Abstract
A substantial literature demonstrates that the main ultrafine particles found in ambient urban air are combustion-derived nanoparticles (CDNP) which originate from a number of sources and pose a hazard to the lungs. For CDNP, three properties appear important-surface area, organics and metals. All of these can generate free radicals and so induce oxidative stress and inflammation. Inflammation is a process involved in the diseases exhibited by the individuals susceptible to the effects of PM-development and exacerbations of airways disease and cardiovascular disease. It is therefore possible to implicate CDNP in the common adverse effects of increased PM. The adverse effects of increases in PM on the cardiovascular system are well-documented in the epidemiological literature and, as argued above, these effects are likely to be driven by the combustion-derived NP. The epidemiological findings can be explained in a number of hypotheses regarding the action of NP:-1) Inflammation in the lungs caused by NP causes atheromatous plaque development and destabilization; 2) The inflammation in the lungs causes alteration in the clotting status or fibrinolytic balance favouring thrombogenesis; 3) The NP themselves or metals/organics released by the particles enter the circulation and have direct effects on the endothelium, plaques, the clotting system or the autonomic nervous system/ heart rhythm. Environmental nanoparticles are accidentally produced but they provide a toxicological model for a new class of purposely 'engineered' NP arising from the nanotechnology industry, whose effects are much less understood. Bridging our toxicological knowledge between the environmental nanoparticles and the new engineered nanoparticles is a considerable challenge.
Keywords: Nanoparticles, nanotubes, particulate matter, inflammation, cardiovascular, endothelium, atherothrombosis
INTRODUCTION
The production of new forms of manufactured/engineered nanoparticles is of increasing concern as nanotechnology continues to develop and manufacture them.1 These form a plethora of particle types that include nanotubes, fullerenes, quantum dots and compound particles of various types. Whilst information on the toxicity of new types of nanoparticles (NP) is accumulating these are mostly in vitro studies, with few animal or human studies. The existing toxicology knowledge regarding NP is almost entirely based on combustion-derived nanoparticles (CDNP) present in environmental air. Evolving from the 'ultrafine hypothesis',2,3 this strand of research has focused on CDNP like diesel soot since this component of particulate matter (PM) is seen as a key component mediating adverse health effects. The mechanism at the cellular level is understood in terms of the ability of particles to cause oxidative stress and inflammation and translocate from the site of deposition.4 This review builds on environmental NP and their mechanisms, as a basic paradigm and then moves on to discuss toxicology of engineered NP.
The portal of entry for NP discussed here is the lungs and the toxic effects seen there are discussed. In addition the lungs and the cardiovascular system are intimately linked and the PM10 literature indicates clearly that the cardiovascular system is a lead target system for the adverse effects of PM.
COMBUSTION-DERIVED NANOPARTICLES IN ENVIRONMENTAL AIR POLLUTION
PM10 and its adverse effects
The adverse health effects of air pollution have been recognised throughout much of recorded time and are now documented in large international epidemiological studies. In the UK, fossil fuel combustion in towns and cities, during periods of cold weather, where there is little mixing of air have been associated with the generation of smog episodes. These smogs consisted largely of sulphur dioxide and particles and could very high concentrations in urban air. The particle component or PM represents a key part of the air pollution cocktail present in ambient air, which also comprises gases such as ozone, nitrogen dioxide etc. PM in ambient air is measured as the mass of particles collected using the PM10 or PM2.5 sampling conventions.5 The adverse health effects of PM are seen at the levels that pertain in UK and other cities today and there is often no threshold. In other words there is a background of ill health being caused by PM that increases when the ambient particle cloud increases in concentration and goes down when the amount of particles in the air decreases.6
These adverse health effects of air pollution have been measured in hundreds of studies and there is good coherence between the acute effects seen in time series and panel studies, and the chronic effects seen in environmental studies.
Nanoparticles as the most toxic component of PM10
PM is a complex mixture of particle types that depend on season, time of day, site of sampler etc. CDNP are present in PM from conurbations and are a major toxicologically important component. CDNP originates principally from car exhausts although there are other sources.4 Sulphates tend to be very low in toxicity in experimental studies, 7 but do show a relationship with adverse effects in some epidemiological studies;8 this apparent anomaly may be explained by a correlation between sulphates and some more potent component of the air pollution mix which is actually driving the adverse effect, such as fine particles.
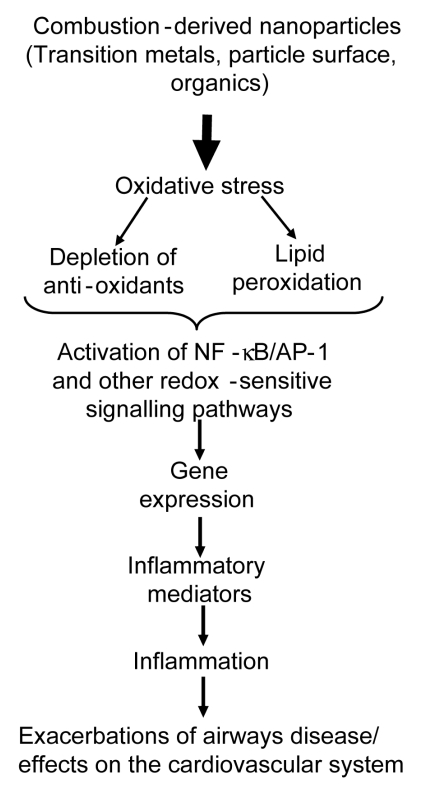
NP number, likely to be principally comprised of CDNP, ranged from 15,000 to 18,000 particles per cm3 in 3 European cities9 and 10,000 to 50,000 particles per 3 in a busy London street.10 In a study on US highways exposure in a vehicle travelling in busy traffic was reported to be 200 to 560 × 103 particles per cm3, (predominantly NP).11,12 Indoor air also contains NP and cooking, vacuuming and burning wax candles produce NP of soot.13 NP also produced during combustion of domestic gas and in one study 3 gas rings produced around 50,000 particles per cm3 which underwent rapid aggregation within a few minutes, as evidenced by increases in particle size and decrease in apparent number.14 Secondary NP also arise from environmental chemistry, e.g. nitrates; but these are unlikely to be as toxicologically potent as CDNP (see below). The molecular mechanisms of the adverse effects of CDNP has been extensively reviewed by the authors4,15,16 and the pro-inflammatory mechanism is summarised in Fig. 1.
Fig. 1.
Hypothetical series of events leading from combustion-derived NP such as diesel soot interactions with lung cells leading to inflammatory gene expression.
CDNP AND THE LUNGS
The present understanding of CDNP activity in the lungs is that the surfaces, organics and metals can all produce free radicals with the potential to produce oxidative stress and contribute to inflammation. Diesel exhaust particles (DEP) are one of the main CDNP to which individuals are exposed. DEP causes inflammation in rat17,18 and human lungs19 following short-term, high level exposure. Oxidative stress is demonstrable as increased levels of 8-OHdG, the oxidative DNA adduct of the hydroxyl radical, in the lungs of rats following exposure and in cells in culture treated with DEP.20,21 The component of DEP responsible for the oxidative stress and subsequent pro-inflammatory signalling is principally the organic fraction,22-25 although transition metals may also be involved especially for welding fume.26 The oxidative stress then causes activation of signalling pathways for pro-inflammatory gene expression, including MAPK22,27-29 and NF-κB activation22,30 and histone acetylation that favours pro-inflammatory gene expression.31 Activation of these pathways culminates in transcription of a number of pro-inflammatory genes such as interleukin-8 (IL-8) in epithelial cells treated in vitro32 and in human lungs exposed by inhalation.33 Tumour necrosis factor-alpha (TNF-α) has been reported to be increased in macrophages exposed to DEP in vitro34 and interleukin-6 (IL-6) is released by primed human bronchial epithelial cells exposed to DEP.35
CARDIOVASCULAR EFFECTS OF PM AND CDNP-POTENTIAL EFFECTS ON ENDOTHELIUM AND ATHEROSCLEROTIC PLAQUE STABILITY
The adverse cardiovascular events associated with increases in PM36,37 could be mediated through the effects of CDNP. In a mixture of studies which have used PM, CAPS and model NP, evidence is accumulating that NP cause inflammation that could adversely affect the cardiovascular system. There is evidence of systemic inflammation following increases in PM, as shown by elevated C-reactive protein, blood leukocytes, platelets, fibrinogen and increased plasma viscosity (reviewed in reference 38). Atherothrombosis is the principle cause of cardiovascular morbidity and mortality.39 Atherosclerosis is an inflammatory process, initiated via endothelial injury and producing systemic markers of inflammation that are risk factors for myocardial and cerebral infarction.39-41 Repeated exposure to PM10 may, by increasing systemic inflammation, exacerbate the vascular inflammation of atherosclerosis and promote plaque development or rupture. Experimental studies with animal models susceptible to atherosclerosis confirm the ability of particle exposure to enhance atherosclerosis.42,43
Normally the endothelial monolayer delicately balances regulatory pathways controlling vasomotion, thrombosis, cellular proliferation, inflammation and oxidative stress. Endothelial dysfunction or denudation is one of the earliest pathological features of atherosclerosis.44 Loss of endothelial function results in expression of leukocyte adhesion proteins, reduced anticoagulant activity and the release of growth factors, inflammatory mediators and cytokines. Chronic inflammation results in leukocyte and monocyte recruitment, induction of atheroma formation and further arterial damage. Plaque expansion and disruption can lead to angina, crescendo angina and acute coronary syndromes, including myocardial infarction.44-46
Inhaled PM may influence the vasculature through indirect effects mediated by pulmonary inflammation or through the direct action of particles that have become blood-borne. Whether inhaled NP can access the circulation is currently the subject of intense research47-49 and there is conflicting reports on whether Technegas-radioactive carbon NP-can reach the blood following inhalation in humans.50,51 Certainly, injured arteries can take up blood borne NP, a fact exploited by the nanotechnology industry for both diagnostic and therapeutic purposes in cardiovascular medicine. The intra-arterial infusion of carbon black NP has a detrimental effect on the mouse microcirculation with up regulation of von Willebrand factor expression and enhanced fibrin deposition on the endothelial surface.52 These prothrombotic effects are in keeping with toxicological evidence from instillation studies, which suggest particle exposure may promote thrombogenesis.53,54
The endothelium plays a vital role in the control of blood flow, coagulation, fibrinolysis and inflammation. Following the seminal work of Furchgott and Zawadski,55 it is widely recognised that an array of mediators including cigarette smoking can influence vascular tone through endothelium-dependent actions, and there is now extensive evidence of abnormal endothelium-dependent vasomotion in patients with atherosclerosis.56-58 Mild systemic inflammation also causes a profound, but temporary suppression of endothelium-dependent vasodilatation.59 However, whilst endothelium-dependent vasomotion is important, it may not be representative of other aspects of endothelial function, such as the regulation of fibrinolysis.
The fibrinolytic factor tissue plasminogen activator (t-PA) regulates the degradation of intravascular fibrin and is released from the endothelium through the translocation of a dynamic intracellular storage pool.59,60 If endogenous fibrinolysis is to be effective, then the rapid mobilization of t-PA from the endothelium is essential because thrombus dissolution is much more effective if t-PA is incorporated during, rather than after, thrombus formation.61,62 The efficacy of plasminogen activation and fibrin degradation is further determined by the relative balance between the acute local release of t-PA and its subsequent inhibition through formation of complexes with plasminogen activator inhibitor type 1 (PAI-1). This dynamic aspect of endothelial function and fibrinolytic balance may be directly relevant to the pathogenesis of atherothrombosis.
CDNP and endothelial dysfunction
In order to investigate the potential role of the endothelium in triggering of acute myocardial infarction following CDNP exposure we investigated the effects of diesel exhaust inhalation on vascular and endothelial function in humans.63 In a double-blind, randomized, cross-over study, 30 healthy men were exposed to diluted diesel exhaust at 300µg/m3 particulate, or air, for 1 hour with intermittent exercise. Two and six hours after exposure, bilateral forearm blood flow was measured following infusions of the endothelium- dependent vasodilators bradykinin (BK) and acetylcholine (ACh), and endothelium-independent vasodilators sodium nitroprusside (SNP) and verapamil. Inflammatory mediators in blood were measured concomitantly. We showed no differences in resting forearm blood flow or inflammatory markers after exposure to diesel exhaust or air. There was a dose-dependent increase in blood flow with each vasodilator, but this vasomotor response was significantly attenuated to BK, ACh and SNP (p < 0.001) infusions 2 hours after exposure to diesel exhaust, and remained impaired at 6 hours.
In addition, BK caused a dose-dependent increase in plasma t-PA that was suppressed 6 hours after exposure to diesel (p < 0.001; area under the curve decreased by 34%). We concluded that, at levels encountered in an urban environment, inhalation of dilute diesel exhaust impairs two important and complementary aspects of vascular function in humans: the regulation of vascular tone and endogenous fibrinolysis. The likely mechanism- is shown in Fig. 2 and is based on the central role of nitric oxide (NO) in the maintenance of vascular tone.
Fig. 2.
Hypothetical scheme to explain the observed effects of DEP exposure on vasomotor function63 see text for explanation.
The vasodilator drugs ACh and BK act on receptors in the endothelium to stimulate calcium increase and endothelial nitric oxide synthase (eNOS) activation leading to local nitric oxide (NO) levels that activate gunayl cyclase in subjacent smooth muscle cells stimulating relaxation. SNP is a NO donor and increases vascular NO directly by an endothelial-independent pathway, while Verapamil causes smooth muscle relaxation by an endothelium-independent and NO-independent pathway. The observed blunting of the vasomotor response to ACh, BK and SNP could be explained by oxidative stress from pulmonary inflammation or from particles that gain access to the blood. In this scenario, superoxide anions in the vessel wall, resulting from oxidative stress, rapidly combine with NO to form peroxynitrite. Therefore less NO is available in the smooth muscle cells and relaxation is blunted.
This explanation combines the well-known oxidative stressing and inflammatory effects of CDNP with key endothelial functions and so provides a potential mechanism that links air pollution to the pathogenesis of atherothrombosis and acute myocardial infarction.63
Engineered nanoparticles
One of the major reasons behind the rapid expansion in industrial use of nanotechnology and in particular engineered NP themselves, are their unique properties due to small size and large reactive surface area. NP come in a wide variety of shapes, sizes and chemical compositions. In addition to the spherical shapes observed for particles such as titanium dioxide (TiO2), shape varieties also include carbon nanotubes, nanowhiskers and nanofibres. Engineered NP vary considerably in their size and composition and so would be anticipated to vary in toxicity. Nanotubes and nanowires can range from less than 100 nm diameter to tens of µm in length. The variety of chemical composition range from substances considered traditionally to be relatively inert (e.g. carbon and gold) to substances associated with significant toxicity (e.g. cadmium and other heavy metals). Since the NP size imparts heightened reactivity to the 'inert' materials, it is interesting to consider the impact of small size on toxic materials, especially since reactivity might relate to toxicity.
Engineered nanoparticles and the lungs
Carbon black (CB) and TiO2 along with alumina and silica have been studied for some time with regard to their pro-inflammatory effects but none of these studies have explicitly addressed effects on the cardiovascular system. Nano-size CB has been intensively studied with regard to the issue of low toxicity dust and the confounding effect of rat lung overload.64-67 In view of the very high surface area per unit volume of NP and the identification of surface area as the driver for overload,68-70 attention has been focused on the nanoparticulate form of these nuisance dusts. These would be anticipated to produce lung overload at lower mass lung burdens than seen with the larger particles and in fact, was indeed shown to be the case.70,71 However, even at low, non-overload exposures to nano-sized CB, there was a pro-inflammatory effect not seen with the larger CB particles.72 Instillation studies have also shown that the nanoparticulate form of CB and TiO2 produce more inflammation than an equal mass of larger, yet respirable particles73,74 of the same material and across a range of NP of nuisance dust the surface area was found to be the driver of the inflammation.75
The molecular mechanism of the increased inflammatory effects of nanoparticle CB have demonstrated that they generate reactive oxygen species (ROS) in cell-free systems76-78 and cause alterations in calcium signaling79,80 in exposed cells. Oxidative stress from the nanoparticulate CB can also activate the EGF-receptor81 and redox-responsive transcription factors such as NF-κB80 and AP-182 leading to the transcription of pro-inflammatory cytokines and lipid mediators.78,80
Carbon nanotubes
Carbon nanotubes (CNT) are long sheets of graphite rolled in the form of a tube, that can range from a few nm thick (single-walled-SWCNT) up to a few hundred nm thick (multi-walled-MWCNT). The needle-like structure implies that a paradigm related to fibres, such as asbestos, might be appropriate in considering their toxicity. The potential pathogenicity of a conventional fibre is dictated by length greater than 20µm, thinness and biopersistence.83 Biopersistence is an important determinant of mineral fibres and Synthetic Vitreous Fibre pathogenicity. Long biopersistent fibres are the biologically effective dose that drives pathogenic effects83 whilst non-biopersistent fibres undergo dissolution processes that can be enhanced at the acid pH of 5.0 existing inside macrophage phagolysosomes.84 Long, biosoluble fibres undergo leaching of key structural molecules leading to breakage into short fibres that are readily phagocytosed by macrophages.83,85
In one study,86 the authors addressed the important issue of biopersistence of CNT using both un-ground and ground nanotubes, 0.7 and 5.9µm long respectively. These were assessed for biopersistence and the longer, un-ground nanotubes were more biopersistent than the short ones. This is consistent with the greater biopersistence of long fibres seen in studies with asbestos and other mineral fibres although these 'long' nanotubes were much shorter than those mineral fibres defined as 'long', which are in the region of 20µm and greater.83 A 20µm diameter rat macrophage is able to enclose and transport fibres less than its own diameter from the lungs,83 and the length-dependent inhibition of clearance seen with 5.9µm long nanotubes is thus rather unexpected. It may be that the well-documented tendency for nanotubes to form bundles and wires87 is important in impairment of clearance.
Nanotubes have been used in a number of rat lung instillation studies.83,88-90 All of these used high dose and dose-rate which raises questions about physiological relevance; no study has addressed the role of length by comparing long (> -20µm) with short (< 10µm) CNT. However all of the studies mentioned above showed an increased ability of CNT to cause granulomatous fibrosis in the absence of severe inflammation.
CNT have also been tested in a range of different cell types in vitro to assess their potential toxicity. Treatment of human keratinocytes have shown that both SWCNT and MWCNT are capable of being internalized, causing cellular toxicity.91,92 In a study with alveolar macrophages, SWCNT were more cytotoxic than MWCNT after exposure at equal mass dose.93 Human T cells exposed to oxidised MWCNT were killed in a time- and dose-dependent manner, through mechanisms involving apoptosis94 as was also the case in kidney cells exposed to SWCNTs.95 Manna et al., demonstrated dose-dependent oxidative stress and NF-κB activation in human keratinocytes along with IκB depletion and MAPK phosphorylation.96 In vitro, CNT can produce free radicals by the role of iron via Fenton-type reactions.97
It is difficult to draw general conclusions on CNT toxicity because of the scarcity of data and CNT variability-they can vary in length and composition including metal contamination. CNT are often kinked and tangled into aggregates of varying size and shape. This kind of variability is found both between and within samples. All of these factors could impact on toxicity. More rigid CNT are likely to disperse more efficiently than tangled CNT. However the tangles are more easily taken up by cells in culture and could therefore be more readily cleared from the lungs by macrophages. A programme of research is warranted to define the factors that control CNT toxicity.
Engineered NP and the cardiovascular system
Radomski et al., examined the role of new NP on the clotting system98 studying the effects of multi-walled and single-walled nanotubes, C60 fullerenes and mixed carbon black NP on human platelet aggregation in vitro and rat vascular thrombosis in vivo. Standard urban PM was used as a control. Nanotubes and carbon black particles but not C60, stimulated platelet aggregation and the same ranking was observed in their ability to affect the rate of vascular thrombosis in rat carotid arteries; urban dust had low activity in these assays. Thus, there are differences between different carbon NP to activate platelets and enhance vascular thrombosis. Yamawaki et al., have shown that carbon back of aggregate size 248nm are cytostatic, cytotoxic and pro-inflammatory in endothelial cells.99
Engineered NP and Quantitative Structure Activity Relationships
The numbers of new NP that are being produced pose a special problem in testing. NP are relatively easy to alter in terms of physicochemistry, size, coating, composition etc and this makes for even more particles to be tested. Other factors are also important-the average small company that is developing a new NP type is unlikely to have the funds to carry out proper toxicology testing and the current climate against animal testing makes this type of testing not viable.
In the pharmacology and toxicology worlds, the term QSAR (Quantitative structure activity relationship) is used to describe the attempt to relate chemical structure to pharmacological or toxicological activity. This idea could be used to categorise NP on the basis of physicochemistry, if physicochemical markers could be related to toxicity. The most obvious candidates for structural markers that could be related to toxicity markers at the moment are size/surface area and oxidative stress. For insoluble particles the surface area times the surface reactivity describes a biologically effective dose (the dose that drives adverse effects) and so particle size is likely to be important. For NP, the quantum effect changes in the physicochemical nature of the surface of a very small particle compared to a larger one and could plausibly impact on toxicology. The dominant hypothesis for the action of harmful particles on cells is oxidative stress and the oxidative stressing activity of particles is a physicochemical parameter that may well be important in structure activity considerations.
The QSAR idea is likely to be achievable for predicting lung inflammation and so could be predictive for cardiovascular effects if they are driven by pulmonary inflammation. For translocation from the lungs to the blood or for effects in the blood, a different QSAR may be necessary.
Potential carcinogenic effects of manufactured NP
The ability of PM to cause cancer is well documented and other types of particles, such as asbestos and silica, are also known to be carcinogenic. The mechanisms are, however, not completely understood and may involve both direct genotoxic effects of the particles themselves and indirect genotoxic effects mediated through the particles ability to cause inflammation. Direct genotoxic effects of particles involve the particles entering cells and delivering damage to DNA. The chemical composition, as well as the structural composition of the particles, both plays a role in this and so CDNP certainly have the potential to mediate this type of effect.100 Transition metals have been shown to redox cycle inside the cell and generate damaging hydroxyl radicals that form mutagenic adducts with DNA.101 Organic molecules adsorbed on to the surface of CDNP, such as polycyclic aromatic hydrocarbons (PAHs), can also form adducts102 whilst large surface areas on NP are capable of generating oxidative stress. 100 In addition, the inflammatory effects of CDNP, as discussed above, can play an important role in the genotoxic and carcinogenic processes and the products of the leukocyte oxidative burst can form adducts within target cells.103 The effects of oxidative stress inside the cell may cause lipid peroxide production in the cell and the products of lipid peroxidation are longer-lived than the ROS themselves and may therefore mediate adduct formation.104
Certain types of NP appear to be able to enter the nucleus in cell culture systems, to a much greater extent than larger particles of the same material105 and NP in general seem to be cable of crossing biological membranes.106 If NP generally gain access to the nucleus rather than being retained in the cytoplasm like larger particles, then by virtue of being closer to the DNA, the oxidative products they produce may be more likely to cause genotoxic effects.
CONCLUSION
A paradigm has evolved arising from experience with environmental CDNP, exemplified by diesel soot. In this paradigm, oxidative stress and inflammation are identified as key processes in the local effects in the lungs. In addition, inflammatory effects and blood translocation could explain adverse cardiovascular effects observed in epidemiology studies with air pollution particles. Support for this contention comes from a number of studies using model NP and CDNP, where adverse cardiovascular effects such as clotting, plaque development and endothelial dysfunction are enhanced after NP exposures in a number of different models. In parallel with these studies, an increasing number of toxicology studies using bulk NP, such as TiO2 and CB, have identified a key role for the large surface area of NP and its ability to produce oxidative stress.78,107,108 It is not known whether the same paradigm can be used for new engineered NP and nanotubes. In the limited studies so far published, engineered NP, such as the CNT are also reported to induce oxidative stress, cell death and inflammation. However, there are differences in the magnitude of the adverse effects caused by NP between models and not all NP are likely to have the same toxic potency. This is to be anticipated since the total toxicity of any particle sample is the complex sum of the surface reactivity times the surface area plus releasable toxic moieties, along with shape, and all modified by the degree of biopersistence. There is a strong likelihood that these factors will differ considerably and so the cumulative toxicity will vary between different particle types.
References
- 1.Royal Society and Royal Academy of Engineering. Nanoscience and nanotechnologies: opportunities and uncertainties. The Royal Society; 2004. [Google Scholar]
- 2.Seaton A, MacNee W, Donaldson K, Godden D. Particulate air pollution and acute health effects. Lancet. 1995;345:176–178. doi: 10.1016/s0140-6736(95)90173-6. [DOI] [PubMed] [Google Scholar]
- 3.Utell MJ, Frampton MW. Acute health effects of ambient air pollution: the ultrafine particle hypothesis. J Aerosol Med. 2000;13:355–359. doi: 10.1089/jam.2000.13.355. [DOI] [PubMed] [Google Scholar]
- 4.Donaldson K, Tran L, Jimenez L, Duffin R, Newby DE, Mills N, et al. Combustion-derived nanoparticles: A review of their toxicology following inhalation exposure. Part Fibre Toxicol. 2005;2:10. doi: 10.1186/1743-8977-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Quality of Urban Air Review Group. Airborne particulate matter in the United Kingdom: third report of the Quality of Urban Air Review Group. Quality of Urban Air Review Group. Quality of Urban Air Review Group; 1996. [Google Scholar]
- 6.Brunekreef B, Holgate ST. Air pollution and health. Lancet. 2002;360:1233–1242. doi: 10.1016/S0140-6736(02)11274-8. [DOI] [PubMed] [Google Scholar]
- 7.Schlesinger RB, Cassee F. Atmospheric secondary inorganic particulate matter: the toxicological perspective as a basis for health effects risk assessment. Inhal Toxicol. 2003;15:197–235. doi: 10.1080/08958370304503. [DOI] [PubMed] [Google Scholar]
- 8.Pope CA, Thun MJ, Namboodiri MM, Dockery DW, Evans JS, Speizer FE, et al. Particulate air pollution as a predictor of mortality in a prospective study of U.S. adults. Am J Respir Crit Care Med. 1995;151:669–674. doi: 10.1164/ajrccm/151.3_Pt_1.669. [DOI] [PubMed] [Google Scholar]
- 9.de Hartog JJ, Hoek G, Mirme A, Tuch T, Kos GP, ten Brink HM, et al. Relationship between different size classes of particulate matter and meteorology in three European cities. J Environ Monit. 2005;7:302–310. doi: 10.1039/b415153d. [DOI] [PubMed] [Google Scholar]
- 10.Air Quality Expert Group. Particulate matter in the United Kingdom. London: Published by the Department of Environment, Food and Rural Affairs; 2005. [Google Scholar]
- 11.Elder A, Gelein R, Finkelstein J, Phipps R, Frampton M, Utell M, et al. On-road exposure to highway aerosols. 2. Exposures of aged, compromised rats. Inhal Toxicol. 2004;16(Suppl 1):41–53. doi: 10.1080/08958370490443222. [DOI] [PubMed] [Google Scholar]
- 12.Kittelson DB, Watts WF, Johnson JP, Remerowki ML, Ische EE, Oberdorster G, et al. On-road exposure to highway aerosols. 1. Aerosol and gas measurements. Inhal Toxicol. 2004;16(Suppl 1):31–39. doi: 10.1080/08958370490443024. [DOI] [PubMed] [Google Scholar]
- 13.Afshari A, Matson U, Ekberg LE. Characterization of indoor sources of fine and ultrafine particles: a study conducted in a full-scale chamber. Indoor Air. 2005;15:141–150. doi: 10.1111/j.1600-0668.2005.00332.x. [DOI] [PubMed] [Google Scholar]
- 14.Dennekamp M, Howarth S, Dick CA, Cherrie JW, Donaldson K, Seaton A. Ultrafine particles and nitrogen oxides generated by gas and electric cooking. Occup Environ Med. 2001;58:511–516. doi: 10.1136/oem.58.8.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Donaldson K, Jimenez LA, Rahman I, Faux SP, MacNee W, Gilmour PS, et al. Respiratory health effects of ambient air pollution particles: Role of reactive species. In: Vallyathan V, Castranova V, Shi X, editors. Oxygen/nitrogen radicals: lung injury and disease. New York: Marcel Dekker; 2004. pp. 257–288. (Vol 187 in Lung Biology in Health and Disease. Executive Editor: Lenfant C.) [Google Scholar]
- 16.Donaldson K, Stone V, Borm PJ, Jimenez LA, Gilmour PS, Schins RP, et al. Oxidative stress and calcium signaling in the adverse effects of environmental particles (PM10) Free Radic Biol Med. 2003;34:1369–1382. doi: 10.1016/s0891-5849(03)00150-3. [DOI] [PubMed] [Google Scholar]
- 17.Miyabara Y, Yanagisawa R, Shimojo N, Takano H, Lim HB, Ichinose T, et al. Murine strain differences in airway inflammation caused by diesel exhaust particles. Eur Respir J. 1998;11:291–298. doi: 10.1183/09031936.98.11020291. [DOI] [PubMed] [Google Scholar]
- 18.Tsurudome Y, Hirano T, Yamato H, Tanaka I, Sagai M, Hirano H, et al. Changes in levels of 8-hydroxyguanine in DNA, its repair and OGG1 mRNA in rat lungs after intratracheal administration of diesel exhaust particles. Carcinogenesis. 1999;20:1573–1576. doi: 10.1093/carcin/20.8.1573. [DOI] [PubMed] [Google Scholar]
- 19.Nordenhall C, Pourazar J, Blomberg A, Levin JO, Sandstrom T, Adelroth E. Airway inflammation following exposure to diesel exhaust: a study of time kinetics using induced sputum. Eur Respir J. 2000;15:1046–1051. doi: 10.1034/j.1399-3003.2000.01512.x. [DOI] [PubMed] [Google Scholar]
- 20.Ichinose T, Yajima Y, Nagashima M, Takenoshita S, Nagamachi Y, Sagai M. Lung carcinogenesis and formation of 8-hydroxy-deoxyguanosine in mice by diesel exhaust particles. Carcinogenesis. 1997;18:185–192. doi: 10.1093/carcin/18.1.185. [DOI] [PubMed] [Google Scholar]
- 21.Arimoto T, Yoshikawa T, Takano H, Kohno M. Generation of reactive oxygen species and 8-hydroxy-2'-deoxyguanosine formation from diesel exhaust particle components in L1210 cells. Jpn J Pharmacol. 1999;80:49–54. doi: 10.1254/jjp.80.49. [DOI] [PubMed] [Google Scholar]
- 22.Bonvallot V, Baeza-Squiban A, Baulig A, Brulant S, Boland S, Muzeau F, et al. Organic compounds from diesel exhaust particles elicit a proinflammatory response in human airway epithelial cells and induce cytochrome p450 1A1 expression. Am J Respir Cell Mol Biol. 2001;25:515–521. doi: 10.1165/ajrcmb.25.4.4515. [DOI] [PubMed] [Google Scholar]
- 23.Hirano S, Furuyama A, Koike E, Kobayashi T. Oxidative-stress potency of organic extracts of diesel exhaust and urban fine particles in rat heart microvessel endothelial cells. Toxicology. 2003;187:161–170. doi: 10.1016/s0300-483x(03)00053-2. [DOI] [PubMed] [Google Scholar]
- 24.Li N, Venkatesan MI, Miguel A, Kaplan R, Gujuluva C, Alam J, et al. Induction of heme oxygenase-1 expression in macrophages by diesel exhaust particle chemicals and quinones via the antioxidant-responsive element. J Immunol. 2000;165:3393–3401. doi: 10.4049/jimmunol.165.6.3393. [DOI] [PubMed] [Google Scholar]
- 25.Nel AE, Diaz-Sanchez D, Li N. The role of particulate pollutants in pulmonary inflammation and asthma: evidence for the involvement of organic chemicals and oxidative stress. Curr Opin Pulm Med. 2001;7:20–26. doi: 10.1097/00063198-200101000-00004. [DOI] [PubMed] [Google Scholar]
- 26.McNeilly JD, Jimenez LA, Clay MF, MacNee W, Howe A, Heal MR, et al. Soluble transition metals in welding fumes cause inflammation via activation of NF-kappaB and AP-1. Toxicol Lett. 2005;158:152–157. doi: 10.1016/j.toxlet.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 27.Marano F, Boland S, Bonvallot V, Baulig A, Baeza-Squiban A. Human airway epithelial cells in culture for studying the molecular mechanisms of the inflammatory response triggered by diesel exhaust particles. Cell Biol Toxicol. 2002;18:315–320. doi: 10.1023/a:1019548517877. [DOI] [PubMed] [Google Scholar]
- 28.Hiura TS, Kaszubowski MP, Li N, Nel AE. Chemicals in diesel exhaust particles generate reactive oxygen radicals and induce apoptosis in macrophages. J Immunol. 1999;163:5582–5591. [PubMed] [Google Scholar]
- 29.Hashimoto S, Gon Y, Takeshita I, Matsumoto K, Jibiki I, Takizawa H, et al. Diesel exhaust particles activate p38 MAP kinase to produce interleukin 8 and RANTES by human bronchial epithelial cells and N-acetylcysteine attenuates p38 MAP kinase activation. Am J Respir Crit Care Med. 2000;161:280–285. doi: 10.1164/ajrccm.161.1.9904110. [DOI] [PubMed] [Google Scholar]
- 30.Takizawa H, Ohtoshi T, Kawasaki S, Kohyama T, Desaki M, Kasama T, et al. Diesel exhaust particles induce NF-kappa B activation in human bronchial epithelial cells in vitro: importance in cytokine transcription. J Immunol. 1999;162:4705–4711. [PubMed] [Google Scholar]
- 31.Gilmour PS, Rahman I, Donaldson K, MacNee W. Histone acetylation regulates epithelial IL-8 release mediated by oxidative stress from environmental particles. Am J Physiol Lung Cell Mol Physiol. 2003;284:L533–L540. doi: 10.1152/ajplung.00277.2002. [DOI] [PubMed] [Google Scholar]
- 32.Terada N, Hamano N, Maesako KI, Hiruma K, Hohki G, Suzuki K, et al. Diesel exhaust particulates upregulate histamine receptor mRNA and increase histamine-induced IL-8 and GM-CSF production in nasal epithelial cells and endothelial cells. Clin Exp Allergy. 1999;29:52–59. doi: 10.1046/j.1365-2222.1999.00406.x. [DOI] [PubMed] [Google Scholar]
- 33.Salvi SS, Nordenhall C, Blomberg A, Rudell B, Pourazar J, Kelly FJ, et al. Acute exposure to diesel exhaust increases IL-8 and GRO-alpha production in healthy human airways. Am J Respir Crit Care Med. 2000;161:550–557. doi: 10.1164/ajrccm.161.2.9905052. [DOI] [PubMed] [Google Scholar]
- 34.Yang HM, Ma JY, Castranova V, Ma JK. Effects of diesel exhaust particles on the release of interleukin-1 and tumor necrosis factor-alpha from rat alveolar macrophages. Exp Lung Res. 1997;23:269–284. doi: 10.3109/01902149709087372. [DOI] [PubMed] [Google Scholar]
- 35.Steerenberg PA, Zonnenberg JA, Dormans JA, Joon PN, Wouters IM, van Bree L, et al. Diesel exhaust particles induced release of interleukin 6 and 8 by (primed) human bronchial epithelial cells (BEAS 2B) in vitro. Exp Lung Res. 1998;24:85–100. doi: 10.3109/01902149809046056. [DOI] [PubMed] [Google Scholar]
- 36.Brook RD, Franklin B, Cascio W, Hong Y, Howard G, Lipsett M, et al. Air pollution and cardiovascular disease: a statement for healthcare professionals from the Expert Panel on Population and Prevention Science of the American Heart Association. Circulation. 2004;109:2655–2671. doi: 10.1161/01.CIR.0000128587.30041.C8. [DOI] [PubMed] [Google Scholar]
- 37.Brook RD, Brook JR, Rajagopalan S. Air pollution: the "Heart" of the problem. Curr Hypertens Rep. 2003;5:32–39. doi: 10.1007/s11906-003-0008-y. [DOI] [PubMed] [Google Scholar]
- 38.Donaldson K, Mills N, MacNee W, Robinson S, Newby D. Role of inflammation in cardiopulmonary health effects of PM. Toxicol Appl Pharmacol. 2005;207(2 Suppl):483–488. doi: 10.1016/j.taap.2005.02.020. [DOI] [PubMed] [Google Scholar]
- 39.Viles-Gonzalez JF, Anand SX, Valdiviezo C, Zafar MU, Hutter R, Sanz J, et al. Update in atherothrombotic disease. Mt Sinai J Med. 2004;71:197–208. [PubMed] [Google Scholar]
- 40.Libby P, Ridker PM, Maseri A. Inflammation and atherosclerosis. Circulation. 2002;105:1135–1143. doi: 10.1161/hc0902.104353. [DOI] [PubMed] [Google Scholar]
- 41.Van Lente F. Markers of inflammation as predictors in cardiovascular disease. Clin Chim Acta. 2000;293:31–52. doi: 10.1016/s0009-8981(99)00236-3. [DOI] [PubMed] [Google Scholar]
- 42.Suwa T, Hogg JC, Quinlan KB, Ohgami A, Vincent R, van Eeden SF. Particulate air pollution induces progression of atherosclerosis. J Am Coll Cardiol. 2002;39:935–942. doi: 10.1016/s0735-1097(02)01715-1. [DOI] [PubMed] [Google Scholar]
- 43.Sun Q, Wang A, Jin X, Natanzon A, Duquaine D, Brook RD, et al. Long-term air pollution exposure and acceleration of atherosclerosis and vascular inflammation in an animal model. JAMA. 2005;294:3003–3010. doi: 10.1001/jama.294.23.3003. [DOI] [PubMed] [Google Scholar]
- 44.Ross R. Atherosclerosis--an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 45.Blum A, Miller HI. The role of inflammation in atherosclerosis. Isr J Med Sci. 1996;32:1059–1065. [PubMed] [Google Scholar]
- 46.Brand K, Page S, Rogler G, Bartsch A, Brandl R, Knuechel R, et al. Activated transcription factor nuclear factor-kappa B is present in the atherosclerotic lesion. J Clin Invest. 1996;97:1715–1722. doi: 10.1172/JCI118598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nemmar A, Vanbilloen H, Hoylaerts MF, Hoet PH, Verbruggen A, Nemery B. Passage of intratracheally instilled ultrafine particles from the lung into the systemic circulation in hamster. Am J Respir Crit Care Med. 2001;164:1665–1668. doi: 10.1164/ajrccm.164.9.2101036. [DOI] [PubMed] [Google Scholar]
- 48.Kreyling WG, Semmler M, Erbe F, Mayer P, Takenaka S, Schulz H, et al. Translocation of ultrafine insoluble iridium particles from lung epithelium to extrapulmonary organs is size dependent but very low. J Toxicol Environ Health A. 2002;65:1513–1530. doi: 10.1080/00984100290071649. [DOI] [PubMed] [Google Scholar]
- 49.Oberdorster G, Sharp Z, Atudorei V, Elder A, Gelein R, Lunts A, et al. Extrapulmonary translocation of ultrafine carbon particles following whole-body inhalation exposure of rats. J Toxicol Environ Health A. 2002;65:1531–1543. doi: 10.1080/00984100290071658. [DOI] [PubMed] [Google Scholar]
- 50.Nemmar A, Hoet PH, Vanquickenborne B, Dinsdale D, Thomeer M, Hoylaerts MF, et al. Passage of inhaled particles into the blood circulation in humans. Circulation. 2002;105:411–414. doi: 10.1161/hc0402.104118. [DOI] [PubMed] [Google Scholar]
- 51.Mills N, Amin N, Robinson SD, Anand A, Davies J, Patel D, et al. Do Inhaled 99mTechnetium-labeled carbon nanoparticles do not translocate into the circulation in man. Am J Resp Crit Care Med. 2005 doi: 10.1164/rccm.200506-865OC. submitted. [DOI] [PubMed] [Google Scholar]
- 52.Khandoga A, Stampfl A, Takenaka S, Schulz H, Radykewicz R, Kreyling W, et al. Ultrafine particles exert prothrombotic but not inflammatory effects on the hepatic microcirculation in healthy mice in vivo. Circulation. 2004;109:1320–1325. doi: 10.1161/01.CIR.0000118524.62298.E8. [DOI] [PubMed] [Google Scholar]
- 53.Nemmar A, Nemery B, Hoet PH, Vermylen J, Hoylaerts MF. Pulmonary inflammation and thrombogenicity caused by diesel particles in hamsters: role of histamine. Am J Respir Crit Care Med. 2003;168:1366–1372. doi: 10.1164/rccm.200306-801OC. [DOI] [PubMed] [Google Scholar]
- 54.Nemmar A, Hoet PH, Dinsdale D, Vermylen J, Hoylaerts MF, Nemery B. Diesel exhaust particles in lung acutely enhance experimental peripheral thrombosis. Circulation. 2003;107:1202–1208. doi: 10.1161/01.cir.0000053568.13058.67. [DOI] [PubMed] [Google Scholar]
- 55.Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980;288:373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- 56.Ludmer PL, Selwyn AP, Shook TL, Wayne RR, Mudge GH, Alexander RW, et al. Paradoxical vasoconstriction induced by acetylcholine in atherosclerotic coronary arteries. N Engl J Med. 1986;315:1046–1051. doi: 10.1056/NEJM198610233151702. [DOI] [PubMed] [Google Scholar]
- 57.Celermajer DS, Adams MR, Clarkson P, Robinson J, McCredie R, Donald A, et al. Passive smoking and impaired endothelium-dependent arterial dilatation in healthy young adults. N Engl J Med. 1996;334:150–154. doi: 10.1056/NEJM199601183340303. [DOI] [PubMed] [Google Scholar]
- 58.Newby DE, Wright RA, Labinjoh C, Ludlam CA, Fox KA, Boon NA, et al. Endothelial dysfunction, impaired endogenous fibrinolysis, and cigarette smoking: a mechanism for arterial thrombosis and myocardial infarction. Circulation. 1999;99:1411–1415. doi: 10.1161/01.cir.99.11.1411. [DOI] [PubMed] [Google Scholar]
- 59.Hingorani AD, Cross J, Kharbanda RK, Mullen MJ, Bhagat K, Taylor M, et al. Acute systemic inflammation impairs endothelium-dependent dilatation in humans. Circulation. 2000;102:994–999. doi: 10.1161/01.cir.102.9.994. [DOI] [PubMed] [Google Scholar]
- 60.van den Eijnden-Schrauwen Y, Kooistra T, de Vries RE, Emeis JJ. Studies on the acute release of tissue-type plasminogen activator from human endothelial cells in vitro and in rats in vivo: evidence for a dynamic storage pool. Blood. 1995;85:3510–3517. [PubMed] [Google Scholar]
- 61.Brommer EJ. The level of extrinsic plasminogen activator (t-PA) during clotting as a determinant of the rate of fibrinolysis; inefficiency of activators added afterwards. Thromb Res. 1984;34:109–115. doi: 10.1016/0049-3848(84)90067-7. [DOI] [PubMed] [Google Scholar]
- 62.Fox KA, Robison AK, Knabb RM, Rosamond TL, Sobel BE, Bergmann SR. Prevention of coronary thrombosis with subthrombolytic doses of tissue-type plasminogen activator. Circulation. 1985;72:1346–1354. doi: 10.1161/01.cir.72.6.1346. [DOI] [PubMed] [Google Scholar]
- 63.Mills NL, Tornqvist H, Robinson SD, Gonzalez M, Darnley K, MacNee W, et al. Diesel exhaust inhalation causes vascular dysfunction and impaired endogenous fibrinolysis. Circulation. 2005;112:3930–3936. doi: 10.1161/CIRCULATIONAHA.105.588962. [DOI] [PubMed] [Google Scholar]
- 64.Bellmann B, Muhle H, Creutzenberg O, Mermelstein R. Irreversible pulmonary changes induced in rat lung by dust overload. Environ Health Perspect. 1992;97:189–191. doi: 10.1289/ehp.9297189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Morrow PE. Dust overloading of the lungs: update and appraisal. Toxicol Appl Pharmacol. 1992;113:1–12. doi: 10.1016/0041-008x(92)90002-a. [DOI] [PubMed] [Google Scholar]
- 66.Mauderly JL, Cheng YS, Snipes MB. Particle overload in toxicological studies: friend or foe, and how can we know? J Aerosol Med. 1990;3(Suppl 1):169–187. [Google Scholar]
- 67.Morrow PE. Possible mechanisms to explain dust overloading of the lungs. Fundam Appl Toxicol. 1988;10:369–384. doi: 10.1016/0272-0590(88)90284-9. [DOI] [PubMed] [Google Scholar]
- 68.Tran CL, Buchanan D, Cullen RT, Searl A, Jones AD, Donaldson K. Inhalation of poorly soluble particles. II. Influence Of particle surface area on inflammation and clearance. Inhal Toxicol. 2000;12:1113–1126. doi: 10.1080/08958370050166796. [DOI] [PubMed] [Google Scholar]
- 69.Cullen RT, Tran CL, Buchanan D, Davis JM, Searl A, Jones AD, et al. Inhalation of poorly soluble particles. I. Differences in inflammatory response and clearance during exposure. Inhal Toxicol. 2000;12:1089–1111. doi: 10.1080/08958370050166787. [DOI] [PubMed] [Google Scholar]
- 70.Driscoll KE, Carter JM, Howard BW, Hassenbein DG, Pepelko W, Baggs RB, et al. Pulmonary inflammatory, chemokine, and mutagenic responses in rats after subchronic inhalation of carbon black. Toxicol Appl Pharmacol. 1996;136:372–380. doi: 10.1006/taap.1996.0045. [DOI] [PubMed] [Google Scholar]
- 71.Oberdorster G, Ferin J, Soderholm S, Gelein R, Cox C, Baggs R, et al. Increased pulmonary toxicity of inhaled ultrafine particles: due to lung overload alone?. In: Dogston J, McCallum RI, editors. Inhaled Part VII, Proceedings of the 7th International Symposium; 1991. pp. 295–302. [Google Scholar]
- 72.Gilmour PS, Ziesenis A, Morrison ER, Vickers MA, Drost EM, Ford I, et al. Pulmonary and systemic effects of short-term inhalation exposure to ultrafine carbon black particles. Toxicol Appl Pharmacol. 2004;195:35–44. doi: 10.1016/j.taap.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 73.Renwick LC, Brown D, Clouter A, Donaldson K. Increased inflammation and altered macrophage chemotactic responses caused by two ultrafine particle types. Occup Environ Med. 2004;61:442–447. doi: 10.1136/oem.2003.008227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hohr D, Steinfartz Y, Schins RP, Knaapen AM, Martra G, Fubini B, et al. The surface area rather than the surface coating determines the acute inflammatory response after instillation of fine and ultrafine TiO2 in the rat. Int J Hyg Environ Health. 2002;205:239–244. doi: 10.1078/1438-4639-00123. [DOI] [PubMed] [Google Scholar]
- 75.Duffin R, Tran L, Brown D, Stone V, Donaldson K. Pro-inflammogenic effects of low-toxicity and metal nanoparticles in vivo and in vitro: highlighting the role of particle surface area and surface reactivity. Inhal Toxicol. 2007;19:849–856. doi: 10.1080/08958370701479323. [DOI] [PubMed] [Google Scholar]
- 76.Wilson MR, Lightbody JH, Donaldson K, Sales J, Stone V. Interactions between ultrafine particles and transition metals in vivo and in vitro. Toxicol Appl Pharmacol. 2002;184:172–179. doi: 10.1006/taap.2002.9501. [DOI] [PubMed] [Google Scholar]
- 77.Brown DM, Stone V, Findlay P, MacNee W, Donaldson K. Increased inflammation and intracellular calcium caused by ultrafine carbon black is independent of transition metals or other soluble components. Occup Environ Med. 2000;57:685–691. doi: 10.1136/oem.57.10.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Beck-Speier I, Dayal N, Karg E, Maier KL, Schumann G, Schulz H, et al. Oxidative stress and lipid mediators induced in alveolar macrophages by ultrafine particles. Free Radic Biol Med. 2005;38:1080–1092. doi: 10.1016/j.freeradbiomed.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 79.Stone V, Tuinman M, Vamvakopoulos JE, Shaw J, Brown D, Petterson S, et al. Increased calcium influx in a monocytic cell line on exposure to ultrafine carbon black. Eur Respir J. 2000;15:297–303. doi: 10.1034/j.1399-3003.2000.15b13.x. [DOI] [PubMed] [Google Scholar]
- 80.Brown DM, Donaldson K, Borm PJ, Schins RP, Dehnhardt M, Gilmour P, et al. Calcium and ROS-mediated activation of transcription factors and TNF-alpha cytokine gene expression in macrophages exposed to ultrafine particles. Am J Physiol Lung Cell Mol Physiol. 2004;286:L344–L353. doi: 10.1152/ajplung.00139.2003. [DOI] [PubMed] [Google Scholar]
- 81.Tamaoki J, Isono K, Takeyama K, Tagaya E, Nakata J, Nagai A. Ultrafine carbon black particles stimulate proliferation of human airway epithelium via EGF receptor-mediated signaling pathway. Am J Physiol Lung Cell Mol Physiol. 2004;287:L1127–L1133. doi: 10.1152/ajplung.00241.2004. [DOI] [PubMed] [Google Scholar]
- 82.Timblin CR, Shukla A, Berlanger I, Berube KA, Churg A, Mossman BT. Ultrafine airborne particles cause increases in protooncogene expression and proliferation in alveolar epithelial cells. Toxicol Appl Pharmacol. 2002;179:98–104. doi: 10.1006/taap.2001.9343. [DOI] [PubMed] [Google Scholar]
- 83.Donaldson K, Tran CL. An introduction to the short-term toxicology of respirable industrial fibres. Mutat Res. 2004;553:5–9. doi: 10.1016/j.mrfmmm.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 84.Nyberg K, Johansson U, Rundquist I, Camner P. Estimation of pH in individual alveolar macrophage phagolysosomes. Exp Lung Res. 1989;15:499–510. doi: 10.3109/01902148909069614. [DOI] [PubMed] [Google Scholar]
- 85.Hesterberg TW, Miiller WC, Musselman RP, Kamstrup O, Hamilton RD, Thevenaz P. Biopersistence of man-made vitreous fibers and crocidolite asbestos in the rat lung following inhalation. Fundam Appl Toxicol. 1996;29:267–279. doi: 10.1006/faat.1996.0031. [DOI] [PubMed] [Google Scholar]
- 86.Muller J, Huaux F, Moreau N, Misson P, Heilier JF, Delos M, et al. Respiratory toxicity of multi-wall carbon nanotubes. Toxicol Appl Pharmacol. 2005;207:221–231. doi: 10.1016/j.taap.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 87.Maynard AD, Baron PA, Foley M, Shvedova AA, Kisin ER, Castranova V. Exposure to carbon nanotube material: aerosol release during the handling of unrefined single-walled carbon nanotube material. J Toxicol Environ Health A. 2004;67:87–107. doi: 10.1080/15287390490253688. [DOI] [PubMed] [Google Scholar]
- 88.Lam CW, James JT, McCluskey R, Hunter RL. Pulmonary toxicity of single-wall carbon nanotubes in mice 7 and 90 days after intratracheal instillation. Toxicol Sci. 2004;77:126–134. doi: 10.1093/toxsci/kfg243. [DOI] [PubMed] [Google Scholar]
- 89.Warheit DB, Laurence BR, Reed KL, Roach DH, Reynolds GA, Webb TR. Comparative pulmonary toxicity assessment of single-wall carbon nanotubes in Rats. Toxicol Sci. 2004;77:117–125. doi: 10.1093/toxsci/kfg228. [DOI] [PubMed] [Google Scholar]
- 90.Shvedova AA, Kisin ER, Mercer R, Murray AR, Johnson VJ, Potapovich AI, et al. Unusual inflammatory and fibrogenic pulmonary responses to single-walled carbon nanotubes in mice. Am J Physiol Lung Cell Mol Physiol. 2005;289:L698–L708. doi: 10.1152/ajplung.00084.2005. [DOI] [PubMed] [Google Scholar]
- 91.Monteiro-Riviere NA, Nemanich RJ, Inman AO, Wang YY, Riviere JE. Multi-walled carbon nanotube interactions with human epidermal keratinocytes. Toxicol Lett. 2005;155:377–384. doi: 10.1016/j.toxlet.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 92.Shvedova AA, Castranova V, Kisin ER, Schwegler-Berry D, Murray AR, Gandelsman VZ, et al. Exposure to carbon nanotube material: assessment of nanotube cytotoxicity using human keratinocyte cells. J Toxicol Environ Health A. 2003;66:1909–1926. doi: 10.1080/713853956. [DOI] [PubMed] [Google Scholar]
- 93.Jia G, Wang H, Yan L, Wang X, Pei R, Yan T, et al. Cytotoxicity of carbon nanomaterials: single-wall nanotube, multi-wall nanotube, and fullerene. Environ Sci Technol. 2005;39:1378–1383. doi: 10.1021/es048729l. [DOI] [PubMed] [Google Scholar]
- 94.Bottini M, Bruckner S, Nika K, Bottini N, Bellucci S, Magrini A, et al. Multi-walled carbon nanotubes induce T lymphocyte apoptosis. Toxicol Lett. 2006;160:121–126. doi: 10.1016/j.toxlet.2005.06.020. [DOI] [PubMed] [Google Scholar]
- 95.Cui D, Tian F, Ozkan CS, Wang M, Gao H. Effect of single wall carbon nanotubes on human HEK293 cells. Toxicol Lett. 2005;155:73–85. doi: 10.1016/j.toxlet.2004.08.015. [DOI] [PubMed] [Google Scholar]
- 96.Manna SK, Sarkar S, Barr J, Wise K, Barrera EV, Jejelowo O, et al. Single-walled carbon nanotube induces oxidative stress and activates nuclear transcription factor-kappaB in human keratinocytes. Nano Lett. 2005;5:1676–1684. doi: 10.1021/nl0507966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kagan VE, Tyurina YY, Tyurin VA, Konduru NV, Potapovich AI, Osipov AN, et al. Direct and indirect effects of single walled carbon nanotubes on RAW 264.7 macrophages: role of iron. Toxicol Lett. 2006;165:88–100. doi: 10.1016/j.toxlet.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 98.Radomski A, Jurasz P, Alonso-Escolano D, Drews M, Morandi M, Malinski T, et al. Nanoparticle-induced platelet aggregation and vascular thrombosis. Br J Pharmacol. 2005;146:882–893. doi: 10.1038/sj.bjp.0706386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yamawaki H, Iwai N. Mechanisms underlying nano-sized air-pollution-mediated progression of atherosclerosis: carbon black causes cytotoxic injury/inflammation and inhibits cell growth in vascular endothelial cells. Circ J. 2006;70:129–140. doi: 10.1253/circj.70.129. [DOI] [PubMed] [Google Scholar]
- 100.Don Porto CA, Hoet PH, Verschaeve L, Schoeters G, Nemery B. Genotoxic effects of carbon black particles, diesel exhaust particles, and urban air particulates and their extracts on a human alveolar epithelial cell line (A549) and a human monocytic cell line (THP-1) Environ Mol Mutagen. 2001;37:155–163. doi: 10.1002/em.1023. [DOI] [PubMed] [Google Scholar]
- 101.Schins RP. Mechanisms of genotoxicity of particles and fibers. Inhal Toxicol. 2002;14:57–78. doi: 10.1080/089583701753338631. [DOI] [PubMed] [Google Scholar]
- 102.de Kok TM, Hogervorst JG, Briede JJ, van Herwijnen MH, Maas LM, Moonen EJ, et al. Genotoxicity and physicochemical characteristics of traffic-related ambient particulate matter. Environ Mol Mutagen. 2005;46:71–80. doi: 10.1002/em.20133. [DOI] [PubMed] [Google Scholar]
- 103.Knaapen AM, Borm PJ, Albrecht C, Schins RP. Inhaled particles and lung cancer. Part A: Mechanisms. Int J Cancer. 2004;109:799–809. doi: 10.1002/ijc.11708. [DOI] [PubMed] [Google Scholar]
- 104.Esterbauer H, Schaur RJ, Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic Biol Med. 1991;11:81–128. doi: 10.1016/0891-5849(91)90192-6. [DOI] [PubMed] [Google Scholar]
- 105.Chen M, von Mikecz A. Formation of nucleoplasmic protein aggregates impairs nuclear function in response to SiO2 nanoparticles. Exp Cell Res. 2005;305:51–62. doi: 10.1016/j.yexcr.2004.12.021. [DOI] [PubMed] [Google Scholar]
- 106.Geiser M, Rothen-Rutlshauser B, Kapp N, Schurch S, Kreyling W, Schulz H, et al. Ultrafine particles cross cellular membranes by non-phagocytic mechanisms in lungs and in cultured cells. Environ Health Perspect. 2005;113:1555–1560. doi: 10.1289/ehp.8006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Dick CA, Brown DM, Donaldson K, Stone V. The role of free radicals in the toxic and inflammatory effects of four different ultrafine particle types. Inhal Toxicol. 2003;15:39–52. doi: 10.1080/08958370304454. [DOI] [PubMed] [Google Scholar]
- 108.Donaldson K, Beswick PH, Gilmour PS. Free radical activity associated with the surface of particles: a unifying factor in determining biological activity? Toxicol Lett. 1996;88:293–298. doi: 10.1016/0378-4274(96)03752-6. [DOI] [PubMed] [Google Scholar]