Abstract
Purpose
Homograft benefits include excellent hemodynamics, resistance to infection, decreased thromboembolic events, ease of handling, and lack of need for anticoagulation. We examined the short and mid-term results of right ventricular outflow tract (RVOT) reconstruction using cryopreserved homografts.
Patients and Methods
From May 1998 to May 2005, 20 patients (male:female = 10:10) underwent RVOT reconstruction using cryopreserved homografts. The median age was 23.8 years (range, 0.9 to 43.3 years) and the median body weight was 57kg (range, 7.3 to 80kg). Eighteen patients underwent re-operation after shunt or corrective operations. Homograft failure was defined as either re-operation for homograft replacement or patient death. Homograft dysfunction was defined as grade 3 or more than 3 of graft regurgitation and more than 40mmHg of transvalvular pressure gradient under echocardiographic examination.
Results
No operative mortality occurred and there were three major complications. Graft failure was observed in one male patient with tetralogy of Fallot. The 8-year freedom from graft failure was 87.5 ± 11.7% and the 7-year freedom from graft dysfunction was 62.3 ± 17.9%. Multivariable analysis revealed that the independent factor for graft dysfunction was age less than 10 years. In the analysis according to age group, the 7-year freedom from graft dysfunction in the group of patients older than 10 years was 100% and 25.0 ± 21.7% in patients age 10 or younger (p = 0.03).
Conclusion
Right ventricular outflow reconstruction using cryopreserved homografts provided excellent short and mid-term results in most patients in this study. However, in patients younger than 10 years old, homografts for RVOT reconstruction showed a high dysfunction rate at mid-term.
Keywords: Homograft, pulmonary regurgitation, right ventricular outflow
INTRODUCTION
Use of an extracardiac conduit between the right ventricle and the pulmonary arteries has made possible the routine repair of pulmonary atresia, complex tetralogy of Fallot, truncus arteriosus, transposition of the great arteries with ventricular septal defect and pulmonary stenosis, and other complex forms of congenital heart disease.1-3
Cryopreserved homografts became the conduit of choice for right ventricular outflow tract (RVOT) reconstruction in the middle 1980s. Cryopreservation solved the storage and availability problems that plagued the early use of homografts. Indeed, cryopreserved aortic and pulmonary homografts have superior event-free survival in the right ventricle (RV) to pulmonary artery (PA) position when compared with porcine valve conduits.4 Homograft benefits include excellent hemodynamics, resistance to infection, lack of need for anticoagulation, ease of handling, option of using pulmonary artery branches, and decreased thromboembolic events.5
The use of valved homografts in the restoration of continuity from the RV to PA was first reported by Ross and Somerville in 1966.1 In Asia, however, homograft usage has not been popular due to a lack of organ donations. Since the establishment of the Yonsei Cardiovascular Tissue Bank at 1998 in our institute, homografts have been used for RVOT reconstruction, the Ross operation, and aortic valve replacement for infective endocarditis. The aim of this study was to evaluate the clinical results and efficacy of cryopreserved homografts for RVOT reconstruction.
PATIENTS AND METHODS
Patients
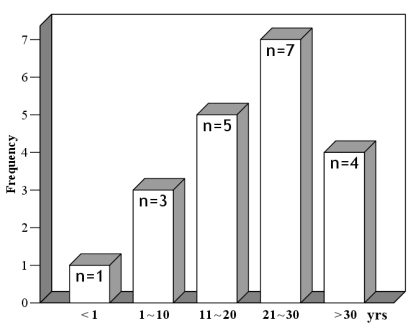
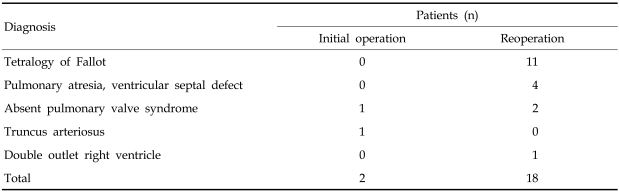
From May 1998 to May 2005, 20 patients (10 male, 10 female) underwent RVOT reconstruction using a cryopreserved homograft by a single surgeon. The median age was 23.8 years (range, 0.9 to 43.3 years) (Fig. 1). The median body weight was 57.0kg (range, 7.3 to 80kg) and the median body surface area was 1.56m2 (range, 0.39 to 1.77m2). Preoperative diagnoses are listed on Table 1. Fifty-five percent of the patients had tetralogy of Fallot. Eighteen patients were re-operated, and the mean time from the previous operation was 13.9 ± 8.0 years (1-32.7 years). Homografts were used initially in two patients. One was an 11 month-old female with truncus arteriosus and the other was a 7 year-old male with absent pulmonary valve syndrome. In the infant, the homograft was trimmed to a diameter of 15mm from 25mm because of the size mismatch of the native RVOT according to the bicuspidalization method described by Santini.6
Fig. 1.
Age distribution at the time of operation.
Table 1.
Preoperative Diagnosis
Homografts
The implanted homografts were sterile cryopreserved aortic and pulmonary valve conduits processed by the Yonsei Cardiovascular Tissue Bank. Homografts were harvested according to the protocol of the Yonsei Cardiovascular Tissue Bank. After sterile packaging, the homografts were frozen gradually (-1℃/min) down to -80℃ and then stored in the vapor phase of liquid nitrogen.
Nineteen patients received pulmonary homografts and one patient received an aortic homograft because a pulmonary homograft of the appropriate size was not available.
The mean preservation time was 579.6 ± 513.4 days (18-1820 days) and the internal diameter of the homografts ranged from 22mm to 38mm (mean diameter 25.5 ± 3.3mm).
Surgical technique
Through a median sternotomy, the heart and the great vessels were dissected free from adhesions to obtain proper exposure. Cardiopulmonary bypass was established with aortic and bicaval cannulation. During moderate hypothermia (28-32℃), the aorta was cross-clamped and myocardial protection was ensured by intermittent infusion of cold blood cardioplegic solution. A longitudinal incision was made through the RV outflow tract and extended to the main PA. If needed, the incision was extended to the right and left pulmonary arteries for angioplasty. The cryopreserved pulmonary homograft was inserted between the main PA and the RV at the level of the crista supraventricularis. A xeno-pericardial patch was also inserted as a hood to cover the enlarged right ventriculotomy.
Additive operations included augmentation of the PA branches (n = 2), patch closure of a ventricular septal defect (n = 2), coronary arterial bypass grafting (n = 1) due to injury of the right coronary artery across the RVOT in the patient with an anomalous origin of the coronary artery, patch closure of an atrial septal defect (n = 1), tricuspid annuloplasty (n = 1), mitral valvuloplasty (n = 1), plication of the main PA (n = 1), and patch aortoplasty (n = 1).
Data collection
Preoperative, postoperative, and late follow-up data were collected, including New York Heart Association (NYHA) functional class, left ventricular ejection fraction, RV end-diastolic dimension, ratio of RV systolic pressure and LV systolic pressure, duration of QRS wave, and transvalvular pressure gradient of homograft.
Follow-up and definition of terms
All patients (100%) were followed up through clinic visits and telephone interviews. The mean follow-up time was 4.1 ± 1.9 years (range, 1.6 to 8.3 years). Echocardiography and electrocardiogram were performed postoperatively (between 4-10 days after operation) and at the time of follow-up. The mean duration of echocardiographic follow-up was 3.2 ± 1.9 years (range, 1.2-7.4 years). During follow-up, symptomatic patients were investigated by echocardiography and occasionally by cardiac catheterization, and patients with arrhythmia underwent Holter monitoring.
Indications for replacement of the homograft were pulmonary stenosis with a minimum gradient of 50mm Hg, RV pressures greater than or equal to 75% of left ventricular pressure, or evidence of RV dilation and failure due to pulmonary homograft valve insufficiency.
Homograft failure was defined as all re-operations linked to the homograft and all valve-related deaths.7,8 Moderate (3+) pulmonary regurgitation (PR) and a transvalvular gradient greater than 40 mm Hg were defined as homograft dysfunction.7,9
Statistical methods
All analyses were performed with the SPSS 13.0 software package for Windows (SPSS Inc., Chicago, IL, USA). Mean values are presented ± 1 SD. Continuous variables were analyzed with the Wilcoxon Signed-Ranks method. Actuarial survival, homograft dysfunction, and homograft failure were generated using the Kaplan-Meier method and were compared using the log-rank test. Confidence limits of 95% were used to report survival.
Potential risk factors evaluated in the multivariable analysis of late conduit dysfunction included age, weight, sex, date of operation, diagnosis, previous cardiac operations, type of homograft (either aortic or pulmonary), size of homograft, duration of cryopreservation, and age of the homograft donor. The analysis of risk factors was performed using Cox's proportional hazards methods. A forward stepwise selection method was used to add variables to the model, requiring significance at p < 0.10 for entry and p < 0.05 for retention in the model. Statistical significance was defined as a p value of less than or equal to 0.05.
RESULTS
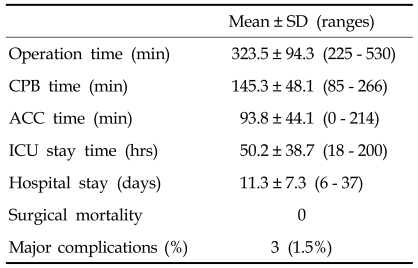
No In-hospital mortality occurred, but there were three complications: deep sternal infection, respiratory failure due to pneumonia, and injury of the right coronary artery across the RVOT in the patient with an anomalous origin of the coronary artery. In the latter instance, the 19 year-old male patient underwent coronary artery bypass grafting due to the injury of the right coronary artery. The coronary artery was a single coronary artery pattern and the right coronary artery was across the RVOT. We did not identify the coronary artery due to the severe adhesion. The right coronary artery was bypassed with the left internal thoracic artery. Other operative data are listed in Table 2. Preoperatively, most patients had right bundle branch block (n = 15, 75%), two had idiopathic ventricular tachyarrhythmia, and two had atrial arrhythmia. During follow-up, idiopathic ventricular tachyarrhythmia had disappeared in the two patients and did not recur.
Table 2.
Operative Data and Results
CPB, cardiopulmonary bypass; ACC, aortic cross clamp; ICU, intensive care unit.
There was no late death. One re-operation was performed in which the patient underwent a corrective operation for tetralogy of Fallot with angioplasty of both pulmonary arteries when he was one year old. At the age of 7 years, he underwent pulmonary valve replacement using a homograft, and 7.3 years later mechanical valve replacement of the pulmonary valve was performed due to severe homograft stenosis and regurgitation that was aggravated by branchial pulmonary artery stenoses. The 8-year freedom from graft failure was 87.5 ± 11.7%.
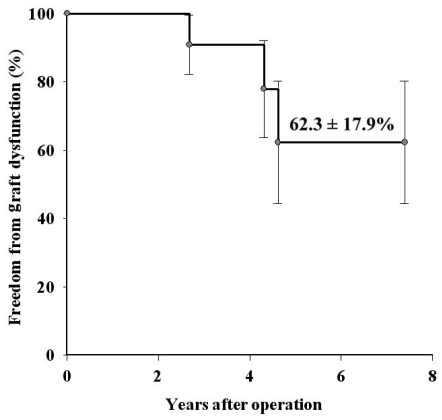
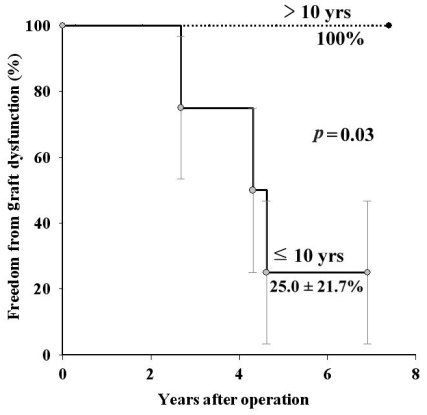
Graft dysfunction occurred in three patients, all less than 10 years old, due to homograft valve stenosis (n = 1), regurgitation (n = 1), and a mixed condition (n = 1). The 7-year freedom from graft dysfunction was 62.3 ± 17.9% (Fig. 1). The mean transvalvular pressure gradient was 14.3 ± 7.5 mmHg except in the patients with graft dysfunction. A multivariate Cox regression hazard model revealed that the independent factor for graft dysfunction was age less than 10 years (OR 2.23, CI 1.12-4.30, p = 0.048). The 7-year freedom from graft dysfunction according to age was 100% in patients older than 10 years of age, and 25.0 ± 21.7% in patients age 10 or under (p = 0.03).
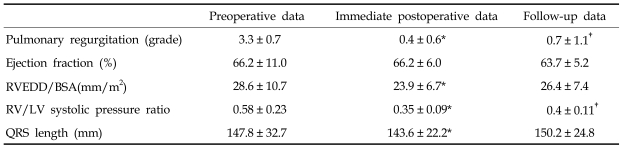
At follow-up, NYHA functional class significantly decreased from 1.95 ± 0.52 to 1.05 ± 0.23 (p < 0.001) and the degree of PR also decreased. LV ejection fractions were similar. RV end-diastolic dimension and duration of QRS wave significantly decreased in the postoperative period, but increased later so that ultimately there was no difference between preoperative and follow-up measurements (Table 3).
Table 3.
Echocardiographic Data and QRS Lengths
RVEDD, right ventricular end-diastolic dimension; BSA, body surface area; RV, right ventricular; LV, left ventricular.
*p < 0.05 when compared between preoperative and immediate postoperative data.
†p < 0.05 when compared between preoperative and follow-up data.
DISCUSSION
Many authors have reported that severe long-term PR and related RV overload may occur, despite the lack of symptoms, in progressive RV dilatation and dysfunction10 and poor exercise tolerance.11 The added effects of the abnormal right ventricle of patients with tetralogy of Fallot who underwent repair of RVOT obstruction (hypertrophy, conduction disturbances, and resection of muscle during repair) may contribute to RV dysfunction.12 The relationship between long-term PR and the increased risk for ventricular arrhythmias and sudden death is also of major concern.13,14
Optimal timing of surgery is essential for the treatment of PR to improve long-term outcomes. The most important surgical indication of PR is the hemodynamic state of the RV rather than patients' symptoms. In this study, despite the absence of evident symptoms, many of our patients had compromised exercise performance and abnormal RV hemodynamic and functional parameters, supporting the assumption that evaluation of symptoms alone does not reflect the functional RV derangement after valveless repair of RVOT obstruction. We evaluated the patients serially after previous RVOT reconstruction and decided on the operation after exact hemodynamic study with cardiac catheterization, echocardiography, and magnetic resonance imaging.
In 95% of patients, pulmonary homografts were inserted. Bando and colleagues 8 reported that although use of an aortic homograft is a significant risk factor for homograft failure, type of homograft is not correlated with patient mortality. Further, pulmonary homografts in RVOT reconstruction are more durable than aortic homografts because they have less calcification and obstruction. Because of the small size of the cohort in our study, we did not reach any conclusions about homograft type. We prefer pulmonary homografts for RVOT reconstruction. Only one aortic homograft was inserted due to the unavailability of a properly sized pulmonary homograft.
Even though the degree of preoperative PR was significantly lower at late follow-up, the indexed RV end-diastolic diameter by body surface area, the ratio of RV systolic pressure/LV systolic pressure, and the duration of QRS complex decreased postoperatively and then increased at late follow-up, such that there was no difference with the preoperative measurements. We assumed that the late RV dilatation may result from anomalies of the distal pulmonary vasculature and residual pulmonary hypertension. We suggest that patients who have severe pulmonary hypertension or underdevelopment of the pulmonary vasculature need regular and more frequent follow-up for evaluation of RV dysfunction.
Known risk factors for failure of cryopreserved homograft include small diameter of the homograft, young age, and low body weight.15,16 On the base of this knowledge, we tried to place a homograft with a larger diameter than that of original annulus of the pulmonary valve to reduce failure rates. In our study, multivariate analysis revealed that the independent factor of late homograft dysfunction was age less than 10 years. It may not be surprising that homografts in small patients fail sooner than in fully grown patients, as the patients outgrow them. We may reconsider the use of homografts in younger patients, especially in view of the lack of supply. We think that safer substitutes for RVOT with greater longevity should be developed. Nowadays, innovative techniques for pulmonary valve replacement such as percutaneous and off-pump approaches have been introduced, and these techniques may prove useful in small patients who should undergo re-operation.17
In conclusion, RV outflow reconstruction using cryopreserved homografts provided excellent short and mid-term results in most patients we studied. However, in patients younger than 10 years old, homografts for RVOT reconstruction showed a high dysfunction rate at mid-term.
Fig. 2.
Overall freedom from graft dysfunction.
Fig. 3.
Freedom from graft dysfunction according to age. Straight line-age 10 years or less, dotted line-More than 10 years old.
References
- 1.Ross DN, Somerville J. Correction of pulmonary atresia with a homograft aortic valve. Lancet. 1966;2:1446–1447. doi: 10.1016/s0140-6736(66)90600-3. [DOI] [PubMed] [Google Scholar]
- 2.Klinner W, Zenker R. Experience with correction of Fallot's tetralogy in 178 cases. Surgery. 1965;57:353–357. [PubMed] [Google Scholar]
- 3.McGoon DC, Rastelli GC, Ongley PA. An operation for the correction of truncus arteriosus. JAMA. 1968;205:69–73. [PubMed] [Google Scholar]
- 4.Albert JD, Bishop DA, Fullerton DA, Campbell DN, Clarke DR. Conduit reconstruction of the right ventricular outflow tract. Lessons learned in a twelve-year experience. J Thorac Cardiovasc Surg. 1993;106:228–236. [PubMed] [Google Scholar]
- 5.Jonas RA, Freed MD, Mayer JE, Castaneda AR. Long-term follow-up of patients with synthetic right heart conduits. Circulation. 1985;72:II77–II83. [PubMed] [Google Scholar]
- 6.Santini F, Mazzucco A. Bicuspid homograft reconstruction of the right ventricular outflow tract in infants. Ann Thorac Surg. 1995;60:S624–S625. doi: 10.1016/0003-4975(96)81282-3. [DOI] [PubMed] [Google Scholar]
- 7.Bando K, Danielson GK, Schaff HV, Mair DD, Julsrud PR, Puga FJ. Outcome of pulmonary and aortic homografts for right ventricular outflow tract reconstruction. J Thorac Cardiovasc Surg. 1995;109:509–518. doi: 10.1016/S0022-5223(95)70282-2. [DOI] [PubMed] [Google Scholar]
- 8.Daenen W, Gewillig M. Factors influencing medium-term performance of right-sided cryopreserved homografts. J Heart Valve Dis. 1997;6:347–353. [PubMed] [Google Scholar]
- 9.Edmunds LH, Clark RE, Cohn LH, Grunkemeier GL, Miller DC, Weisel RD. Guidelines for reporting morbidity and mortality after cardiac valvular operations. The American Association for Thoracic Surgery, Ad Hoc Liasion Committee for Standardizing, Definitions of Prosthetic Heart Valve Morbidity. Ann Thorac Surg. 1996;62:932–935. doi: 10.1016/s0003-4975(96)00531-0. [DOI] [PubMed] [Google Scholar]
- 10.Gatzoulis MA, Clark AL, Cullen S, Newman CG, Redington AN. Right ventricular diastolic function 15 to 35 years after repair of tetralogy of Fallot. Restrictive physiology predicts superior exercise performance. Circulation. 1995;91:1775–1781. doi: 10.1161/01.cir.91.6.1775. [DOI] [PubMed] [Google Scholar]
- 11.Jonsson H, Ivert T, Jonasson R, Holmgren A, Björk VO. Work capacity and central hemodynamics thirteen to twenty-six years after repair of tetralogy of Fallot. J Thorac Cardiovasc Surg. 1995;110:416–426. doi: 10.1016/S0022-5223(95)70238-5. [DOI] [PubMed] [Google Scholar]
- 12.Bove EL, Kavey RE, Byrum CJ, Sondheimer HM, Blackman MS, Thomas FD. Improved right ventricular function following late pulmonary valve replacement for residual pulmonary insufficiency or stenosis. J Thorac Cardiovasc Surg. 1985;90:50–55. [PubMed] [Google Scholar]
- 13.Yemets IM, Williams WG, Webb GD, Harrison DA, McLaughlin PR, Trusler GA, et al. Pulmonary valve replacement late after repair of tetralogy of Fallot. Ann Thorac Surg. 1997;64:526–530. doi: 10.1016/S0003-4975(97)00577-8. [DOI] [PubMed] [Google Scholar]
- 14.Joung B, Xu Z, Kim I, Lee MH, Kim S. The effect of cryoinjury on ventricular tachycardia in the swine right ventricle. Yonsei Med J. 2006;47:672–679. doi: 10.3349/ymj.2006.47.5.672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bielefeld MR, Bishop DA, Campbell DN, Mitchell MB, Grover FL, Clarke DR. Reoperative homograft right ventricular outflow tract reconstruction. Ann Thorac Surg. 2001;71:482–488. doi: 10.1016/s0003-4975(00)02521-2. [DOI] [PubMed] [Google Scholar]
- 16.Baskett RJ, Ross DB, Nanton MA, Murphy DA. Factors in the early failure of cryopreserved homograft pulmonary valves in children: preserved immunogenicity? J Thorac Cardiovasc Surg. 1996;112:1170–1179. doi: 10.1016/S0022-5223(96)70130-7. [DOI] [PubMed] [Google Scholar]
- 17.Khambadkone S, Coats L, Taylor A, Boudjemline Y, Derrick G, Tsang V, et al. Percutaneous pulmonary valve implantation in humans: results in 59 consecutive patients. Circulation. 2005;112:1189–1197. doi: 10.1161/CIRCULATIONAHA.104.523266. [DOI] [PubMed] [Google Scholar]