there is a lack of anti-inflammatory IL-10 and increased IL-23 production by DCs, thus promoting a pro-inflammatory Th17 response in the colon milieu, as demonstrated in our in vitro colon cultures.
Activation of p38 MAP kinase is prolonged in the Gαi2-/- DC
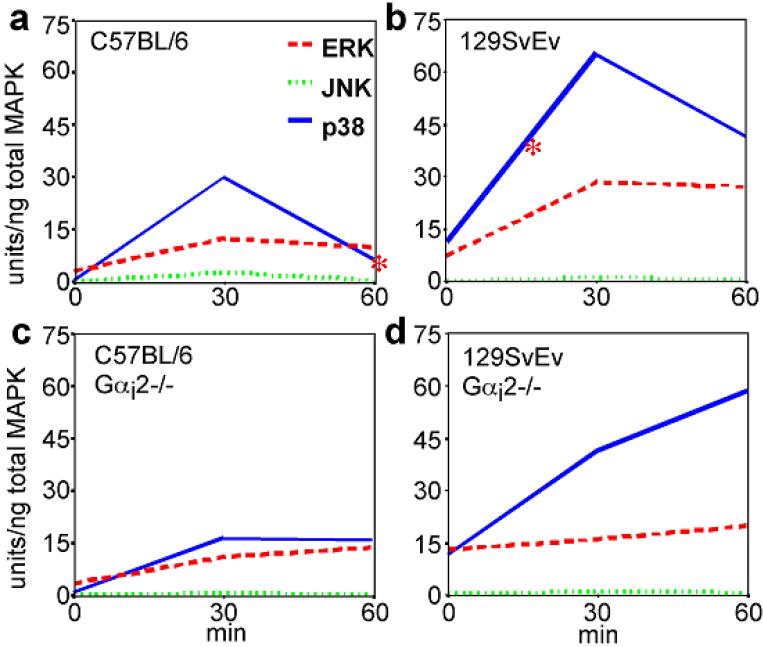
The response of Gαi2-/- DCs to TLR ligands leads to an increase in inflammatory cytokines that could be responsible for the inflammation in the colons of the Gαi2-/- mouse. Since the heterotrimeric G protein linked receptors are known to activate a number of different signaling pathways including the activation of MAP kinases,40 we examined the activation of 3 families of kinases: ERK, JNK and p38 in CpG stimulated 129SvEv and C57BL/6 CD11c-enriched BMDC cultures.
Wild-type BMDCs, from both 129 and B6 mice, responded to TLR stimulation primarily by p38 MAPK activation that peaked at 30 min post-stimulation, followed by a decrease by 60 min. BMDCs from 129 mice displayed increased p38 MAPK activation following CpG stimulation (Figure 10), likely contributing to increased inflammatory cytokine output. Additionally, BMDCs from Gαi2-/- 129 mice showed a continuous elevation of p38 MAPK activation, unlike Gαi2-/- B6 BMDCs, even after 60 min. We detected no differences in ERK1/2 activation between WT and Gαi2-/- BMDCs or between strains and no JNK activation in BMDCs stimulated with either CpG or LPS. The lack of Gαi2 in the 129 mouse affects the p38 signal transduction pathway and could account for the differences in the Gαi2-/- DC activation (e.g. cytokine production) state following TLR9 ligand stimulation.
Figure 10.
MAP kinase activation in CD11c-enriched BMDCs following stimulation with CpG-ODN. Note that p38 MAP kinase is activated and peaks at 30 min following TLR9 activation with subsequent decrease in WT BMDCs. In Gαi2-deficient BMDCs, however, p38 MAP kinase activation persists beyond 30 minutes.
Discussion
Although the exact etiology of IBD is not completely understood at this time, accumulating evidence shows that these diseases represent complex interactions of several genetic phenotypes that contribute to the susceptibility. In human patients with IBD, it is hypothesized that abnormal DC responses to the microflora play a role in inflammation.18, 41, 42
The use of mouse models to dissect the individual genetic and cellular components that contribute to the pathogenesis of IBD has been very useful in gaining some understanding of these complex diseases. Studies with the IL-10-deficient mouse have been successful in correlating a strong T helper type 1 immune response with the development of IBD. Similar to the Gαi2-/- mouse, the disease susceptibility of the IL-10-deficient mouse is strain dependent. Indeed, one of the major loci that correlates with IBD susceptibility, CdcS1, is shared by both the IL-10- and Gαi2-deficient mouse models of IBD.8-10
All previous reports on the Gαi2-deficient mouse model for IBD utilized hybrid mouse backgrounds.12, 28, 43, 44 In those studies, it was difficult to appreciate the potential for strain differences to influence the immune system. Our study was designed to further the analysis of complex genetic components that contribute to IBD susceptibility by breeding congenic strains of mice checked for the IBD susceptibility locus, CdcS1.9
Gαi2-deficient mice on a 129 background develop earlier and more severe disease than those on a B6 background. The inflammation in the colon of Gαi2-deficient mice is accompanied by expression of inflammatory cytokines. We compared colon cytokine production from these two strains at ages when one normally observes inflammation beginning (4-6 m for 129 versus >9 m for B6). We found both Th1 and Th17-type cytokine production in colon cultures correlated well with the inflammatory state of the Gαi2-/- 129 mice.
Since dendritic cells play a significant role in the maintenance of tolerance as well as pathogen recognition in the gut,45 our interest focused on the possible role of DCs in the development of colitis in these strains of Gαi2-/- mice. We demonstrated that Gαi2-/- 129 mice contain significantly more DCs in the cecum and colon compared to WT 129 and Gαi2-/- B6 mice. Additionally, DCs displayed a disorderly pattern in the GALT of Gαi2-deficient mice. In WT mice, DCs are primarily situated in the lymphoid follicles in the colon. This is in contrast to Gαi2-deficient mice, where DCs are abundant throughout the lamina propria as well as the follicular regions of the colon. This may impact the normal antigen processing and presenting function of these cells.
Using naïve, immature DCs from bone marrow, we have analyzed DC responses to the TLR9 ligand, CpG-ODN. TLR9 activation was previously demonstrated to mediate homeostatic effects of probiotics in the gut.46 We chose to study immature DCs derived from bone marrow precursors as these precursors have been shown to migrate to the gut and give rise to intestinal lamina propria DCs.47
We found that Gαi2-/- BMDCs both strains of mice show enhanced production of pro-inflammatory cytokines such as TNF-α, IL-6, IL-12p 40 and IL-23 in response to CpG-ODN. However, we also observed that Gαi2-/- 129 DCs produce twice as much IL-23 compared with Gαi2-/- B6 DCs in response to CpG-ODN stimulation. An increase in IL-23 production may explain the increase in mucosal production of IL-17 (as a marker for Th17 response) in the Gαi2-/- 129 mice that correlates with the degree of inflammation found in the colon. Since it is known that the presence of IL-23 supports the proliferation and viability of the Th17 T cells that contribute to the pathology of colitis,21, 22, 48 our observation support the theory that DCs in the Gαi2-deficient gut have a major impact on the development of the disease. Indeed, a rare allele of the IL-23 receptor gene has been correlated with resistance to Crohn's disease in humans.49
Significantly, DCs in the Gαi2-/- B6 mouse produce more of the anti-inflammatory cytokine, IL-10, than Gαi2-/- 129 mice, in response to CpG-ODN stimulation. The increase in cytokine expression correlates with an increased activation of p38 MAP kinase in the Gαi2-/- DCs in both mouse strains relative to WT counterparts. Most significantly, a sustained, elevated activation of p38 MAPK in Gαi2-deficient 129 DCs suggests that these DCs may fail to down-regulate responses to TLR9 stimulation.
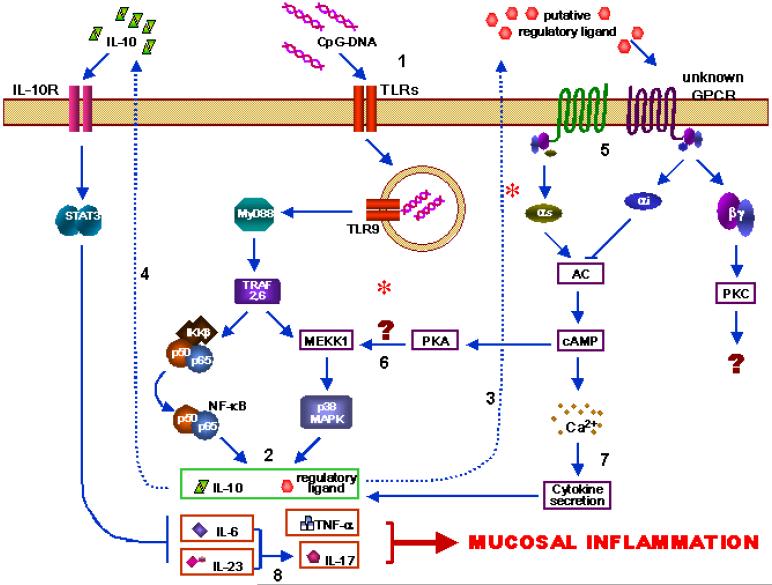
These findings lend support to our conjecture that the absence of Gαi2 leads to dysfunctional DCs, which not only fail to migrate to their appropriate gut mucosal destination, but also respond differently to microbial-associated molecular patterns (MAMPs), such as CpG-ODN, which activate components of innate immunity. Due to Gαi2 insufficiency, the abnormality in signal transduction in DCs result in exaggerated, chronic inflammation, mediated by Th1- and Th17-type cytokines. In B6 mice lacking Gαi2, DCs are able to keep this damaging inflammation in check by simultaneous production of IL-10, while 129 mice cannot (proposed model, Figure 11).
Figure 11.
Proposed pathway. Innate immune stimulation by bacterial DNA (and possibly other TLRs) lead to activation of both NF-kB and MAP kinase pathways, especially p38 (1). Multiple cytokines are elaborated by DCs (2). In wild-type mice, there may be a yet-unknown putative regulatory ligand that is signals via G protein coupled receptor (3). In B6 mice, IL-10 is also produced following TLR stimulation which serves to decrease inflammatory signals (4). This pathway lacking in the Gαi1-deficient 129 mouse. Putative regulatory signals go through G protein-coupled receptors which would normally serve to dampen the inflammatory signals (5). In the Gαi2-deficient mouse, this pathway is altered, with possible continuous activation of adenylate cyclase (AC), leading to increased MAPK activation (6) and cytokine secretion (7). Absence of (4) and (5) leads to over-production of inflammatory cytokines, especially IL-6 and IL-23 which promote a Th17 phenotype (8) and ultimately inflammation.
The expression of Gαi2 is ubiquitous and is not surprising that several immune cell defects in the Gαi2-/- mouse have been described. T cell defects include an increase in the proportion of single-positive CD4+8- and CD4-8+ thymocytes with a hyper-response to T cell receptor signaling in the Gαi2-/- thymus. Gαi2-/- thymocytes produce significantly more Th1 cytokines (IL-2, IFN-γ and TNF-α) after activation with immobilized CD3-ε.30, 31 Thymic DCs have a major impact on the development and maturation of T regulatory cells within the thymus.50 Purified naïve splenic T cells from Gαi2-/- mice are also hyper-responsive to T cell receptor-mediated signaling.51 However, the specific T cell responsible for the pathogenesis of the disease in the Gαi2-/- mouse is not clear, since it has been shown that adoptive transfer of T cells from the spleen of Gαi2-/- mice but not T cells from the mesenteric lymph node (MLN) of Gαi2-/- mice induces disease in T cell deficient RAG2-/- mice. Furthermore, CD4+ T cells alone or CD8+ T cells alone from the spleen of Gαi2-/- mice could not induce disease in adoptive transfer models of colitis,52 indicating that at least both types of T cells in the spleen are needed to induce colitis. In another study, adoptive transfer of Gαi2-/- CD4+ splenic T cells alone was able to induce colitis in a T cell deficient RAG2-/- mouse. In this model, CD4+ T cell-mediated colitis could be prevented by simultaneous adoptive transfer of wild type CD8α+ T cells plus wild type B cells,53 another indication of the complexity of the colitis in the Gαi2-/-mouse. The discrepancies between these prior findings are mostly likely explained by a lack of a pure, defined background mouse strain and the variation in resident microflora from one animal housing facility to another. Most of the signals from commensal bacteria and antigens are presented to the mucosal immune system through the DCs within the GALT.
The central role of DCs as a link between the innate and the antigen-specific responses is an accepted paradigm. DCs, expressing high levels of MHC Class II, are the major APC in the immune system and are the only APCs known to present new antigens to naïve lymphocytes. T cells isolated from mesenteric lymph nodes or Peyer's Patches from Gαi2-/- mice produce IFN-γ in response to soya dietary antigen.29 This production of the inflammatory Th1 response is MHC class II dependent, implicating the possible role of antigen presentation by DCs in the development of these Th1 T cells. The significant role that DCs might play in initiating an inappropriate inflammatory response to commensal bacteria in the development of IBD may be under-appreciated.
Thus, understanding the interactions between DCs and microbes, and the early molecular events occurring during this interaction, should shed some light on the mechanisms of initiation of the immune response in IBD,54 its progression, and ultimately, lead to the development of novel treatments for the disease. Appropriate discrimination between pathogens and normal commensal flora require PRRs, including TLRs, to recognize MAMPs such as LPS, lipopeptides, peptidoglycans and bacterial DNA.55 The combination of these MAMPs allows the DCs to recognize lumenal microbes as pathogenic56 or components of the normal microflora. Indeed, dendritic cells are known to respond differently to specific TLR signals alone or in combination57 and secrete different cytokines in a bacterial class-specific manner.58 The response of mucosal DCs within the GALT to specific TLR ligand signal combinations then creates a unique cytokine environment within the tissue that reflects the microbes present. At the same time, DCs are programming commensal antigen recognition and tolerance within the mucosal adaptive immune system, including T helper cell and B cell responses. Consequently, lumenal bacteria modify DC innate responses that potentially affect subsequent T cell mediated adaptive responses to become Th1, Th2, or Th17.54, 59-64 Improper immune responses to commensal flora or dietary antigen result in gut inflammation where these antigens are most abundant. In our hands, the Gai2-/- mouse favors both the development Th1 (IFN-γ) and Th17 (IL-17) T helper phenotypes, as evidenced by colon explant cytokine production and CpG stimulation of BMDCs.
An aberrant DC population also influences the environment within the Peyer's patches and lymphoid follicles. Normal myeloid DCs in the Peyer's patches (CD11b+, CD11c+, CD8α-) have been shown to polarize T cells towards production of Th2-type cytokines, including IL-10.65 The DCs in the Peyer's patches are also essential for programming T cells to home to the colon by up-regulating expression of α4β7 selectin on the T cells.66 The absence of Gαi2 in DCs may affect normal development and function of T cells within the Peyer's patches leading to the reported defect in Peyer's patch sizes and numbers.29, 67 We propose that the small lymphoid follicles and fewer Peyer's patches in the Gαi2-/- mouse represents a defective myeloid DC population lacking the ability to induce Th2 T cells. Indeed, our data would indicate that the large increase in production of IL-6 by the CD11c+CD11b+ DCs from Gαi2-/- mice favors the development of Th17 T cells that then home to the colon and contribute to the development of colitis.
These findings may serve as a paradigm for understanding multi-factorial inflammatory disorders. We show that at least two distinct processes act in concert to produce colitis in the Gαi2-deficient mouse. One involves the initial inflammatory response to gut flora or pathogens and the second, involves factors required to attenuate the response. In our model, Gαi2-deficiency leads to hyperactive responses to microbial sensing in both B6 and 129 strains. However, the difference in signaling pathways in DCs between these two strains of mice, involving p38 MAPK activation, leads to more inflammatory cytokine production in the 129 strain and more anti-inflammatory IL-10 production in the B6 strain. Finally, there is an apparent higher threshold for activation of inflammation in the B6 strain of mice as they do eventually develop colitis as they age and as their microflora undergoes succession.
Further studies on the kinetics of the development of disease in the B6 mouse with an emphasis on identifying factors that delay inflammation. This includes the potential role of IP-10, which is increased in Gαi2-/- B6 BMDCs compared to B6 WT, in DC homing. Although comparisons of cross-bred mice have already been performed, with the subsequent identification of the CdcS1 locus, additional genetic studies could include screening of BMDC differences in MAPK or IL-6 production, to further identify other contributory genetic loci. A comparison of the lamina propria DCs and MLN DCs isolated from normal versus Gαi2-/- mice would further establish any major functional differences in tissue DCs that contribute to disease. Finally, restoration of Gαi2 expression in DCs of 129 Gαi2-/- mouse, by transgenic delivery using a DC-specific promoter, should impart disease resistance. If these mice are resistant to IBD, then our hypothesis is confirmed.
FIGURE 7a.
Cytokine production by sorted BMDCs following CpG-ODN stimulation. BMDCs were sorted according to CD11c and CD11b expression. Experiments represent at least 3 animals per strain and genotype. Three distinct subtypes: (1) single-positive/CD11c+ CD11b- (c+), (2) double-hi/CD11c+hi CD11b+hi, and (3) double-lo/ CD11c+lo CD11b+lo. Cell culture supernatants were assayed for cytokine production 24h post-stimulation. Data in box-plots with median (heavy line inside box), interquartile range (box) and range (error bars). Intergroup comparisons yielding significantly different levels of cytokines are represented (*red bars, P<0.05)
Figure 7b.
Cytokine production by sorted BMDCs following CpG-ODN stimulation. BMDCs were sorted according to CD11c and CD11b expression. Experiments represent at least 3 animals per strain and genotype. Three distinct subtypes: (1) single-positive/CD11c+ CD11b- (c+), (2) double-hi/CD11c+hi CD11b+hi, and (3) double-lo/ CD11c+lo CD11b+lo. Cell culture supernatants were assayed for cytokine production 24h post-stimulation. Data in box-plots with median (heavy line inside box), interquartile range (box) and range (error bars). Intergroup comparisons yielding significantly different levels of cytokines are represented (*red bars, P<0.05)
Acknowledgement
The authors would like to acknowledge G.J. Snipes, for reading the manuscript, Z. Coskun, K. Bustos, C. Nicholson, L. Chen and X. Shi for technical support. Support for this work included access to the Core Laboratories and a Pilot-feasibility Award from the NIH-funded Texas Gulf Coast Digestive Disease Center (DK 56338) and the Baylor College of Medicine Department of Pathology Moran Foundation to LT-S.
Sources of support: Core Laboratories and a Pilot-feasibility Award from the NIH-funded Texas Gulf Coast Digestive Disease Center (DK 56338) and the Baylor College of Medicine Department of Pathology Moran Foundation.
References
- 1.Chieppa M, Rescigno M, Huang AY, Germain RN. Dynamic imaging of dendritic cell extension into the small bowel lumen in response to epithelial cell TLR engagement. J Exp Med. 2006;203:2841–52. doi: 10.1084/jem.20061884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fort MM, Leach MW, Rennick DM. A role for NK cells as regulators of CD4+ T cells in a transfer model of colitis. J Immunol. 1998;161:3256–61. [PubMed] [Google Scholar]
- 3.Bouma G, Strober W. The immunological and genetic basis of inflammatory bowel disease. Nat Rev Immunol. 2003;3:521–33. doi: 10.1038/nri1132. [DOI] [PubMed] [Google Scholar]
- 4.Sartor RB. Targeting enteric bacteria in treatment of inflammatory bowel diseases: why, how, and when. Curr Opin Gastroenterol. 2003;19:358–65. doi: 10.1097/00001574-200307000-00006. [DOI] [PubMed] [Google Scholar]
- 5.Strober W, Fuss I, Mannon P. The fundamental basis of inflammatory bowel disease. J Clin Invest. 2007;117:514–21. doi: 10.1172/JCI30587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rudolph U, Finegold MJ, Rich SS, Harriman GR, Srinivasan Y, Brabet P, Boulay G, Bradley A, Birnbaumer L. Ulcerative colitis and adenocarcinoma of the colon in G alpha i2-deficient mice. Nat Genet. 1995;10:143–50. doi: 10.1038/ng0695-143. [DOI] [PubMed] [Google Scholar]
- 7.Bjursten M, Hultgren OH, Hultgren Hornquist E. Enhanced proinflammatory cytokine production in Galphai2-deficient mice on colitis prone and colitis resistant 129Sv genetic backgrounds. Cell Immunol. 2004;228:77–80. doi: 10.1016/j.cellimm.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 8.Borm ME, He J, Kelsall B, Pena AS, Strober W, Bouma G. A major quantitative trait locus on mouse chromosome 3 is involved in disease susceptibility in different colitis models. Gastroenterology. 2005;128:74–85. doi: 10.1053/j.gastro.2004.10.044. [DOI] [PubMed] [Google Scholar]
- 9.Beckwith J, Cong Y, Sundberg JP, Elson CO, Leiter EH. Cdcs1, a major colitogenic locus in mice, regulates innate and adaptive immune response to enteric bacterial antigens. Gastroenterology. 2005;129:1473–84. doi: 10.1053/j.gastro.2005.07.057. [DOI] [PubMed] [Google Scholar]
- 10.Farmer MA, Sundberg JP, Bristol IJ, Churchill GA, Li R, Elson CO, Leiter EH. A major quantitative trait locus on chromosome 3 controls colitis severity in IL-10-deficient mice. Proc Natl Acad Sci U S A. 2001;98:13820–5. doi: 10.1073/pnas.241258698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Edwards RA, Smock AZ. Defective arachidonate release and PGE2 production in Gi alpha2-deficient intestinal and colonic subepithelial myofibroblasts. Inflamm Bowel Dis. 2006;12:153–65. doi: 10.1097/01.MIB.0000201100.72191.19. [DOI] [PubMed] [Google Scholar]
- 12.Dalwadi H, Wei B, Schrage M, Spicher K, Su TT, Birnbaumer L, Rawlings DJ, Braun J. B cell developmental requirement for the G alpha i2 gene. J Immunol. 2003;170:1707–15. doi: 10.4049/jimmunol.170.4.1707. [DOI] [PubMed] [Google Scholar]
- 13.Leithauser F, Trobonjaca Z, Moller P, Reimann J. Clustering of colonic lamina propria CD4(+) T cells to subepithelial dendritic cell aggregates precedes the development of colitis in a murine adoptive transfer model. Lab Invest. 2001;81:1339–49. doi: 10.1038/labinvest.3780348. [DOI] [PubMed] [Google Scholar]
- 14.Morise K, Yamaguchi T, Kuroiwa A, Kanayama K, Matsuura T, Shinoda M, Yamamoto H, Horiuchi Y, Furusawa A, Iwase H. Expression of adhesion molecules and HLA-DR by macrophages and dendritic cells in aphthoid lesions of Crohn's disease: an immunocytochemical study. J Gastroenterol. 1994;29:257–64. doi: 10.1007/BF02358363. [DOI] [PubMed] [Google Scholar]
- 15.Malmstrom V, Shipton D, Singh B, Al-Shamkhani A, Puklavec MJ, Barclay AN, Powrie F. CD134L expression on dendritic cells in the mesenteric lymph nodes drives colitis in T cell-restored SCID mice. J Immunol. 2001;166:6972–81. doi: 10.4049/jimmunol.166.11.6972. [DOI] [PubMed] [Google Scholar]
- 16.Seldenrijk CA, Drexhage HA, Meuwissen SG, Pals ST, Meijer CJ. Dendritic cells and scavenger macrophages in chronic inflammatory bowel disease. Gut. 1989;30:486–91. doi: 10.1136/gut.30.4.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karlis J, Penttila I, Tran TB, Jones B, Nobbs S, Zola H, Flesch IE. Characterization of colonic and mesenteric lymph node dendritic cell subpopulations in a murine adoptive transfer model of inflammatory bowel disease. Inflamm Bowel Dis. 2004;10:834–47. doi: 10.1097/00054725-200411000-00018. [DOI] [PubMed] [Google Scholar]
- 18.Baumgart DC, Metzke D, Schmitz J, Scheffold A, Sturm A, Wiedenmann B, Dignass AU. Patients with active inflammatory bowel disease lack immature peripheral blood plasmacytoid and myeloid dendritic cells. Gut. 2005;54:228–36. doi: 10.1136/gut.2004.040360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Becker C, Wirtz S, Blessing M, Pirhonen J, Strand D, Bechthold O, Frick J, Galle PR, Autenrieth I, Neurath MF. Constitutive p40 promoter activation and IL-23 production in the terminal ileum mediated by dendritic cells. J Clin Invest. 2003;112:693–706. doi: 10.1172/JCI17464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yen D, Cheung J, Scheerens H, Poulet F, McClanahan T, McKenzie B, Kleinschek MA, Owyang A, Mattson J, Blumenschein W, Murphy E, Sathe M, Cua DJ, Kastelein RA, Rennick D. IL-23 is essential for T cell-mediated colitis and promotes inflammation via IL-17 and IL-6. J Clin Invest. 2006;116:1310–6. doi: 10.1172/JCI21404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Becker C, Dornhoff H, Neufert C, Fantini MC, Wirtz S, Huebner S, Nikolaev A, Lehr HA, Murphy AJ, Valenzuela DM, Yancopoulos GD, Galle PR, Karow M, Neurath MF. Cutting edge: IL-23 cross-regulates IL-12 production in T cell-dependent experimental colitis. J Immunol. 2006;177:2760–4. doi: 10.4049/jimmunol.177.5.2760. [DOI] [PubMed] [Google Scholar]
- 22.Uhlig HH, McKenzie BS, Hue S, Thompson C, Joyce-Shaikh B, Stepankova R, Robinson N, Buonocore S, Tlaskalova-Hogenova H, Cua DJ, Powrie F. Differential activity of IL-12 and IL-23 in mucosal and systemic innate immune pathology. Immunity. 2006;25:309–18. doi: 10.1016/j.immuni.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 23.Veldhoen M, Hocking RJ, Atkins CJ, Locksley RM, Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–89. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 24.Weaver CT, Harrington LE, Mangan PR, Gavrieli M, Murphy KM. Th17: an effector CD4 T cell lineage with regulatory T cell ties. Immunity. 2006;24:677–88. doi: 10.1016/j.immuni.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 25.Aggarwal S, Gurney AL. IL-17: prototype member of an emerging cytokine family. J Leukoc Biol. 2002;71:1–8. [PubMed] [Google Scholar]
- 26.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–8. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 27.Fossiez F, Djossou O, Chomarat P, Flores-Romo L, Ait-Yahia S, Maat C, Pin JJ, Garrone P, Garcia E, Saeland S, Blanchard D, Gaillard C, Das Mahapatra B, Rouvier E, Golstein P, Banchereau J, Lebecque S. T cell interleukin-17 induces stromal cells to produce proinflammatory and hematopoietic cytokines. J Exp Med. 1996;183:2593–603. doi: 10.1084/jem.183.6.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hornquist CE, Lu X, Rogers-Fani PM, Rudolph U, Shappell S, Birnbaumer L, Harriman GR. G(alpha)i2-deficient mice with colitis exhibit a local increase in memory CD4+ T cells and proinflammatory Th1-type cytokines. J Immunol. 1997;158:1068–77. [PubMed] [Google Scholar]
- 29.Bjursten M, Hultgren Hornquist E. Dietary antigen-specific T-cell responses: switch from an interleukin-10-dominated response in normal mice to a T-helper 1 cytokine profile in Galphai2-deficient mice prior to colitis. Scand J Immunol. 2005;61:29–35. doi: 10.1111/j.0300-9475.2005.01535.x. [DOI] [PubMed] [Google Scholar]
- 30.Rudolph U, Finegold MJ, Rich SS, Harriman GR, Srinivasan Y, Brabet P, Bradley A, Birnbaumer L. Gi2 alpha protein deficiency: a model of inflammatory bowel disease. J Clin Immunol. 1995;15:101S–105S. doi: 10.1007/BF01540899. [DOI] [PubMed] [Google Scholar]
- 31.Zhang Y, Finegold MJ, Jin Y, Wu MX. Accelerated transition from the double-positive to single-positive thymocytes in G alpha i2-deficient mice. Int Immunol. 2005;17:233–43. doi: 10.1093/intimm/dxh204. [DOI] [PubMed] [Google Scholar]
- 32.He J, Gurunathan S, Iwasaki A, Ash-Shaheed B, Kelsall BL. Primary role for Gi protein signaling in the regulation of interleukin 12 production and the induction of T helper cell type 1 responses. J Exp Med. 2000;191:1605–10. doi: 10.1084/jem.191.9.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Berg DJ, Davidson N, Kuhn R, Muller W, Menon S, Holland G, Thompson-Snipes L, Leach MW, Rennick D. Enterocolitis and colon cancer in interleukin-10-deficient mice are associated with aberrant cytokine production and CD4(+) TH1-like responses. J Clin Invest. 1996;98:1010–20. doi: 10.1172/JCI118861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ohman L, Franzen L, Rudolph U, Harriman GR, Hultgren Hornquist E. Immune activation in the intestinal mucosa before the onset of colitis in Galphai2-deficient mice. Scand J Immunol. 2000;52:80–90. doi: 10.1046/j.1365-3083.2000.00759.x. [DOI] [PubMed] [Google Scholar]
- 35.Ardavin C. Dendritic cell heterogeneity: developmental plasticity and functional diversity. Semin Immunol. 2005;17:251–2. doi: 10.1016/j.smim.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 36.Ito T, Liu YJ, Kadowaki N. Functional diversity and plasticity of human dendritic cell subsets. Int J Hematol. 2005;81:188–96. doi: 10.1532/IJH97.05012. [DOI] [PubMed] [Google Scholar]
- 37.Liu YJ, Kanzler H, Soumelis V, Gilliet M. Dendritic cell lineage, plasticity and cross-regulation. Nat Immunol. 2001;2:585–9. doi: 10.1038/89726. [DOI] [PubMed] [Google Scholar]
- 38.Krieg AM. Signal transduction induced by immunostimulatory CpG DNA. Springer Semin Immunopathol. 2000;22:97–105. doi: 10.1007/s002810050019. [DOI] [PubMed] [Google Scholar]
- 39.Ewaschuk JB, Backer JL, Churchill TA, Obermeier F, Krause DO, Madsen KL. Surface expression of Toll-like receptor 9 is upregulated on intestinal epithelial cells in response to pathogenic bacterial DNA. Infect Immun. 2007;75:2572–9. doi: 10.1128/IAI.01662-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lopez-Ilasaca M. Signaling from G-protein-coupled receptors to mitogen-activated protein (MAP)-kinase cascades. Biochem Pharmacol. 1998;56:269–77. doi: 10.1016/s0006-2952(98)00059-8. [DOI] [PubMed] [Google Scholar]
- 41.Kelsall BL, Leon F. Involvement of intestinal dendritic cells in oral tolerance, immunity to pathogens, and inflammatory bowel disease. Immunol Rev. 2005;206:132–48. doi: 10.1111/j.0105-2896.2005.00292.x. [DOI] [PubMed] [Google Scholar]
- 42.Niess JH, Reinecker HC. Dendritic cells: the commanders-in-chief of mucosal immune defenses. Curr Opin Gastroenterol. 2006;22:354–60. doi: 10.1097/01.mog.0000231807.03149.54. [DOI] [PubMed] [Google Scholar]
- 43.Wu JY, Jin Y, Edwards RA, Zhang Y, Finegold MJ, Wu MX. Impaired TGF-beta responses in peripheral T cells of Galphai2-/- mice. J Immunol. 2005;174:6122–8. doi: 10.4049/jimmunol.174.10.6122. [DOI] [PubMed] [Google Scholar]
- 44.Bjursten M, Bland PW, Willen R, Hornquist EH. Long-term treatment with anti-alpha 4 integrin antibodies aggravates colitis in G alpha i2-deficient mice. Eur J Immunol. 2005;35:2274–83. doi: 10.1002/eji.200526022. [DOI] [PubMed] [Google Scholar]
- 45.Coombes JL, Powrie F. Dendritic cells in intestinal immune regulation. Nat Rev Immunol. 2008;8:435–46. doi: 10.1038/nri2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee J, Rachmilewitz D, Raz E. Homeostatic effects of TLR9 signaling in experimental colitis. Ann N Y Acad Sci. 2006;1072:351–5. doi: 10.1196/annals.1326.022. [DOI] [PubMed] [Google Scholar]
- 47.Varol C, Landsman L, Fogg DK, Greenshtein L, Gildor B, Margalit R, Kalchenko V, Geissmann F, Jung S. Monocytes give rise to mucosal, but not splenic, conventional dendritic cells. J Exp Med. 2007;204:171–80. doi: 10.1084/jem.20061011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Iwakura Y, Ishigame H. The IL-23/IL-17 axis in inflammation. J Clin Invest. 2006;116:1218–22. doi: 10.1172/JCI28508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dubinsky MC, Wang D, Picornell Y, Wrobel I, Katzir L, Quiros A, Dutridge D, Wahbeh G, Silber G, Bahar R, Mengesha E, Targan SR, Taylor KD, Rotter JI. IL-23 receptor (IL-23R) gene protects against pediatric Crohn's disease. Inflamm Bowel Dis. 2007;13:511–5. doi: 10.1002/ibd.20126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Watanabe N, Wang YH, Lee HK, Ito T, Cao W, Liu YJ. Hassall's corpuscles instruct dendritic cells to induce CD4+CD25+ regulatory T cells in human thymus. Nature. 2005;436:1181–5. doi: 10.1038/nature03886. [DOI] [PubMed] [Google Scholar]
- 51.Huang TT, Zong Y, Dalwadi H, Chung C, Miceli MC, Spicher K, Birnbaumer L, Braun J, Aranda R. TCR-mediated hyper-responsiveness of autoimmune Galphai2(-/-) mice is an intrinsic naive CD4(+) T cell disorder selective for the Galphai2 subunit. Int Immunol. 2003;15:1359–67. doi: 10.1093/intimm/dxg135. [DOI] [PubMed] [Google Scholar]
- 52.Bjursten M, Willen R, Hultgren Hornquist E. Transfer of colitis by Galphai2-deficient T lymphocytes: impact of subpopulations and tissue origin. Inflamm Bowel Dis. 2005;11:997–1005. doi: 10.1097/01.mib.0000185401.27170.22. [DOI] [PubMed] [Google Scholar]
- 53.Wei B, Velazquez P, Turovskaya O, Spricher K, Aranda R, Kronenberg M, Birnbaumer L, Braun J. Mesenteric B cells centrally inhibit CD4+ T cell colitis through interaction with regulatory T cell subsets. Proc Natl Acad Sci U S A. 2005;102:2010–5. doi: 10.1073/pnas.0409449102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rescigno M, Granucci F, Ricciardi-Castagnoli P. Molecular events of bacterial-induced maturation of dendritic cells. J Clin Immunol. 2000;20:161–6. doi: 10.1023/a:1006629328178. [DOI] [PubMed] [Google Scholar]
- 55.Kawai T, Akira S. Pathogen recognition with Toll-like receptors. Curr Opin Immunol. 2005;17:338–44. doi: 10.1016/j.coi.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 56.Svensson L, Wenneras C. Human eosinophils selectively recognize and become activated by bacteria belonging to different taxonomic groups. Microbes Infect. 2005;7:720–8. doi: 10.1016/j.micinf.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 57.Warger T, Osterloh P, Rechtsteiner G, Fassbender M, Heib V, Schmid B, Schmitt E, Schild H, Radsak MP. Synergistic activation of dendritic cells by combined Toll-like receptor ligation induces superior CTL responses in vivo. Blood. 2006;108:544–50. doi: 10.1182/blood-2005-10-4015. [DOI] [PubMed] [Google Scholar]
- 58.Scott K, Manunta M, Germain C, Smith P, Jones M, Mitchell P, Dessi D, Branigan Bamford K, Lechler RI, Fiori PL, Foster GR, Lombardi G. Qualitatively distinct patterns of cytokines are released by human dendritic cells in response to different pathogens. Immunology. 2005;116:245–54. doi: 10.1111/j.1365-2567.2005.02218.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pulendran B. Modulating TH1/TH2 responses with microbes, dendritic cells, and pathogen recognition receptors. Immunol Res. 2004;29:187–96. doi: 10.1385/IR:29:1-3:187. [DOI] [PubMed] [Google Scholar]
- 60.Pulendran B, Palucka K, Banchereau J. Sensing pathogens and tuning immune responses. Science. 2001;293:253–6. doi: 10.1126/science.1062060. [DOI] [PubMed] [Google Scholar]
- 61.Lohr J, Knoechel B, Wang JJ, Villarino AV, Abbas AK. Role of IL-17 and regulatory T lymphocytes in a systemic autoimmune disease. J Exp Med. 2006;203:2785–91. doi: 10.1084/jem.20061341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Park ST, Kim KE, Na K, Kim Y, Kim TY. Effect of dendritic cells treated with CpG ODN on atopic dermatitis of Nc/Nga mice. J Biochem Mol Biol. 2007;40:486–93. doi: 10.5483/bmbrep.2007.40.4.486. [DOI] [PubMed] [Google Scholar]
- 63.Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, Weaver T. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6:1123–32. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 64.Langrish CL, Chen Y, Blumenschein WM, Mattson J, Basham B, Sedgwick JD, McClanahan T, Kastelein RA, Cua DJ. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201:233–40. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Iwasaki A, Kelsall BL. Unique functions of CD11b+, CD8 alpha+, and double-negative Peyer's patch dendritic cells. J Immunol. 2001;166:4884–90. doi: 10.4049/jimmunol.166.8.4884. [DOI] [PubMed] [Google Scholar]
- 66.Mora JR, Bono MR, Manjunath N, Weninger W, Cavanagh LL, Rosemblatt M, Von Andrian UH. Selective imprinting of gut-homing T cells by Peyer's patch dendritic cells. Nature. 2003;424:88–93. doi: 10.1038/nature01726. [DOI] [PubMed] [Google Scholar]
- 67.Ohman L, Franzen L, Rudolph U, Birnbaumer L, Hornquist EH. Regression of Peyer's patches in G alpha i2 deficient mice prior to colitis is associated with reduced expression of Bcl-2 and increased apoptosis. Gut. 2002;51:392–7. doi: 10.1136/gut.51.3.392. [DOI] [PMC free article] [PubMed] [Google Scholar]