Abstract
Purpose
To analyze the effect of allogeneic blood transfusion on clinical outcome in 119 patients with stage IIB cervical cancer who were treated with radiotherapy ± chemotherapy.
Patients and Methods
Medical records were examined for hemoglobin levels before and during radiotherapy, history of allogeneic blood transfusions and the time point when transfusions were given. These factors were retrospectively analyzed along with other clinical risk factors for influences on the patients' clinical outcomes.
Results
Thirty-two patients (26.9%) received packed red blood cell transfusion (mean, 3.4 units; range, 1 - 12 units) before or during radiotherapy. Median follow-up period was 39.3 months (range, 7.6 - 58.4 months). Patients with history of transfusion showed poorer metastasis-free survival and a trend toward poorer overall survival than non-transfused patients. When patients who received transfusions were sub-divided by the time of transfusion, those who received transfusions before radiotherapy had significantly poorer clinical outcome than those who received transfusions during radiotherapy. In a multivariable analysis, patients with pretreatment transfusion showed a higher risk of distant metastasis (HR = 3.75, 95% CI: 1.28 - 12.15, p = 0.017) and decreased overall survival rates (HR = 4.62, 95% CI: 1.15-18.54, p = 0.031) compared with those of other patients.
Conclusion
Our results suggest that allogeneic blood transfusions given before radiotherapy may be associated with higher incidence of distant metastases and decreased survival in patients with stage IIB cervical cancer.
Keywords: Anemia, blood transfusion, cervical cancer, metastasis, radiotherapy
INTRODUCTION
One of the most commonly encountered medical conditions among cancer patients is anemia, which is frequently caused by tumor bleeding, malnutrition, and other tumor-associated metabolic processes, as well as the myelosuppressive effects of various anticancer agents. In women with cervical cancer, blood loss due to acute or chronic vaginal bleeding is the most frequent cause of anemia. Incidence of anemia at the time of diagnosis (hemoglobin level < 12 g/dL) is 25 - 40%, and this incidence is higher in more advanced stage diseases.1,2 It was shown by Vaupel et al. that the median pO2 in cervical and vulvar cancers increases significantly at a mean hemoglobin (Hb) of 13 ± 0.1 g/dL, and decreases toward either side of the value, suggesting that an optimal Hb level for maximal oxygen saturation in the tumor tissues is around 13 g/dL.3 Patients with low Hb have been treated with allogeneic blood transfusions (ABT) before radiotherapy because anemia was thought to have adverse effects on treatment outcome by aggravating tumor hypoxia and resistance to radiotherapy. This belief was supported by the findings from the largest retrospective review of over 600 patients treated at 7 different cancer centers in Canada which showed Hb below 12 g/dL was associated with bad prognosis after radiotherapy.4-6 More recently, anemia has been reported to cause chronic fatigue in patients with cancer, and the importance of preventing and treating anemia has been emphasized in the context of improving the quality of life of cancer patients.7 Use of human recombinant erythropoietin compounds has been suggested as a solution, especially to prevent treatment-related anemia, however, administration of these agents to patients with cervical cancer is a topic of debate due to the reports of an association of these compounds with unfavorable treatment outcomes.8,9 In addition, these agents do not rectify anemia as rapidly as blood transfusions, and hence, blood transfusions are still needed in patients who have insufficient physiologic compensatory mechanisms for low hemoglobic level and tissue hypoxia.10-12 On the other hand, ABT is associated with the development of transfusion-related immunomodulation and the role of ABT has been challenged, because of several reports showing poor oncologic outcomes in patients who received blood transfusions.13-17 In the background of these conflicting aspects of ABT in the management of anemia, we retrospectively analyzed the effect of ABT on clinical outcomes in patients with FIGO stage IIB uterine cervical cancer.
PATIENTS AND METHODS
Patients and treatment
The patient population consisted of 119 women with FIGO stage IIb cervical cancer who were treated with radiotherapy between July 2001 and March 2005. One hundred seven of these women (90%) also received weekly cisplatin, whereas 12 patients (10%) were treated with radiotherapy alone. Radiotherapy consisted of 45 - 50 Gy external beam radiotherapy in 1.8 Gy fractions with high-dose-rate brachytherapy beginning during week 4 - 5 of external beam radiotherapy. Point A was irradiated up to total 85 - 90 Gy and intravenous cisplatin (40 mg/m2 body surface area) was administered once weekly during radiotherapy. All patients completed treatment within 42 to 79 days (median, 52 days).
Anemia and allogeneic blood transfusion
It has been our treatment policy to maintain patient Hb levels above 10 g/dL before or during radiotherapy, and transfusion of packed RBC was recommended for patients with Hb less than 10g/dL at any given time before or during radiotherapy. This resulted in 32 cases of transfusions, among which 15 cases received pre-treatment transfusion. Filters were not used for transfusion on a routine basis, and leukocyte-depleted blood component was not used in any patient during this period in our clinic. To evaluate the effect of anemia and transfusion, we examined the effect of 2 different values of Hb; i.e., baseline Hb (BHb) and average weekly nadir Hb (AWNHb). BHb represents the lowest Hb value before radiotherapy, whereas weekly nadir Hb (WNHb) was the lowest Hb value during each week of radiotherapy, with AWNHb being the average of WNHbs. The effects of ABT were compared in patients who did or did not receive transfusions, as well as whether transfusions were given before radiotherapy began or during radiotherapy. We further examined whether patients with Hb less than 10 g/dL had microcytic or normocytic anemia by looking at the mean corpuscular volume (MCV) and red blood cell distribution width (RDW). Microcytic anemia was defined as MCV less than 80 fL. According to the recent review of diagnosis of anemia,18 iron deficiency anemia (IDA) is microcytic anemia which is mostly associated with increased RDW. If normocytic or macrocytic, the presence of elevated serum ferritin level favors diagnosis of anemia of chronic disease (ACD), especially when associated with normal RDW (reference of RDW, 11.5 - 14.5%).
Statistical analysis
Distribution of the patients' characteristics was presented by median (range) or mean (SD) for continuous variables, and frequency (%) for categorical variables including tumor size, histology type, pelvic lymph node status, laparoscopic lymph node staging (LLND), BHb, and AWNHb; transfusion vs. non-transfusion; pretreatment transfusion (PTf) vs. transfusion only during treatment (DTf) vs. non-transfusion group. Student's t-test was performed to test for distribution differences in BHb and AWNHb values for various clinical parameters. Locoregional recurrence-free survival (LRRFS), metastasis-free survival (MFS), and overall survival (OS) were evaluated by the Kaplan-Meier method and were tested using logrank test. To account for potential confounding effects on survival outcomes, Cox proportional hazards model was employed using the general strategy for model selection involving the hierarchic principle with the likelihood ratio chi-square test. All reported p values were 2 sided and the results were considered as significant at p < 0.05. All statistical analyses were performed with SAS statistical software (ver. 8.01, SAS Institute Inc., Cary, NC, USA).
RESULTS
Patient characteristics
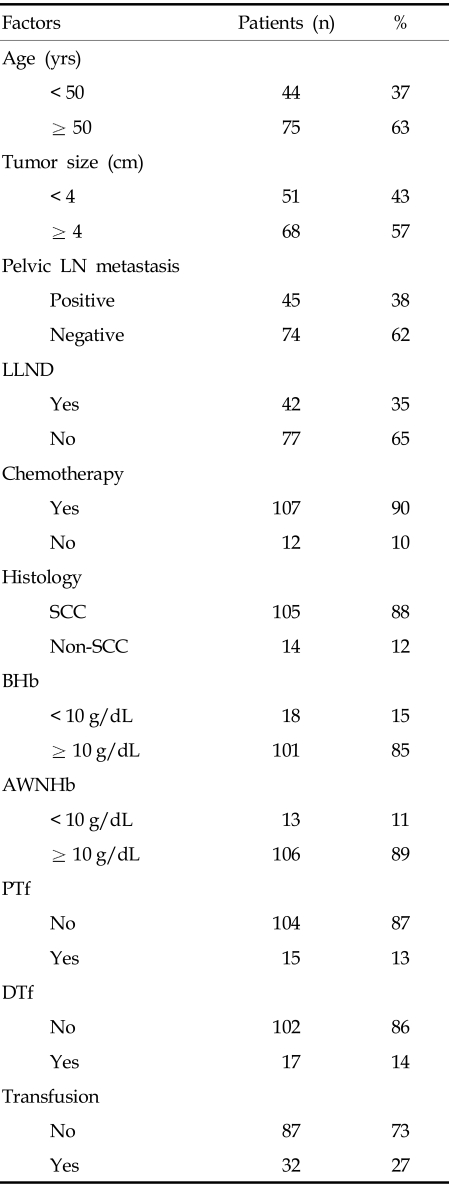
The median follow-up for survivors was 39.3 months (range, 7.6 - 58.4 months), and median age of the patients was 60 years (range, 23 - 80 years). Of the 119 patients, 105 (88%) had histologic diagnosis of squamous cell carcinoma, with the remainder having adenocarcinoma or adenosquamous cell carcinoma. Median tumor diameter was 4.0 cm (range, 2.0 - 12.3 cm). Forty-five (38%) patients had metastases to the pelvic LNs, as shown by LLND or radiological imaging, including MRI and/or PET-CT. Patients found to have nodal metastases outside the pelvis were excluded from this study. Laparoscopic lymph node staging was performed in 42 (35%) patients prior to radiotherapy (Table 1).
Table 1.
Patient Characteristics
AWNHb, average weekly nadir hemoglobin; BHb, baseline hemoglobin; DTf, transfusion only during treatment; LN, lymph node; LLND, laparoscopic lymph node dissection; PTf, pretreatment transfusion; SCC, squamous cell carcinoma
Incidence of anemia and frequency of blood transfusions
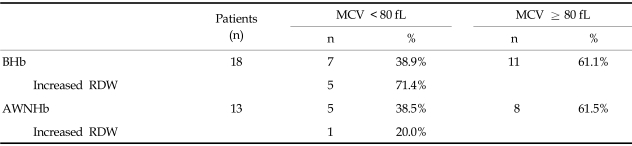
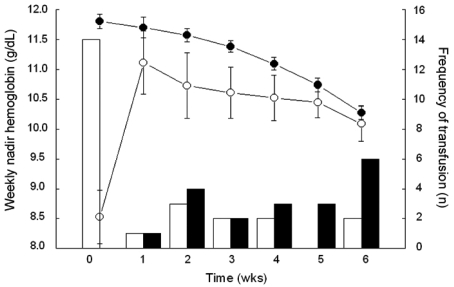
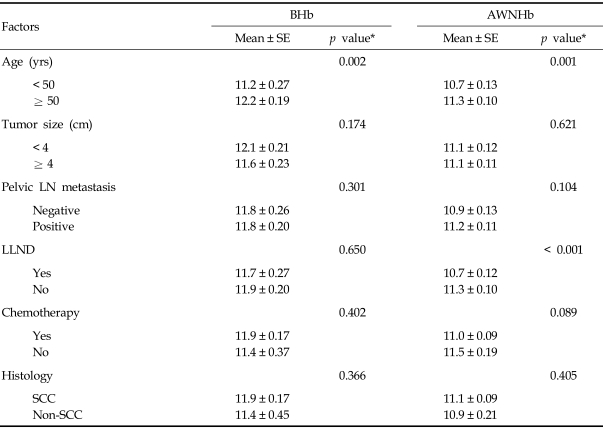
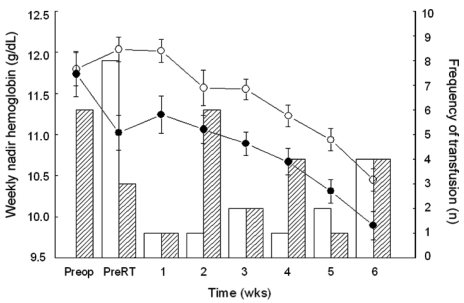
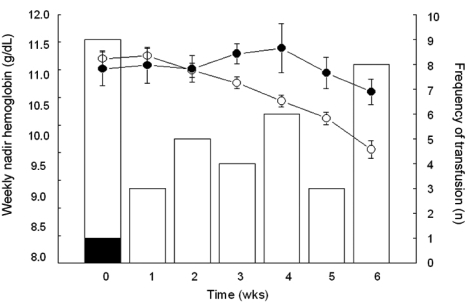
The median values for BHb and AWNHb were 12.0 g/dL (range, 6.0 - 14.7 g/dL) and 11.1 g/dL (range, 9.0 - 13.4 g/dL), respectively. In our series, the proportion of microcytic anemia in 18 patients whose BHb was less than 10g/dL was 7 patients with the rest of the patients having MCV over 80 fL (Table 2). Five of these patients had increased RDW, suggesting iron deficiency anemia. For 11 patients who were normocytic, there were possibilities that they had anemia due to chronic disease, caused by cancer or other chronic systemic disease. In our patients, there was higher incidence of normocytic anemia in older patients (5/9 in < 50 vs. 3/4 in ≥ 50), suggesting the contribution of chronic condition promoting cytokine-mediated process, which leads to poor RBC production or interference of erythropoietin production in this age group. Overall, 32 patients (27%) were given at least one transfusion before or during radiotherapy, with 23 (23/32, 72%) receiving a single transfusion and 9 (9/32, 28%) receiving more than 1. Patients who were given transfusion received an average of 3.4 units of packed red blood cells (range, 1 - 12 units). Fifteen patients (13%) received transfusions before treatment and 17 (14%) only during treatment (Table 1). Fig. 1 shows the numbers of patients who received ABT before and during the course of radiotherapy and their Hb values. Average BHb was 8.5 g/dL (n = 15) for patients who had PTf and 11.8 g/dL (n = 104) for the remainder. This difference in mean Hb levels decreased during the course of radiotherapy, and the significance of mean Hb differences was lost by the second week after initiation of treatment (Fig. 1). When the Hb variables were analyzed in relation to clinical parameters, we found that BHb and AWNHb below 10 g/dL were significantly associated with younger age (p = 0.002 and 0.001, respectively, Table 3). Patients who underwent LLND showed a decrease in Hb levels after surgery, and the AWNHbs remained lower through the entire treatment period than in those who did not undergo LLND (Fig. 2 and Table 3), although BHb did not differ between the two groups. Overall, patients who underwent LLND received more frequent transfusions than the remainder of the patients, before and during radiotherapy (27/42, 64.3% vs. 19/77, 24.7%, respectively). Fig. 3 shows that the patients who were treated with weekly cisplatin chemotherapy concurrently with to radiotherapy had a significant decrease in Hb levels after the fourth week of radiotherapy (p = 0.008, 0.015, and 0.009 for 4th, 5th, and 6th week respectively).
Table 2.
Type of Anemia
AWNHb; average weekly nadir hemoglobin, BHb; baseline hemoglobin, MCV; mean corpuscular volume, RDW; red blood cell distribution width.
Fig. 1.
Average weekly nadir hemoglobin values in patients who received blood transfusions before the initiation of radiotherapy (open circles) and in patients who did not receive transfusions (closed circles). Transfusion frequency in patients with (white column) and without (black column) pretreatment transfusions.
Table 3.
Factors Influencing Hemoglobin Level
AWNHb, average weekly nadir hemoglobin; BHb, baseline hemoglobin; LN, lymph node; LLND, laparoscopic lymph node dissection; SCC, squamous cell carcinoma; SE, mean of standard error.
*Student's t-test was performed to compare the differences in hemoglobin level by 2 groups.
Fig. 2.
Effect of laparoscopic lymph node dissection on anemia and transfusion. Closed circle and shaded column represent patients with laparoscopic surgery (n = 42). Open circle and white column represent patients without laparoscopic surgery (n = 77). PreOp, Hemoglobin at initial presentation; PreRT, Nadir hemoglobin at the time of initiation of radiotherapy.
Fig. 3.
Effect of chemotherapy on hemoglobin and transfusion. Open circle and white column represent patients undergoing chemotherapy (n = 107), whereas, closed circle and black column for patients without chemotherapy (n = 12).
Analysis of prognostic parameters and clinical outcomes
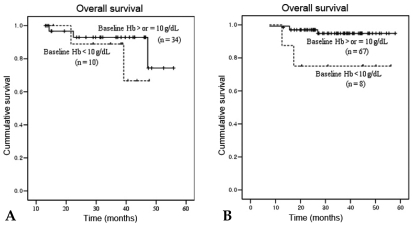
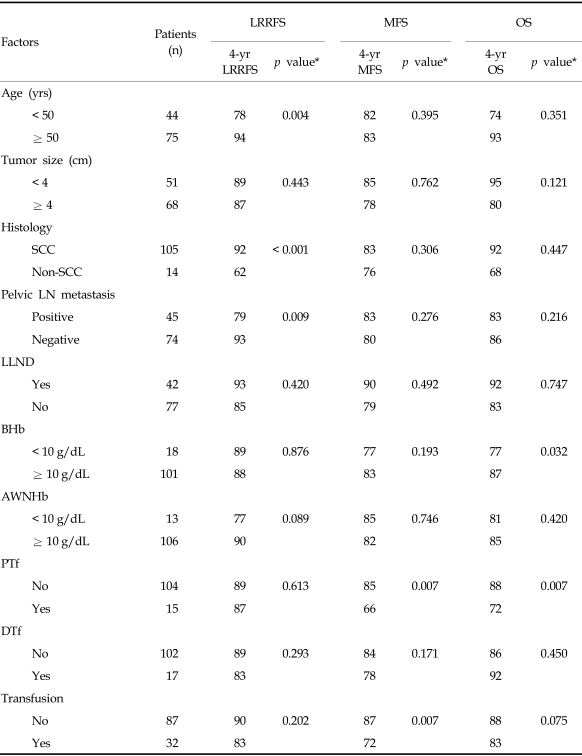
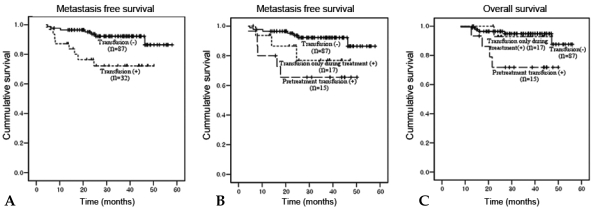
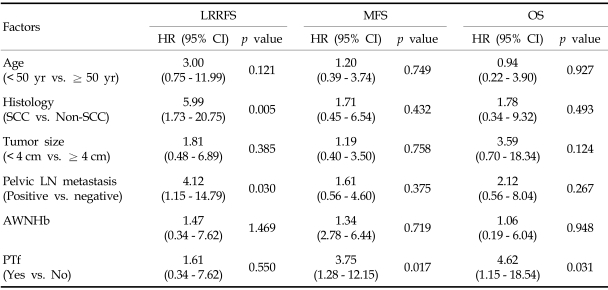
Locoregional recurrence developed in 12 patients (10%). Sites of recurrence included the cervix (n = 5), pelvic LN (n = 6), and pelvic peritoneum (n = 2). Distant metastases were observed in 14 patients (12%), located in the para-aortic LN (n = 10), liver (n = 1), lung (n = 3), abdominal peritoneum (n = 2), and supraclavicular LN (n = 1). Ten patients had combined locoregional recurrence and distant metastasis, with 4 of them having recurrent pelvic and para-aortic lymphadenopathies. Univariate analysis of clinical outcomes showed that BHb was associated with significantly poorer OS (p = 0.032). When the effect of BHb was further analyzed by the patients' age, BHb was significant for overall survival in patient of age ≥ 50 years (p = 0.028), but not for patients under 50 years (Fig. 4). Lower AWNHb level showed a tendency to be associated with higher locoregional recurrence, but this result did not reach a statistical significance (p = 0.089). Age below 50 years, non-squamous histology, the presence of metastatic pelvic LN and lower AWNHb level were other significant factors associated with the higher rate of locoregional recurrences (Table 4). From multivariable analyses, the presence of metastatic pelvic LN and non-squamous histology remained as prognostic factors for locoregional recurrence. Patients with a history of transfusion showed a significantly poorer metastasis-free survival than patients without transfusion (4-years metastasis-free survival, 72% vs. 87%, p = 0.007, Fig. 5a) and about 5.7 fold higher risk of distant metastases (HR 5.70, CI 1.26 - 10.73, p = 0.017, data not shown), although it did not reach statistical significance in terms of OS rates (4-years OS 83% vs. 88%, p = 0.075, Table 4 and Fig. 5c; HR=2.821, CI 0.72 - 11.11, p = 0.138, data not presented). The transfusion group was subdivided into the PTf and DTf, and the survival rates of the latter were compared with those who did not receive transfusion at all. When the PTf and DTf subgroups were further compared with the non-transfusion group, the 4-year MFS and OS rates were 66% vs. 78%, and 72% vs. 92%, respectively, while the corresponding figures were 87% and 88% for the non-transfusion group (Fig. 5b and c). Univariate analysis showed that DTf did not significantly affect outcome, whereas PTf resulted in a higher incidence of distant metastases and decreased survival. Furthermore, the history of PTf was the most significant predictor for the development of distant metastasis and OS on multivariable analysis (HR = 3.75, 95% CI: 1.28 - 12.15, p = 0.017; HR = 4.62, 95% CI: 1.15 - 18.54, p = 0.031, respecstively, Table 5).
Fig. 4.
Overall survival rate according to the age group, stratified by Baseline Hb 10g/dL. (A) Patients < 50 yrs, p = 0.36, (B) Patients ≥ 50 yrs, p = 0.028, p value calculated by log rank test. Solid line BHb ≥ 10 g/dL, Broken line BHb < 10 g/dL
Table 4.
Univariable Analysis of Clinical Outcome with Variables
AWNHb, average weekly nadir hemoglobin; BHb, baseline Hb; DTf, transfusion only during treatment; LN, lymph node; LLND, laparoscopic lymph node dissection; LRRFS, locoregional recurrence free survival; MFS, metastasis free survival; OS, overall survival; PTf, pretreatment transfusion; SCC, squamous cell carcinoma.
*p values were obtained from log-rank tests.
Fig. 5.
(A) Metastasis-free survival in patients with transfusion vs. non-transfusion (4-year MFS rates 72% vs. 87%, p = 0.007, p value calculated by log rank test). (B) Metastasis-free survival in patients with no transfusion, with transfusion only during treatment, or with transfusion before treatment (4-year MFS rates 87%, 78%, and 66%, respectively). (C) Overall survival in patients with no transfusion, with transfusion only during treatment, or with transfusion before treatment (4-year OS rates 88%, 92%, and 72%).
Table 5.
Multivariable Analysis of Clinical Outcome with Variables
AWNHb, average weekly nadir hemoglobin; LN, lymph node; LRRFS, locoregional recurrence free survival; MFS, metastasis free survival; OS, overall survival; PTf, pretreatment transfusion; SCC, squamous cell carcinoma.
DISCUSSION
Low Hb values in cancer are generally associated with poor prognosis.1 Therefore we examined the impact of anemia using Hb level lower than 10 g/dL, which is more severe anemia than in many other studies1-4 which used Hb value lower than 11.5 - 12.5 g/dL. Among the clinical parameters included in this study, tumor histology and the presence of metastatic pelvic LN were highly associated with locoregional recurrence on univariate analysis, whereas none of the Hb parameters used in this study showed a significant effect.
Baseline Hb and AWNHb were both associated with younger age in our study. This is interpreted as due to the presence of regular menstrual bleeding in the age below 50 years and also the difficulty in separating periodic bleeding from abnormal bleeding in this group of patients. In support of this speculation, the patient of over age 50 years had more normocytic anemia, suggesting the contribution of chronic disease to the low Hb compared to the younger patients. Also found in subgroup analysis was that BHb was significant for overall survival in patient age ≥ 50 year. Serum ferritin is known to be one of adverse prognostic marker which activates inflammatory signal and promotes cancer cell survival.19 The effect of anemia could be confounded by the ferritin or transferrin. Serum iron storage profiles such as serum iron, total iron-binding capacity, and ferritin level were not routinely checked in our series, therefore, we could not evaluate the role of those factors in our result. In our study, we also found that lower AWNHb was more frequently distributed in patients with LLND before radiotherapy. The relationship between LLND, lower AWNHb, and greater need for blood transfusion in this group of patients presently observed require cautious interpretation, because the number of patients with laparoscopy was small. Although ABT was successful in maintaining Hb levels above 10 g/dL during radiotherapy, Hb levels remained lower during the course of treatment in patients who underwent LLND compared with those who did not. Since the median and mean Hb values at initial presentation were similar in the LLND and non-LLND groups, the higher overall transfusion rate in both the LLND group may reflect that this surgical procedure itself as well as the preference of surgeons for ABT in patients who will undergo surgical procedure contribute to lowering the Hb level.
We found that patients who received ABT had a poorer prognosis and this adverse effect was distinctively apparent in the higher rate of distant metastases in the subset of patients who were given ABT before treatment started (PTf). We speculate that this finding might suggest that ABT negatively affected patient's outcome more seriously because it was given during the phase when the tumors were composed of large number of viable cancer cells and were more under the control of paracrine effect of tumor microenvironment and had disrupted tumor vasculature, whereas DTf was given mostly for correction of cisplatin-induced anemia in the later part of the treatment course. Once definitive radiotherapy with or without chemotherapy given and begins to induce the tumor bed effect,20-22 and once the tumors starts to regress, the ABT may no longer activate the tumor cells.23,24 Several mechanisms, based on pre-clinical findings, have been suggested to support the adverse effect of ABT, and the leukocyte component of donor blood is considered mostly responsible for the altered immune function. Decreased natural killer cell activity was observed in blood-transfused mice in an in vivo metastasis model and also in the plasma of the transfused patients along with the increased interleukin (IL)-10 level.14,25 In one study, higher median number of metastases was observed when there were higher white blood cell counts in the blood.15 In addition, the presence of allogeneic leukocytes in a co-culture of murine endothelial cells and lung carcinoma cells increased tumor cell adhesion, suggesting that allogeneic leukocytes influence metastatic potential by modulating intercellular adhesion molecules.26
However, our study has several limitations in showing that this adverse effect was solely influenced by allogeneic blood transfusion, although multivariable analysis revealed that history of ABT remained the strongest parameter in predicting distant metastases. First, our analysis was retrospective in nature and the implementation of transfusion could have been affected by the preference and robustness of individual physicians in recommending transfusion as well as by the choice of patients. Second, more importantly, there was a possibility that the presently observed effect of ABT was confounded by the presence of severe anemia because there were more patients with Hb below 9 g/dL in patients with ABT before treatment (data not shown). Hence, there is a possibility of a multicollinearity problem remaining between anemia and transfusion, which we could not fully identify with the small number of our patients. Nevertheless, our results indicate that our past practice in the management of anemia may have been erroneous.
The adverse effect of ABT is extremely important in the treatment of patients with cervical cancer because these patients are considered as a subset of patients who already have immune defense mechanisms attenuated. The development of invasive cervical cancer is due to interaction between HPV infection, the host immunity, and genetic factors.27 Treatment outcome in patients with cervical cancer was shown to correlate with plasma and cervical residual HPV viral load,28,29 again suggesting that the disruption of the local host immune barrier could be an important factor associated with poorer treatment outcome in this disease. Heavy bleeding may also contribute to a deterioration of immune function in these patients because isolated hemorrhages, even without tissue injury, have been shown to cause immunosuppression and higher mortality rate in mouse model.30
Although it is not clear whether the use of pre-storage leukocyte-depleted blood products would prevent these adverse effects, the use of leukocyte-free blood compound is strongly recommended in situations requiring blood transfusions, thus minimizing the immune modulating effects of ABT in patients with cervical cancer.15,31 It is of utmost importance, however, that ABT should be saved for the patients with acute blood loss who are considered in need of immediate correction of anemia, or for those patients who are chronically ill and cachexic, and therefore, tissue hypoxia is not likely to be compensated by normal physiologic mechanisms.
Footnotes
This work was supported by grants (061051-3) from the National Cancer Center of Korea.
References
- 1.Fyles AW, Milosevic M, Pintilie M, Syed A, Hill RP. Anemia, hypoxia and transfusion in patients with cervix cancer: a review. Radiother Oncol. 2000;57:13–19. doi: 10.1016/s0167-8140(00)00245-0. [DOI] [PubMed] [Google Scholar]
- 2.Marchal C, Rangeard L, Brunaud C. [Anemia impact on treatments of cervical carcinomas] Cancer Radiother. 2005;9:87–95. doi: 10.1016/j.canrad.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 3.Vaupel P, Thews O, Mayer A, Höckel S, Höckel M. Oxygenation status of gynecologic tumors: what is the optimal hemoglobin level? Strahlenther Onkol. 2002;178:727–731. doi: 10.1007/s00066-002-1081-x. [DOI] [PubMed] [Google Scholar]
- 4.Varlotto J, Stevenson MA. Anemia, tumor hypoxemia, and the cancer patient. Int J Radiat Oncol Biol Phys. 2005;63:25–36. doi: 10.1016/j.ijrobp.2005.04.049. [DOI] [PubMed] [Google Scholar]
- 5.Koukourakis MI, Giatromanolaki A, Sivridis E, Pastorek J, Karapantzos I, Gatter KC, et al. Hypoxia-activated tumor pathways of angiogenesis and pH regulation independent of anemia in head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2004;59:67–71. doi: 10.1016/j.ijrobp.2003.10.016. [DOI] [PubMed] [Google Scholar]
- 6.Dunst J. Management of anemia in patients undergoing curative radiotherapy. Erythropoietin, transfusions, or better nothing? Strahlenther Onkol. 2004;180:671–681. doi: 10.1007/s00066-004-9191-2. [DOI] [PubMed] [Google Scholar]
- 7.Obermair A, Cheuk R, Horwood K, Neudorfer M, Janda M, Giannis G, et al. Anemia before and during concurrent chemoradiotherapy in patients with cervical carcinoma: Effect on progression-free survival. Int J Gynecol Cancer. 2003;13:633–639. doi: 10.1046/j.1525-1438.2003.13395.x. [DOI] [PubMed] [Google Scholar]
- 8.Lavey RS, Liu PY, Greer BE, Robinson WR, 3rd, Chang PC, Wynn RB, et al. Recombinant human erythropoietin as an adjunct to radiation therapy and cisplatin for stage IIB-IVA carcinoma of the cervix: a Southwest Oncology Group study. Gynecol Oncol. 2004;95:145–151. doi: 10.1016/j.ygyno.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 9.Wun T, Law L, Harvey D, Sieracki B, Scudder SA, Ryu JK. Increased incidence of symptomatic venous thrombosis in patients with cervical carcinoma treated with concurrent chemotherapy, radiation, and erythropoietin. Cancer. 2003;98:1514–1520. doi: 10.1002/cncr.11700. [DOI] [PubMed] [Google Scholar]
- 10.Günter P. Practice guidelines for blood component therapy. Anesthesiology. 1996;85:1219–1220. doi: 10.1097/00000542-199611000-00052. [DOI] [PubMed] [Google Scholar]
- 11.Birgegård G, Bokemeyer C. New guidelines on anaemia management in patients with cancer: How do these affect clinical practice? Oncology. 2005;69 Suppl 2:17–21. doi: 10.1159/000088284. [DOI] [PubMed] [Google Scholar]
- 12.Murphy MF, Wallington TB, Kelsey P, Boulton F, Bruce M, Cohen H, et al. Guidelines for the clinical use of red cell transfusions. Br J Haematol. 2001;113:24–31. doi: 10.1046/j.1365-2141.2001.02701.x. [DOI] [PubMed] [Google Scholar]
- 13.Okuno K, Ozaki M, Shigeoka H, Nakajima I, Nakamura K, Hirohata T, et al. Effect of packed red cell and whole blood transfusion on liver-associated immune function. Am J Surg. 1994;168:340–344. doi: 10.1016/s0002-9610(05)80161-8. [DOI] [PubMed] [Google Scholar]
- 14.Clarke PJ, Burton RC, Wood KJ. Allogeneic blood transfusion reduces murine pulmonary natural killer (NK) activity and enhances lung metastasis of a syngeneic tumour. Int J Cancer. 1993;55:996–1002. doi: 10.1002/ijc.2910550620. [DOI] [PubMed] [Google Scholar]
- 15.Blajchman MA. Immunomodulatory effects of allogeneic blood transfusions: clinical manifestations and mechanisms. Vox Sang. 1998;74 Suppl 2:315–319. doi: 10.1111/j.1423-0410.1998.tb05437.x. [DOI] [PubMed] [Google Scholar]
- 16.Blajchman MA. Transfusion immunomodulation or TRIM: what does it mean clinically? Hematology. 2005;10 Suppl 1:208–214. doi: 10.1080/10245330512331390447. [DOI] [PubMed] [Google Scholar]
- 17.Yamamoto J, Kosuge T, Takayama T, Shimada K, Yamasaki S, Ozaki H, et al. Perioperative blood transfusion promotes recurrence of hepatocellular carcinoma after hepatectomy. Surgery. 1994;115:303–309. [PubMed] [Google Scholar]
- 18.Tefferi A. Anemia in adults: a contemporary approach to diagnosis. Mayo Clin Proc. 2003;78:1274–1280. doi: 10.4065/78.10.1274. [DOI] [PubMed] [Google Scholar]
- 19.Kwok JC, Richardson DR. The iron metabolism of neoplastic cells: alterations that facilitate proliferation? Crit Rev Oncol Hematol. 2002;42:65–78. doi: 10.1016/s1040-8428(01)00213-x. [DOI] [PubMed] [Google Scholar]
- 20.Clifton KH, Jirtle R. Mammary carcinoma cell population growth in preirradiated and unirradiated transplant sites. Viable tumor growth, vascularity, and the tumor-bed effect. Radiology. 1975;117:459–465. doi: 10.1148/117.2.459. [DOI] [PubMed] [Google Scholar]
- 21.Zips D, Eicheler W, Brüchner K, Jackisch T, Geyer P, Petersen C, et al. Impact of the tumour bed effect on microenvironment, radiobiological hypoxia and the outcome of fractionated radiotherapy of human FaDu squamous-cell carcinoma growing in the nude mouse. Int J Radiat Biol. 2001;77:1185–1193. doi: 10.1080/09553000110073402. [DOI] [PubMed] [Google Scholar]
- 22.Petersen C, Eicheler W, Baisch H, Zips D, Baumann M. Impact of preirradiation of the tumour bed on cell production rate and cell loss of human FaDu squamous cell carcinoma growing in nude mice. Int J Radiat Biol. 1999;75:1293–1297. doi: 10.1080/095530099139458. [DOI] [PubMed] [Google Scholar]
- 23.Kaminski JM, Shinohara E, Summers JB, Niermann KJ, Morimoto A, Brousal J. The controversial abscopal effect. Cancer Treat Rev. 2005;31:159–172. doi: 10.1016/j.ctrv.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 24.Demaria S, Ng B, Devitt ML, Babb JS, Kawashima N, Liebes L, et al. Ionizing radiation inhibition of distant untreated tumors (abscopal effect) is immune mediated. Int J Radiat Oncol Biol Phys. 2004;58:862–870. doi: 10.1016/j.ijrobp.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 25.Santin AD, Bellone S, Palmieri M, Bossini B, Dunn D, Roman JJ, et al. Effect of blood transfusion during radiotherapy on the immune function of patients with cancer of the uterine cervix: role of interleukin-10. Int J Radiat Oncol Biol Phys. 2002;54:1345–1355. doi: 10.1016/s0360-3016(02)03757-4. [DOI] [PubMed] [Google Scholar]
- 26.Quigley RL. The effect of leukocytes on adhesion molecules. An explanation of blood transfusion enhancement of tumor growth. Arch Surg. 1996;131:438–441. doi: 10.1001/archsurg.1996.01430160096021. [DOI] [PubMed] [Google Scholar]
- 27.zur Hausen H. Papillomaviruses in anogenital cancer as a model to understand the role of viruses in human cancers. Cancer Res. 1989;49:4677–4681. [PubMed] [Google Scholar]
- 28.Yang HJ, Liu VW, Tsang PC, Yip AM, Tam KF, Wong LC, et al. Quantification of human papillomavirus DNA in the plasma of patients with cervical cancer. Int J Gynecol Cancer. 2004;14:903–910. doi: 10.1111/j.1048-891X.2004.014528.x. [DOI] [PubMed] [Google Scholar]
- 29.Nagai Y, Toma T, Moromizato H, Maehama T, Asato T, Kariya K, et al. Persistence of human papillomavirus infection as a predictor for recurrence in carcinoma of the cervix after radiotherapy. Am J Obstet Gynecol. 2004;191:1907–1913. doi: 10.1016/j.ajog.2004.06.088. [DOI] [PubMed] [Google Scholar]
- 30.Hill GE, Frawley WH, Griffith KE, Forestner JE, Minei JP. Allogeneic blood transfusion increases the risk of postoperative bacterial infection: a meta-analysis. J Trauma. 2003;54:908–914. doi: 10.1097/01.TA.0000022460.21283.53. [DOI] [PubMed] [Google Scholar]
- 31.Bilgin YM, van de Watering LM, Eijsman L, Versteegh MI, Brand R, van Oers MH, et al. Double-blind, randomized controlled trial on the effect of leukocyte-depleted erythrocyte transfusions in cardiac valve surgery. Circulation. 2004;109:2755–2760. doi: 10.1161/01.CIR.0000130162.11925.21. [DOI] [PubMed] [Google Scholar]