Abstract
The prognosis for gastric cancer with liver metastasis continues to be poor. We present our preliminary findings from 4 cases of liver metastasis from gastric adenocarcinomas treated using radiofrequency ablation (RFA). Between 1995 and 2004, the clinical history and course of 4 patients who underwent radiofrequency ablation for liver metastases from gastric cancer were reviewed. Two patients with smaller metachronous metastasis are currently alive without recurrence at 16 and 14 months and the other patients with larger synchronous metastatic lesions died after 4 and 12 months after RFA. Although this study was limited to a few cases and had a short follow-up duration, our findings suggest that RFA may provide an alternative treatment modality for liver metastasis resulting from gastric adenocarcinoma. Additional study is needed with a larger group of patients and longer follow up to evaluate the efficacy of RFA.
Keywords: Liver, metastasis, stomach, neoplasms, radiofrequency ablation
INTRODUCTION
Although there have been reports of long term survival after liver resection for liver metastasis from gastric adenocarcinoma, the prognosis for most cases remains poor and there is no established and effective management.1,2 Radiofrequency ablation (RFA) has become a popular alternative to surgery for tumor ablation due to its safety, availability and wide applicability to primary or secondary hepatic malignancies.3-5 However, the treatment efficacy of RFA for liver metastasis from gastric cancer remains unknown; only a few small series have been reported to date.6,7 Here we report our findings using RFA for liver metastasis from gastric cancer in four cases.
CASE REPORT
Case 1
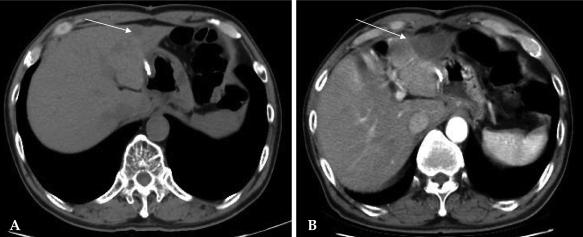
A 72-year-old man who presented with intermittent melena for a month was admitted to our hospital. He had a past medical history of myocardial infarction with congestive heart failure. Endoscopic examination and upper gastrointestinal series revealed a Borrmann type II tumor involving the greater curvature of the lower third to antrum of the stomach. Abdominal computed tomography (CT) showed a mass encircling the gastric antrum with perigastric fat infiltration and regional lymph node enlargement. There was no distant metastasis noted. Radical subtotal gastrectomy with Billroth-I reconstruction was performed in April 2002. The final pathology demonstrated a 5.5 × 5 cm papillary adenocarcinoma with no lymphovascular or neural involvement. The tumor was staged as pT2N0M0 (stage Ib) according to the sixth edition of the UICC TNM classification.8 The patient was discharged at postoperative day eight without postoperative morbidity. Adjuvant chemotherapy with oral 5-fluorouracil was administered. Forty months after surgery, the follow-up endoscopic examination revealed no tumor recurrence in the gastric remnant, but diffuse hyperemic and edematous mucosa was noted. Follow-up CT performed at the same time uncovered a 1.7 × 1.5 cm ill-defined low attenuated lesion on the left lateral segment of the liver. Liver ultrasonography revealed a solid mass at the subcapsular area in segment III which was confirmed to be a metastatic adenocarcinoma by liver biopsy. RFA, using 3 cm internally cooled electrodes (Cool-tip; Radionics Corporation; Burlington, MA, USA) was performed percutaneously with ultrasound guidance for 12 minutes at 100℃ under local anesthesia. There were no complications after RFA. A CT scan obtained two months after RFA showed that the area of the tumor was completely coagulated and necrotic (Fig. 1). Chemotherapy with TS-1 was administered after RFA. The patient is still alive without intrahepatic or local recurrence 16 months after RFA.
Fig. 1.
Case 1: Radiofrequency ablation of a hepatic metastasis from gastric cancer. (A) Preprocedural CT scan revealing a 1.7 cm sized liver metastasis in segment III. (B) CT scan 2 months after RFA showing complete tumor ablation.
Case 2
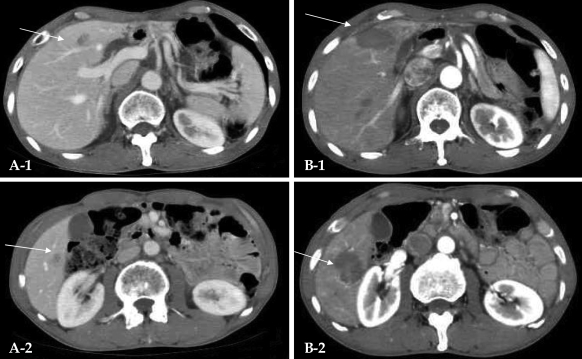
A 62-year-old man who had undergone radical subtotal gastrectomy with Billroth-I reconstruction in March 2003 and had a 3.5 × 3 cm well-differentiated adenocarcinoma classified as pT3N0M0 (stage II) confirmed 30 months previously was admitted to our hospital for the evaluation of a hepatic mass. There were two masses noted at segment IV (2.4 cm) and VI (2.3 cm) of the liver which were confirmed to be well-differentiated adenocarcinomas. Under local anesthesia, ultrasound- guided percutaneous RFA using 3 cm internally cooled electrodes (Cool-tip) was performed for each lesion for six and ten minutes. Three months later, a follow up CT scan showed no viable tumor or new lesions (Fig. 2). The patient received no adjuvant therapy and is alive without local or distant recurrence 15 months after RFA.
Fig. 2.
Case 2: Radiofrequency ablation of hepatic metastases from gastric cancer. (A-1) Preprocedural CT scan showing a 2.4 cm sized hepatic mass in segment IV. (A-2) Preprocedural CT scan showing a 2.3 cm sized hepatic mass in segment VI. (B-1 and B-2) CT scans one month after RFA. Both metastatic lesions in segments IV and VI were completely ablated.
Case 3
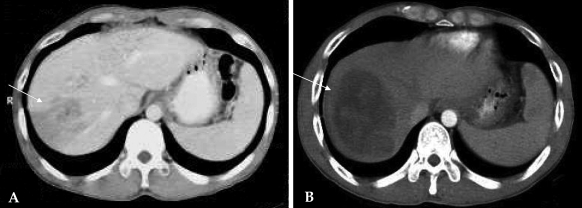
A 37-year-old man who had been diagnosed with gastric cancer at a local clinic was admitted to our hospital presenting with intermittent cramping abdominal pain for three months in April 1999. The endoscopy, upper gastrointestinal series and abdominal CT revealed a Borrmann type II gastric cancer located in the lower body of the stomach without any evidence of metastatic disease. He underwent radical subtotal gastrectomy with Billroth-II reconstruction. The pathological findings confirmed the diagnosis of an 8.5 × 8.5 cm ulcerofungating mass composed of undifferentiated adenocarcinoma. There were no lymphovascular tumor emboli and there was no neural invasion. The cancer was staged as pT2N1M0 (stage II) according to the sixth edition of the UICC TNM classification system. Three months after surgery, follow-up CT revealed a 6.2 × 4 cm liver metastasis at segment VIII. Liver biopsy was performed and microscopic findings revealed an undifferentiated adenocarcinoma compatible with a metastatic tumor. RFA was performed percutaneously because the patient did not want to undergo liver resection. A 50-watt monopolar RF generator (model 500 series; Radiofrequency Interstitial Thermal Ablation Medical System, Mountain View, CA, USA) and an active expandable RF needle electrode with 7 retractable lateral prongs were used. A needle probe with a 7-deployable array was inserted into the tumor under ultrasound guidance. Coagulation, 6 times for 10 minutes each, was performed at 100℃. Near the portal vein, coagulation for 7 minutes at 90℃ was performed. One month later, follow-up liver CT scan indicated that the mass was larger with heterogeneous enhancement, suggesting a viable tumor and portal vein thrombosis (Fig. 3). The patient died four months after RFA.
Fig. 3.
Case 3: Radiofrequency ablation of a hepatic metastasis from gastric cancer. (A) Preprocedural computed tomography (CT) scan revealing a 6.2 cm sized hepatic metastasis in segment VIII. (B) CT scan obtained one month after RFA of tumor. The hepatic metastasis is larger and shows heterogeneous enhancement.
Case 4
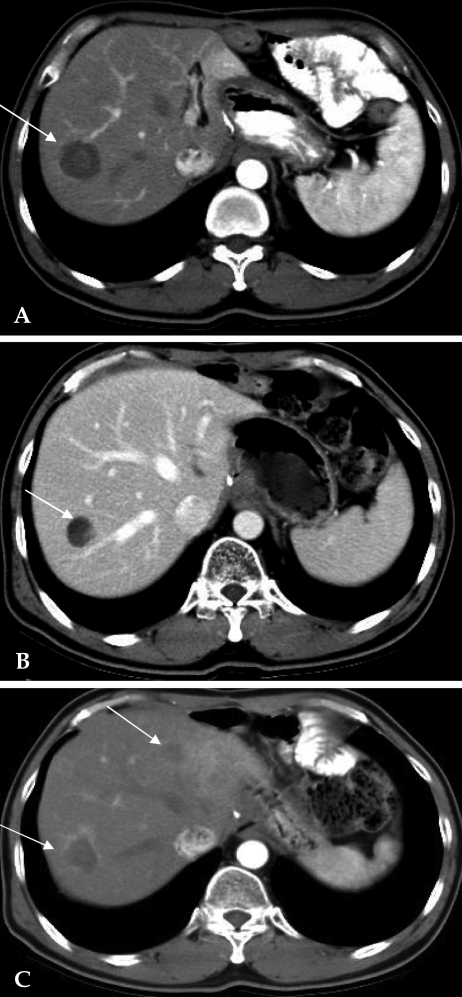
A 53-year-old man underwent radical subtotal gastrectomy with Billroth-I reconstruction in March 2000 and was diagnosed with a pT2N1M0 (stage II), 3.5 × 3 cm, poorly-differentiated adenocarcinoma. Nine months later, he returned with liver metastasis at segment VII. Liver biopsy confirmed a metastatic adenocarcinoma. After five cycles of chemotherapy with taxotere and cisplatin over five months there was no change in the 4 cm hepatic mass. A percutaneous RFA using a 3 cm internally cooled electrode (Cool-tip) was performed twice for 12 minutes at 100℃. There were no RFA-related complications. The patient received chemotherapy with irinotecan, leucovorin and 5-fluorouracil. A CT scan performed one month after RFA showed a hypodense lesion without contrast enhancement consistent with complete ablation and no residual intrahepatic metastases (Fig. 4). Four months after the RFA, however, multiple new liver metastases were detected. The patient died one year after RFA.
Fig. 4.
Case 4: Radiofrequency ablation of a hepatic metastasis from gastric cancer. (A) Preprocedural CT scan displaying a 4 cm sized hepatic metastasis in segment VII. (B) CT scan one month after RFA. A hypodense lesion without contrast enhancement covers the site of metastasis. (C) CT scan obtained after four months after RFA showing slight enhancement on the margin of the tumor and multiple hepatic metastases.
DISCUSSION
Liver metastasis is one of the most frequent forms of hematogenous spread of gastric cancers; the proportion of gastric cancers metastasizing to the liver ranges from 3.7% to 11%.2,9,10 Most cases of liver metastases from gastric cancer involve both lobes of the liver and are accompanied by peritoneal dissemination, extensive lymph node metastases or direct invasion to adjacent organs.11,12 The rate of hepatic resection in such cases ranges from only 0.5% to 2.3%.2,9,10 Moreover, the survival outcomes for hepatic resection of gastric cancer liver metastases have been unsatisfactory,13,14 although there have been isolated reports of long-term improved survival after aggressive surgical treatments for this disease in highly selected patients.9,15-17
RFA has been used for the treatment of primary and secondary hepatic malignancies, especially in hepatocellular carcinoma and colorectal cancer, and has provided a safe and effective alternative for unresectable tumors.3,5,18 RFA can eliminate tumors with minimal injury to the surrounding hepatic parenchyma, and percutaneous, laparoscopic and open surgical approaches have all been proven to be safe.19 The reported therapeutic response rate is 66% for metastatic colorectal cancer.18 Tumor control rates at one year have reached 94% and 90% for intraoperative and percutaneous RFA, respectively.20 However, there have only been a few reports on the results of RFA therapy used for liver metastasis from gastric cancer.6,7,21 Based on these considerations, we applied RFA to liver metastasis from gastric cancer without extrahepatic metastasis.
In April 1999, we started RF ablations for hepatic tumors in our institution. We used four kinds of commercially available RF devices manufactured by three different companies. Among them, two RF devices were used in our study. We selected the type of devices on a case-by-case basis, depending on the availability of the electrodes in stock and the size and location of the tumors. Our strategy during RFA was to include a peripheral margin of 0.5 - 1.0 cm of normal hepatic parenchyma surrounding the tumor as well as the entire tumor itself, regardless of the RF device used or the size of the tumor. According to manufacturers' recommendations, we usually ablated a small (less than 3 cm in diameter) hepatic tumor for 12 minutes with either a set power or target temperature. However, we sometimes decreased the ablation time when injury of the adjacent organs or blood vessels was a concern. For tumors larger than 3 cm in diameter, whenever possible, we performed multiple overlapping ablations.
Zacherl et al. reported that curative resection for metachronous liver metastases might allow long-term survival in selected patients.15 In addition, Rho et al. reported that the median survival for metachronous hepatic metastases was better than that of synchronous metastases (74.3 months vs. 13.0 months), and that surgical management should be considered for solitary hepatic lesions as a treatment option.17 In our cases, both of the patients with synchronous metastasis (cases 3 and 4) showed poor therapeutic response after RFA, whereas the therapeutic response to RFA for cases 1 and 2 was relatively favorable. These findings are in agreement with the above reports despite the short follow-up. Therefore, accurate evaluation for the presence of liver metastasis preoperatively is important to predict patient prognosis and to determine the treatment plan. In these four cases, we performed a preoperative CT scan, which can readily detect the presence of visceral metastatic diseases, and no patient has evidence of liver metastasis. However, the accuracy of diagnosis in the patient discussed in case 3 in whom follow-up CT revealed a liver metastasis three months after surgery is questionable.
The size of the hepatic metastasis to be treated is the most important factor in determining whether complete local ablation can be achieved.5 In general, lesions measuring less than 2.5 cm in diameter have been reported to have a greater than 90% chance of being destroyed, and less than 50% of tumors measuring greater than 5 cm are likely to be completely ablated.5 Gannon et al. recommended not treating tumors > 5 cm in maximal diameter with RFA.19 The tumor sizes in our cases ranged from 1.7 to 6.2 cm. The hepatic lesions were smaller in cases 1 and 2 than in cases 3 and 4. In case 3, RFA was performed six times due to the large size (6.2 cm) of the tumor and its location next to an adjacent major vessel, but successful tumor control was still not achieved.
The number of tumors present is another significant prognostic factor in patients with liver metastasis. Gannon et al. reported that tumor biology and behavior indicate that it is unlikely that RFA would be effective for more than five metastases with regard to a survival benefit for patients.19 Shirabe et al. reported that the presence of more than three hepatic metastases was an independent poor prognostic factor based on observations in patients after hepatic resection of liver metastasis from gastric cancer.22 Three patients in our sample had a solitary liver metastasis while one had two lesions. Further study is needed to compare the outcome of patients according to the number of metastatic lesions in the liver.
With improvements in ablation techniques and instruments, the number and extent of RFA treatments is increasing. However the efficacy, indications and limitations of this therapy for liver metastasis from gastric adenocarcinomas have not been studied in a large series of patients to date. In our small number of cases, the complementary role of RFA as a modality of treatment for hepatic metastasis from gastric cancer should be considered as an option for such patients. Further evaluation is needed to determine the appropriate inclusion criteria for RFA and to achieve improved survival benefits. In addition, study of survival benefits with RFA compared to hepatic resection should be pursued.
References
- 1.Ambiru S, Miyazaki M, Ito H, Nakagawa K, Shimizu H, Yoshidome H, et al. Benefits and limits of hepatic resection for gastric metastases. Am J Surg. 2001;181:279–283. doi: 10.1016/s0002-9610(01)00567-0. [DOI] [PubMed] [Google Scholar]
- 2.Saiura A, Umekita N, Inoue S, Maeshiro T, Miyamoto S, Matsui Y, et al. Clinicopathological features and outcome of hepatic resection for liver metastasis from gastric cancer. Hepatogastroenterology. 2002;49:1062–1065. [PubMed] [Google Scholar]
- 3.Chen MH, Yang W, Yan K, Gao W, Dai Y, Wang YB, et al. Treatment efficacy of radiofrequency ablation of 338 patients with hepatic malignant tumor and the relevant complications. World J Gastroenterol. 2005;11:6395–6401. doi: 10.3748/wjg.v11.i40.6395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Baere T, Elias D, Ducreux M, Dromain C, Kuach V, Gamal El Din M, et al. [Percutaneous radiofrequency ablation of hepatic metastases. Preliminary experience] Gastroenterol Clin Biol. 1999;23:1128–1133. [PubMed] [Google Scholar]
- 5.Iannitti DA, Dupuy DE, Mayo-Smith WW, Murphy B. Hepatic radiofrequency ablation. Arch Surg. 2002;137:422–426. doi: 10.1001/archsurg.137.4.422. discussion 427. [DOI] [PubMed] [Google Scholar]
- 6.Carditello A, Scisca C, Stilo F, Parisi A, Basile M. The possible role of radiofrequency as complementary treatment of locally advanced gastric cancer. Ann Ital Chir. 2005;76:39–41. [PubMed] [Google Scholar]
- 7.Yamakado K, Nakatsuka A, Takaki H, Mori Y, Tonouchi H, Kusunoki M, et al. Prospective study of arterial infusion chemotherapy followed by radiofrequency ablation for the treatment of liver metastasis of gastric cancer. J Vasc Interv Radiol. 2005;16:1747–1751. doi: 10.1097/01.RVI.0000188738.84911.3B. [DOI] [PubMed] [Google Scholar]
- 8.Sobin LH, Wittekind CH, editors. TNM: classification of malignant tumors. 6th ed. New York, NY: Wiley-Liss; 2002. pp. 65–68. [Google Scholar]
- 9.Okano K, Maeba T, Ishimura K, Karasawa Y, Goda F, Wakabayashi H, et al. Hepatic resection for metastatic tumors from gastric cancer. Ann Surg. 2002;235:86–91. doi: 10.1097/00000658-200201000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sakamoto Y, Ohyama S, Yamamoto J, Yamada K, Seki M, Ohta K, et al. Surgical resection of liver metastases of gastric cancer: an analysis of a 17-year experience with 22 patients. Surgery. 2003;133:507–511. doi: 10.1067/msy.2003.147. [DOI] [PubMed] [Google Scholar]
- 11.Koga S, Kawaguchi H, Kishimoto H, Tanaka K, Miyano Y, Kimura O, et al. Therapeutic significance of noncurative gastrectomy for gastric cancer with liver metastasis. Am J Surg. 1980;140:356–359. doi: 10.1016/0002-9610(80)90167-1. [DOI] [PubMed] [Google Scholar]
- 12.Adam YG, Efron G. Trends and controversies in the management of carcinoma of the stomach. Surg Gynecol Obstet. 1989;169:371–385. [PubMed] [Google Scholar]
- 13.Ercolani G, Grazi GL, Ravaioli M, Ramacciato G, Cescon M, Varotti G, et al. The role of liver resections for noncolorectal, nonneuroendocrine metastases: experience with 142 observed cases. Ann Surg Oncol. 2005;12:459–466. doi: 10.1245/ASO.2005.06.034. [DOI] [PubMed] [Google Scholar]
- 14.Taniguchi H, Takahashi T, Sawai K, Yamaguchi T, Hagiwara A, Kitamura K, et al. Comparison in survival between hepatic metastases of gastric and colorectal cancers. Hepatogastroenterology. 1997;44:897–900. [PubMed] [Google Scholar]
- 15.Zacherl J, Zacherl M, Scheuba C, Steininger R, Wenzl E, Mühlbacher F, et al. Analysis of hepatic resection of metastasis originating from gastric adenocarcinoma. J Gastrointest Surg. 2002;6:682–689. doi: 10.1016/s1091-255x(01)00075-0. [DOI] [PubMed] [Google Scholar]
- 16.Morise Z, Yamafuji K, Takahashi T, Asami A, Takeshima K, Hayashi N, et al. Successful treatment of recurrent liver metastases from gastric cancer by repeated hepatic resections: report of a case. Surg Today. 2000;30:1041–1045. doi: 10.1007/s005950070031. [DOI] [PubMed] [Google Scholar]
- 17.Roh HR, Suh KS, Lee HJ, Yang HK, Choe KJ, Lee KU. Outcome of hepatic resection for metastatic gastric cancer. Am Surg. 2005;71:95–99. [PubMed] [Google Scholar]
- 18.Solbiati L, Livraghi T, Goldberg SN, Ierace T, Meloni F, Dellanoce M, et al. Percutaneous radio-frequency ablation of hepatic metastases from colorectal cancer: long-term results in 117 patients. Radiology. 2001;221:159–166. doi: 10.1148/radiol.2211001624. [DOI] [PubMed] [Google Scholar]
- 19.Gannon CJ, Curley SA. The role of focal liver ablation in the treatment of unresectable primary and secondary malignant liver tumors. Semin Radiat Oncol. 2005;15:265–272. doi: 10.1016/j.semradonc.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 20.de Baere T, Elias D, Dromain C, Din MG, Kuoch V, Ducreux M, et al. Radiofrequency ablation of 100 hepatic metastases with a mean follow-up of more than 1 year. AJR Am J Roentgenol. 2000;175:1619–1625. doi: 10.2214/ajr.175.6.1751619. [DOI] [PubMed] [Google Scholar]
- 21.Kosaka T, Imaizumi H, Kamei K, Usami K, Nakano Y, Ueno K, et al. [A case of gastric cancer patient with liver metastasis treated by radiofrequency ablation therapy combined with intra-arterial chemotherapy] Gan To Kagaku Ryoho. 2004;31:1737–1739. [PubMed] [Google Scholar]
- 22.Shirabe K, Shimada M, Matsumata T, Higashi H, Yakeishi Y, Wakiyama S, et al. Analysis of the prognostic factors for liver metastasis of gastric cancer after hepatic resection: a multi-institutional study of the indications for resection. Hepatogastroenterology. 2003;50:1560–1563. [PubMed] [Google Scholar]