Abstract
Background
It has been hypothesized that ambient particulate air pollution is able to modify the autonomic nervous control of the heart, measured as heart rate variability (HRV). Previously we reported heterogeneous associations between particulate matter with aerodynamic diameter < 2.5 μm (PM2.5) and HRV across three study centers.
Objectives
We evaluated whether exposure misclassification, effect modification by medication, or differences in particle composition could explain the inconsistencies.
Methods
Subjects with coronary heart disease visited clinics biweekly in Amsterdam, the Netherlands; Erfurt, Germany; and Helsinki, Finland for 6–8 months. The standard deviation (SD) of NN intervals on an electrocardiogram (ECG; SDNN) and high frequency (HF) power of HRV was measured with ambulatory ECG during paced breathing. Outdoor levels of PM2.5 were measured at a central site. In Amsterdam and Helsinki, indoor and personal PM2.5 were measured during the 24 hr preceding the clinic visit. PM2.5 was apportioned between sources using principal component analyses. We analyzed associations of indoor/personal PM2.5, elements of PM2.5, and source-specific PM2.5 with HRV using linear regression.
Results
Indoor and personal PM2.5 were not associated with HRV. Increased outdoor PM2.5 was associated with decreased SDNN and HF at lags of 2 and 3 days only among persons not using beta-blocker medication. Traffic-related PM2.5 was associated with decreased SDNN, and long-range transported PM2.5 with decreased SDNN and HF, most strongly among persons not using beta blockers. Indicators for PM2.5 from traffic and long-range transport were also associated with decreased HRV.
Conclusions
Our results suggest that differences in the composition of particles, beta-blocker use, and obesity of study subjects may explain some inconsistencies among previous studies on HRV.
Keywords: absorbance, air pollution, cardiovascular health, elements of PM2.5, heart rate variability, medication, PM2.5, source-specific particulate matter
Increased cardiovascular mortality and morbidity have been reported in association with increases in daily ambient levels of particulate matter (PM) in epidemiologic studies (Analitis et al. 2006; Le Tertre et al. 2002; Samet et al. 2000). However, it is not known which constituents of particles are responsible for the effects associated with particle mass. The source of particles defines their composition. Recent epidemiologic studies suggest that particles from combustion sources are especially harmful (Laden et al. 2000; Lanki et al. 2006). Transition metals and organic carbon compounds were shown to be toxic in a toxicologic study (Pagan et al. 2003). These can be found in abundance in combustion particles.
The relative importance of different pathways from particle exposure to effects on the cardiovascular system is not clear, but exposure to particles has been associated both with increased systemic inflammation and changes in autonomic nervous control of the heart (Brook et al. 2004). The latter is most often measured indirectly as heart rate variability (HRV) (Task Force 1996). A decreased overall HRV has proven to be a strong independent predictor of cardiac mortality in subjects with existing cardiovascular disease (La Rovere et al. 1998; Nolan et al. 1998). Several studies have shown decreased indices of HRV on days with increased outdoor levels of respirable particles [aerodynamic diameter < 10 μm (PM10)] (Liao et al. 2004; Lipsett et al. 2006) and fine particles [< 2.5 μm (PM2.5)] (Holguín et al. 2003; Schwartz et al. 2005).
In the Exposure and Risk Assessment for Fine and Ultrafine Particles in Ambient Air (ULTRA) study, levels of outdoor air pollution were monitored for 6–8 months in 1998–1999 in three European cities. At the same time, panels of patients with coronary heart disease were followed up with measurements of HRV. We previously reported that the levels of ultrafine particles (PM < 0.1 μm) were associated with changes in the balance between sympathetic and vagal nervous input to the heart (Timonen et al. 2006). However, PM2.5 in particular showed different associations with HRV in the different study centers. In Helsinki, Finland, elevated concentrations of PM2.5 were associated with decreased high frequency (HF) and increased low frequency (LF)/HF ratio, whereas the opposite was true in Erfurt, Germany. No such associations were observed in Amsterdam, the Netherlands.
In the present article, we evaluate whether exposure misclassification, effect modification by medication, or variable particle composition could explain these inconsistencies. Personal and indoor PM2.5 were measured in Amsterdam and Helsinki to obtain more accurate estimates of exposure. Possible effect modification by beta-blocker (β-adrenergic antagonist) medication or obesity was evaluated in light of the limited number of earlier studies (Chen et al. 2007; Park et al. 2005; Wheeler et al. 2006). For comparison, we also tested other medication possibly modifying the effect of particulate air pollution on HRV.
Finally, we linked source-specific PM2.5 with HRV to evaluate the importance of particle composition for cardiovascular effects of PM.
Methods
Heart rate variability was measured at biweekly clinic visits in panels of elderly subjects with coronary heart disease in Amsterdam, Erfurt, and Helsinki in 1998–1999. In Amsterdam, 37 panelists were followed for 8 months, and in Erfurt and Helsinki, 47 panelists were followed for 6 months. The visits of every subject were always scheduled for the same weekday and for the same time. The medication of the subjects was not changed for the clinic visits. Outdoor levels of PM2.5 were measured concurrently with the visits at one central site in each city. In Helsinki and Amsterdam, indoor and personal measurements of PM2.5 were also performed during the 24 hr preceding the clinic visit. All measurements in the study were performed according to standard operating procedures (Brunekreef et al. 2005; Pekkanen et al. 2000).
The main inclusion criteria for the study were a self-report of a physician-diagnosed coronary heart disease, being a nonsmoker, and age = 50 years. Ethical committees in each study center approved the study protocol. A written informed consent was obtained from all subjects.
At the clinic visits, HRV was recorded with an ambulatory electrocardiogram (ECG) recorder (Medilog MR 63 recorder; Oxford Instruments, Abington, UK) using a standardized protocol (Timonen et al. 2006). Breathing frequency strongly affects HRV, and for that reason, HRV recorded during a 5-min period of paced breathing in supine position (frequency 0.2 Hz; 2.5-sec inhalation and 2.5-sec exhalation) has been used for the analyses. Two-channel ambulatory ECG recordings were performed with analog ambulatory ECG recorders (Medilog MR 63 recorder; Oxford Instruments) using standard electrode position for leads V1 and V5. The recordings were analyzed with ambulatory ECG analysis software (Exel Medilog II V7.5 system; Oxford Instruments). The recordings were digitized with a sampling rate of 128 Hz. The software used an interpolation algorithm to refine the R wave fiducial point and to improve the resolution in R-peak detection. Details of the analyses have been published previously (Tarkiainen et al. 2005; Timonen et al. 2006).
We were mainly interested in explaining the heterogeneous results in the main end points [the SD of NN intervals (SDNN), HF, and LF/HF ratio] of a previous ULTRA paper (Timonen et al. 2006). Therefore, we used two common indices of HRV in the present analyses: SDNN, which is a time-domain measure of overall HRV, and HF power (0.15–0.4 Hz) of HRV, which is a frequency domain measure believed to reflect mainly the vagal (parasympathetic) part of the autonomic nervous input to the heart. HF is highly correlated with r-MSSD, a commonly used time-domain variable (Task Force 1996). The LF/HF ratio was left out of the paper, because the interpretation and physiological basis are more controversial.
Information on physician-administrated daily medication was collected at baseline visit. Medication categories tested for effect modification were beta-blockers, calcium (Ca2+) channel blockers, statins, angiotensin-converting enzyme (ACE) inhibitors, angiotensin receptor blockers, and acetylsalicylic acid (ASA). Antiarrhythmic medication was not included in the analyses because of limited use among study participants (7%).
Harvard impactors (BGI, Inc., Waltham, MA, USA) were used to collect filter samples of outdoor PM2.5; GK2.05 cyclones and battery-operated AFC400S pumps (BGI, Inc.) were used for the collection of personal PM2.5 samples. The filters were weighted to determine mass of PM2.5, and reflectance was measured with a reflectometer (Model 43, Diffusion Systems Ltd., London, UK). The reflectance was transformed into absorbance [absorption coefficient (ABS)], which is an indicator for elemental carbon. Finally, elemental composition of the samples was determined using energy-dispersive X-ray fluorescence spectrometry. All methods have been described in detail in previous papers (Brunekreef et al. 2005; de Hartog et al. 2005; Janssen et al. 2000, 2005).
We used principal-component analysis and multivariate linear regression to apportion PM2.5 mass to different sources (Vallius et al. 2005), thereby obtaining estimates of daily source-specific PM2.5 concentrations. Besides components of PM2.5 (elemental concentrations and absorbance), daily data on ultrafine (diameter < 0.1 μm) and accumulation mode particles (0.1–1.0 μm), nitrogen dioxide, and sulfur dioxide were used to identify sources.
We identified four to six main source categories in each city: local traffic (with contribution from other local combustion sources), long-range transported (secondary) air pollution, industry, crustal, oil combustion, and salt (Vallius et al. 2005).
We analyzed data using the SAS statistical package and mixed models (PROC MIXED) (SAS Institute Inc., Cary, NC, USA) taking into account repeated observations and assuming constant correlation between observations within a subject. A basic model was first built without including particulate air pollution in the model. Criteria for building the basic model were Akaike’s information criterion and covariate-response plots. The same basic models as in the previous paper have been used (Timonen et al. 2006). Lag 0 was defined as the 24-hr period from noon of the day of the clinic visit to noon of the previous day, lag 1 was the previous 24-hr period, and so on. In Amsterdam, the model included linear terms for time trend, temperature (lag 2), relative humidity (lag 3), and barometric pressure. In Erfurt, the model included linear terms for time trend, relative humidity (lag 3), and barometric pressure (lag 2). Temperature (lag 3) was modeled with linear, squared, and cubic terms. The basic model for Helsinki included linear terms for time trend, relative humidity (lag 1), and barometric pressure (lag 1). Temperature (lag 3) was modeled with linear and squared terms. In all cities, the model included weekday as a categorical variable. Results were insensitive to alternative model specifications.
For comparison of the effects of outdoor, indoor, and personal PM2.5 on HRV, only the days with all three types of measurements were included in the analyses. We analzed associations of source-specific PM2.5 with HRV using multipollutant models that included at the same time all identified sources and the fraction. Multipollutant unidentified PM2.5 models were not used for elements of PM2.5 because of high intercorrelations. We analyzed data only for the elements that are either indicators for the PM2.5 sources or that have been found harmful in toxicologic studies. The indicators were chosen based on the elemental profiles of sources (Vallius et al. 2005): absorbance for local traffic; sulfur for long-range transported particles; vanadium for oil combustion (not used for Erfurt because oil source was not identified there, and > 50% of concentrations were below detection limit); Ca for soil particles; and chloride for salt particles (not in Erfurt). Elements considered because of potential toxicity were the transition metals copper, iron, and zinc.
HF was log-transformed for the analyses, and the effect of particulate air pollution on the end point was estimated as percent change: [e(β × IQR)−1] × 100%, where β is the estimated regression coefficient and IQR is the interquartile range. Effect estimates for the elements are presented for increases that are close to study mean interquartile ranges (IQRs)—the differences between the 25th and 75th percentiles of the exposure distributions. Pooled effect estimates were calculated as a weighted average of the center-specific estimates using the inverse of center-specific variances as weights. The heterogeneity of effect estimates between centers was tested with a chi-square test (Normand 1999).
Effect of extreme source-specific PM2.5 values on the results was evaluated by excluding at each lag the concentrations that were more than three times the IQR.
Results
There were 424 clinical visits in Amsterdam, 491 in Erfurt, and 519 in Helsinki. Although special care was given to attachment of the electrodes, some ECG recordings were unsuccessful. There were 366 successful recordings (from 33 patients) in Amsterdam, 432 (44) in Erfurt, and 468 (45) in Helsinki.
In Helsinki, the proportion of males and females was almost equal, but in Amsterdam the panel contained mostly males and in Erfurt almost exclusively males (Table 1). Obesity was common in Helsinki, where one-third of the study subjects were obese (17 persons). There were clearly fewer obese persons in Amsterdam and Erfurt (7 in both). The most commonly used medication was ASA. About two-thirds of the study subjects in Erfurt and Helsinki had daily beta-blocker medication, whereas only about one-third of the subjects were on medication in Amsterdam. Except for SDNN in Amsterdam, HRV indices were lower among beta-blocker users than among nonusers.
Table 1.
Characteristics of three study panels.
| Characteristic | Amsterdam (n = 33)a | Erfurt (n = 44)a | Helsinki (n = 45)a |
|---|---|---|---|
| Sex/female [no. (%)] | 11 (34) | 4 (9) | 21 (47) |
| Mean age (range) | 70.9 (54–83) | 64.3 (40–78) | 68.2 (54–83) |
| Obeseb [no. (%)] | 7 (19) | 7 (15) | 16 (34) |
| Past myocardial infarction [no. (%)] | 22 (69) | 30 (68) | 27 (60) |
| Angina pectoris [no. (%)] | 21 (66) | 24 (55) | 29 (64) |
| CABG or PTCA conducted [no. (%)] | 17 (53) | 31 (70) | 23 (51) |
| Daily beta-blocker medication [no. (%)] | 13 (39) | 34 (77) | 31 (69) |
| Ca2+ channel blockers [no. (%)] | 11 (30) | 18 (38) | 13 (28) |
| Statins [no. (%)] | 12 (32) | 20 (43) | 21 (45) |
| ACE inhibitors and angiotensin receptor blockers [no. (%)] | 12 (32) | 25 (53) | 10 (21) |
| ASA [no. (%)] | 22 (59) | 36 (77) | 36 (77) |
| Meanc SDNN (SD), msec | |||
| Beta-blocker users | 46.7 (17.8) | 29.9 (8.8) | 35.3 (10.7) |
| Nonusers | 40.0 (11.9) | 41.2 (12.1) | 38.8 (11.7) |
| Meanc HF (SD), msec2 | |||
| Beta-blocker users | 414 (289) | 280 (237) | 600 (376) |
| Nonusers | 504 (307) | 547 (396) | 629 (409) |
Abbreviations: CABG, coronary artery bypass graft; PTCA, percutaneous transluminal coronary angioplasty.
Number of patients available for analyses.
Obese = body mass index ≥ 30 kg/m2.
Average of individual means and SDs.
Outdoor levels of PM2.5 were lower in Helsinki than in Amsterdam and Erfurt (Table 2). In Helsinki, about half of PM2.5 was of secondary origin, that is, could be considered long-range transported; in Amsterdam and Erfurt, this was about one-third. Industrial sources of PM2.5 were not identified in Helsinki (Vallius et al. 2005). Oil combustion and salt as sources of PM2.5 were not identified in Erfurt, and the indicator elements for these sources have not been included.
Table 2.
Daily outdoor levels of PM2.5, its components, and temperature at central measurement sites in three cities.
| Amsterdam (n = 223)
|
Erfurt (n = 156)
|
Helsinki (n = 164)
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| p25 | p50 | P75 | p95 | p25 | p50 | p75 | p95 | p25 | p50 | p75 | p95 | |
| PM2.5 (μg/m3) | 10.4 | 16.7 | 23.9 | 47.0 | 10.8 | 16.3 | 26.7 | 62.3 | 8.3 | 10.6 | 15.9 | 25.8 |
| Source-specific PM2.5 (μg/m3) | ||||||||||||
| Local traffic | 3.5 | 6.1 | 9.3 | 20.4 | 4.1 | 7.0 | 10.0 | 18.4 | 1.7 | 2.6 | 3.4 | 6.5 |
| Long-range transported | 0.3 | 5.1 | 11.6 | 21.8 | 3.1 | 5.4 | 9.8 | 31.9 | 2.2 | 5.5 | 9.8 | 15.9 |
| Oil combustion | 0.9 | 1.6 | 3.1 | 5.9 | NA | NA | NA | NA | 0.6 | 1.3 | 2.3 | 4.2 |
| Industry | −2.6 | −0.5 | 3.0 | 9.2 | −3.6 | −1.6 | 2.2 | 24.7 | NA | NA | NA | NA |
| Crustal | 0.7 | 1.4 | 2.1 | 3.6 | 1.8 | 2.7 | 4.8 | 13.8 | 0.0 | 0.4 | 1.1 | 2.2 |
| Salt | 0.1 | 0.2 | 0.8 | 1.8 | NA | NA | NA | NA | 0.3 | 0.8 | 1.2 | 2.4 |
| Absorbance (m−1 × 10−5) | 0.9 | 1.5 | 2.2 | 3.4 | 1.3 | 2.0 | 3.4 | 5.1 | 1.4 | 1.89 | 2.47 | 3.56 |
| Elements (ng/m3) | ||||||||||||
| S | 936 | 1,340 | 2,240 | 3,650 | 600 | 862 | 1,530 | 3,740 | 839 | 1,380 | 2,080 | 3,400 |
| V | 2.5 | 4.1 | 7.8 | 14.7 | NA | NA | NA | NA | 3.2 | 6.69 | 9.8 | 16.4 |
| Zn | 8.7 | 18.2 | 33.9 | 65.2 | 22.6 | 40.2 | 75.1 | 199 | 11.3 | 16.8 | 25.1 | 47.3 |
| Ca | 26.9 | 37.1 | 51.8 | 76.9 | 34.6 | 47.0 | 72.2 | 193 | 22.7 | 32.3 | 47.3 | 87.0 |
| Cl | 33.8 | 116 | 432 | 990 | NA | NA | NA | NA | 8.1 | 36.4 | 102 | 386 |
| Fe | 47.1 | 70.7 | 107 | 175 | 38.6 | 59.9 | 112 | 248 | 39.3 | 66.7 | 100 | 165 |
| Cu | 1.4 | 2.5 | 4.7 | 9.0 | 0.6 | 2.5 | 4.9 | 10.4 | 0.6 | 1.6 | 2.8 | 5.1 |
| Temperature (°C) | 4.4 | 7.9 | 12.3 | 16.9 | −0.1 | 3.9 | 6.7 | 11.2 | −5.0 | −0.6 | 2.5 | 8.8 |
Abbreviations: NA, not available; p25, 25th percentile; p50, 50th percentile (median); p75, 75th percentile; p95, 95th percentile.
PM2.5 (total) correlated most strongly with long-range transported PM2.5, and the correlation with S, the indicator element for this source, was even higher (Table 3). Transition metals Zn, Fe, and Cu correlated highly with absorbance, with the correlation highest for Cu in Amsterdam (r = 0.83) and lowest for Fe in Helsinki (r = 0.49) (data not shown).
Table 3.
Correlation (Spearman’s correlation coefficients.) of total PM2.5 with source-specific PM2.5 and elements at central sites in three cities.
| Source-specific PM2.5 |
Elements of PM2.5 |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Traffic | LRT | Oil | Industry | Crustal | Salt | ABS | S | V | Zn | Ca | Cl | Fe | Cu | |
| PM2.5 | ||||||||||||||
| Amsterdam (n = 223) | 0.50 | 0.62 | 0.18 | 0.27 | −0.15 | 0.04 | 0.73 | 0.84 | 0.27 | 0.81 | 0.04 | 0.14 | 0.68 | 0.63 |
| Erfurt (n = 156) | 0.32 | 0.57 | NA | 0.41 | 0.19 | NA | 0.81 | 0.85 | NA | 0.82 | 0.51 | 0.63 | 0.81 | 0.70 |
| Helsinki (n = 164) | 0.26 | 0.82 | 0.35 | NA | −0.01 | 0.19 | 0.70 | 0.85 | 0.59 | 0.77 | 0.17 | −0.03 | 0.38 | 0.42 |
Abbreviations: NA, not available; LRT, long-range transported.
The medians of individual averages (number of measurements) of outdoor, indoor, and personal PM2.5 in Amsterdam were 21.0 (417), 14.9 (411), and 15.3 (338) μg/m3, respectively. The respective PM2.5 levels in Helsinki were 12.0 (478), 10.2 (503), and 10.0 (336) μg/m3 (Janssen et al. 2000).
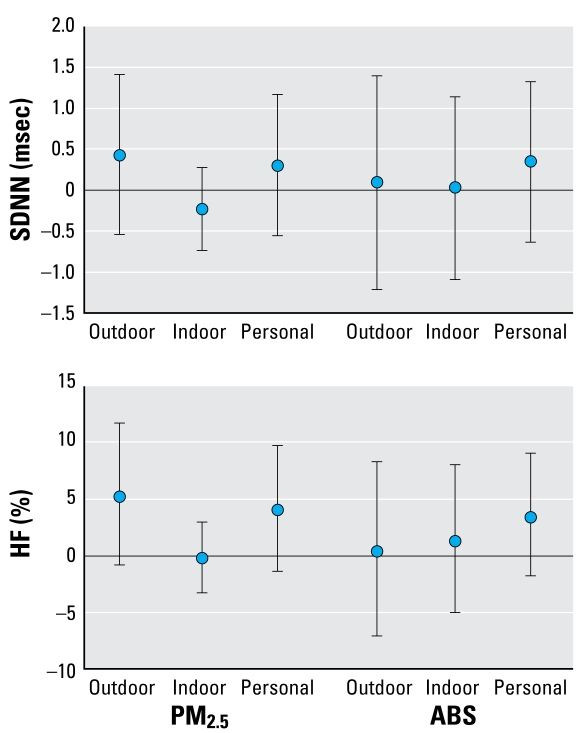
Outdoor, indoor, and personal PM2.5 were not associated with SDNN at lag 0 (Figure 1). Indoor and personal PM2.5 measurements were not available at lags 1, 2, or 3. There was a suggestive positive association of outdoor and personal PM2.5 with HF.
Figure 1.
Pooled effect estimates (95% CIs) for two study panels (Amsterdam and Helsinki) for the association outdoor, indoor, and personal PM2.5at 0-day lag with HRV (SDNN and HF). Effect estimates are calculated for an increase of 10 μg/m3 for PM2.5 and 1 m−1 × 10−5 for absorbance.
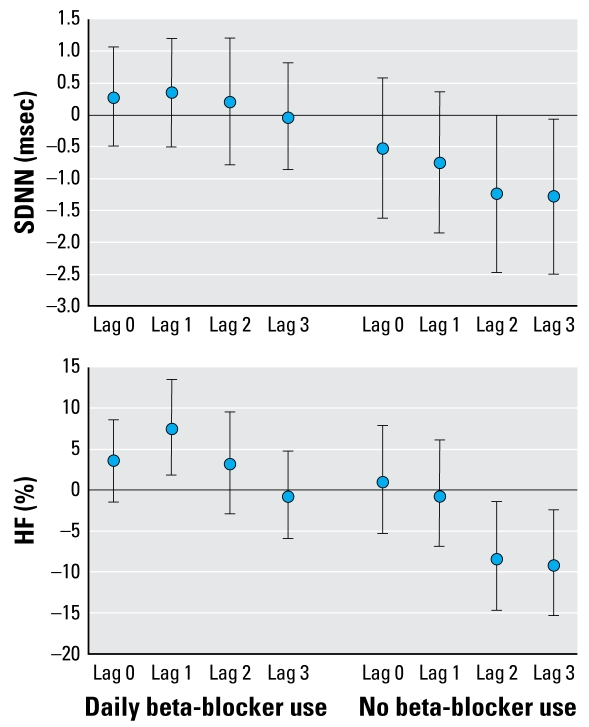
Among study subjects not on daily beta-blocker medication, increased concentrations of PM2.5 were associated with decreased SDNN and HF, especially at longer lags (Figure 2). For this group the city-specific estimates were homogeneous. There was a positive association at single (1-day) lag between PM2.5 and HF among subjects who were on medication.
Figure 2.
Pooled effect estimates (95% CIs) for three study panels for the association of outdoor PM2.5 with HRV (SDNN and HF) stratified by beta-blocker use. Effect estimates are calculated for an increase of 10 μg/m3 for PM2.5
There was no consistent modification of the effects of PM sources by medication other than beta-blockers (results not shown). Those not using ACE inhibitors or angiotensin receptor blockers had more clearly decreased HF in association with long-range transported PM than all subjects [at lag 2: −1.25; 95% confidence interval (CI), −2.09 to −0.41; at lag 3: −1.1; 95% CI, −2.04 to −0.26], but same kind of modifying effect was not observed for other sources or SDNN. On the other hand, those not using statins had decreased HF in association with PM2.5 at a 3-day lag (−6.45; 95% CI, −11.63 to −0.96), but no modifying effect of statins was observed for source-specific PM2.5 or SDNN.
Obesity was not associated with beta-blocker use: 60.0% of obese and 60.4% of non-obese persons used beta-blockers. However, obesity itself seemed to modify the effects of PM2.5. At a 3-day lag, PM 2.5 was associated with SDNN (−1.99; 95% CI, −3.69 to −0.30) and HF (−12.50; 95% CI, −20.1 to −4.24) among obese persons, whereas such an effect was not observed among all subjects. Effects of long-range transported PM2.5 were similarly modified by obesity (results not shown), obviously because of substantial correlation between PM2.5 and long-range transported PM2.5. However, no such effect modification was observed for PM2.5 from traffic or other sources of PM2.5.
Increases in PM2.5 originating from local traffic were consistently associated with decreased SDNN, somewhat more strongly among study subjects not using beta-blockers than in the whole study panel (Table 4). Long-range transported PM2.5 was associated with decreased SDNN and HF at lags 2 and 3 among persons not having daily beta-blocker medication. Among all subjects, there was heterogeneity in the effect estimate for long-range transported PM at a 2-day lag for HF because of negative estimates in Amsterdam (−0.91; 95% CI, −2.02 to 0.22) and Helsinki (−1.92; 95% CI, −3.26 to −0.57) and a positive estimate in Erfurt (0.25; 95% CI, –0.81 to 1.31). There was evidence of the effect of PM2.5 from oil combustion only for SDNN among nonmedicated subjects. Crustal PM2.5 was associated with increased HF irrespective of medication use at lag 2. Associations between 5-day average (lags 0–4) particulate air pollution and HRV were weaker than for individual lags (data not shown).
Table 4.
Pooled effect estimates in three study panels [β (95% CIs)]a for the associations of source-specific PM2.5 with HRV in multipollutant models.b
| SDNN (msec)
|
HF (%)
|
|||
|---|---|---|---|---|
| All subjects | Subjects without beta-blockers | All subjects | Subjects without beta-blockers | |
| Local traffic | ||||
| Lag 0 | −0.05 (−0.26 to 0.15) | 0.11 (−0.23 to 0.44) | 0.11 (−1.05 to 1.28) | 0.31 (−1.65 to 2.30) |
| Lag 1 | −0.12 (−0.36 to 0.12) | −0.27 (−0.59 to 0.05) | 0.43 (−0.91 to 1.79) | −0.21 (−2.16 to 1.77) |
| Lag 2 | −0.28 (−0.57 to 0.01) | −0.45 (−0.90 to 0.01) | −0.13 (−1.74 to 1.50) | −0.67 (−3.34 to 2.07) |
| Lag 3 | −0.20 (−0.45 to 0.06) | −0.35 (−0.69 to 0.00) | −0.64 (−2.03 to 0.78) | −1.43 (−3.40 to 0.58) |
| Long-range transport | ||||
| Lag 0 | 0.00 (−0.10 to 0.09) | −0.03 (−0.19 to 0.14) | 0.12 (−0.43 to 0.67) | −0.18c (−1.13 to 0.77) |
| Lag 1 | −0.04 (−0.14 to 0.06) | 0.00 (−0.15 to 0.16) | 0.19 (−0.38 to 0.77) | 0.06 (−0.86 to 0.99) |
| Lag 2 | −0.05 (−0.17 to 0.07) | −0.11 (−0.30 to 0.07) | −0.69c (−1.35 to −0.02) | −1.06 (−2.14 to 0.03) |
| Lag 3 | 0.00 (−0.13 to 0.12) | −0.20 (−0.39 to −0.01) | −0.54 (−1.23 to 0.15) | −1.98 (−3.07 to −0.88) |
| Oil combustiond | ||||
| Lag 0 | −0.02 (−0.74 to 0.70) | −0.46 (−1.34 to 0.41) | 3.20 (−0.48 to 7.03) | 1.43 (−3.83 to 6.97) |
| Lag 1 | −0.29 (−1.04 to 0.45) | −1.08 (−2.09 to −0.06) | 1.05 (−2.70 to 4.94) | −3.04 (−8.80 to 3.08) |
| Lag 2 | 0.36 (−0.42 to 1.13) | 0.22 (−0.89 to 1.33) | 1.50 (−2.36 to 5.51) | 0.10 (−6.34 to 6.98) |
| Lag 3 | 0.00 (−0.77 to 0.77) | −0.43 (−1.27 to 0.42) | 0.49 (−3.25 to 4.38) | −0.42 (−5.32 to 4.73) |
| Industryd | ||||
| Lag 0 | −0.07 (−0.23 to 0.09) | −0.17 (−0.43 to 0.10) | 0.13 (−0.80 to 1.07) | 0.08 (−1.44 to 1.62) |
| Lag 1 | 0.03 (−0.12 to 0.19) | −0.14 (−0.44 to 0.16) | 0.62 (−0.34 to 1.59) | −0.08 (−1.79 to 1.65) |
| Lag 2 | 0.02 (−0.12 to 0.16) | −0.08 (−0.34 to 0.18) | 0.05 (−0.82 to 0.94) | −1.03 (−2.53 to 0.49) |
| Lag 3 | −0.04 (−0.17 to 0.09) | 0.12 (−0.19 to 0.42) | −0.05 (−0.87 to 0.77) | 0.68 (−0.98 to 2.37) |
| Crustal | ||||
| Lag 0 | −0.02 (−0.36 to 0.31) | −0.05 (−0.84 to 0.75) | 0.01 (−2.07 to 2.15) | 0.80 (−3.47 to 5.26) |
| Lag 1 | 0.11 (−0.35 to 0.56) | 0.07 (−0.97 to 1.11) | 1.57 (−1.28 to 4.50) | 1.93 (−3.86 to 8.06) |
| Lag 2 | 0.18 (−0.37 to 0.73) | 0.35 (−0.82 to 1.52) | 4.72 (1.16 to 8.41) | 5.67 (−1.11 to 12.91) |
| Lag 3 | 0.11 (−0.43 to 0.66) | 0.20 (−1.05 to 1.45) | 0.93 (−2.43 to 4.41) | 2.68 (−4.02 to 9.84) |
| Saltd | ||||
| Lag 0 | 1.07 (−0.66 to 2.80) | −0.03 (−2.61 to 2.55) | 5.20 (−3.83 to 15.08) | 4.33 (−10.46 to 21.56) |
| Lag 1 | −0.19 (−1.92 to 1.55) | −0.64 (−3.29 to 2.00) | −1.43 (−9.86 to 7.78) | 4.33 (−10.68 to 21.87) |
| Lag 2 | −0.33 (−2.13 to 1.47) | −0.44 (−2.88 to 2.00) | −1.06 (−9.69 to 8.38) | −6.55 (−18.85 to 7.62) |
| Lag 3 | 1.47 (−0.28 to 3.22) | 2.17 (−0.07 to 4.41) | 6.70 (−2.30 to 16.52) | 2.74 (−9.65 to 16.83) |
β, effect estimate for an increase of 1 μg/m−3 in source-specific PM2.5.
The number of observations in the analyses was 1,195 for SDNN and 1,183 for HF.
Pooled effect estimates have heterogeneous underlying center-specific effect estimates (significance test < 0.05).
Oil combustion source and salt source of PM2.5 were not identified in Erfurt; industrial source of PM2.5 was not identified in Helsinki; estimates of only two cities pooled.
The fraction of PM2.5 that could not be linked to any particular source category was positively associated at 0-day lag with SDNN (estimate 0.18; 95% CI, 0.00 to 0.35) and HF (1.53; CI, 0.48 to 2.59) among all study subjects, but the association was not evident among subjects not using beta-blockers. The positive association between unidentified PM2.5 fraction and SDNN disappeared when extreme source-specific PM2.5 concentrations were excluded from the analyses. Overall, exclusions of extreme values did not change the interpretation of the results. After exclusion, the city-specific estimates were no longer heterogeneous for the association of long-range transported PM2.5 with HF at lag 2 among all study centers.
Among persons not having daily beta-blocker medication, increases in absorbance (local traffic) and S (long-range transport) were consistently associated with decreased SDNN and HF (Table 5). The associations between V (oil combustion) and HRV were less consistent, and for the other source indicators there was no evidence of an effect. However, for the transition metals (Cu, Fe, and Zn) included because of potential toxicity, there was some evidence of negative associations with HRV at longer lags.
Table 5.
Pooled effect estimates [β (95% CIs)]a in three study panels for the associations of elements of PM with HRV among study subjects in single-pollutant models.
| SDNN (msec)
|
HF (%)
|
|||
|---|---|---|---|---|
| All subjects | Subjects without beta-blockers | All subjects | Subjects without beta-blockers | |
| ABS | ||||
| Lag 0 | −0.54 (−1.39 to 0.31) | −0.64 (−2.25 to 0.97) | 0.45 (−4.48 to 5.64) | −2.54 (−11.15 to 6.91) |
| Lag 1 | −0.52 (−1.46 to 0.41) | −1.59 (−3.11 to −0.06) | 2.91 (−2.54 to 8.67) | −4.94 (−13.04 to 3.91) |
| Lag 2 | −0.78 (−1.72 to 0.16) | −1.36 (−2.99 to 0.27) | −1.42 (−6.76 to 4.22) | −7.13 (−15.51 to 2.08) |
| Lag 3 | −0.31 (−1.23 to 0.62) | −1.44 (−3.15 to 0.27) | −2.57 (−7.75 to 2.90) | −7.83 (−16.27 to 1.45) |
| S | ||||
| Lag 0 | −0.25 (−1.06 to 0.55) | −0.71 (−1.98 to 0.56) | 0.74 (−3.76 to 5.46) | −2.70 (−9.71 to 4.84) |
| Lag 1 | −0.51 (−1.36 to 0.33) | −0.76 (−1.99 to 0.47) | 0.25b (−4.42 to 5.14) | −3.61 (−10.36 to 3.64) |
| Lag 2 | −0.43 (−1.39 to 0.52) | −1.44 (−2.84 to −0.04) | −4.78b (−9.69 to 0.40) | −10.56 (−17.63 to −2.87) |
| Lag 3 | 0.10 (−0.88 to 1.08) | −1.54 (−3.02 to −0.06) | −4.02 (−9.01 to 1.25) | −13.05 (−20.18 to −5.29) |
| Vc | ||||
| Lag 0 | −0.12 (−1.14 to 0.91) | −0.46 (−1.85 to 0.93) | 4.58 (−0.89 to 10.36) | 3.68 (−4.72 to 12.82) |
| Lag 1 | −0.66 (−1.73 to 0.41) | −1.97 (−3.56 to −0.39) | 0.73 (−4.74 to 6.53) | −6.24 (−14.71 to 3.07) |
| Lag 2 | 0.40 (−0.66 to 1.46) | −0.16b (−1.72 to 1.39) | 1.40 (−4.02 to 7.13) | −4.58 (−12.97 to 4.61) |
| Lag 3 | 0.04 (−0.99 to 1.07) | −0.43 (−1.78 to 0.91) | −1.92 (−7.00 to 3.45) | −2.09 (−9.64 to 6.09) |
| Zn | ||||
| Lag 0 | −0.19 (−0.79 to 0.41) | −0.69 (−1.83 to 0.46) | 1.68 (−1.97 to 5.46) | 0.40 (−5.91 to 7.13) |
| Lag 1 | 0.12 (−0.55 to 0.79) | −0.78 (−2.11 to 0.54) | 3.85 (−0.26 to 8.13) | 0.13 (−6.92 to 7.72) |
| Lag 2 | 0.06 (−0.58 to 0.70) | −0.92 (−2.20 to 0.37) | 2.28 (−1.71 to 6.43) | −3.78 (−10.41 to 3.35) |
| Lag 3 | −0.13 (−0.72 to 0.46) | −0.28 (−1.53 to 0.96) | −1.43 (−4.95 to 2.22) | −6.41 (−12.60 to 0.22) |
| Ca | ||||
| Lag 0 | −0.23 (−0.85 to 0.38) | −0.72 (−2.17 to 0.73) | −0.77 (−4.50 to 3.10) | −0.37 (−8.12 to 8.04) |
| Lag 1 | 0.27 (−0.58 to 1.11) | −0.47 (−2.16 to 1,21) | 3.39 (−1.80 to 8.86) | 2.10 (−7.18 to 12.31) |
| Lag 2 | 0.62 (−0.36 to 1.60) | 0.35 (−1.47 to 2.18) | 7.89 (1.70 to 14.46) | 5.60 (−4.98 to 17.35) |
| Lag 3 | 0.03 (−0.93 to 1.00) | 0.01 (−2.03 to 2.05) | 0.61 (−5.01 to 6.56) | −0.01 (−10.58 to 11.81) |
| Clc | ||||
| Lag 0 | 0.25 (−0.25 to 0.76) | 0.07 (−0.48 to 0.61) | 2.36 (−0.15 to 4.94) | 2.34 (−1.00 to 5.79) |
| Lag 1 | 0,14 (−0.39 to 0.67) | 0.00 (−0.63 to 0.64) | 1.13 (−1.48 to 3.81) | 1.46 (−2.39 to 5.46) |
| Lag 2 | 0.38 (−0.12 to 0.88) | 0.32 (−0.22 to 0.85) | 1.40 (−1.06 to 3.94) | 1.71 (−1.56 to 5.08) |
| Lag 3 | 0.31 (−0.17 to 0.80) | 0.37 (−0.13 to 0.87) | 1.21 (−1.15 to 3.61) | 0.81 (−2.21 to 3.91) |
| Fe | ||||
| Lag 0 | −0.32 (−1.25 to 0.61) | −0.32 (−1.94 to 1.29) | 0.72 (−4.49 to 6.22) | −0.12 (−9.19 to 9.86) |
| Lag 1 | 0.15 (−1.00 to 1.30) | −1.09 (−2.85 to 0.67) | 6.69 (0.11 to 13.69) | 0.13 (−9.70 to 11.04) |
| Lag 2 | −0.44 (−1.72 to 0.84) | −1.51 (−3.58 to 0.56) | 1.50 (−5.52 to 9.04) | −3.31 (−14.26 to 9.05) |
| Lag 3 | −0.44 (−1.67 to 0.79) | −1.77 (−3.86 to 0.32) | −3.45 (−9.90 to 3.46) | −9.93 (−20.26 to 1.72) |
| Cu | ||||
| Lag 0 | −0.18 (−0.73 to 0.36) | −0.29 (−1.26 to 0.68) | 1.56 (−1.65 to 4.87) | −0.97 (−6.40 to 4.77) |
| Lag 1 | −0.08 (−0.74 to 0.57) | −0.20 (−1.19 to 0.78) | 3.00 (−0.85 to 7.00) | 2.34 (−3.49 to 8.52) |
| Lag 2 | −0.43 (−1.10 to 0.24) | −1.55 (−2.71 to −0.39) | 1.71 (−2.30 to 5.88) | −4.16 (−10.53 to 2.67) |
| Lag 3 | 0.12 (−0.56 to 0.80) | −0.54 (−1.59 to 0.51) | −1.97 (−5.76 to 1.98) | −4.41 (−9.91 to 1.43) |
β, effect estimate, calculated for an increase of 1 m−1 × 10−5 in absorbance, 1 μg/m3 in S, 4 ng/m3 in V, 30 ng/m3 in Ca and Zn, 100 ng/m3 in Cl, 70 ng/m3 in Fe, and 2 ng/m3 in Cu.
Pooled effect estimates have heterogeneous underlying center-specific effect estimates (significance test < 0.05).
V and Cl were not used in Erfurt; estimates of only two cities pooled.
Discussion
In this panel study conducted among persons with coronary heart disease in three European cities, personal, indoor, or outdoor PM2.5 measured during the 24 hr preceding clinic visit (lag 0) were not associated with HRV. However, at 2- and 3-day lags, we observed that daily increases in outdoor levels of PM2.5 were associated with decreased HRV, but only among persons not on beta-blocker medication. When we linked source-specific PM2.5to HRV, we observed increases in traffic-related PM2.5 to be associated with decreased SDNN, especially among persons who were not on beta-blocker medication. Daily increases in the long-range transported PM2.5 were associated both with decreased HF and SDNN, more strongly or exclusively among nonmedicated persons. In separate analyses, indicator elements for these two sources, absorbance and S, were also negatively associated with HRV among persons not on medication. There was also evidence for a negative association of transition metals with HRV.
We reported previously that outdoor levels of PM2.5 were not consistently associated with HRV in the three study panels (Timonen et al. 2006). However, people spend most of their time indoors, and persons with compromised health, like the panel members in our study, even more so (Brunekreef 2005). Consequently, outdoor levels of particulate air pollution measured at a central site may not be perfect proxies for variation in personal PM exposure. However, we did not find personal or indoor PM2.5 to be associated with decreased HRV. Unfortunately, we had only personal and indoor measurements in the 24 hr preceding the clinic visit, and PM2.5 mass and composition during that time period were not associated with HRV. Our observation thus indicates only that the lack of association at 0-day lag for outdoor PM2.5 was not due to exposure misclassification. In some studies, the effects of PM on HRV have been observed even within hours of exposure (Devlin et al. 2003; Gold et al. 2000). However, the use of daily averages to measure PM2.5 exposure in our study prevented us from detecting possible immediate effects of PM.
Beta-blockers have been shown to enhance HRV in patients with coronary heart disease (Niemela et al. 1994; Sandrone et al. 1994). Consistent with this, we observed increased outdoor levels of PM2.5 to be associated with decreased SDNN and HF (at 2- and 3-day lags) only among persons not using beta-blockers. Effect modification by medication use thus seems to explain the lack of associations between PM2.5 and HRV in our previous analysis (Timonen et al. 2006). There was little evidence of effect modification by any other medication group in the present study.
The interpretation of earlier studies evaluating the importance of beta-blocker use for the effects of ambient particles on HRV is somewhat difficult because of the differences in disease status between users and nonusers of beta-blockers. In a study by Park et al. (2005) conducted among veteran men, beta-blocker users were all hypertensive, whereas only half of the nonusers had hypertension. No clear effect of PM2.5 (adjusted for ozone) on SDNN or HF was observed in either medication group. However, the low-frequency component of HRV decreased in association with PM2.5 only among persons not using beta-blockers. In a study by Wheeler and coworkers (2006), all but one of the beta-blocker users were myocardial infarction survivors, whereas most nonusers had chronic obstructive pulmonary disease. Effect modification by beta-blocker use was reported only for SDNN, which decreased in association with PM2.5 among users and increased among nonusers. In the present study, all patients had coronary heart disease, and our results suggest that the use of beta-blockers modifies the effect of PM on HRV even in this more homogeneous patient group.
Medication use is obviously never independent of health status. Consequently, the suggestive increase in HF in association with PM2.5 among beta-blocker users in our study may indicate either that the use of medication changes the direction of the association, or that those with less severe heart disease differ in their response to particulate air pollution. Obesity has been suggested to modify the effects of PM on HRV (Chen et al. 2007), which was confirmed by our results. PM2.5 seemed to be more strongly associated with HRV among obese persons. In our study, obesity was not associated with beta-blocker use.
Clinical studies have related decreased HRV in cardiac patients with increased risk of mortality over relatively long periods of follow-up (Task Force 1996). The extent to which short-term decreases in HRV measures predict short-term mortality is not known. However, vagal withdrawal is observed a few minutes before transient ischemic events (Kochiadakis et al. 2000; Kop et al. 2001), suggesting that short-term changes in HRV are not harmless. In a large study among elderly subjects (de Bruyne et al. 1999), increased HRV has been even more strongly associated with decreased survival than decreased HRV. Taking this into account, our study cannot be straightforwardly interpreted as showing that beta-blocker use is protective against the effects of particulate air pollution on cardiovascular health, because there was a suggestive increase in HF in association with PM2.5 among medicated persons.
There was some indication of the effects of traffic-related PM2.5 on SDNN, and long-range transported PM2.5 on HF and SDNN even before taking medication into account, but after considering beta-blocker use, the associations became stronger. Some earlier studies have evaluated the effects of traffic-related particles on HRV without conducting source apportionment. Absorbance, considered as an indicator for traffic-originating particles, has been more strongly associated with HRV among elderly subjects than PM2.5 (Schwartz et al. 2005). In-vehicle PM2.5 was more strongly associated with HRV in healthy young men than were ambient or roadside PM2.5 (Riediker et al. 2004a). In-vehicle PM2.5 was further apportioned among different sources (Riediker et al. 2004b), and strongest associations were observed between PM2.5 from brake wear and engine emissions and HRV.
Schwartz et al. (2005) evaluated indirectly the effects of secondary particles on HRV and found no effect. They regressed PM2.5 against black carbon concentrations and interpreted residuals to represent the fraction of secondary particles that varied independently from primary combustion particles. It is possible that the effects of long-range transported PM2.5 on HRV in our study are related to primary combustion particles generated, for example, by regional traffic. In our study, the effect estimates (for SDNN) per microgram of particle mass were clearly higher for local traffic-related PM2.5 than for long-range transported particles. However, there was also some evidence of the effects of PM2.5 from oil combustion on SDNN. The results are consistent with our previous study, where PM2.5 from traffic and other local combustion was most strongly associated with the occurrence of ST segment depressions in Helsinki, but long-range transported particles and possibly oil combustion were also contributing to the effects of PM2.5 (Lanki et al. 2006).
In the last part of our analyses, we evaluated the associations of HRV with elements of PM2.5 and absorbance, a proxy for elemental carbon content of particles. In these analyses, decreased HRV was associated with absorbance and S, which were considered markers for local traffic and long-range transported PM2.5, respectively. The finding thus confirmed the analyses conducted using source-specific PM 2.5. However, long-range transported PM2.5 also contains traffic-originating PM and elemental carbon. There was also evidence of the negative associations of V (oil combustion), Zn (e.g., industry), Fe, and Cu with HRV, but the associations were mostly nonsignificant. Transition metals are typically associated with combustion processes, so it was not a surprise that absorbance was highly correlated with Zn, Fe, and Cu. It has been suggested that organic carbon compounds and transition metals attached to elemental carbon core (approximated by absorbance) are responsible for the effects of PM on health (Obot et al. 2002).
Toxicologic studies have often observed cellular defenses to be even more responsive to the coarse particle fraction (PM10–PM2.5) than to finer-size fractions (Hetland et al. 2004; Soukup and Becker 2001). The ambient levels of coarse particles are typically dominated by crustal material, whereas PM2.5 levels are more influenced by combustion emissions. In a recent study (Lipsett et al. 2006), coarse particles were associated with decreased HRV, whereas PM2.5 was not. Interestingly, we found increases in HF in association with increased outdoor levels of crustal PM2.5. On the other and, the chosen indicator element for crustal PM2.5—Ca—was not associated with HF.
Our study has both strengths and weaknesses. The study had rather stringent inclusion criteria for the study subjects to obtain a homogeneous cardiac panel presumably vulnerable for the effects of air pollution (von Klot et al. 2005). The three study centers used common standard operating procedures and standardized equipment, and Holter recordings were analyzed in a single lab. HRV was recorded during a paced breathing period to avoid influence of breathing patterns on the results. However, because we measured out- door levels of source-specific PM 2.5 instead of actual exposure, exposure misclassification may have biased the results. We previously reported considerable longitudinal correlations between outdoor and personal PM2.5, absorbance (traffic), and S (long-range transport), but correlations were lower for Ca (soil), Cl (salt), and Cu (Janssen et al. 2005). Finally, our source-specific PM2.5 levels are not always products of homogeneous sources but rather of broader source categories.
In conclusion, we found PM2.5 originating from local traffic and other local combustion and also long-range transported PM 2.5 to be associated with decreased indices of HRV. The effects were stronger among persons not using beta-blocker medication and among obese persons. Differences in the composition of particles and medication use or disease severity of study subjects may explain some inconsistencies between previous studies on HRV.
Footnotes
This study was supported by the Health Effects Institute (HEI research agreement 98-16) and was conducted within the framework of the exposure and risk assessment ULTRA Study (Exposure and Risk Assessment for Fine and Ultrafine Particles in Ambient Air), funded by the European Union Environment and Climate Research Programme (contract ENV4-CT97-0568).
References
- Analitis A, Katsouyanni K, Dimakopoulou K, Samoli E, Nikoloulopoulos AK, Petasakis Y, et al. Short-term effects of ambient particles on cardiovascular and respiratory mortality. Epidemiology. 2006;17:230–233. doi: 10.1097/01.ede.0000199439.57655.6b. [DOI] [PubMed] [Google Scholar]
- Brook RD, Franklin B, Cascio W, Hong Y, Howard G, Lipsett M, et al. Air pollution and cardiovascular disease: a statement for health care professionals from the Expert Panel on Population and Prevention Science of the American Heart Association. Circulation. 2004;109:2655–2671. doi: 10.1161/01.CIR.0000128587.30041.C8. [DOI] [PubMed] [Google Scholar]
- Brunekreef B, Janssen NAH, de Hartog JJ, Oldenwening M, Meliefste K, Hoek G, et al. Personal, indoor, and outdoor exposures to PM2.5 and its components for groups of cardiovascular patients in Amsterdam and Helsinki. Res Rep Health Eff Inst. 2005;127:1–70. 71–79. [PubMed] [Google Scholar]
- Chen JC, Cavallari JM, Stone PH, Christiani DC. Obesity is a modifier of autonomic cardiac responses to fine metal particulates. Environ Health Perspect. 2007;115:1002–1006. doi: 10.1289/ehp.9609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Bruyne MC, Kors JA, Hoes AW, Klootwijk P, Dekker JM, Hofman A, et al. Both decreased and increased heart rate variability on the standard 10-second electrocardiogram predict cardiac mortality in the elderly: the Rotterdam Study. Am J Epidemiol. 1999;150:1282–1288. doi: 10.1093/oxfordjournals.aje.a009959. [DOI] [PubMed] [Google Scholar]
- de Hartog JJ, Hoek G, Mirme A, Tuch T, Kos GPA, ten Brink HM, et al. Relationship between different size classes of particulate matter and meteorology in three European cities. J Environ Monit. 2005;4(7):302–310. doi: 10.1039/b415153d. [DOI] [PubMed] [Google Scholar]
- Devlin RB, Ghio AJ, Kehrl H, Sanders G, Cascio W. Elderly humans exposed to concentrated air pollution particles have decreased heart rate variability. Eur Respir J. 2003;21(suppl 40):76–80. doi: 10.1183/09031936.03.00402403. [DOI] [PubMed] [Google Scholar]
- Gold DR, Litonjua A, Schwartz J, Lovett E, Larson A, Nearing B, et al. Ambient pollution and heart rate variability. Circulation. 2000;101:1267–1273. doi: 10.1161/01.cir.101.11.1267. [DOI] [PubMed] [Google Scholar]
- Hetland RB, Cassee FR, Refsnes M, Schwarze PE, Lag M, Boere AJ, et al. Release of inflammatory cytokines, cell toxicity and apoptosis in epithelial lung cells after exposure to ambient air particles of different size fractions. Toxicol In Vitro. 2004;18:203–212. doi: 10.1016/s0887-2333(03)00142-5. [DOI] [PubMed] [Google Scholar]
- Holguín F, Téllez-Rojo MM, Hernández M, Cortez M, Chow JC, Watson JG, et al. Air pollution and heart rate variability among the elderly in Mexico city. Epidemiology. 2003;14:521–527. doi: 10.1097/01.ede.0000081999.15060.ae. [DOI] [PubMed] [Google Scholar]
- Janssen NA, de Hartog JJ, Hoek G, Brunekreef B, Lanki T, Timonen KL, et al. Personal exposure to fine particulate matter in elderly subjects: relation between personal, indoor and outdoor concentrations. J Air Waste Manag Assoc. 2000;50:1133–1143. doi: 10.1080/10473289.2000.10464159. [DOI] [PubMed] [Google Scholar]
- Janssen NAH, Lanki T, Hoek G, Vallius M, de Hartog JJ, van Grieken R, et al. Associations between ambient, personal, and indoor exposure to fine particulate matter constituents in Dutch and Finnish panels of cardiovascular patients. Occup Environ Med. 2005;62:868–877. doi: 10.1136/oem.2004.016618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochiadakis GE, Marketou ME, Igoumenidis NE, Simantirakis EN, Parthenakis FI, Manios EG, et al. Autonomic nervous system activity before and during episodes of myocardial ischemia in patients with stable coronary artery disease during daily life. Pacing Clin Electrophysiol. 2000;23:2030–2039. doi: 10.1111/j.1540-8159.2000.tb00772.x. [DOI] [PubMed] [Google Scholar]
- Kop WJ, Verdino RJ, Gottdiener JS, O’Leary ST, Bairey Merz CN, Krantz DS. Changes in heart rate and heart rate variability before ambulatory ischemic events. J Am Coll Cardiol. 2001;38:742–749. doi: 10.1016/s0735-1097(01)01451-6. [DOI] [PubMed] [Google Scholar]
- Laden F, Neas LM, Dockery DW, Schwartz J. Association of fine particulate matter from different sources with daily mortality in six U.S. cities. Environ Health Perspect. 2000;108:941–947. doi: 10.1289/ehp.00108941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanki T, de Hartog JJ, Heinrich J, Hoek G, Janssen NAH, Peters A, et al. Can we identify sources of fine particles responsible for exercise-induced ischemia on days with elevated air pollution? The ULTRA study. Environ Health Perspect. 2006;114:655–660. doi: 10.1289/ehp.8578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Rovere MT, Bigger JT, Jr, Marcus FI, Mortara A, Schwartz PJ. Baroreflex sensitivity and heart-rate variability in prediction of total cardiac mortality after myocardial infarction. ATRAMI (Autonomic Tone and Reflexes After Myocardial Infarction) Investigators. Lancet. 1998;351:478–484. doi: 10.1016/s0140-6736(97)11144-8. [DOI] [PubMed] [Google Scholar]
- Le Tertre A, Medina S, Samoli E, Forsberg B, Michelozzi P, Boumghar A, et al. Short-term effects of particulate air pollution on cardiovascular diseases in eight European cities. J Epidemiol Community Health. 2002;56:773–779. doi: 10.1136/jech.56.10.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao D, Duan Y, Whitsel EA, Zheng ZJ, Heiss G, Chinchilli VM, et al. Association of higher levels of ambient criteria pollutants with impaired cardiac autonomic control: a population-based study. Am J Epidemiol. 2004;159:768–777. doi: 10.1093/aje/kwh109. [DOI] [PubMed] [Google Scholar]
- Lipsett MJ, Tsai FC, Roger L, Woo M, Ostro BD. Coarse particles and heart rate variability among older adults with coronary artery disease in the Coachella Valley, California. Environ Health Perspect. 2006;114:1215–1220. doi: 10.1289/ehp.8856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niemela MJ, Airaksinen KE, Huikuri HV. Effect of beta-blockade on heart rate variability in patients with coronary artery disease. J Am Coll Cardiol. 1994;23:1370–1377. doi: 10.1016/0735-1097(94)90379-4. [DOI] [PubMed] [Google Scholar]
- Nolan J, Batin PD, Andrews R, Lindsay SJ, Brooksby P, Mullen M, et al. Prospective study of heart rate variability and mortality in chronic heart failure: results of the United Kingdom heart failure evaluation and assessment of risk trial (UK-heart) Circulation. 1998;98:1510–1516. doi: 10.1161/01.cir.98.15.1510. [DOI] [PubMed] [Google Scholar]
- Normand S-L. Meta-analysis: formulating, evaluating, combining, and reporting. Stat Med. 1999;18:321–359. doi: 10.1002/(sici)1097-0258(19990215)18:3<321::aid-sim28>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Obot CJ, Morandi MT, Beebe TP, Hamilton RF, Holian A. Surface components of airborne particulate matter induce macrophage apoptosis through scavenger receptors. Toxicol Appl Pharmacol. 2002;184:98–106. [PubMed] [Google Scholar]
- Pagan I, Costa DL, McGee JK, Richards JH, Dye JA. Metals mimic airway epithelial injury induced by in vitro exposure to Utah Valley ambient particulate matter extracts. J Toxicol Environ Health A. 2003;66:1087–1112. doi: 10.1080/15287390390213908. [DOI] [PubMed] [Google Scholar]
- Park SK, O’Neill MS, Vokonas PS, Sparrow D, Schwartz J. Effects of air pollution on heart rate variability: the VA Normative Aging Study. Environ Health Perspect. 2005;113:304–309. doi: 10.1289/ehp.7447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pekkanen J, Timonen KL, Tiittanen P, Vallius M, Lanki T, Sinkko H, et al. Study Manual and Data Book. Kuopio: National Public Health Institute; 2000. [[accessed 1 November 2007]]. ULTRA: Exposure and Risk Assessment for Fine and Ultrafine Particles in Ambient Air. Available: http://www.ktl.fi/ultra. [Google Scholar]
- Riediker M, Cascio WE, Griggs TR, Herbst MC, Bromberg PA, Neas L, et al. Particulate matter exposure in cars is associated with cardiovascular effects in healthy young men. Am J Respir Crit Care Med. 2004a;169:934–940. doi: 10.1164/rccm.200310-1463OC. [DOI] [PubMed] [Google Scholar]
- Riediker M, Devlin RB, Griggs TR, Herbst MC, Bromberg PA, Williams RW, et al. Cardiovascular effects in patrol officers are associated with fine particulate matter from brake wear and engine emissions. Part Fibre Toxicol. 2004b;1:2. doi: 10.1186/1743-8977-1-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samet JM, Dominici F, Curriero FC, Coursac I, Zeger SL. Fine particulate air pollution and mortality in 20 U.S. cities, 1987–1994. N Engl J Med. 2000;343:1742–1749. doi: 10.1056/NEJM200012143432401. [DOI] [PubMed] [Google Scholar]
- Sandrone G, Mortara A, Torzillo D, La Rovere MT, Malliani A, Lombardi F. Effects of beta blockers (atenolol or metoprolol) on heart rate variability after acute myocardial infarction. Am J Cardiol. 1994;74:340–345. doi: 10.1016/0002-9149(94)90400-6. [DOI] [PubMed] [Google Scholar]
- Schwartz J, Litonjua A, Suh H, Verrier M, Zanobetti A, Syring M, et al. Traffic related pollution and heart rate variability in a panel of elderly subjects. Thorax. 2005;60:455–461. doi: 10.1136/thx.2004.024836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soukup JM, Becker S. Human alveolar macrophage responses to air pollution particulates are associated with insoluble components of coarse material, including particulate endotoxin. Toxicol Appl Pharmacol. 2001;171:20–26. doi: 10.1006/taap.2000.9096. [DOI] [PubMed] [Google Scholar]
- Tarkiainen TH, Timonen KL, Tiittanen P, Hartikainen JE, Pekkanen J, Hoek G, et al. Stability over time of short-term heart rate variability. Clin Auton Res. 2005;15:394–399. doi: 10.1007/s10286-005-0302-7. [DOI] [PubMed] [Google Scholar]
- Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability. Standards of measurement, physiological interpretation, and clinical use. Eur Heart J. 1996;17:354–381. [PubMed] [Google Scholar]
- Timonen KL, Vanninen E, de Hartog J, Ibald-Mulli A, Brunekreef B, Gold DR, et al. Effects of ultrafine and fine particulate and gaseous air pollution on cardiac autonomic control in subjects with coronary artery disease. The ULTRA study. J Expo Sci Environ Epidemiol. 2006;16:332–341. doi: 10.1038/sj.jea.7500460. [DOI] [PubMed] [Google Scholar]
- Vallius M, Janssen NA, Heinrich J, Hoek G, Ruuskanen J, Cyrys J, et al. Sources and elemental composition of ambient PM2.5 in three European cities. Sci Total Environ. 2005;337:147–162. doi: 10.1016/j.scitotenv.2004.06.018. [DOI] [PubMed] [Google Scholar]
- von Klot S, Peters A, Aalto P, Bellander T, Berglind N, D’Ippoliti D, et al. Ambient air pollution is associated with increased risk of hospital cardiac readmissions of myocardial infarction survivors in European cities. Circulation. 2005;112:3073–3079. doi: 10.1161/CIRCULATIONAHA.105.548743. [DOI] [PubMed] [Google Scholar]
- Wheeler A, Zanobetti A, Gold DR, Schwartz J, Stone P, Suh HH. The relationship between ambient air pollution and heart rate variability differs for individuals with heart and pulmonary disease. Environ Health Perspect. 2006;114:560–566. doi: 10.1289/ehp.8337. [DOI] [PMC free article] [PubMed] [Google Scholar]