Abstract
Blastocystis is a prevalent enteric protozoan that infects a variety of vertebrates. Infection with Blastocystis in humans has been associated with abdominal pain, diarrhea, constipation, fatigue, skin rash, and other symptoms. Researchers using different methods and examining different patient groups have reported asymptomatic infection, acute symptomatic infection, and chronic symptomatic infection. The variation in accounts has lead to disagreements concerning the role of Blastocystis in human disease, and the importance of treating it. A better understanding of the number of species of Blastocystis that can infect humans, along with realization of the limitations of the existing clinical laboratory diagnostic techniques may account for much of the disagreement. The possibility that disagreement was caused by the emergence of particular pathogenic variants of Blastocystis is discussed, along with the potential role of Blastocystis infection in irritable bowel syndrome (IBS). Findings are discussed concerning the role of protease-activated receptor-2 in enteric disease which may account for the presence of abdominal pain and diffuse symptoms in Blastocystis infection, even in the absence of fever and endoscopic findings. The availability of better diagnostic techniques and treatments for Blastocystis infection may be of value in understanding chronic gastrointestinal illness of unknown etiology.
Review
Introduction
Blastocystis is a prevalent enteric protist that infects a variety of vertebrates. Researchers have described asymptomatic and symptomatic infection in humans. Infection with Blastocystis is termed blastocystosis and has been associated with abdominal pain, diarrhea, constipation, fatigue[1], skin rash [2-4], and other symptoms. Although Blastocystis is commonly referred to as a protozoal parasite, small subunit (SSU) rRNA analysis has placed Blastocystis in the phylum Stramenopile, along with other organisms such as diatoms, brown algae, slime nets, and water moulds [2]. Blastocystis is non-motile, and is the only Stramenopile known to commonly cause infection in humans [2].
One of the earliest reports of symptomatic blastocystosis occurred in 1899 and was followed by sporadic reports through the 20th century, that accelerated in 1984 [3]. In the United States, a 1987 survey by the Center for Disease Control showed the frequency of occurrence of Blastocystis in US clinical lab samples to be a relatively low 2.6% [4]. A study published by two physicians in California in 1988 reported symptoms of blastocystosis could usually be attributed to another cause and suggested that Blastocystis was non-pathogenic [5]. However, this finding was not in agreement with the experience of US researchers treating patients with international travel history [6,7] or with many researchers outside the United States [1,8].
A debate followed in 1990 between the Californian physicians and others in which some noted a lack of historical study identifying Blastocystis as pathogenic, and suggested that resolution of symptoms in patients following antiprotozoal therapy was due to the treatment of an undetected pathogen [9]. Objections notwithstanding, the clinical community has diagnosed and treated the infection frequently since then [6,10-12], although the research community describes Blastocystis pathogenicity as controversial [13]. Blastocystis is now by far the most prevalent mono-infection in symptomatic patients in the United States [14] and was found 28.5 times more often than Giardia lamblia as a mono-infection in symptomatic patients in a 2000 study [14]. Informal communications suggest the controversy surrounding this protozoan has made it an unpopular research topic in some countries (unpublished data, Kenneth Boorom).
The problem of irritable bowel syndrome
Irritable bowel syndrome (IBS) is a highly prevalent gastrointestinal disorder characterized by abdominal pain with diarrhea and/or constipation. The etiology of IBS has not been definitively established. IBS was originally thought to be a psychosomatic disorder [15], but more recent studies have identified chronic immune activation in IBS patients [16]. The annual direct and indirect costs of IBS in the United States may be as high as $30 billion [17], making it one of the most expensive gastrointestinal diseases in the US and other developed countries. Unlike viral and bacterial gastroenteritis, symptoms of IBS may last indefinitely [18]. The chronic nature of the disease may limit ordinary activities (Figure 1[19]). The disease raises health costs by 49% and much of the additional cost is due to extra-intestinal co-morbidities [20]. The prevalence of IBS in some developing countries is in the 35–43% range [21,22].
Figure 1.
Patients adapt to chronic gastrointestinal illness, which is found at an increasingly high rate in the United Kingdom[117]. The "Mobilet" was developed by an IBS patient and consists of a toilet in an enclosed structure which can be towed behind a vehicle to facilitate travel by persons with severe chronic diarrhea. The UK journal Gut Reaction reported that the device sells for £1349 [19].
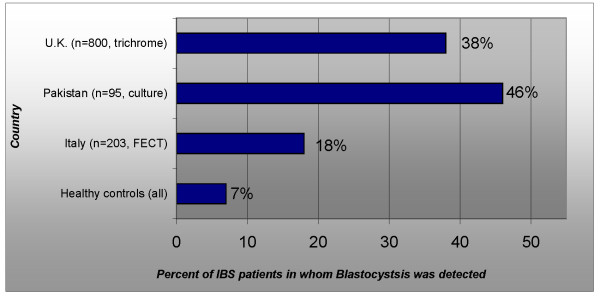
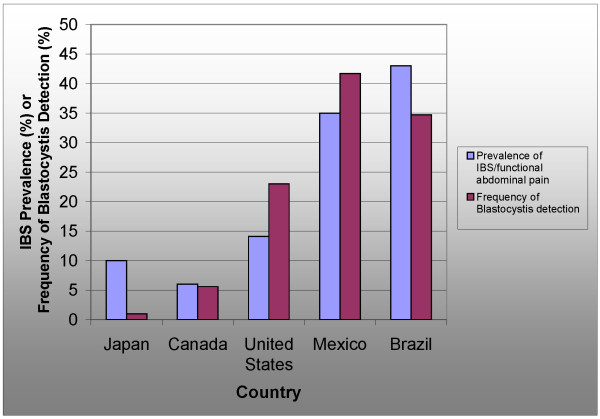
In medical practice, a functional disorder can be described as "a set of symptoms not explained by structural or biochemical abnormalities" [23]. IBS is the only functional bowel disorder where a protozoal infection has been found in almost half of diagnosed cases using simple methods such as culture and staining (Figure 2[24-27]). The relative prevalence of symptoms of abdominal pain, diarrhea, and constipation in Blastocystis infection and IBS show a remarkable similarity (Figure 3[28,29]). High Blastocystis infection rates seem to accompany a high prevalence of IBS (Figure 4[30-35]). Researchers from Pakistan and other countries have suggested that IBS may be caused by Blastocystis [36,37]. However, the indeterminacy of Blastocystis' pathogenicity may have made it difficult to address this infection with a systematic approach used in other infectious diseases. The use of modern tools for identification and classification of Blastocystis isolates from humans, along with a better understanding of the pathogenesis of enteric protozoal infections may explain conflicting reports from researchers.
Figure 2.
Although IBS is ostensibly a functional disorder, IBS patients have been found to be infected with Blastocystis at statistically significant levels in Italy[37], Pakistan[25], the United Kingdom[24,26]but not Thailand [27]. A study from Pakistan identified an elevated serum antibody response to Blastocystis in patients from whom Blastocystis could not be cultured [36]. The figure for the UK includes IBS and chronic GI illness. Numbers shown represent the total number of participants in the study (symptomatic and asymptomatic).
Figure 3.
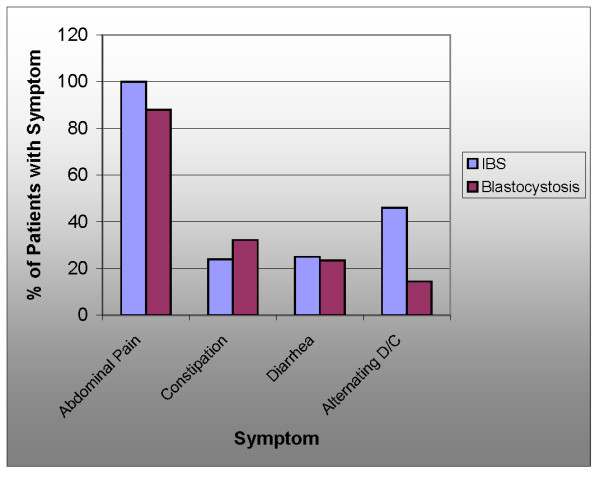
Comparison of the frequency of symptoms seen in blastocystosis[1]to those seen in IBS[28]. Host genetics may influence expression of symptoms in IBS [29].
Figure 4.
Comparison of the prevalence of IBS and chronic abdominal pain to the frequency of detection of Blastocystis in Japan[22,30], Canada[31,32], United States[14,33], Mexico[21,34], and Brazil[22,35].
Phylogeny
Following the discovery of Blastocystis in humans, it was assumed that humans carried a unique species of Blastocystis, which was given the name Blastocystis hominis [38]. Animals and birds were thought to carry different species, which were subsequently assigned names such as Blastocystis ratti for isolates from rats [39] and Blastocystis galli for isolates from chickens [40]. Phylogenetic analysis of SSU rRNA gene sequences of Blastocystis isolates from humans and animals has shown no evidence of a species of Blastocystis unique to humans [12]. Rather, humans acquire infection with the same species of Blastocystis acquired by rats, dogs, horses, cows, pigs, birds and other animals [12]. All nine of the subtypes of Blastocystis found in mammals and birds are found in humans as well. As a reflection of the low host specificity of Blastocystis, a classification system has been developed in which isolates are identified by subtype numbers rather than host names [41]. The nomenclature proposed describes such isolates as Blastocystis sp. subtype n where n is a number from 1 to 9. The genetic diversity that exists within the isolates previously identified as Blastocystis hominis is similar to the diversity that exists within the entire Cryptosporidium genus (Unpublished data, Dr. Eric Viscogliosi). The genomes of the mitochondrion-like organelle (MLO) of Blastocystis sp. subtypes 4 and 1 have been sequenced. The divergence seen between the MLO genomes of these two subtypes was greater than that seen between the MLO genomes of many congeneric species and even the MLO genomes from some species of differing genera [42].
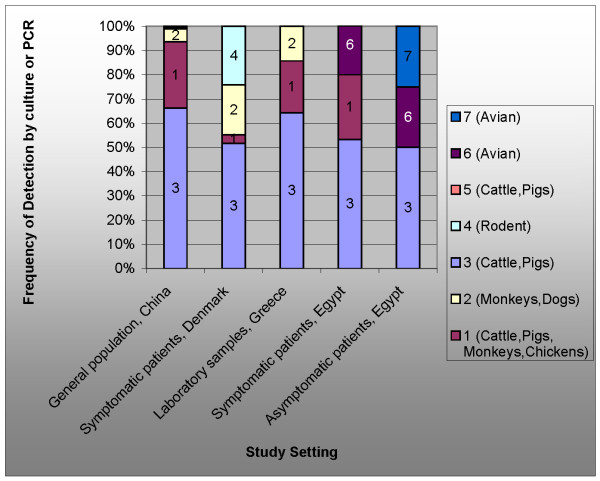
Blastocystis sp. subtypes 3 and 1 make up most chronic infections in humans [43-45] (Table 1 and Figure 5[46]). These types are carried by cows, pigs, chickens, and horses [12]. Blastocystis sp. subtypes 3 and 1 are also associated with most symptomatic mono-infections [44,47-49]. Researchers have identified Blastocystis sp. subtype 2 infection as frequently asymptomatic [50] or occurring primarily as a co-infection in symptomatic patients [44] or carried primarily by older adults [51]. Expression of symptoms does not equate with ease of detection – Blastocystis sp. subtype 2 is the most readily detected [44] while Blastocystis sp. subtype 3 is difficult to detect [52] and may remain undetected in symptomatic patients despite extensive laboratory testing [49].
Table 1.
Based on a classification system published in January 2007, Blastocystis isolates from humans, animals, and birds are identified as Blastocystis sp. subtypes 1 to 9, as determined by analysis of SSU rRNA sequences [41].
| Sub-type # | Yoshikawa subtype # (used in some earlier studies) [41] | % of Blastocystis isolates in population belonging to this subtype from study in China adjusted for co-infections [43] | Results from animal study from Egypt [48] | Non-human carriers [12,74] | Characteristics in human infection | Detectability +=Difficult +++=Readily Detected [44,74] |
| Blastocystis sp. subtype 3 | (3) | 66% | Illness | Cows, Pigs | Associated with most chronic symptomatic infections [44,47,51] | + |
| Blastocystis sp. subtype 1 | (1) | 28% | Illness Death | Cows, Pigs, Chickens, Monkeys | Associated with many chronic symptomatic infections [44,47,51] | ++ |
| Blastocystis sp. subtype 2 | (5) | 5% | Not done | Dogs, Monkeys | Usually asymptomatic [50] or co-infection in symptomatic patients [44] | +++ |
| Blastocystis sp. subtype 4 | (7) | 0.5% | Not done | Rodents | Rare in one population study [43], but was more common in a study of symptomatic patients at clinic [52] | ++ |
| Blastocystis sp. subtype 6 | (4) | 0.5% | Illness | Birds | Rare in one population study [43], but found in a study of symptomatic patients at a clinic [48] | Unknown |
| Blastocystis sp. subtype 5 | (6) | 0% | Not done | Pigs | Rare in one population study [43] | Unknown |
| Blastocystis sp. subtype 7 | (2) | 0% | No Illness | Birds | Rare in one population study [43], but found in a study of asymptomatic individuals [48] | Unknown |
China has completed the only large study on the prevalence of different Blastocystis subtypes in the general population, and that study found most infections were associated with subtypes 1, 2 and 3.
Figure 5.
Distribution of subtypes inBlastocystisfound in the general Chinese population[43], patients at a clinical lab in Denmark[44], samples from a hospital in Greece[46], patients in Egyptian lab [48]. These subtypes are associated with various non-human hosts [12,74].
Subtypes 4, 6, and 7 appear to show some degree of host specificity, in that their only established non-human hosts are rodents (subtype 4) and birds (subtypes 6 and 7) [12]. This host specificity may be responsible for the low prevalence of these subtypes in studies of the human population [53], although they appear more frequently in clinical laboratory studies where samples are collected from symptomatic patients [48,52], suggesting the possibility that these may be associated with acute infection in humans. However, the number of studies performed to date is small, and additional research is needed to understand potential differences in the course of infection associated with various subtypes.
Some isolates from the American Type Culture Collection (ATCC) have been genotyped, and belong to subtype 1 (ATCC 50177, 50609, 50610, 50751, 50752), subtype 3 (ATCC 50587, 50629, 50754), subtype 4 (ATCC 50608, 50753) while ATCC 50588 and 50613 are mixed [54]. The ATCC's records show that the most recently accessioned strain of Blastocystis was acquired in 1995 [55].
Symptoms
A 1989 Saudi study of over 200 symptomatic patients described symptoms of abdominal pain, constipation, diarrhea, nausea, and anorexia [1]. The study also noted a number of extra-intestinal symptoms, such as headaches, depression, and fatigue. The inclusion of these as symptoms was criticized in the 1980's [9], but subsequently found to be co-morbidities in IBS [56]. A 1991 study from a researcher at the US National Institutes of Health described the unusual clinical presentation of blastocystosis [3]:
"The most usual complaint of blastocystosis patients is of intense abdominal discomfort accompanied by pain. Diarrhea is not standard, and constipation is common. The symptoms gleaned from the literature include abdominal pain, discomfort, anorexia, bloating, cramps, diarrhea, constipation, alternating diarrhea and constipation, watery diarrhea, mucus diarrhea, vomiting, dehydration, sleeplessness, nausea, weight loss, inability to work, lassitude, dizziness, flatus, pruritis, and tenesmus. Blood in the stool as well as excessive mucus and leukocytes have been reported."
In a study from Jordan, the most common symptoms reported in preschool children were abdominal pain, recurrent diarrhea, cramps, anorexia, and fatigue [57]. In older school children, additional symptoms were seen, such as mild diarrhea, nausea, bloating, and alternating diarrhea and constipation [58]. A 1989 study of 16,545 specimens from a clinical laboratory in Vancouver, Canada found Blastocystis as a mono-infection in 342 patients [10]. In this study, the symptoms found in 143 patients whose physicians responded to a survey were diarrhea, pain, nausea, gas, malaise, fever, weight loss, and chills. A frequency of bowel movements ranging from 1 to 25 per day was noted. Of the 69 patients responding to a detailed survey, 8 reported bloody diarrhea [10]. In a smaller study of patients in Oregon with chronic gastrointestinal illness and findings of infection with Blastocystis sp. subtype 3, intermittent bloody diarrhea was reported by one patient as well [49].
Testimony to the Oregon State Legislature by physician-diagnosed patients with blastocystosis included descriptions of severe fatigue, with one patient noting the inability to walk more than 15 minutes [59]. Symptoms reported in a collection of reports from patients in the United States included those from a returnee from Nepal were described as "constipation, spasms in ascending colon area, large loss of weight, occasional loose stools, fatigue, mental fogginess, increase in symptoms after eating, the need to eat every few hours, fast heartbeat, pale skin and others [60]." Upper gastrointestinal symptoms, such as dyspepsia, are also seen in Blastocystis infection, particularly in females according to one study [51]. Food intolerance was noted in the original Saudi study [1]. Patients with blastocystosis may develop elaborate exclusion diets to control symptoms (unpublished data, Ken Boorom).
Dermatological involvement
Reports of skin rash began appearing in the literature in 1993 [61], with additional reports in the following years [62-65]. Recent clinical studies have reported the association of skin rash with Blastocystis infection [51,66]. A Greek case report of one patient with Blastocystis associated rash identified the genotype infecting the patient as Blastocystis sp subtype 3 [67]. Dermatologists have found Blastocystis infection at a statistically significant rate in patients with allergic skin conditions [68]. Figure 6 show such a rash.
Figure 6.
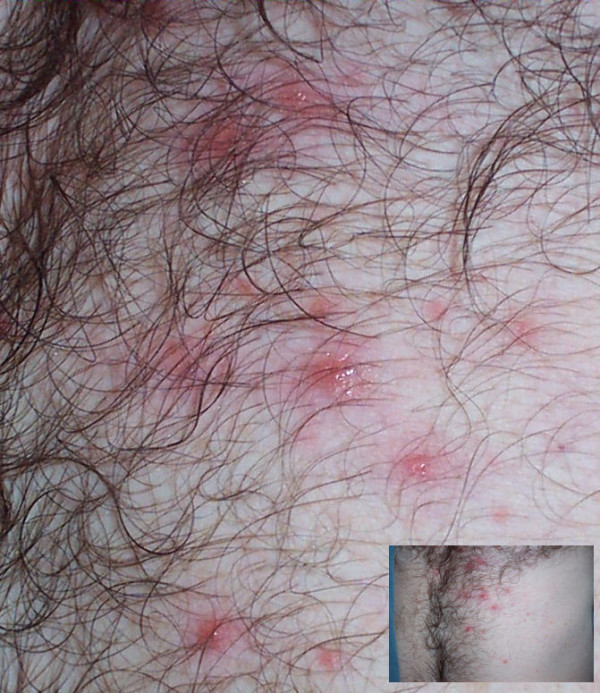
Rash from 39-year old US male diagnosed with chronic blastocystosis acquired domestically[60]. Skin rash in Blastocystis infection has been described as recurrent [65] and intensely pruritic [67]. Diagnosis of blastocystosis was by exclusion: eleven ova and parasite exams (trichrome staining) performed at clinical laboratories from 2003–2006 were negative except for findings of Blastocystis. Colonoscopy, endoscopy, and gluten challenge test were negative. The infection was unresponsive to metronidazole, nitazoxanide, and TMP/SMX. The isolate was genotyped as Blastocystis sp. subtype 3 in a 2007 study [49].
Diagnostic techniques
Diagnosis of Blastocystis infection involves the detection of the organism, and a determination as to whether its presence is responsible for symptoms. Methods which have been used clinically and in research studies for this purpose are described below.
Detection of the organism in stool specimens
Most clinical methods endeavor to identify infection by finding the organism in stool specimens. Variations on this method may involve concentration, staining, culturing, and extraction of DNA followed by polymerase chain reaction (PCR) testing. Most methods have been found to have low sensitivity [69] (Table 2).
Table 2.
Sensitivities of detection of Blastocystis reported in various studies.
| Detection Method | compared to... | reported a sensitivity of | Year and country of study |
| Formol ethyl acetate concentration technique (FECT) | Culture | 0% | 2004, UK [116] |
| FECT | Culture | 19.6% | 2002, Thailand [72] |
| Simple Smear | Culture | 16.7% | 2004, Thailand [69] |
| Simple Smear | Culture | 42.5% | 2002, Thailand [72] |
| Trichrome Staining | Culture | 40.2% | 2004, Thailand [69] |
| FECT | PCR | 50% | 2007, Denmark [44] |
| Sodium acetate-acetic acid-formalin (SAF) concentration technique | PCR | 82% | 2007, Denmark [44] |
| Culture | PCR | 89% | 2007, Denmark [44] |
| ELISA serum antibody | Culture | 92.1% | 2008, China [87] |
| Merthiolate-Iodine-Formalin | -- | (no studies found) |
Blastocystis is one of numerous human protozoan and metazoan intestinal parasites sought for in stools in clinical microbiology laboratories. Therefore, generic, physico-chemical methods such as the formol ethyl acetate concentration technique (FECT) will not necessarily maximize recoveries of all intestinal parasites because of their differing specific gravities and sensitivities to formalin. Both parasite morphology and morphometry may vary. Some isolates are only 6–8 μm in size [70] which makes microscopical detection difficult, especially when parasites are present in small numbers. Vegetative stages can be mistaken for fat globules, leukocytes, or other artifacts in the stool (Figure 7). The quantity of Blastocystis shed has been found to vary significantly between patients, and over time from the same experimental animal [71]. Infection with Blastocystis sp. subtype 3 is less readily detected than infection with Blastocystis sp. subtype 2 using FECT [44]. As Blastocystis sp. subtype 2 is less likely to be associated with symptomatic mono-infection [44,50], this effect may be responsible for producing conflicting results between researchers.
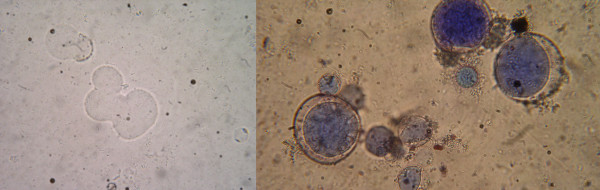
Figure 7.
(LEFT)Blastocystisin simple smear. Researchers have cited the non-descript appearance of Blastocystis as one reason for the low sensitivity of clinical diagnostic methods, along with the possibility that not all stages have been documented [52]. (RIGHT) The classic diagnostic form of Blastocystis found in the stool of patients varies in size from 6 to 40 μm. The parasite is characterized by a central body (blue) that morphologically resembles a vacuole. The central body pushes the nuclei to the periphery of the cell. The central body is a reservoir for proteases [83] and may serve other functions as well. Photos courtesy of Dr. Hanaa Moussa, Cairo University.
To improve detection rates, one study reported the use of a "purged sample" [6], which involves the use of a laxative. Stool culture has been suggested as an additional method, which may offer the best tradeoff between sensitivity and cost and may be less costly than methods currently in use [44]. Xenic cultures of Blastocystis can be produced from stool samples with inexpensive materials, and without the use of an anaerobic chamber [72]. The absolute sensitivity of stool culture as a diagnostic method is unknown. Stool culture may be positive in only 50–70% of infections with Entamoeba histolytica [73]. PCR testing of DNA extracted directly from stool specimens is considered to be the most sensitive method for the detection of Blastocystis, and represents a 10% improvement over culture [52]. It is currently the only way to differentiate subtypes of Blastocystis, and is used in research studies where genotyping is necessary [74-76]. A real time PCR test has been developed [54].
PCR testing of DNA extracted directly from stool specimens is a valuable tool for genotyping Blastocystis in specimens [74-76]. However, complexities associated with this method should be noted to guard against overconfidence in this as a diagnostic tool. Stool specimens contain PCR inhibitors which can interfere with the detection process. The high concentration of nucleases in some protozoa can interfere with detection [77]. Blastocystis' ability to undergo programmed cell death with associated DNA fragmentation [78] may also complicate PCR testing. Finding suitable loci in order to detect and differentiate isolates is a challenge because the entire genome of Blastocystis has not been sequenced and substantial genetic heterogeneity exists among the nine subtypes displayed within the genus. Stool samples have been observed to convert to PCR negative after remaining at room temperature for several hours, although the organism is still visible microscopically (unpublished data, Dr. Gregory Spanakos). Stool specimens have been found to convert to PCR negative after a few months of cold storage at -20C (personal communications, Dr. Morris S. Jones and Dr. Saovanee Leelayoova). Cryopreservation of extracted DNA may offer an alternative.
Significance of detection of the organism in stool specimens
In addition to detection of Blastocystis, there is a need to determine if it is the cause of illness in patients. Some early reports suggested that Blastocystis might be diagnosed as a cause of illness only when present in large numbers in stool samples. Blastocystis is now the only protozoan whose presence is reported along with its quantity by US pathologists, with standardized terms such as "rare, few, moderate, and many" describing its abundance [79]. However, several studies have found no correlation between the quantity of Blastocystis in stool samples and symptoms [10,51,80]. Researchers have found that tests with low sensitivity failed to detect infection with the type of Blastocystis most frequently found in symptomatic patients [44]. Therefore, quantity of organisms identifiable in stool specimens may be a poor indicator of symptomatic status.
Genotyping of Blastocystis isolates may offer useful clinical information, especially as more studies are accumulated [44,47,51]. It may be possible to differentiate between infections which are likely to be asymptomatic, acutely symptomatic, and chronically symptomatic by subtyping Blastocystis. Isolates that have been associated with symptomatic infection in humans have also been found in asymptomatic carriers, so subtyping can not establish Blastocystis as a cause of illness in a particular patient.
One study has reported the ability to distinguish between symptomatic and asymptomatic infections by culturing stool samples and identifying specific forms in culture [81]. Because a limited number of isolates may have been used in that study, additional work would be needed to understand the applicability of this technique in other settings.
Detecting other factors in stool specimens
Some diagnostic tests seek to identify factors in stool specimens which would be uniquely associated with symptomatic infection. These may offer better sensitivity than assays used to detect Blastocystis directly from stool.
One researcher reported success in the use of a fecal IgA assay to discriminate between symptomatic and asymptomatic Blastocystis infections [82]. In that study, the researcher reported that all patients with symptomatic Blastocystis infection tested positive at a titer of 1:400, while all asymptomatic Blastocystis carriers and uninfected patients tested negative at that titer.
As the pathogenesis of Blastocystis has been centered on proteases [83], one approach may be to detect the protease rather than the Blastocystis, on the assumption that Blastocystis may secrete higher levels of protease in symptomatic patients. Although it has not been applied with the intention of detecting blastocystosis, researchers have reported success in using a fecal serine protease assay in the study of diarrhea predominant irritable bowel syndrome (IBS-d) [84]. This study found that patients with IBS-d exhibited significantly higher protease levels in stool specimens, which were not found in patients diagnosed with diarrhea from acute infectious causes.
Detecting serum antibody response
Serum antibody tests using whole cell antigen have been developed many times for research purposes, and generally show a correlation between serum antibody response and symptomatic blastocystosis [36,82,85-87]. A 2007 study from China found that the serum antibody test was almost as sensitive as stool culture [87]. Researchers have found this test to be highly selective and sensitive for symptomatic Blastocystis infection, although additional trials are needed [85]. One study reported the ability to differentiate between most symptomatic and asymptomatic carriers using an ELISA assay quantitatively [82]. A 1991 study noted an effort to make antisera available commercially [3]. A United States company has reported its investigation of this type of assay as a diagnostic product [88].
Detecting infection through biopsies
In one study, colonic biopsies from IBS patients were found to produce elevated levels of serine protease [89]. Since the biopsies, but not necessarily the stool samples, seem to consistently contain the etiological element of such gastrointestinal illness, these may provide a better basis for the development of a "Gold Standard" for assays that detect infectious elements associated with IBS.
How sensitive does an assay need to be?
Early studies suggested Blastocystis produced symptoms by "overgrowing" in the gastrointestinal tract, which lead to the practice of diagnosing blastocystosis only when the organism was found in large quantities in stool samples. A view more consistent with observed data suggests that specific genotypes of Blastocystis [48] interact with host factors, triggering an immune reaction [85] and producing proteases that produce symptoms [83]. As an extracellular enteric protozoan, Blastocystis is theoretically capable of completing its life cycle without damaging host tissue, so symptomatic infection may be considered to be a side effect of infection. The mechanism that produces the symptoms may differ from the mechanism by which Blastocystis reproduces and expresses itself in stool samples.
Blastocystis undergoes programmed cell death in response to antibody exposure [90-93], so it is possible that patient immune response may complicate recovery of Blastocystis from stool specimens. One study found that the inability to culture Blastocystis from patients was correlated with an IgG3 response [36]. That study also found that the ability to culture Blastocystis from symptomatic patients was poorly correlated with positive results in immunological testing [36]. The author attributed the effect to residual immune response from treated Blastocystis. An alternate possibility is that antibiotic treatment converts stool test results from positive to negative, but leaves patients with a residual infection.
Treatment
Modern treatment of Blastocystis has generally been with metronidazole, with studies from the Middle East and US reporting treatment success [1,6]. Co-trimoxazole (TMP/SMX) has been used as well [94,95], as have nitazoxanide [96] and rifaximin [97].
Resistance to metronidazole was reported as early as 1991 [3]. Researchers have reported frequent resistance [98-100] and metronidazole may no longer be useful as a first line treatment [100]. Metronidazole resistance varies geographically [98] and Appendix A summarizes some resistance studies (see additional file 1). One clinic has reported success with a combination of secnidazole, nitazoxanide, and furazolidone [100]. All three drugs are not available in many countries. A 2006 text described a US patient returning from Nepal with chronic blastocystosis who was treated without success over a period of three years with iodoquinol, paromomycin, doxycycline, albendazole, tinidazole, ornidazole, quinacrine, nitazoxanide, rifaximin, furazolidone, co-trimoxazole, itraconazole, ketoconazole, and various combinations of those drugs [60]. A 1916 study described Blastocystis as an "infection that is difficult to get rid of" and noted the use of emetine [101].
A literature survey conducted for this review identified at least 79 studies describing treatment and epidemiology of Blastocystis (see additional file 1), but controlled investigation into the efficacy of drugs used in treatment is almost non-existent. Only two studies have screened drugs in vitro for effectiveness against Blastocystis [102,103]. The most recent in vitro study is over 17 years old, and no study has genotyped the Blastocystis isolates used, so it can not be determined if the isolates used in those studies are the same ones physicians seek to treat today.
The list of issues that complicates development of treatments for Blastocystis is long. Mouse models have existed since 1997 [104], but no animal studies have been published to evaluate treatment efficacy of drugs. The development of antiprotozoal resistance may be a factor in treatment failure, but there is a lack of evidence to show that this drug (or any other) should be effective against Blastocystis. Little is known about the organism's metabolic processes, although a recent study has identified the role of Blastocystis' mitochondrion-like-organelles in the reduction of ferrodoxins [105] that play a role in the conversion of metronidazole into its active state [106]. Metronidazole has been found to be one of the more effective drugs in vitro [102], but in vitro effectiveness may not correlate well with animal study in the treatment of parasitic infections [107]. Other factors in treatment failure, such as treatment compliance, remain uninvestigated. Some patients report an inability to complete metronidazole treatment due to vomiting, or difficulty administering the treatment to children (unpublished data, Kenneth Boorom).
No study has been published to identify agents that would be effective against the variants from IBS patient which exhibit metronidazole and furazolidone resistance in vitro [99]. Many drugs which have been reported to be effective against infection have been removed from common clinical use [3], although it is not known if those drugs would be effective in current infections. Many studies do not provide information about the length of time the patient had been symptomatic or provide follow-up information on the patient's condition. Most treatment studies lack information about the genotype being treated, so it is difficult to assess their significance. The lack of a reliable diagnostic method to determine if the infection has been eradicated complicates the evaluation of treatments.
Prevalence
Before discussing the prevalence of Blastocystis in the population, several points of interest should be addressed. Because diagnostic methods vary widely in sensitivity, figures reported from studies may reflect the testing method as much as the actual frequency of occurrence. Statistics gathered from clinical diagnostic laboratories are often reported as "prevalence," but as laboratory specimens are often submitted with the intention of diagnosing an illness, this practice may overestimate the prevalence in the population. Such numbers should properly be reported as "frequency of detection of Blastocystis in laboratory samples by (method)."
Prevalence in China
The only extensive studies of Blastocystis prevalence have been performed in China. A 2007 study evaluated 2321 samples taken from 4 geographic areas for Blastocystis presence and genotype (Figure 5) [43]. The prevalence of Blastocystis infection ranged from 1.9% in Shanghai municipality (in the East) to 32.6% in Menghai county (in the West).
Frequency of detection in United States and Canada
It has been suggested that case reports of Blastocystis increased in frequency after 1984 [3]. An analysis of studies reporting the frequency of detection of Blastocystis from North American laboratories suggests an increasing trend that began after 1988, followed by a reducing trend that began around 2000 (see Appendix A in additional file 1). Controversy concerning Blastocystis may have been a reflection of the disease's emergence, as the debate peaked in the time period of 1988–1991, with a series of letters exchanged between researchers in the Journal of Clinical Microbiology [5,9,108-110].
Changing genotypes
Studies from the 1980's and early 1990's from the Western United States and Canada indicated that Blastocystis was usually found as a co-infection, or was found in asymptomatic patients [5] and was comparatively rare [10,111]. These are characteristics associated with Blastocystis sp. subtype 2 [43,44,51]. A 2000 study found that Blastocystis was much more prevalent and it was no longer a co-infection in most patients which would be consistent with the characteristics of Blastocystis sp. subtype 3 [44,48]. Patients who were singly infected with Blastocystis were as likely to show symptoms as those singly infected with Cryptosporidium. As such, the increase could have represented the emergence of a new subtype of Blastocystis in the US population during the 1990's.
The distribution shown in current studies usually identifies subtype 3 as the most common, with subtype 1 found at a frequency of 10–60% of subtype 3. This pattern has been found in varying geographic regions, such as China, Greece, Denmark, and Singapore. While this may be a statistical anomaly, the etiology of this relationship may be of interest.
Transmission mechanisms
Regardless of opinions concerning pathogenicity, the existence of long-term trends in the prevalence of Blastocystis may be of scientific interest, especially when this occurred during a time when the frequency of detection of other enteric protozoa, such as Giardia lamblia and Entamoeba histolytica decreased [4,14]. Rising and falling prevalences have been found in propagated source epidemics such as influenza, but these trends occur over shorter time periods. A multi-year trend was reported in Scotland in association with epidemic infection of farm animals with Salmonella typhimurium and subsequent zoonotic transmission to the population [112]. The question of how Blastocystis is able to spread in industrialized countries with modern water treatment facilities remains to be addressed, but researchers have reported potential vectors as contaminated produce [113], sewage effluent [114], and contamination of treated drinking water due to infiltration at points distant from the treatment facility [115].
Global trends
One study noted that case reports of Blastocystis infection began to increase world-wide in 1984 [3]. Was the US trend part of a larger global increase? Studies concerning trends in Blastocystis infection are lacking, and many countries have historically used detection methods that have been found to have a low sensitivity [116]. Trends in Blastocystis infection may be mirrored in the prevalence of chronic lower gastrointestinal illness of unknown etiology. One study noted a statistically significant increase in IBS prevalence in younger patient groups [117], but studies of the prevalence of IBS are lacking as well. Rates of inflammatory bowel disease (IBD) are better documented, possibly because they result in hospital admissions. Researchers noted increases of 25–100% in the incidence or rates of hospitalization for IBD during the 1990's in the United States [118], Ireland [119], Scotland [120] and Italy [121] but not in Canada [122] or Minnesota [123]. In some areas, the increase was most pronounced in individuals 18-years old and younger, with a doubling of the incidence of IBD in Scotland in individuals aged 12–18 years [120]. It is possible that other factors could have contributed to an increase in chronic gastrointestinal illness of unknown etiology during this time period. Researchers have cited the stress of city living [21] and the stress of international travel [124] as factors in development of such illness so it is possible that increased urbanization and travel has lead to greater morbidity. Serious gastrointestinal illness has been attributed to various viral and bacterial causes [125] and the hygiene hypothesis, which suggests that lack of gastrointestinal infection in childhood leads to chronic gastrointestinal illness [126]. Additional research may be valuable in understanding the relationship between trends in the prevalence of unexplained chronic gastrointestinal illness and poorly understood protozoal infections.
Pathogenesis
Studies of the pathogenesis of Blastocystis have focused on immunological reactions of epithelial cells to proteases secreted by Blastocystis. Co-culture studies of Blastocystis have shown Blastocystis initially down-regulates and then up-regulates production of the inflammatory cytokine IL-8 in epithelial cells [127].
Specific in vitro co-culture studies have indicated that Blastocystis possesses pathogenicity mechanisms [83,128]. Pathogenesis was reported to result from interaction between parasite products (e.g. cysteine protease from the zoonotic isolate Blastocystis ratti WR1) and enterocytes that influence host inflammatory and immunological responses. Blastocystis ratti WR1 cysteine protease upregulated interleukin IL-8 gene expression through nuclear factor κB activation, but metronidazole treatment averted IL-8 production. Blastocystis also induced cell apoptosis, possibly in a subtype-dependent manner. An in vitro study also reported that the activation of nuclear factor κB/inhibitor of κB (NF-κB/IκB) signaling system lead to production of pro-inflammatory mediators, including IL-8, regulated upon activation, normal T-cell expressed, and secreted (RANTES) chemokines, and transforming-growth-factor TGF-β resulting in intestinal inflammation [83].
The pathogenesis of Blastocystis has been problematic, since the infection presents with abdominal pain in the absence of endoscopic findings [129]. Recent research into the etiology of abdominal pain, constipation and diarrhea in patients with symptoms attributed to IBS has suggested that the these symptoms are produced by high levels of serine protease produced in the gastrointestinal tract which are capable of exciting neurons directly through the protease-activated receptor-2 (PAR2) pathway [84,89]. This may offer an explanation for the conundrum of patients who experience pain in gastrointestinal illness in the absence of endoscopic findings [129].
While Blastocystis infection may be more serious in immunocompromised patients [130], studies have found that the majority of patients with symptomatic Blastocystis infection are immunocompetent, a pattern that is also present in infection with Giardia and Entamoeba histolytica. A Canadian study found that of 103 symptomatic patients with findings of only Blastocystis infection, only 3 were immunocompromised. Outbreaks of symptomatic Blastocystis infection have occurred in demographic groups not traditionally associated with immunocompromised status, such as groups of schoolchildren in the Middle East [58] and parents and children from the same families [131,132].
Conflicting research results
To better understand conflicting findings concerning Blastocystis pathogenicity, we surveyed studies in the US National Institutes of Health Pubmed Database (see additional file 1). Studies reported pathogenicity in 84% (86/102) of the cases, and non-pathogenicity in 16% (16/102).
Many researchers identified a specific finding which they felt would be inconsistent with the behavior of a pathogen, such as a lack of correlation between the quantity of Blastocystis in stool samples and the patient's symptoms [133] or the absence of endoscopic findings in symptomatic infection [129].
Appendix B (additional file 2) summarizes characteristics of known gastrointestinal pathogens which may be of value. Additional observations follow, with details in additional file 3:
(1) All studies (16/16) finding Blastocystis to be non-pathogenic were conducted on subjects from more affluent countries;
(2) All studies finding Blastocystis to be non-pathogenic performed after 1994 used FECT or MIF for detection [134-136];
(2) Findings of non-pathogenicity were much more likely to have been reported by researchers studying Blastocystis in North America before 1994 (additional file 3, P < 0.0022, Fisher's Exact Test);
(3) North American studies reporting pathogenicity were often conducted in laboratories in coastal cities (Vancouver, New York, Palo Alto) which served individuals with travel history to less developed countries [6] or processed large numbers of samples (> 2,500) from the community [10,111]. Studies conducted on the more insular population of a health maintenance organization found Blastocystis to be non-pathogenic [5,9,137-140].
The variable sensitivity of diagnostic techniques discussed earlier may be responsible for some of the disagreement. Additional causes may include:
Blastocystis sp. subtype 2 may have been the dominant genotype in the United States in the early 1990's : During this time, immigrants from Southeast Asia had a high rate of infection which was difficult to detect [109]. The description of Blastocystis as a co-infection in symptomatic patients from Californian physicians in the 1980's [139] is consistent with the behavior of Blastocystis sp. subtype 2 [44]. Blastocystis acquired overseas was associated with symptomatic infection, while US-acquired blastocystosis was asymptomatic [6]. Following an increase in detection rates of Blastocystis in the 1990's (see Appendix A in additional file 1), US studies now show Blastocystis appearing frequently as a symptomatic mono-infection [14] with characteristics similar to Cryptosporidium parvum and Entamoeba histolytica. (Figure 8).
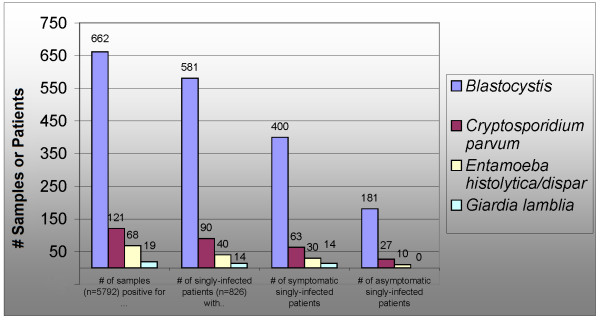
Figure 8.
The characteristics of common enteric protozoa reported in study of 5792 specimens from US patients collected in 2000[14]. Studies from the 1980's reported Blastocystis was usually found as a co-infection with Giardia or Entamoeba histolytica in symptomatic patients [139,140], which was not the case in 2000 [14]. In 2000, the number of symptomatic patients who were found to be singly infected with Blastocystis (400) exceeded the number of samples found positive for Cryptosporidium, Giardia, and Entamoeba histolytica combined. Patients singly infected with Blastocystis were as likely to be symptomatic as patients singly infected with Cryptosporidium (69% vs. 70%) [14].
Symptomatic carriage of enteric protozoa may be mediated by host genetics (see Appendix B in additional file2) : In affluent countries, if symptomatic carriers can seek and receive treatment, they may leave a population of asymptomatic carriers to be studied by epidemiologists.
Studies may have fulfilled many criteria listed in a 1990 communication as necessary to establish the pathogenicity of Blastocystis [110]: fulfillment of Koch's postulates [48,104,141]; identification of an immune response [82,85,86]; definition of the pathogenesis [83,127,128,142]; identification of treatments [94,143,144]; and description of point source outbreak of diarrhea where Blastocystis was the cause [57,131,145].
Conclusion
1. Blastocystis comprises a group of genetically diverse organisms, some of which will cause chronic or acute infection in some immunocompetent humans. The behavior of Blastocystis in humans is consistent with that of Giardia and Entamoeba histolytica – expression of symptoms depends on parasite genotype, host genotype, host immunity, and age. Parasite genotype may vary geographically along with other factors.
2. Most diagnostics and treatments in clinical use for blastocystosis have been shown to have low sensitivity. Stool culture may provide the best short-term option for improving sensitivity, with serum antibody testing being a better option if it becomes available. PCR testing is currently the only way to identify the genotype of Blastocystis isolates.
3. Researchers from less affluent countries consistently report that Blastocystis is pathogenic, while some researchers in more affluent countries have authored contradictory studies. The outcome has been that little clinical research is performed or supported by affluent countries, while less affluent countries now publish sophisticated studies into Blastocystis infection [43,51,53,75,94,146,147].
4. Blastocystis has met all criteria for pathogenicity that are met by Giardia and Entamoeba histolytica, such as fulfillment Koch's Postulates [48,104,141,148], demonstration of a treatment that eliminates the organism and the symptoms, an immune response that is elevated in symptomatic individuals [36], and a positive association with symptoms in most studies. Blastocystis fails to meet criteria which Giardia and Entamoeba histolytica fail to meet. For example, all persons infected are not symptomatic; all researchers in all geographies have not shown an association between infection and symptoms; and the use of inadequate methods in some historical studies has produced the appearance of non-pathogenicity [149,150].
5. The lack of reliable diagnostics and the development of metronidazole resistance in Blastocystis may lead to many undiagnosed infections. Blastocystis may play a significant role in several chronic gastrointestinal illnesses of unknown etiology which can be expensive to manage and debilitating to patients.
Abbreviations
ATCC: American Type Culture Collection; CWM: CONSED™ preservation followed by wet mounting; FECT: Formol Ethyl Acetate Concentration Technique; IBD: Inflammatory Bowel Disease; IBS: Irritable Bowel Syndrome; IBS-c: Constipation predominant Irritable Bowel Syndrome; IBS-d: Diarrhea predominant Irritable Bowel Syndrome; IFA: Indirect Immunofluorescence Assay; MIF: Merthiolate-Iodine Concentration; PAR2: Protease-Activated Receptor-2; PCR: Polymerase Chain Reaction; SAF: Sodium Acetate-acetic acid-formalin; SSU rRNA: Small subunit ribosomal RNA
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
All authors engaged in developing the manuscript and approved the final version.
Supplementary Material
Appendix A – Additional charts.
Appendix B – Characteristics of known gastrointestinal pathogens.
List of Blastocystis studies categorized by researcher finding and geographic location.
Contributor Information
Kenneth F Boorom, Email: director@bhomcenter.org.
Huw Smith, Email: Huw.Smith@NorthGlasgow.Scot.NHS.UK.
Laila Nimri, Email: nimri@just.edu.jo.
Eric Viscogliosi, Email: eric.viscogliosi@pasteur-lille.fr.
Gregory Spanakos, Email: grspan@yahoo.com.
Unaiza Parkar, Email: unaiza@iinet.net.au.
Lan-Hua Li, Email: orchid8@sina.com.
Xiao-Nong Zhou, Email: ipdzhouxn@sh163.net.
Ülgen Z Ok, Email: okulgen@superonline.com.
Saovanee Leelayoova, Email: s_leelayoova@scientist.com.
Morris S Jones, Email: Morris.Jones@travis.af.mil.
References
- Qadri SM, al-Okaili GA, al-Dayel F. Clinical significance of Blastocystis hominis. J Clin Microbiol. 1989;27:2407–2409. doi: 10.1128/jcm.27.11.2407-2409.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silberman JD, Sogin ML, Leipe DD, Clark CG. Human parasite finds taxonomic home. Nature. 1996;380:398. doi: 10.1038/380398a0. [DOI] [PubMed] [Google Scholar]
- Zierdt CH. Blastocystis hominis–past and future. Clin Microbiol Rev. 1991;4:61–79. doi: 10.1128/cmr.4.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kappus KK, Juranek DD, Roberts JM. Results of testing for intestinal parasites by state diagnostic laboratories, United States, 1987. MMWR CDC Surveill Summ. 1991;40:25–45. [PubMed] [Google Scholar]
- Markell EK, Udkow MP. Association of Blastocystis hominis with human disease. J Clin Microbiol. 1988;26:609–610. doi: 10.1128/jcm.26.3.609-610.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan DJ, Raucher BG, McKitrick JC. Association of Blastocystis hominis with signs and symptoms of human disease. J Clin Microbiol. 1986;24:548–550. doi: 10.1128/jcm.24.4.548-550.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Gorman MA, Orenstein SR, Proujansky R, Wadowsky RM, Putnam PE, Kocoshis SA. Prevalence and characteristics of Blastocystis hominis infection in children. Clin Pediatr (Phila) 1993;32:91–96. doi: 10.1177/000992289303200206. [DOI] [PubMed] [Google Scholar]
- Zaki M, Daoud AS, Pugh RN, al-Ali F, al-Mutairi G, al-Saleh Q. Clinical report of Blastocystis hominis infection in children. J Trop Med Hyg. 1991;94:118–122. [PubMed] [Google Scholar]
- Markell EK, Udkow MP. Association of Blastocystis hominis with human disease? J Clin Microbiol. 1990;28:1085–1086. doi: 10.1128/jcm.28.5.1085-1086.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle PW, Helgason MM, Mathias RG, Proctor EM. Epidemiology and pathogenicity of Blastocystis hominis. J Clin Microbiol. 1990;28:116–121. doi: 10.1128/jcm.28.1.116-121.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andiran N, Acikgoz ZC, Turkay S, Andiran F. Blastocystis hominis–an emerging and imitating cause of acute abdomen in children. J Pediatr Surg. 2006;41:1489–1491. doi: 10.1016/j.jpedsurg.2006.04.037. [DOI] [PubMed] [Google Scholar]
- Noel C, Dufernez F, Gerbod D, Edgcomb VP, Delgado-Viscogliosi P, Ho LC, Singh M, Wintjens R, Sogin ML, Capron M, et al. Molecular phylogenies of Blastocystis isolates from different hosts: implications for genetic diversity, identification of species, and zoonosis. J Clin Microbiol. 2005;43:348–355. doi: 10.1128/JCM.43.1.348-355.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedro-Botet J, Auguet T, Rubies-Prat J. Blastocystis hominis: a controversial enteric protozoon. J Clin Gastroenterol. 1992;14:88–89. doi: 10.1097/00004836-199201000-00022. [DOI] [PubMed] [Google Scholar]
- Amin OM. Seasonal prevalence of intestinal parasites in the United States during 2000. Am J Trop Med Hyg. 2002;66:799–803. doi: 10.4269/ajtmh.2002.66.799. [DOI] [PubMed] [Google Scholar]
- Levy RL, Whitehead WE, Von Korff MR, Feld AD. Intergenerational transmission of gastrointestinal illness behavior. Am J Gastroenterol. 2000;95:451–456. doi: 10.1111/j.1572-0241.2000.01766.x. [DOI] [PubMed] [Google Scholar]
- Liebregts T, Adam B, Bredack C, Roth A, Heinzel S, Lester S, Downie-Doyle S, Smith E, Drew P, Talley NJ, et al. Immune activation in patients with irritable bowel syndrome. Gastroenterology. 2007;132:913–920. doi: 10.1053/j.gastro.2007.01.046. [DOI] [PubMed] [Google Scholar]
- Hulisz D. The burden of illness of irritable bowel syndrome: current challenges and hope for the future. J Manag Care Pharm. 2004;10:299–309. doi: 10.18553/jmcp.2004.10.4.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lembo T, Fullerton S, Diehl D, Raeen H, Munakata J, Naliboff B, Mayer EA. Symptom duration in patients with irritable bowel syndrome. Am J Gastroenterol. 1996;91:898–905. [PubMed] [Google Scholar]
- Smith S. The Travelling Toilet Hits the Road. Gut Reaction. 2007;Summer 2007:6. [Google Scholar]
- Levy RL, Von Korff M, Whitehead WE, Stang P, Saunders K, Jhingran P, Barghout V, Feld AD. Costs of care for irritable bowel syndrome patients in a health maintenance organization. Am J Gastroenterol. 2001;96:3122–3129. doi: 10.1111/j.1572-0241.2001.05258.x. [DOI] [PubMed] [Google Scholar]
- Schmulson M, Ortiz O, Santiago-Lomeli M, Gutierrez-Reyes G, Gutierrez-Ruiz MC, Robles-Diaz G, Morgan D. Frequency of functional bowel disorders among healthy volunteers in Mexico City. Dig Dis. 2006;24:342–347. doi: 10.1159/000092887. [DOI] [PubMed] [Google Scholar]
- Quigley EM, Locke GR, Mueller-Lissner S, Paulo LG, Tytgat GN, Helfrich I, Schaefer E. Prevalence and management of abdominal cramping and pain: a multinational survey. Aliment Pharmacol Ther. 2006;24:411–419. doi: 10.1111/j.1365-2036.2006.02989.x. [DOI] [PubMed] [Google Scholar]
- Everhart JENDDDWGUS, National Institute of Diabetes and Digestive and Kidney Diseases (U.S.) Digestive diseases in the United States: epidemiology and impact. [Bethesda, Md.] Washington, DC: US Dept. of Health and Human Services, Public Health Service; 1994. [Google Scholar]
- Windsor JJ. B. hominis and D. fragilis: Neglected human protozoa. The Biomedical Scientist. 2007. pp. 524–527.
- Yakoob J, Jafri W, Jafri N, Khan R, Islam M, Beg MA, Zaman V. Irritable bowel syndrome: in search of an etiology: role of Blastocystis hominis. Am J Trop Med Hyg. 2004;70:383–385. [PubMed] [Google Scholar]
- Windsor JJ, Macfarlane L, Hughes-Thapa G, Jones SK, Whiteside TM. Incidence of Blastocystis hominis in faecal samples submitted for routine microbiological analysis. Br J Biomed Sci. 2002;59:154–157. doi: 10.1080/09674845.2002.11783653. [DOI] [PubMed] [Google Scholar]
- Tungtrongchitr A, Manatsathit S, Kositchaiwat C, Ongrotchanakun J, Munkong N, Chinabutr P, Leelakusolvong S, Chaicumpa W. Blastocystis hominis infection in irritable bowel syndrome patients. Southeast Asian J Trop Med Public Health. 2004;35:705–710. [PubMed] [Google Scholar]
- Wilson S, Roberts L, Roalfe A, Bridge P, Singh S. Prevalence of irritable bowel syndrome: a community survey. Br J Gen Pract. 2004;54:495–502. [PMC free article] [PubMed] [Google Scholar]
- Camilleri M, Carlson P, McKinzie S, Grudell A, Busciglio I, Burton D, Baxter K, Ryks M, Zinsmeister AR. Genetic variation in endocannabinoid metabolism, gastrointestinal motility, and sensation. Am J Physiol Gastrointest Liver Physiol. 2008;294:G13–19. doi: 10.1152/ajpgi.00371.2007. [DOI] [PubMed] [Google Scholar]
- Hirata T, Nakamura H, Kinjo N, Hokama A, Kinjo F, Yamane N, Fujita J. Prevalence of Blastocystis hominis and Strongyloides stercoralis infection in Okinawa, Japan. Parasitol Res. 2007;101:1717–1719. doi: 10.1007/s00436-007-0712-7. [DOI] [PubMed] [Google Scholar]
- Boivin M. Socioeconomic impact of irritable bowel syndrome in Canada. Can J Gastroenterol. 2001;15:8B–11B. doi: 10.1155/2001/401309. [DOI] [PubMed] [Google Scholar]
- Lagace-Wiens PR, VanCaeseele PG, Koschik C. Dientamoeba fragilis: an emerging role in intestinal disease. CMAJ. 2006;175:468–469. doi: 10.1503/cmaj.060265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hungin AP, Chang L, Locke GR, Dennis EH, Barghout V. Irritable bowel syndrome in the United States: prevalence, symptom patterns and impact. Aliment Pharmacol Ther. 2005;21:1365–1375. doi: 10.1111/j.1365-2036.2005.02463.x. [DOI] [PubMed] [Google Scholar]
- Cruz Licea V, Plancarte Crespo A, Moran Alvarez C, Valencia Rojas S, Rodriguez Sasnchez G, Vega Franco L. Blastocystis hominis among food vendors in Xochimilco markets. Rev Latinoam Microbiol. 2003;45:12–15. [PubMed] [Google Scholar]
- Guimaraes S, Sogayar MI. Blastocystis hominis: occurrence in children and staff members of municipal day-care centers from Botucatu, Sao Paulo State, Brazil. Mem Inst Oswaldo Cruz. 1993;88:427–429. doi: 10.1590/s0074-02761993000300012. [DOI] [PubMed] [Google Scholar]
- Hussain R, Jaferi W, Zuberi S, Baqai R, Abrar N, Ahmed A, Zaman V. Significantly increased IgG2 subclass antibody levels to Blastocystis hominis in patients with irritable bowel syndrome. Am J Trop Med Hyg. 1997;56:301–306. doi: 10.4269/ajtmh.1997.56.301. [DOI] [PubMed] [Google Scholar]
- Giacometti A, Cirioni O, Fiorentini A, Fortuna M, Scalise G. Irritable bowel syndrome in patients with Blastocystis hominis infection. Eur J Clin Microbiol Infect Dis. 1999;18:436–439. doi: 10.1007/s100960050314. [DOI] [PubMed] [Google Scholar]
- Brumpt E. Blastocystis hominis N. sp. Et formes voisines. Bull Soc Pathol Exot. 1912;5:725–730. [Google Scholar]
- Chen XQ, Singh M, Ho LC, Tan SW, Ng GC, Moe KT, Yap EH. Description of a Blastocystis species from Rattus norvegicus. Parasitol Res. 1997;83:313–318. doi: 10.1007/s004360050255. [DOI] [PubMed] [Google Scholar]
- Belova LM. [The ultrastructure of Blastocystis galli from chickens] Parazitologiia. 1998;32:553–559. [PubMed] [Google Scholar]
- Stensvold CR, Suresh GK, Tan KS, Thompson RC, Traub RJ, Viscogliosi E, Yoshikawa H, Clark CG. Terminology for Blastocystis subtypes–a consensus. Trends Parasitol. 2007;23:93–96. doi: 10.1016/j.pt.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Perez-Brocal V, Clark CG. Analysis of two genomes from the mitochondrion-like organelle of the intestinal parasite Blastocystis: Complete sequences, gene content and genome organization. Mol Biol Evol. 2008 doi: 10.1093/molbev/msn193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li LH, Zhang XP, Lv S, Zhang L, Yoshikawa H, Wu Z, Steinmann P, Utzinger J, Tong XM, Chen SH, et al. Cross-sectional surveys and subtype classification of human Blastocystis isolates from four epidemiological settings in China. Parasitol Res. 2007;102:83–90. doi: 10.1007/s00436-007-0727-0. [DOI] [PubMed] [Google Scholar]
- Stensvold CR, Arendrup MC, Jespersgaard C, Molbak K, Nielsen HV. Detecting Blastocystis using parasitologic and DNA-based methods: a comparative study. Diagn Microbiol Infect Dis. 2007;59:303–307. doi: 10.1016/j.diagmicrobio.2007.06.003. [DOI] [PubMed] [Google Scholar]
- Wong KH, Ng GC, Lin RT, Yoshikawa H, Taylor MB, Tan KS. Predominance of subtype 3 among Blastocystis isolates from a major hospital in Singapore. Parasitol Res. 2008;102:663–670. doi: 10.1007/s00436-007-0808-0. [DOI] [PubMed] [Google Scholar]
- Menounos PG, Spanakos G, Tegos N, Vassalos CM, Papadopoulou C, Vakalis NC. Direct detection of Blastocystis sp. in human faecal samples and subtype assignment using single strand conformational polymorphism and sequencing. Mol Cell Probes. 2008;22:24–29. doi: 10.1016/j.mcp.2007.06.007. [DOI] [PubMed] [Google Scholar]
- Yoshikawa H, Wu Z, Kimata I, Iseki M, Ali IK, Hossain MB, Zaman V, Haque R, Takahashi Y. Polymerase chain reaction-based genotype classification among human Blastocystis hominis populations isolated from different countries. Parasitol Res. 2004;92:22–29. doi: 10.1007/s00436-003-0995-2. [DOI] [PubMed] [Google Scholar]
- Hussein EM, Hussein AM, Eida MM, Atwa MM. Pathophysiological variability of different genotypes of human Blastocystis hominis Egyptian isolates in experimentally infected rats. Parasitol Res. 2008;102:853–860. doi: 10.1007/s00436-007-0833-z. [DOI] [PubMed] [Google Scholar]
- Jones MS, Whipps CM, Ganac RD, Hudson NR, Boorom K. Association of Blastocystis subtype 3 and 1 with patients from an Oregon community presenting with chronic gastrointestinal illness. Parasitol Res. 2008 doi: 10.1007/s00436-008-1198-7. [DOI] [PubMed] [Google Scholar]
- Dogruman-Al F, Dagci H, Yoshikawa H, Kurt O, Demirel M. A possible link between subtype 2 and asymptomatic infections of Blastocystis hominis. Parasitol Res. 2008 doi: 10.1007/s00436-008-1031-3. [DOI] [PubMed] [Google Scholar]
- Ozyurt M, Kurt O, Molbak K, Nielsen HV, Haznedaroglu T, Stensvold CR. Molecular epidemiology of Blastocystis infections in Turkey. Parasitol Int. 2008;57:300–306. doi: 10.1016/j.parint.2008.01.004. [DOI] [PubMed] [Google Scholar]
- Stensvold R, Brillowska-Dabrowska A, Nielsen HV, Arendrup MC. Detection of Blastocystis hominis in unpreserved stool specimens by using polymerase chain reaction. J Parasitol. 2006;92:1081–1087. doi: 10.1645/GE-840R.1. [DOI] [PubMed] [Google Scholar]
- Li LH, Zhou XN, Du ZW, Wang XZ, Wang LB, Jiang JY, Yoshikawa H, Steinmann P, Utzinger J, Wu Z, et al. Molecular epidemiology of human Blastocystis in a village in Yunnan province, China. Parasitol Int. 2007;56:281–286. doi: 10.1016/j.parint.2007.06.001. [DOI] [PubMed] [Google Scholar]
- Jones Ii MS, Ganac RD, Hiser G, Hudson NR, Le A, Whipps CM. Detection of Blastocystis from stool samples using real-time PCR. Parasitol Res. 2008;103:551–557. doi: 10.1007/s00436-008-1006-4. [DOI] [PubMed] [Google Scholar]
- ATCC Web Site http://www.atcc.org
- Cole JA, Rothman KJ, Cabral HJ, Zhang Y, Farraye FA. Migraine, fibromyalgia, and depression among people with IBS: a prevalence study. BMC Gastroenterol. 2006;6:26. doi: 10.1186/1471-230X-6-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimri LF. Evidence of an epidemic of Blastocystis hominis infections in preschool children in northern Jordan. J Clin Microbiol. 1993;31:2706–2708. doi: 10.1128/jcm.31.10.2706-2708.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nimri L, Batchoun R. Intestinal colonization of symptomatic and asymptomatic schoolchildren with Blastocystis hominis. J Clin Microbiol. 1994;32:2865–2866. doi: 10.1128/jcm.32.11.2865-2866.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- State of Oregon House Legislature Committee on Health Policy, Patient and Physician Testimony in Support of HB 2699 March 1, 2007, Transcript and audio link http://www.bhomcenter.org/news/transcript_2007_03_salem.htm
- Boorom K. Commensal and Pathogenic Blastocystis with Case Studies from Oregon's Willamette Valley. Corvallis: FBH Press, ISBN 9781430309048; 2006. [Google Scholar]
- Armentia A, Mendez J, Gomez A, Sanchis E, Fernandez A, de la Fuente R, Sanchez P. Urticaria by Blastocystis hominis. Successful treatment with paromomycin. Allergol Immunopathol (Madr) 1993;21:149–151. [PubMed] [Google Scholar]
- Biedermann T, Hartmann K, Sing A, Przybilla B. Hypersensitivity to non-steroidal anti-inflammatory drugs and chronic urticaria cured by treatment of Blastocystis hominis infection. Br J Dermatol. 2002;146:1113–1114. doi: 10.1046/j.1365-2133.2002.473212.x. [DOI] [PubMed] [Google Scholar]
- Gupta R, Parsi K. Chronic urticaria due to Blastocystis hominis. Australas J Dermatol. 2006;47:117–119. doi: 10.1111/j.1440-0960.2006.00244.x. [DOI] [PubMed] [Google Scholar]
- Pasqui AL, Savini E, Saletti M, Guzzo C, Puccetti L, Auteri A. Chronic urticaria and blastocystis hominis infection: a case report. Eur Rev Med Pharmacol Sci. 2004;8:117–120. [PubMed] [Google Scholar]
- Micheloud D, Jensen J, Fernandez-Cruz E, Carbone J. [Chronic angioedema and blastocystis hominis infection] Rev Gastroenterol Peru. 2007;27:191–193. [PubMed] [Google Scholar]
- Amin O. Epidemiology of Blastocystis hominis in the United States. Research Journal of Parasitology. 2005;2006:1–10. [Google Scholar]
- Katsarou-Katsari A, Vassalos CM, Tzanetou K, Spanakos G, Papadopoulou C, Vakalis N. Acute urticaria associated with amoeboid forms of Blastocystis sp. subtype 3. Acta Derm Venereol. 2008;88:80–81. doi: 10.2340/00015555-0338. [DOI] [PubMed] [Google Scholar]
- Giacometti A, Cirioni O, Antonicelli L, D'Amato G, Silvestri C, Del Prete MS, Scalise G. Prevalence of intestinal parasites among individuals with allergic skin diseases. J Parasitol. 2003;89:490–492. doi: 10.1645/0022-3395(2003)089[0490:POIPAI]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Termmathurapoj S, Leelayoova S, Aimpun P, Thathaisong U, Nimmanon T, Taamasri P, Mungthin M. The usefulness of short-term in vitro cultivation for the detection and molecular study of Blastocystis hominis in stool specimens. Parasitol Res. 2004;93:445–447. doi: 10.1007/s00436-004-1157-x. [DOI] [PubMed] [Google Scholar]
- Rajah S, Suresh K, Vennila G, Khairual AA. Small forms of Blastocystis hominis. J Int Med Res. 1997;1:93–96. [Google Scholar]
- Vennila GD, Suresh Kumar G, Khairul Anuar A, Rajah S, Saminathan R, Sivanandan S, Ramakrishnan K. Irregular shedding of Blastocystis hominis. Parasitol Res. 1999;85:162–164. doi: 10.1007/s004360050528. [DOI] [PubMed] [Google Scholar]
- Leelayoova S, Taamasri P, Rangsin R, Naaglor T, Thathaisong U, Mungthin M. In-vitro cultivation: a sensitive method for detecting Blastocystis hominis. Ann Trop Med Parasitol. 2002;96:803–807. doi: 10.1179/000349802125002275. [DOI] [PubMed] [Google Scholar]
- Clark CG, Diamond LS. Methods for cultivation of luminal parasitic protists of clinical importance. Clin Microbiol Rev. 2002;15:329–341. doi: 10.1128/CMR.15.3.329-341.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkar U, Traub RJ, Kumar S, Mungthin M, Vitali S, Leelayoova S, Morris K, Thompson RC. Direct characterization of Blastocystis from faeces by PCR and evidence of zoonotic potential. Parasitology. 2007;134:359–367. doi: 10.1017/S0031182006001582. [DOI] [PubMed] [Google Scholar]
- Motazedian H, Ghasemi H, Sadjjadi SM. Genomic diversity of Blastocystis hominis from patients in southern Iran. Ann Trop Med Parasitol. 2008;102:85–88. doi: 10.1179/136485908X252197. [DOI] [PubMed] [Google Scholar]
- Abd-Alla MD, Wahib AA, Ravdin JI. Comparison of antigen-capture ELISA to stool-culture methods for the detection of asymptomatic Entamoeba species infection in Kafer Daoud, Egypt. Am J Trop Med Hyg. 2000;62:579–582. doi: 10.4269/ajtmh.2000.62.579. [DOI] [PubMed] [Google Scholar]
- Chou CF, Tai JH. Simultaneous extraction of DNA and RNA from nuclease-rich pathogenic protozoan Trichomonas vaginalis. Biotechniques. 1996;20:790–791. doi: 10.2144/96205bm11. [DOI] [PubMed] [Google Scholar]
- Nasirudeen AM, Tan KS. Caspase-3-like protease influences but is not essential for DNA fragmentation in Blastocystis undergoing apoptosis. Eur J Cell Biol. 2004;83:477–482. doi: 10.1078/0171-9335-00411. [DOI] [PubMed] [Google Scholar]
- Winn WC, Koneman EW. Koneman's color atlas and textbook of diagnostic microbiology. 6. Philadelphia: Lippincott Williams & Wilkins; 2006. [Google Scholar]
- Carbajal JA, Villar J, Lanuza MD, Esteban JG, Munoz C, Borras R. [Clinical significance of Blastocystis hominis infection: epidemiologic study] Med Clin (Barc) 1997;108:608–612. [PubMed] [Google Scholar]
- Tan TC, Suresh KG. Predominance of amoeboid forms of Blastocystis hominis in isolates from symptomatic patients. Parasitol Res. 2006;98:189–193. doi: 10.1007/s00436-005-0033-7. [DOI] [PubMed] [Google Scholar]
- Mahmoud MS, Saleh WA. Secretory and humoral antibody responses to Blastocystis hominis in symptomatic and asymptomatic human infections. J Egypt Soc Parasitol. 2003;33:13–30. [PubMed] [Google Scholar]
- Puthia MK, Lu J, Tan KS. Blastocystis ratti contains cysteine proteases that mediate interleukin-8 response from human intestinal epithelial cells in an NF-kappaB-dependent manner. Eukaryot Cell. 2008;7:435–443. doi: 10.1128/EC.00371-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gecse K, Roka R, Ferrier L, Leveque M, Eutamene H, Cartier C, Ait-Belgnaoui A, Rosztoczy A, Izbeki F, Fioramonti J, et al. Increased faecal serine protease activity in diarrhoeic IBS patients: a colonic lumenal factor impairing colonic permeability and sensitivity. Gut. 2008;57:591–599. doi: 10.1136/gut.2007.140210. [DOI] [PubMed] [Google Scholar]
- Zierdt CH, Nagy B. Antibody response to Blastocystis hominis infections. Ann Intern Med. 1993;118:985–986. doi: 10.7326/0003-4819-118-12-199306150-00018. [DOI] [PubMed] [Google Scholar]
- Garavelli PL, Zierdt CH, Fleisher TA, Liss H, Nagy B. Serum antibody detected by fluorescent antibody test in patients with symptomatic Blastocystis hominis infection. Recenti Prog Med. 1995;86:398–400. [PubMed] [Google Scholar]
- Su SL, Yan YM, Liao H, Chen GF, Zhang RQ, Xie QJ, Le X, Hu YQ, Zeng XY, Lan HY, et al. [Dot enzyme-linked immunosorbent assay for detection of serum antibody to Blastocystis hominis in humans] Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi. 2007;25:256–258. [PubMed] [Google Scholar]
- Antibodies Incorporated press release http://antibodiesinc.com/PressReleaseDetail.asp?nPrID=12
- Cenac N, Andrews CN, Holzhausen M, Chapman K, Cottrell G, Andrade-Gordon P, Steinhoff M, Barbara G, Beck P, Bunnett NW, et al. Role for protease activity in visceral pain in irritable bowel syndrome. J Clin Invest. 2007;117:636–647. doi: 10.1172/JCI29255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasirudeen AM, Hian YE, Singh M, Tan KS. Metronidazole induces programmed cell death in the protozoan parasite Blastocystis hominis. Microbiology. 2004;150:33–43. doi: 10.1099/mic.0.26496-0. [DOI] [PubMed] [Google Scholar]
- Nasirudeen AM, Tan KS, Singh M, Yap EH. Programmed cell death in a human intestinal parasite, Blastocystis hominis. Parasitology. 2001;123:235–246. doi: 10.1017/s0031182001008332. [DOI] [PubMed] [Google Scholar]
- Tan KS, Ibrahim M, Ng GC, Nasirudeen AM, Ho LC, Yap EH, Singh M. Exposure of Blastocystis species to a cytotoxic monoclonal antibody. Parasitol Res. 2001;87:534–538. doi: 10.1007/s004360000365. [DOI] [PubMed] [Google Scholar]
- Tan SW, Singh M, Ho LC, Howe J, Moe KT, Chen XQ, Ng GC, Yap EH. Survival of Blastocystis hominis clones after exposure to a cytotoxic monoclonal antibody. Int J Parasitol. 1997;27:947–954. doi: 10.1016/S0020-7519(97)00066-0. [DOI] [PubMed] [Google Scholar]
- Moghaddam DD, Ghadirian E, Azami M. Blastocystis hominis and the evaluation of efficacy of metronidazole and trimethoprim/sulfamethoxazole. Parasitol Res. 2005;96:273–275. doi: 10.1007/s00436-005-1363-1. [DOI] [PubMed] [Google Scholar]
- Ok UZ, Girginkardesler N, Balcioglu C, Ertan P, Pirildar T, Kilimcioglu AA. Effect of trimethoprim-sulfamethaxazole in Blastocystis hominis infection. Am J Gastroenterol. 1999;94:3245–3247. doi: 10.1111/j.1572-0241.1999.01529.x. [DOI] [PubMed] [Google Scholar]
- Rossignol JF, Kabil SM, Said M, Samir H, Younis AM. Effect of nitazoxanide in persistent diarrhea and enteritis associated with Blastocystis hominis. Clin Gastroenterol Hepatol. 2005;3:987–991. doi: 10.1016/S1542-3565(05)00427-1. [DOI] [PubMed] [Google Scholar]
- Amenta M, Dalle Nogare ER, Colomba C, Prestileo TS, Di Lorenzo F, Fundaro S, Colomba A, Ferrieri A. Intestinal protozoa in HIV-infected patients: effect of rifaximin in Cryptosporidium parvum and Blastocystis hominis infections. J Chemother. 1999;11:391–395. doi: 10.1179/joc.1999.11.5.391. [DOI] [PubMed] [Google Scholar]
- Haresh K, Suresh K, Khairul Anus A, Saminathan S. Isolate resistance of Blastocystis hominis to metronidazole. Trop Med Int Health. 1999;4:274–277. doi: 10.1046/j.1365-3156.1999.00398.x. [DOI] [PubMed] [Google Scholar]
- Yakoob J, Jafri W, Jafri N, Islam M, Asim Beg M. In vitro susceptibility of Blastocystis hominis isolated from patients with irritable bowel syndrome. Br J Biomed Sci. 2004;61:75–77. doi: 10.1080/09674845.2004.11732647. [DOI] [PubMed] [Google Scholar]
- Stein J. Drug Combination Promising for Enteric Parasitic Infections. Reuters Health (Indexed in Medcape) 2007.
- Low G. Two chronic amoebic dysentery carriers treated by emetine, with some remarks on the treatment of Lamblia, Blastocystis and E. coli infections. J Trop Med Hyg. 1916;19:29–34. [Google Scholar]
- Dunn LA, Boreham PF. The in-vitro activity of drugs against Blastocystis hominis. J Antimicrob Chemother. 1991;27:507–516. doi: 10.1093/jac/27.4.507. [DOI] [PubMed] [Google Scholar]
- Zierdt CH, Swan JC, Hosseini J. In vitro response of Blastocystis hominis to antiprotozoal drugs. J Protozool. 1983;30:332–334. doi: 10.1111/j.1550-7408.1983.tb02925.x. [DOI] [PubMed] [Google Scholar]
- Moe KT, Singh M, Howe J, Ho LC, Tan SW, Chen XQ, Ng GC, Yap EH. Experimental Blastocystis hominis infection in laboratory mice. Parasitol Res. 1997;83:319–325. doi: 10.1007/s004360050256. [DOI] [PubMed] [Google Scholar]
- Lantsman Y, Tan KS, Morada M, Yarlett N. Biochemical characterization of a mitochondrial-like organelle from Blastocystis sp. subtype 7. Microbiology. 2008;154:2757–2766. doi: 10.1099/mic.0.2008/017897-0. [DOI] [PubMed] [Google Scholar]
- Upcroft P, Upcroft JA. Drug targets and mechanisms of resistance in the anaerobic protozoa. Clin Microbiol Rev. 2001;14:150–164. doi: 10.1128/CMR.14.1.150-164.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabensteiner E, Liebhart D, Arshad N, Hess M. Antiprotozoal activities determined in vitro and in vivo of certain plant extracts against Histomonas meleagridis, Tetratrichomonas gallinarum and Blastocystis sp. Parasitol Res. 2008;103:1257–1264. doi: 10.1007/s00436-008-1122-1. [DOI] [PubMed] [Google Scholar]
- Zierdt CH. Pathogenicity of Blastocystis hominis. J Clin Microbiol. 1991;29:662–663. doi: 10.1128/jcm.29.3.662-663.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MJ. Pathogenicity of Blastocystis hominis. J Clin Microbiol. 1991;29:2089. doi: 10.1128/jcm.29.9.2089-.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenblatt JE. Blastocystis hominis. J Clin Microbiol. 1990;28:2379–2380. doi: 10.1128/jcm.28.10.2379-2380.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babb RR, Wagener S. Blastocystis hominis–a potential intestinal pathogen. West J Med. 1989;151:518–519. [PMC free article] [PubMed] [Google Scholar]
- Cotruvo JA, et al. Waterborne Zoonoses: Identification, Causes and Control. IWA Publishing; 2004. [Google Scholar]
- Al-Binali AM, Bello CS, El-Shewy K, Abdulla SE. The prevalence of parasites in commonly used leafy vegetables in South Western, Saudi Arabia. Saudi Med J. 2006;27:613–616. [PubMed] [Google Scholar]
- Suresh K, Smith HV, Tan TC. Viable blastocystis cysts in Scottish and Malaysian sewage samples. Appl Environ Microbiol. 2005;71:5619–5620. doi: 10.1128/AEM.71.9.5619-5620.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basualdo J, Pezzani B, De Luca M, Cordoba A, Apezteguia M. Screening of the municipal water system of La Plata, Argentina, for human intestinal parasites. Int J Hyg Environ Health. 2000;203:177–182. doi: 10.1078/S1438-4639(04)70025-5. [DOI] [PubMed] [Google Scholar]
- Suresh K, Smith H. Comparison of methods for detecting Blastocystis hominis. Eur J Clin Microbiol Infect Dis. 2004;23:509–511. doi: 10.1007/s10096-004-1123-7. [DOI] [PubMed] [Google Scholar]
- Ehlin AG, Montgomery SM, Ekbom A, Pounder RE, Wakefield AJ. Prevalence of gastrointestinal diseases in two British national birth cohorts. Gut. 2003;52:1117–1121. doi: 10.1136/gut.52.8.1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen GC, Tuskey A, Dassopoulos T, Harris ML, Brant SR. Rising hospitalization rates for inflammatory bowel disease in the United States between 1998 and 2004. Inflamm Bowel Dis. 2007;13:1529–1535. doi: 10.1002/ibd.20250. [DOI] [PubMed] [Google Scholar]
- Smyth CM, Picha SB, Rathore O, Deasy J, Patchett SE, Murray FE. Increasing rates and changing patterns of hospital admissions for patients with inflammatory bowel disease in Ireland: 1996–2001. Ir J Med Sci. 2005;174:28–32. doi: 10.1007/BF03168978. [DOI] [PubMed] [Google Scholar]
- Armitage E, Drummond HE, Wilson DC, Ghosh S. Increasing incidence of both juvenile-onset Crohn's disease and ulcerative colitis in Scotland. Eur J Gastroenterol Hepatol. 2001;13:1439–1447. doi: 10.1097/00042737-200112000-00007. [DOI] [PubMed] [Google Scholar]
- Castro M, Papadatou B, Baldassare M, Balli F, Barabino A, Barbera C, Barca S, Barera G, Bascietto F, Berni Canani R, et al. Inflammatory bowel disease in children and adolescents in Italy: Data from the pediatric national IBD register (1996–2003) Inflamm Bowel Dis. 2008;14:1246–1252. doi: 10.1002/ibd.20470. [DOI] [PubMed] [Google Scholar]
- Bernstein CN, Nabalamba A. Hospitalization, surgery, and readmission rates of IBD in Canada: a population-based study. Am J Gastroenterol. 2006;101:110–118. doi: 10.1111/j.1572-0241.2006.00330.x. [DOI] [PubMed] [Google Scholar]
- Loftus CG, Loftus EV, Jr, Harmsen WS, Zinsmeister AR, Tremaine WJ, Melton LJ, 3rd, Sandborn WJ. Update on the incidence and prevalence of Crohn's disease and ulcerative colitis in Olmsted County, Minnesota, 1940–2000. Inflamm Bowel Dis. 2007;13:254–261. doi: 10.1002/ibd.20029. [DOI] [PubMed] [Google Scholar]
- Tuteja AK, Talley NJ, Gelman SS, Alder SC, Thompson C, Tolman K, Hale DC. Development of functional diarrhea, constipation, irritable bowel syndrome, and dyspepsia during and after traveling outside the USA. Dig Dis Sci. 2008;53:271–276. doi: 10.1007/s10620-007-9853-x. [DOI] [PubMed] [Google Scholar]
- Irving PM, Gibson PR. Infections and IBD. Nat Clin Pract Gastroenterol Hepatol. 2008;5:18–27. doi: 10.1038/ncpgasthep1004. [DOI] [PubMed] [Google Scholar]
- Klement E, Lysy J, Hoshen M, Avitan M, Goldin E, Israeli E. Childhood Hygiene Is Associated With the Risk for Inflammatory Bowel Disease: A Population-Based Study. Am J Gastroenterol. 2008 doi: 10.1111/j.1572-0241.2008.01905.x. [DOI] [PubMed] [Google Scholar]
- Long HY, Handschack A, Konig W, Ambrosch A. Blastocystis hominis modulates immune responses and cytokine release in colonic epithelial cells. Parasitol Res. 2001;87:1029–1030. doi: 10.1007/s004360100494. [DOI] [PubMed] [Google Scholar]
- Puthia MK, Sio SW, Lu J, Tan KS. Blastocystis ratti induces contact-independent apoptosis, F-actin rearrangement, and barrier function disruption in IEC-6 cells. Infect Immun. 2006;74:4114–4123. doi: 10.1128/IAI.00328-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuckerman MJ, Watts MT, Ho H, Meriano FV. Blastocystis hominis infection and intestinal injury. Am J Med Sci. 1994;308:96–101. doi: 10.1097/00000441-199408000-00006. [DOI] [PubMed] [Google Scholar]
- Ok UZ, Cirit M, Uner A, Ok E, Akcicek F, Basci A, Ozcel MA. Cryptosporidiosis and blastocystosis in renal transplant recipients. Nephron. 1997;75:171–174. doi: 10.1159/000189527. [DOI] [PubMed] [Google Scholar]
- Guglielmetti P, Cellesi C, Figura N, Rossolini A. Family outbreak of Blastocystis hominis associated gastroenteritis. Lancet. 1989;2:1394. doi: 10.1016/S0140-6736(89)92000-X. [DOI] [PubMed] [Google Scholar]
- Bratt DE, Tikasingh ES. Blastocystis hominis in two children of one family. West Indian Med J. 1990;39:57–58. [PubMed] [Google Scholar]
- Sun T, Katz S, Tanenbaum B, Schenone C. Questionable clinical significance of Blastocystis hominis infection. Am J Gastroenterol. 1989;84:1543–1547. [PubMed] [Google Scholar]
- Leder K, Hellard ME, Sinclair MI, Fairley CK, Wolfe R. No correlation between clinical symptoms and Blastocystis hominis in immunocompetent individuals. J Gastroenterol Hepatol. 2005;20:1390–1394. doi: 10.1111/j.1440-1746.2005.03868.x. [DOI] [PubMed] [Google Scholar]
- Chen TL, Chan CC, Chen HP, Fung CP, Lin CP, Chan WL, Liu CY. Clinical characteristics and endoscopic findings associated with Blastocystis hominis in healthy adults. Am J Trop Med Hyg. 2003;69:213–216. [PubMed] [Google Scholar]
- Junod C. [Blastocystis hominis: a common commensal in the colon. Study of prevalence in different populations of Paris] Presse Med. 1995;24:1684–1688. [PubMed] [Google Scholar]
- Markell EK. Is there any reason to continue treating Blastocystis infections? Clin Infect Dis. 1995;21:104–105. doi: 10.1093/clinids/21.1.104. [DOI] [PubMed] [Google Scholar]
- Markell EK, Udkow MP. Blastocystis hominis. West J Med. 1990;152:721. [PMC free article] [PubMed] [Google Scholar]
- Markell EK, Udkow MP. Blastocystis hominis: pathogen or fellow traveler? Am J Trop Med Hyg. 1986;35:1023–1026. doi: 10.4269/ajtmh.1986.35.1023. [DOI] [PubMed] [Google Scholar]
- Udkow MP, Markell EK. Blastocystis hominis: prevalence in asymptomatic versus symptomatic hosts. J Infect Dis. 1993;168:242–244. doi: 10.1093/infdis/168.1.242. [DOI] [PubMed] [Google Scholar]
- Zhang HW, Li W, Yan QY, He LJ, Su YP. [Impact of blastocystis hominis infection on ultrastructure of intestinal mucosa in mice] Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi. 2006;24:187–191. [PubMed] [Google Scholar]
- Puthia MK, Vaithilingam A, Lu J, Tan KS. Degradation of human secretory immunoglobulin A by Blastocystis. Parasitol Res. 2005;97:386–389. doi: 10.1007/s00436-005-1461-0. [DOI] [PubMed] [Google Scholar]
- Nigro L, Larocca L, Massarelli L, Patamia I, Minniti S, Palermo F, Cacopardo B. A placebo-controlled treatment trial of Blastocystis hominis infection with metronidazole. J Travel Med. 2003;10:128–130. doi: 10.2310/7060.2003.31714. [DOI] [PubMed] [Google Scholar]
- Kaya S, Cetin ES, Aridogan BC, Arikan S, Demirci M. Pathogenicity of Blastocystis hominis, a clinical reevaluation. Turkiye Parazitol Derg. 2007;31:184–187. [PubMed] [Google Scholar]
- Leelayoova S, Rangsin R, Taamasri P, Naaglor T, Thathaisong U, Mungthin M. Evidence of waterborne transmission of Blastocystis hominis. Am J Trop Med Hyg. 2004;70:658–662. [PubMed] [Google Scholar]
- Tan TC, Suresh KG. Amoeboid form of Blastocystis hominis – a detailed ultrastructural insight. Parasitol Res. 2006;99:737–742. doi: 10.1007/s00436-006-0214-z. [DOI] [PubMed] [Google Scholar]
- Tan TC, Suresh KG, Thong KL, Smith HV. PCR fingerprinting of Blastocystis isolated from symptomatic and asymptomatic human hosts. Parasitol Res. 2006;99:459–465. doi: 10.1007/s00436-006-0177-0. [DOI] [PubMed] [Google Scholar]
- Yao FR, Qiao JY, Zhao Y, Zhang X, Yang JH, Li XQ. [Experimental infection of mice with Blastocystis hominis] Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi. 2005;23:444–448. [PubMed] [Google Scholar]
- Allason-Jones E, Mindel A, Sargeaunt P, Williams P. Entamoeba histolytica as a commensal intestinal parasite in homosexual men. N Engl J Med. 1986;315:353–356. doi: 10.1056/NEJM198608073150603. [DOI] [PubMed] [Google Scholar]
- Anand BS, Tuteja AK, Kaur M, Alam SM, Aggarwal DS, Mehta SP, Baveja UK. Entamoeba histolytica cyst passers. Clinical profile and spontaneous eradication of infection. Dig Dis Sci. 1993;38:1825–1830. doi: 10.1007/BF01296105. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix A – Additional charts.
Appendix B – Characteristics of known gastrointestinal pathogens.
List of Blastocystis studies categorized by researcher finding and geographic location.