Abstract
The purpose of current experiment is the generation of insulin-producing human mesenchymal stem cells as therapeutic source for the cure of type 1 diabetes. Type 1 diabetes is generally caused by insulin deficiency accompanied by the destruction of islet β-cells. In various trials for the treatment of type 1 diabetes, cell-based gene therapy using stem cells is considered as one of the most useful candidate for the treatment. In this experiment, human mesenchymal stem cells were transduced with AAV which is containing furin-cleavable human preproinsulin gene to generate insulin-producing cells as surrogate β-cells for the type 1 diabetes therapy. In the rAAV production procedure, rAAV was generated by transfection of AD293 cells. Human mesenchymal stems cells were transduced using rAAV with a various multiplicity of infection. Transduction of recombinant AAV was also tested using β-galactosidse expression. Cell viability was determined by using MTT assay to evaluate the toxicity of the transduction procedure. Expression and production of Insulin were tested using reverse transcriptase-polymerase chain reaction and immunocytochemistry. Secretion of human insulin and C-peptide from the cells was assayed using enzyme-linked immunosorbent assay. Production of insulin and C-peptide from the test group represented a higher increase compared to the control group. In this study, we examined generation of insulin-producing cells from mesenchymal stem cells by genetic engineering for diabetes therapy. This work might be valuable to the field of tissue engineering for diabetes treatment.
Keywords: Cell-based gene therapy, mesenchymal stem cells, adeno-associated virus, type 1 diabetes
INTRODUCTION
Diabetes is caused by an insulin deficiency (type 1) or insulin resistance (type 2). In type 1 diabetes, there is almost an autoimmune destruction of the β-cells, while in type 2 diabetes, the β-cells are unable to keep up with the body's insulin requirements.
A cure for diabetes has long been sought using several different approaches, including islet transplantation, regeneration of beta-cells and insulin gene therapy. However, permanent remission of type 1 diabetes has not yet been satisfactorily achieved.1
In the field of cell-based gene therapy, genetic engineering of multipotent adult bone-marrow mesenchymal stem cells into insulin-expressing cells has been studied for diabetes therapy using viral or non-viral vectors.2,3 Several gene delivery vehicles are being developed for somatic gene therapy and each of these vectors has unique properties which makes them appropriate for different human disease applications. Recombinant adeno-associated viral (rAAV) vectors are proving themselves to be safe and efficacious for the long-term expression of proteins and correction of genetic diseases following a single administration.4 AAV vectors integrate, if at all, at only low frequencies and this decreases the risk of insertional mutagenesis. AAV vectors also do not contain viral genes that could elicit undesirable cellular immune responses and generally appear not to induce inflammatory response.5
Mesenchymal stem cells (MSCs) represent an ideal stem cell source for cell therapies because of their easy purification and amplification, and their multipotency. As an example, clinical assays were initiated in the last years showing MSC-induced improvement of bone growth and mineralization in children affected with osteogenesis imperfecta, a genetic disorder characterized by defective type I collagen. Moreover, MSC multipotency suggests that these cells may be used to cure other human pathologies.6-10
The purpose of this study is generation and characterization of genetically engineered human mesenchymal stem cells producing human insulin using rAAV vector-mediated gene transfer for the treatment of type 1 diabetes. Generation of surrogate β-cells which can be used for cure of diabetes from human mesenchymal stem cells with effectiveness and safety will be the final goal of this study as cell-based gene therapy of type 1 diabetes.
MATERIALS AND METHODS
Culture of cells
The human mesenchymal stem cells (hMSC) were isolated from the bone marrow. The human bone marrow was obtained from the Dep. of Orthopaedic Surgery at the Yonsei Medical Center with permission from the Medical Material Control and Management Committee of Yonsei University, and the donor age was 28 years. MSCs were purified by using Ficoll-Paque™ Plus (Amersham Biosciences, Uppsala, Sweden)-based method. The cells were resuspended in Dulbecco's Minimal Essential Medium containing 10% FBS, 100 U/mL penicillin, 100 µg/mL streptomycin, and 25 µg/mL amphortericin B (Antibiotic-Antimycotic 100 ×; Gibco, Grand Island, NY, USA). All cells were plated to the culture vessels and the non-adherent cells were removed by changing the medium. The cells were maintained at 37℃ in 5% CO2 in air, with an initial medium change at 7 days after initial plating and then medium was changed every 3 or 4 days. AD-293 cells (Stratagene, La Jolla, CA, USA) were cultured for the production of recombinant adeno-associated virus particles in Dulbecco's modified eagle's medium -hg (containing 4.5 g/L glucose and 110 mg/L sodium pyruvate and 2 mM L-glutamine), supplemented with 10% (v/v) heat-inactivated fetal bovine serum (FBS). Cells were maintained at ≤ 50% confluence. HIT-T15 (hamster islet cell line) was purchased from Korea Cell Line Bank in the form of frozen vials (passage 19). They were cultured in RPMI1640 with L-glutamine (300 mg/L), 25 mM HEPES and 25 mM NaHCO3, 90%; heat inactivated fetal bovine serum (FBS), 10%. Subculture was performed with 3 mL/175 cm2 of trypsin-EDTA solution (Gibco, Grand Island, NY, USA) at 70-80% confluence every 3-4 days.11
Plasmids
For the transfection of AD-293 cells, plasmids were extracted and purified from four groups of E. coli. transformed with pAAV-hPPI (human preproinsulin gene, restriction sites of multiple cloning site of pAAV-MCS are BamHI and XbaI, pAAV-RC which encodes replication and capsid gene of AAV, pHelper, and pAAV-LacZ, respectively. These transformed bacterial cells were a gift from Dr. CW. Park (Division of Endocrinology, Department of Internal Medicine, College of Medicine, Yonsei University, Seoul, Korea). QIAprep® Spin Miniprep kit (QIAGEN, Valencia, CA, USA) was used for the preparation of plasmids. These plasmids were precipitated by ethanol and vacuum centrifugation to concentrate plasmid DNA. All the precipitated plasmids were resuspended in TE buffer, pH 7.5 to adjust the concentration of DNA to 1 mg/mL. Agarose-gel electrophoresis was performed with Lambda DNA-BstE II digest (New England BioLabs, Beverly, MA. USA) size marker to verify the plasmids. The structure of plasmids used in this experiment is shown in Fig. 1.
Fig. 1.
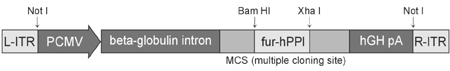
Structure of plasmid used to generate recombinant AAV. Furin-cleavable human preproinsulin gene was inserted into MCS (multiple cloning site) of pAAV-MCS by restriction enzyme treatment.
Production of recombinant adeno-associated virus
Transfection of AD-293 cells was performed according to the instruction manual, AAV Helper-Free System supplied by Stratagene, USA. Production and purification of AAV was performed according to the protocols coordinated by Anne-Marie DOUAR (Genethon, Evry, France). Cells were seeded at a density of 3 × 106 cells/10-cm dishes and maintained in complete DMEM (4.5 g/L Glucose), containing 10% FBS and incubated 48 hours prior to transfection in a humidified 37℃, 5% CO2 incubator. 1h before transfection, cells were at approximately 70 to 80% of confluence and media was changed to serum-free DMEM for the optimal transfection. 10 µL of each of the three plasmid DNA solutions (10 µg of each plasmid) was mixed with 1mL of 0.3 M CaCl2 1 mL and 2 × HBS (Hepes-buffered saline). Prepared mixture suspension was applied to the plate of cells immediately, followed by incubation at 37℃ for 6 hours. At the end of the incubation period, the medium was removed from the plate and replaced with 10 mL of fresh DMEM growth medium, followed by incubation at 37℃ for 72 hours. The phenotypic changes of the AD-293 were monitored for 3 days using inverted light microscope (IX2-SLP, Olympus, Japan). All cells were harvested at three days post-transfection and centrifuged for 10 min at 700 g, 8℃. The pellet was dissolved with 2 mL of lysis buffer (50 mM Hepes; 150 mM NaCl; 1 mM MgCl2; 1 mM CaCl2), followed by repeated freezing and thawing by transferring the tubes between a dry ice/ethanol bath and a 37℃ water bath with a vigorous vortexing between each cycle. The lysate was treated with Benzonase (250 U/µL, Sigma, St. Louis, MO, USA), followed by centrifugation 20 min at 10,000 g, 4℃. To the supernatant, 1 volume of ice-cold saturated ammonium sulfate was added and precipitated on ice for 1 hour, followed by centrifugation 30 min at 12,000 g, 4℃. Then the pellet was dissolved in PBS containing calcium and magnesium ion, followed by cesium chloride (CsCl) gradient-based purification. The suspension was placed between 1.5 g/mL and 1.35 g/mL CsCl solutions, followed by centrifugation at 67,000 rpm, 8℃ for 6 hours. The next day, AAVs were aspirated from the middle of the tube, followed by dialysis in MWCO 10,000 Slide-A-Lyzer dialysis cassettes (Pierce, Rockford, IL, USA) against three 500-mL changes of sterile 1 × PBS containing calcium and magnesium ion for three hours each at 4℃.12-14
Determination of AAV titer was performed by treatment of proteinase K and 10% SDS with AAV at 55℃. After 1 hour incubation, 1 volume of phenolchloroform was added to the mixture, followed by centrifugation at 14,000 g for 10 seconds. To the supernatant, centrifugation in the 0.1 volume of 3 M NaCl plus 2.5 volume of EtOH was performed. The pellet was dissolved in 200 µL of distilled water to measure the absorbance at 260 nm in the spectrophotometer.
Transduction of human mesenchymal stem cells with the recombinant AAV
Human mesenchymal stem cells at passage 5 were treated with 100 multiplicity of infection (MOI) (2 × 105 particle-forming unit (PFU) of rAAV/2 × 105 cells of 6-well plate) and 200 MOI of rAAV-hPPI in serum-free/antibiotics-free DMEM-lg at 37℃ in the CO2 incubator for 24 hours. Negative control group of hMSCs was treated with equal volume of medium without AAV at 37℃ in the CO2 incubator for 24 hours. At the end of incubation periods, media was removed from the each culture plate and fresh complete DMEM-lg (containing 10% FBS and 1% antibiotics antimycotics) was applied to the culture plates.15
β-Galactosidase staining
Human MSCs were infected with rAAV-LacZ at the identical condition and allowed expression of β-galactosidase for 24 hours. At the end of incubation period, cells were washed with D-PBS (Dulbecco's phosphate-buffered saline, Gibco, USA) and fixed with fixative solution (D-PBS containing 2% formaldehyde, 0.05% glutaraldehyde) for 5 minutes at room temperature. Fixed cells were washed with D-PBS and treated with substrate (X-gal)/stain solution (D-PBS containing 5 mM potassium ferricyanide, 5 mM potassium ferrocyanide, 2 mM MgCl2) at 37℃ for 12 hours (overnight). At the end of treatment, cells were washed with D-PBS and observed using inverted light microscope (IX2-SLP, Olympus, Tokyo, Japan).
Reverse-transcriptase polymerase-chain reaction (RT-PCR)
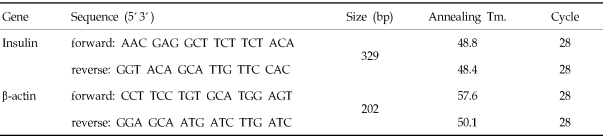
Total RNA was isolated using the RNeasy Mini Kit (Cat. #74106 QIAGEN, USA) as described by the supplier. The final concentration of RNA measured by spectrophotometer was 11.2 µg/mL for AAV-infected hMSCs and 15.2 µg/mL for control hMSCs at 260 nm wavelength. This template RNA was mixed with Oligo DT (12-18mer, 0.5 µg/mL, Invitrogen, USA) and distilled water. The mixture was incubated at 70℃ for 5 min, followed by incubation at 0℃ for 5 min. Reverse-transcriptase PCR was performed using AccuPower RT PreMix Kit (Bioneer, Rockville, MD, USA) as described by supplier at 42℃ for 60 min, followed by RTase inactivation at 94℃ for 5 min. Real-time PCR was performed using PCR premix, Sapphire (SuperBio, Seoul, Korea) as described by supplier. Primers were purchased from Bioneer (Rockville, MD, USA) and shown in Table 1.
Table 1.
Primer Sequences Used in the Polymerase Chain Reactions
Immunocytochemistry
Immunocytochemistry was performed using streptavidin-based 3-step staining kit (Biomeda, Foster City, CA, USA). AAV-infected hMSCs and uninfected hMSCs were fixed with 4% paraformaldehyde at room temperature for 2 hours, followed by washing with PBS. Fixed cells were blocked with 20% BSA (bovine serum albumin) at room temperature for 30 min. Primary antibody (Guinea Pig Anti-Insulin) was treated to the cells at 37℃ for 2 hours and secondary antibody was treated at room temperature for 30 min. Peroxidase reagent was treated at room temperature and chromogen solution was treated at 37℃ for 10 min. Cells were counterstained using hematoxylin at room temperature for 2 min, followed by mounting with crystal/mount (Biomeda, Foster City, CA, USA).
Enzyme-linked immunosorbent assay (ELISA)
Human mesenchymal stem cells transduced with rAAV-(fur)hPPI and untransduced cells were cultured at a density of 4 × 104 cells/well in 12-well plate. Culture media were changed to serum-free media at three days post-infection and cultured to 3 weeks post-transduction. Each culture medium was collected at 1, 2, and 3 week(s) post-transduction, and the concentration of mature insulin and C-peptide was measured using Human insulin-ELISA Kit (BioSource, Camarillo, CA, USA) and Human C-peptide ELISA kit (Linco Research, St. Charles, MO, USA) according to the manufacturer's instruction. Reactivity was measured at 450 nm using ELISA reader. Measured values were converted to the insulin concentrations using the references supplied by the manufacturer and positive control.
MTT assay
To evaluate the cytotoxicity of the AAV used in this experiment, MTT assay was performed. The incubation medium in the test wells were replaced with a volume of 1mL of tetrazolium dye 3-(4,5-dimethylthiazole-2-yl)-2,5-diphenyltetrazolium bromide (MTT) solution at 0.2 mg/mL in D-PBS, and the cells were incubated for 2 h at 37℃. After incubation, the MTT solution was discarded, and 2 mL of 2-propanol was added. The extraction process was performed during 20 min at room temperature. The optical density (OD) was then recorded at 570 nm using a spectrophotometer. The mean OD 570 of the control cells exposed to test compound-free culture medium was set to represent 100% of viability and the results were expressed as percentage of these controls.16
Statistical analysis
Values were calculated as means ± S.E.M. One-way ANOVA was used to compare the insulin/C-peptide ELISA and viability test.
RESULTS
Cell culture
Human MSCs have grown up to 15-passage with adhering to culture flask, and the cells exceeding 15-passage were cryopreserved for next use. When thawed, human MSCs were recovered with more than 90% viability. Frozen cells were plated at a density of 2 × 104 cells/cm2 and formed heterogeneous cell populations after 4-7 days in culture, which consisted of polygonal and spindle-shaped cells. In the initial passage of culture, the cells proliferated slowly and gave rise to confluence in 14-21 days. When the cells were subcultured, the heterogeneous cell populations changed into a homogeneous one with flat and fibroblast-like shape. HIT-T15 cells thawed at 19-passage and plated at a density of 2 × 104 cells/cm2. They were less adhesive than human MSCs. HIT-T15 cells grew slowly, in the initial passage of culture. They appeared to have rounded shapes forming cell-clusters (Fig. 2).
Fig. 2.
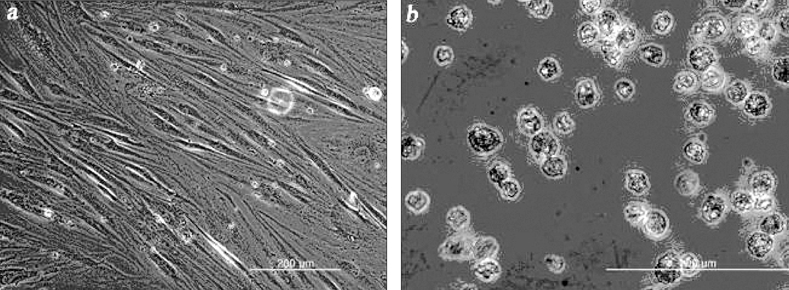
Cells used in the experiment. Human mesenchymal stem cells (a, magnification × 100) and hamster islet cells (b, magnification × 400) are observed with light microscope.
AAV plasmids
For the plasmids used in this study, agarose-gel electrophoresis was performed to confirm the size of plasmids. 0.5% Agarose gel consists of Agarose-LE (USB, Spain) was used. At a marker size of 4.8 kb, a band of pAAV-hPPI consists of AAV's inverted terminal repeats (ITRs) and furin-cleavable hPPI (human preproinsulin gene) was appeared. At a marker size of 7.3 kb, two plasmids, pAAV-RC consisting of replication/capsid expression gene and pAAV-LacZ containing β-galactosidase gene, were appeared. Also, at a marker size of 11.6 kb, pHelper containing adenoviral gene was observed. In the production of rAAV-hPPI, pHelper, pAAV-RC, and pAAV-hPPI were used for rAAV-hPPI production. In the same manner, for the production of rAAV-LacZ, three of plasmids, pHelper, pAAV-RC, and pAAV-LacZ were used. Gel-electrophoresis of each plasmid was performed at a total volume of 6 µL with 1 µL of 6 × loading buffer (Fig. 3).
Fig. 3.
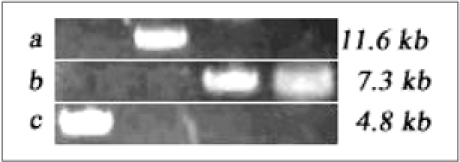
Agarose-gel electrophoresis of plasmids. (A) pHelper (11.6kb), (B) pAAV-RC and pAAV-LacZ (7.3kb), (C) pAAV-hPPI (4.8kb).
Transfection of AD-293 cells and rAAV production
AD-293 cells grown at high confluence may lose the increased adherence phenotype. Care was taken to maintain cells propagated to establish a liquid nitrogen stock at ≤ 50% confluence to ensure the integrity of the stock. The sign of viral production was a color change in the medium from red to orange or yellow (compare to negative control). As viral production proceeds, some of the cells rounded up and detached from the plate, and could be seen floating in the medium (Fig. 4). Scanning electron microscopy shows the spherical shaped rAAV (Fig. 5).
Fig. 4.
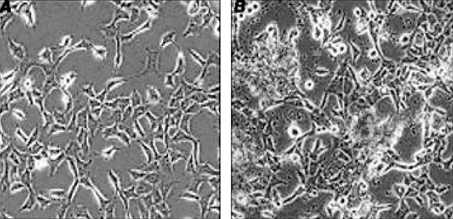
AAV production from the transfected AD-293 cells. AD-293 cells before transfection (A) and at 3 days post-transfection (B). Arrows indicate the floated cells by rAAV production. Magnification × 100.
Fig. 5.
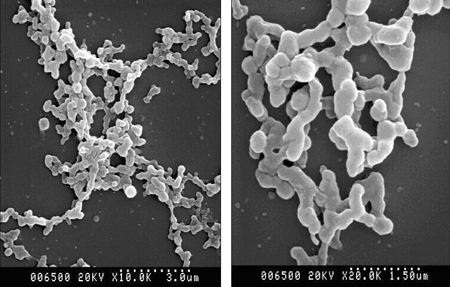
Scanning electron microscopy of rAAV-(fur)hPPI. Magnification × 10K (left panel) and × 20K (right panel).
LacZ expression
Human mesenchymal stem cells were transduced also using rAAV-LacZ to test gene expression of the β-galactosidase gene. Cells were counted and plated to 6-well plate. Recombinant AAV-LacZ was transduced to the cells. β-Galactosidase staining was performed following the procedure in Materials and Methods. After 24-hours incubation, the portion of blue stained cells was appeared to approximately 25 to 30%, indicating the transfection of rAAV-LacZ and expression of β-galactosidase gene (Fig. 6).
Fig. 6.
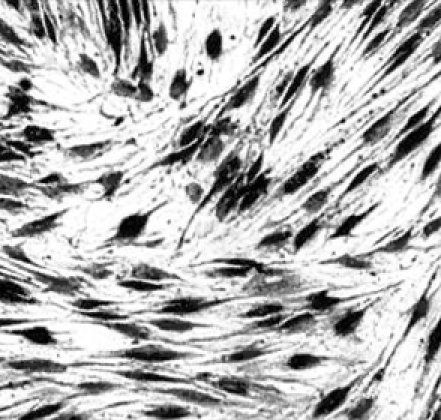
β-Galactosidase staining of the hMSCs infected with rAAV-LacZ. Magnification × 100.
RNA analysis
To further analyze the insulin expression that occurred in transduced hMSCs during the culture, the insulin expression was determined by RT-PCR. 100 bp-DNA ladder was used as size marker. Data represent the insulin expression (329 bp) from mRNA of transduced hMSCs, but not from mRNA of control hMSCs (Fig. 7). β-Actin was expressed in both groups (202bp).
Fig. 7.
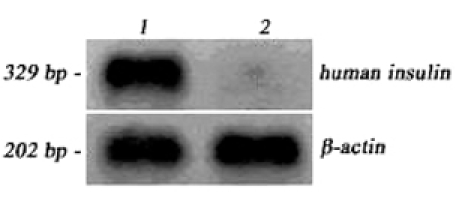
RT-PCR analysis. AAV-hPPI-transduced (lane 1) and control (lane 2) hMSCs.
Immuonocytochemistry
Immunocytochemistry of AAV-hPPI-transduced hMSCs (a, b), control hMSCs (c, d), HIT-T15 cells (e, f) are shown in Fig. 8. Cells were plated at a density of 2 × 105 cell/cm2 and tested about insulin expression using an insulin detection kit as described in Materials and Methods. Some noises (non-specific binding) were inevitably observed following incomplete blocking using bovine serum albumin (BSA). After mounting with mount/crystal solution, microscopic observation showed stained cells with a dark-blue color and brown-red colored portions. Brown-red dots (insulin) were appeared in the transduced hMSCs (Fig. 8, a and b), and HIT-T15 cells (Fig. 8, e and f). Control hMSCs represented no insulin-positive staining (Fig. 8, c and d). Cells were counterstained with hematoxylin before mounting.
Fig. 8.
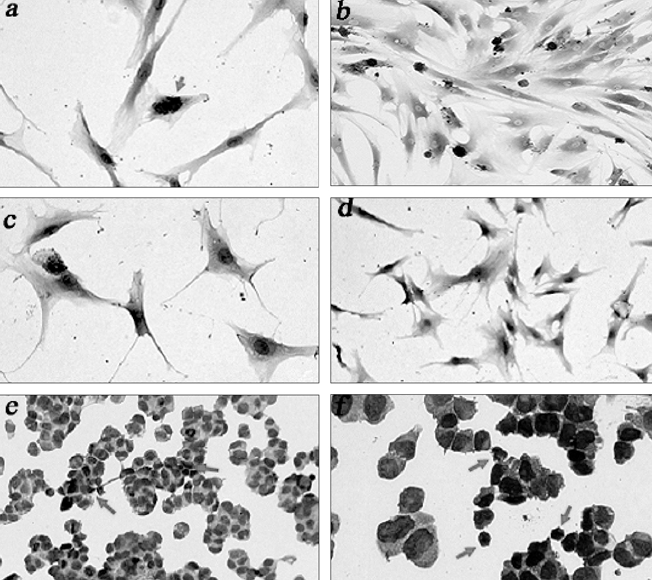
Immunocytochemistry. Arrows indicate insulin-positive staining (brown-red).
Insulin production
In vitro insulin and C-peptide production was measured using ELISA (Fig. 9). Cells were cultured to 3 weeks post-transduction and assays were performed at each weekend. Insulin production level of each group of transduced hMSCs was higher than control hMSCs but lower than HIT-T15 cells. Human MSCs transduced at 500 MOI represented the highest insulin production, especially at 3 weeks post-transduction (Fig. 9e). Production of C-peptide showed as increasement in the patterns at all groups but represented irregular values within the same group. Stable C-peptide production was observed in HIT-T15 cells.
Fig. 9.
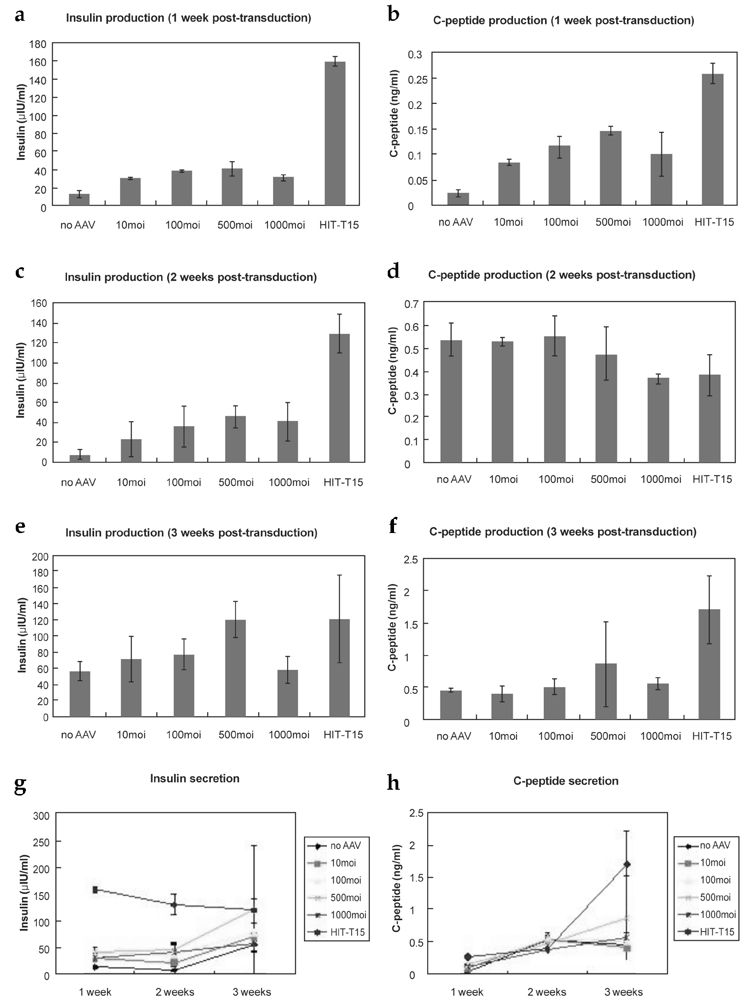
Insulin and C-peptide production. AAV-transduced hMSCs, untransduced hMSCs, and HIT-T15 cells are shown. Insulin production of cells at 1 week (a), 2 weeks (c), and 3 weeks (e) post-transduction and C-peptide production at 1 week (b), 2 weeks (d), and 3 weeks (f) post-transduction were tested. Insulin secretion from 1-3 week(s) post-transduction maintained a similar level or decreased slightly (c). C-peptide secretion from 1 week to 3 weeks post-transduction (d). Data were tested using ANOVA (a~g, p < 0.005).
Viability of the engineered hMSCs
The viability of transduced hMSCs and control hMSCs was tested by using MTT assay (Fig. 10). Recombinant AAV was produced through dialysis and filtering. Therefore, there is little possibility of contamination. In this test, it was tested that the possible hindrance effect of rAAV to the metabolism of transducing cells. Relative cell viability was decreased with an increasement of AAV MOI, however, not so significantly. Therefore, it can be regarded as the amount of AAV is not the criteria of cytotoxicity.
Fig. 10.
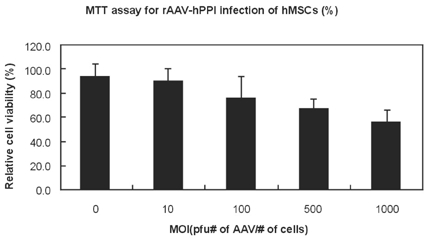
Relative viability of AAV-infected cells. MTT assay was used (p < 0.005).
DISCUSSION
Increasing the number of basic residues in the furin consensus sequence would facilitate processing and greater yields of insulin.17 AAV production procedures have shown compatible results with the manufacturer's manual. In this study, transduction of AAV into MSCs was tested by beta-galactosidase staining and approximately 20% of MSCs were infected by rAAV and expressed LacZ gene (Fig. 6). RT-PCR analysis explained mRNA synthesis of hPPI from the transduced hMSCs (Fig. 7). The procedure was performed just for a detection of insulin mRNA so that quantification of total isolated cell mass was almost ignored. As a result, the bands of each groups are not be identical to real masses of insulin mRNA.
Production of insulin on the transduced cells was represented by Immunocytochemistry analysis (Fig. 8). Insulin positive staining was observed around nucleus of AAV-hMSC and HIT-T15 cells. Relatively, it appeared that insulin positive staining of HIT-T15 cells was scarce. It was supposed that HIT-T15 cells and hMSCs were cultured for just 1 day after trypsinization until performing immunocytochemistry so that these cells might not be adapted to a new culture condition. Generally, non-β-cells cannot produce and process insulin. In this system, the produced proinsulin from genetically engineered hMSCs by tranducing AAV can be processed to be matured insulin by endogenous furin.18 Secretion of insulin and C-peptide was assayed in different groups of cells (Fig. 9). Maximal insulin levels developed in cultures infected with 500 MOI of rAAV-hPPI. At 3 weeks posttransduction, however, hMSCs of 500 MOI showed a similar insulin production level although it was lower than insulin production of HIT-T15 cells until 2 weeks post-transduction. The reason may be because of a higher proliferation rate of hMSCs. It was observed that HIT-T15 cells grew very slowly. It was also supposed that insulin production of MOI dependency might be determined by experimental conditions.19 Between 2 and 3 weeks after transduction with 1000 MOI of rAAV-PPI, there was no increase in insulin production. Brower-Toland et al. suggested that adenovirus and its transgene might have a transient adverse effect on infected cells.20
In this experiment, C-peptide production did not show compatible patterns with insulin production. The reason is probably that the expression of endogenous furin in the AAV plasmid was not stable and effected by condition. In contrast, HIT-T15 cells have prehormone convertase so that C-peptide may be expressed stably.
Insulin specificity of the ELISA kit used in this experiment about human proinsulin represented as 100% in the instruction manual with no cross-reaction. Standard curves showed reliable values in the experiment, however, there were slight differences in the absorbance values even among the groups of identical condition. Unlike standard solutions, media from the cell culture contain various materials and this might be the reason of lower specificity in culture media than standard solutions.
The toxicity of rAAV transduction of hMSCs was different according to MOI (Fig. 10). This result suggests that too many AAV particles could disturb the viability of hMSCs.
The introduction of the insulin gene under the control of the CMV promoter appears to be responsible for activating the gene(s) required for the expression of preproinsulin in this genetically engineered hMSCs from the experimental results. Generally, CMV promoter represents a efficient yield in a various kind of cell lines.21 while shows low activity in embryonic stem cells. In the results from this study, CMV promoter used in the hMSCs seems to be effective in the point of production of insulin and C-peptide. However, to understand the accurate effectiveness and mechanism of action in human mesenchymal stem cells, further analyses should be performed in many studies with a comparison between CMV promoter and other promoters. Several factors, such as relatively crude preparations of vector stocks, heterogeneous population of target cells, suboptimal promoter and transgene cassette, and so on, were the most likely contributing factors for the expression of promoter and proinsulin gene in human mesenchymal stem cells.22
Gluose-induced in vitro insulin secretion was not tested in this study. Human mesenchymal stem cells have no glucose receptors. For regulation of glucose-dependent insulin secretion, proper gene should be transduced such as GLUT-2 gene to generate receptors. However, as it is shown in Introduction, glucose regulation mechanism is so complicated that gene transduction may not be enough. It is supposed that differentiation based on multipotency of hMSCs may be required to have a closer performance of β-cells. GLUT-2 containing cell lines, such as hepatic cells, showed glucose regulation ability in many studies.23
For the establishment of surrogate β-cells, selection of transduced hMSCs might be required in further study. Although genetic engineering of cells in vitro before cell implantation is more controllable than direct injection of genes and allows retrievability if unwanted events arise,24,25 the in vivo safety in terms of genetic alterations within implanted cells should be evaluated in further study.
In conclusion, human mesenchymal stem cells were successfully transduced with AAV containing furin-cleavable human preproinsulin gene in this study. Production of insulin and C-peptide from the infected group represented a higher inverse compared to the control group. However, implantation of engineered cells using animal models and evaluation of therapeutic effect shall be performed with more tests of efficacy and safety of engineered human mesenchymal stem cells as surrogate β-cells in further study.
References
- 1.Lee HC, Kim SJ, Kim KS, Shin HC, Yoon JW. Remission in models of type 1 diabetes by gene therapy using a single-chain insulin analogue. Nature. 2000;408:483–488. doi: 10.1038/35044106. [DOI] [PubMed] [Google Scholar]
- 2.Jiang Y, Jahagirdar BN, Reinhardt RL, Schwartz RE, Keene CD, Ortiz-Gonzalez XR, et al. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418:41–49. doi: 10.1038/nature00870. [DOI] [PubMed] [Google Scholar]
- 3.Jahr H, Bretzel RG. Insulin-positive cells in vitro generated from rat bone marrow stromal cells. Transplant Proc. 2003;35:2140–2141. doi: 10.1016/s0041-1345(03)00747-4. [DOI] [PubMed] [Google Scholar]
- 4.Snyder RO. Adeno-associated virus-mediated gene delivery. J Gene Med. 1999;1:166–175. doi: 10.1002/(SICI)1521-2254(199905/06)1:3<166::AID-JGM34>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 5.Carter BJ. Adeno-associated virus vectors in clinical trials. Hum Gene Ther. 2005;16:541–550. doi: 10.1089/hum.2005.16.541. [DOI] [PubMed] [Google Scholar]
- 6.Tropel P, Noel D, Platet N, Legrand P, Benabid AL, Berger F. Isolation and characterization of mesenchymal stem cells from adult mouse bone marrow. Exp Cell Res. 2004;295:395–406. doi: 10.1016/j.yexcr.2003.12.030. [DOI] [PubMed] [Google Scholar]
- 7.Horwitz EM, Prockop DJ, Fitzpatrick LA, Koo WW, Gordon PL, Neel M, et al. Transplantability and therapeutic effects of bone marrow-derived mesenchymal cells in children with osteogenesis imperfecta. Nat Med. 1999;5:309–313. doi: 10.1038/6529. [DOI] [PubMed] [Google Scholar]
- 8.Horwitz EM, Gordon PL, Koo WK, Marx JC, Neel MD, McNall RY, et al. Isolated allogenic bone marrow-derived mesenchymal cells engraft and stimulate growth in children with osteogenesis imperfecta: implications for cell therapy of bone. Proc Natl Acad Sci USA. 2002;99:8932–8937. doi: 10.1073/pnas.132252399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997;276:71–74. doi: 10.1126/science.276.5309.71. [DOI] [PubMed] [Google Scholar]
- 10.Pittenger MF, Marshak DR. Mesenchymal stem cells of human adult bone marrow. In: Marshak DR, Gardner RL, Gottlieb D, editors. Stem Cell Biology. New York: Cold Spring Harbor Laboratory Press; 2001. pp. 349–373. [Google Scholar]
- 11.Wang RN, Paraskevas S, Rosenberg L. Characterization of integrin expression in islets isolated from hamster, canine, porcine, and human pancreas. J Histochem Cytochem. 1999;47:499–506. doi: 10.1177/002215549904700408. [DOI] [PubMed] [Google Scholar]
- 12.Samulski RJ, Chang LS, Shenk T. Helper-free stocks of recombinant adeno-associated viruses: normal integration does not require viral gene expression. J Virol. 1989;63:3822–3828. doi: 10.1128/jvi.63.9.3822-3828.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xiao X, Li J, Samulski RJ. Production of high-titer recombinant adeno-associated virus vectors in the absence of helper adenovirus. J Virol. 1998;72:2224–2232. doi: 10.1128/jvi.72.3.2224-2232.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Salvetti A, Oreve S, Chadeuf G, Favre D, Cherel Y, Champion-Arnaud P, et al. Factors influencing recombinant adeno-associated virus production. Hum Gene Ther. 1998;9:695–706. doi: 10.1089/hum.1998.9.5-695. [DOI] [PubMed] [Google Scholar]
- 15.Moriscot C, de Fraipont F, Richard MJ, Marchand M, Savatier P, Bosco D, et al. Human bone marrow mesenchymal stem cells can express insulin and key transcription factors of the endocrine pancreas developmental pathway upon genetic and/or microenvironmental manipulation in vitro. Stem Cells. 2005;23:594–604. doi: 10.1634/stemcells.2004-0123. [DOI] [PubMed] [Google Scholar]
- 16.Denizot F, Lang R. Rapid colorimetric assay for cell growth and survival. Modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. J Immunol Methods. 1986;89:271–277. doi: 10.1016/0022-1759(86)90368-6. [DOI] [PubMed] [Google Scholar]
- 17.Hay CW, Docherty K. Enhanced expression of a furin-cleavable proinsulin. J Mol Endocrinol. 2003;31:597–607. doi: 10.1677/jme.0.0310597. [DOI] [PubMed] [Google Scholar]
- 18.Yang YW, Hsieh YC. Regulated secretion of proinsulin/insulin from human hepatoma cells transduced by recombinant adeno-associated virus. Biotechnol Appl Biochem. 2001;33:133–140. doi: 10.1042/ba20000096. [DOI] [PubMed] [Google Scholar]
- 19.Groskreutz DJ, Sliwkowski MX, Gorman CM. Genetically engineered proinsulin constitutively processed and secreted as mature, active insulin. J Biol Chem. 1994;269:6241–6245. [PubMed] [Google Scholar]
- 20.Brower-Toland BD, Saxer RA, Goodrich LR, Mi Z, Robbins PD, Evans CH, et al. Direct adenovirus-mediated insulin-like growth factor I gene transfer enhances transplant chondrocyte function. Hum Gene Ther. 2001;12:117–129. doi: 10.1089/104303401750061186. [DOI] [PubMed] [Google Scholar]
- 21.Tuch BE, Szymanska B, Yao M, Tabiin MT, Gross DJ, Holman S, et al. Function of a genetically modified human liver cell line that stores, processes and secretes insulin. Gene Ther. 2003;10:490–503. doi: 10.1038/sj.gt.3301911. [DOI] [PubMed] [Google Scholar]
- 22.Srivastava A. Hematopoietic stem cell transduction by recombinant adeno-associated virus vectors: problems and solutions. Hum Gene Ther. 2005;16:792–798. doi: 10.1089/hum.2005.16.792. [DOI] [PubMed] [Google Scholar]
- 23.Hamra FK, Chapman KM, Nguyen DM, Williams-Stephens AA, Hammer RE, Garbers DL. Self renewal, expansion, and transfection of rat spermatogonial stem cells in culture. Proc Natl Acad Sci U S A. 2005;102:17430–17435. doi: 10.1073/pnas.0508780102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lehrman S. Virus treatment questioned after gene therapy death. Nature. 1999;401:517–518. doi: 10.1038/43977. [DOI] [PubMed] [Google Scholar]
- 25.Hacein-Bey-Abina S, von Kalle C, Schmidt M, Le Deist F, Wulffraat N, McIntyre E, et al. A serious adverse event after successful gene therapy for X-linked severe combined immunodeficiency. N Engl J Med. 2003;348:255–256. doi: 10.1056/NEJM200301163480314. [DOI] [PubMed] [Google Scholar]