Abstract
Purpose
Typically, a diagnosis of erythema nodosum (EN) is based on clinical features. However, other diseases manifest with inflammatory nodules of the lower limbs in addition to EN, such as the EN-like lesions of Behçet's disease (BD). The purpose of this retrospective study was to investigate the frequency of histologically proven EN among diseases diagnosed clinically as EN, to determine underlying causes of EN, and to compare clinical and histologic features between EN and other diseases.
Patients and Methods
We selected 99 patients diagnosed clinically with EN and performed skin biopsies. All pathologic slides were evaluated and diagnosed; and after histologic diagnoses were made we reviewed the patients' medical records.
Results
Among the 99 patients diagnosed clinically with EN, 47 were biopsy-verified EN. The EN-like lesions of BD and nodular vasculitis were both in the primary differential diagnosis of EN. No definite difference in clinical features exists among these three diseases. Histologically, EN demonstrated septal panniculitis in the majority of patients. Lobular panniculitis was frequently observed in NV, and mixed or mostly lobular panniculitis was observed in the EN-like lesion. Vasculitis was rarely observed in EN; however lymphocytic vasculitis was observed frequently in EN-like lesions and neutrophilic vasculitis was observed in NV. The frequency of granulomatous inflammation was highest in NV. Some cases of patients with typical BD demonstrated classic EN lesions.
Conclusion
It was extremely difficult to clinically differentiate EN from EN-like lesions or NV. We feel skin biopsy is mandatory for the diagnosis of lower extremity erythematous nodular lesions.
Keywords: Erythema nodosum, erythema nodosum-like lesion in Behçet's disease, nodular vasculitis, histologic diagnosis
INTRODUCTION
Erythema nodosum (EN) is a cutaneous reaction pattern characterized clinically by the presence of erythematous tender nodules and raised plaques, which are located predominantly over the extensor aspects of the legs.1,2 Usually the diagnosis of EN is clinical,3 though other diseases including EN-like lesions in Behçet's disease (BD), nodular vasculitis (NV), cutaneous polyarteritis nodosa (PAN), superficial thrombophlebitis, subcutaneous granuloma annulare, erythema nodosum leprosum, neutrophilic lobular panniculitis associated with rheumatoid arthritis, Crohn's disease, traumatic panniculitis, cytophagic histiocytic panniculitis, lupus panniculitis, and membranous lipodystrophy can manifest as inflammatory nodules of the legs.4-6 These diseases share some clinical features, and therefore histopathologic study is required for a specific diagnosis.4
The purpose of this retrospective study was to investigate the frequency of histologically proven EN among diseases diagnosed clinically as EN, determine the underlying causes of EN, and compare the clinical and histologic features of EN as compared to other diseases manifesting clinically as EN.
MATERIALS AND METHODS
Selection of patients
Clinical photographs were taken of all patients presenting with inflammatory nodules located on the lower limbs. Every patient then had a biopsy for diagnosis at the Department of Dermatology, Ajou University Hospital, Suwon, Korea, from January 2000 through August 2005. We reviewed the photographs and selected 118 patients unanimously diagnosed by three dermatologists with EN based on clinical features.
Histopathologic examination
Pathologic slides of all 118 patients were evaluated by one dermatopathologist. Of those, 19 patients were subsequently excluded because subcutaneous tissue specimens were insufficient for histopathologic evaluation. The degree of inflammatory cell infiltration in the dermis, fat lobules, and subcutaneous septa was graded as absent (-), mild (+), moderate (++), or marked (+++). The pattern of dermal inflammation was classified as perivascular, interstitial, or periappendageal infiltration. Panniculitis was classified as mostly septal, mostly lobular, or mixed septal and lobular when no clear compartmentalization of inflammatory cell infiltrates were observed. When the number of neutrophils was greater than the number of lymphocytes, the infiltrate was considered predominantly neutrophilic or vice versa. We also assessed the presence and type of vasculitis by using the diagnostic criteria for leukocytoclastic vasculitis as described by Barnhill et al.7 For lymphocytic vasculitis, we used the criteria of Carlson et al.,8 which requires morphologic evidence of venule damage. We also evaluated the presence of granulomatous inflammation and fat necrosis.
We reclassified all cases according to the results of histologic evaluation; however, the diagnosis of BD was made by the International Study Group for Behçet's disease criteria.9 Thus we referred to the previous medical history for diagnosis of EN-like lesions in BD. Nodular vasculitis was diagnosed by the following histopathologic findings: vasculitis of arteries and veins, focal or extensive necrosis of fat lobules, predominantly lobular or septolobular panniculitis consisting of neutrophils, lymphocytes, and granulomatous inflammation. A consensus was reached that erythema induratum and nodular vasculitis would be considered the same entity.1,5 NV is likely a reactive, immune complex-mediated vasculitis, with tuberculosis as one of its etiologies. Therefore, we used the term NV and did not distinguish NV from erythema induratum.
Review of medical records
After histologic evaluation we collected the following data from past medical records: gender, age at diagnosis, duration (from the onset of skin lesions until the time of diagnosis), distribution (bilateral or unilateral), location of lesion, concurrent disease, presence of associated diseases, and relapse.
Laboratory studies included complete blood count (CBC), erythrocyte sedimentation rate (ESR), anti-streptolysin O (ASO), C-reactive protein (CRP), liver function test, blood urea nitrogen (BUN), creatinine, blood glucose, venereal disease research laboratory (VDRL), antinuclear anibody (ANA), antineutrophil cytoplasmic antibodies (ANCAs), anti-herpes simplex virus (HSV) antibodies (IgM and IgG), hepatitis B virus antigen, tuberculin test, chest X-ray, and bone scan.
RESULTS
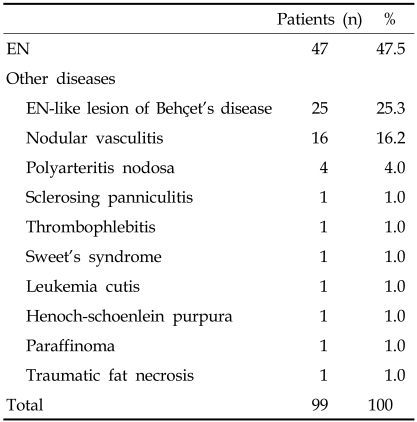
Ninety-nine patients diagnosed clinically with EN were distributed as follows: 23 males (23%) and 76 females (77%) with a mean age (± SD) of 34.1 years (± 12.5) (range 1 to 69). All patients were classified based on histologic diagnoses, as summarized in Table 1. Of 99 total patients, 47 patients (48%) had histologically proven EN, 25 patients (25%) had EN-like lesions in BD, and 16 patients (16%) had NV.
Table 1.
Classification Based on Histologic Diagnosis
EN, erythema nodosum.
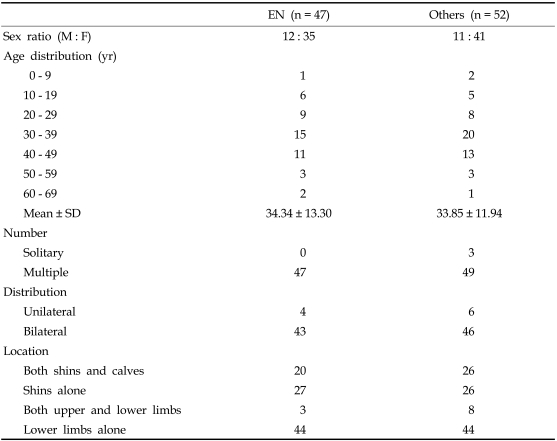
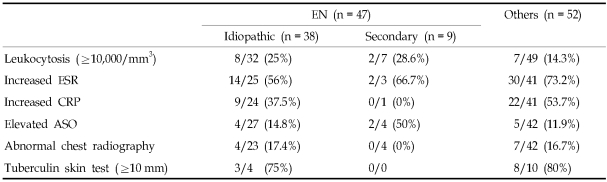
To investigate differences in demographic and clinical characteristics between EN and other diseases resembling EN, we pooled all patients with histologically proven EN into a single group and compared them to the group of patients with other diseases (Table 2). Forty-seven patients had EN and 52 patients had other diseases. No significant differences were observed between the two groups. In both groups the frequency of any disease was three to four times higher in females and highest in the fourth decade of life. The fifth decade of life had the next highest frequency. The mean age (± SD) was 34.3 years (± 13.3) in the group of patients with EN and 33.9 years (± 11.9) in the group with other diseases. Multiple, bilateral lesions were observed in the majority of patients in both groups. Involvement of shins and calves was observed in 20 of 47 patients (43%) with EN and in 26 of 52 patients (50%) with other diseases. Laboratory data are summarized in Table 3, with no statistically significant results observed between the EN group and the group with other diseases.
Table 2.
Demographic Data and Clinical Findings of All Patients
EN, erythema nodosum.
Table 3.
Comparison of Summarized Laboratory Data between EN and Other Diseases
EN, erythema nodosum; ESR, erythrocyte sedimentation rate; CRP, C-reactive protein; ASO, anti-streptolysin O.
The numerators indicate the number of patients who had positive results or increased levels.
The denominators indicate the number of patients who were tested.
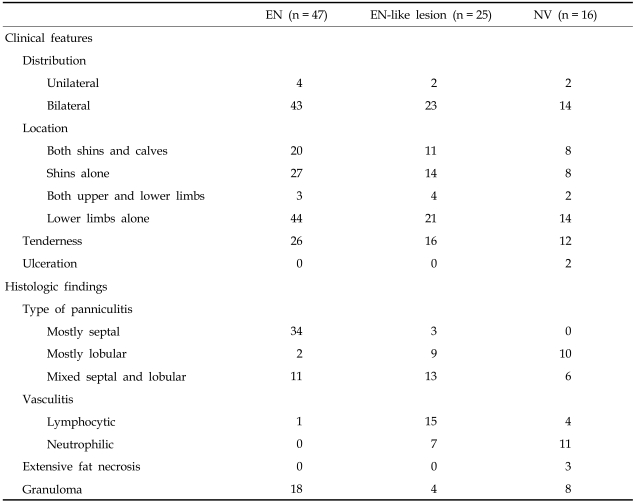
We classified EN into two groups; EN with an unidentified etiology (idiopathic EN) and EN with an etiologic factor (secondary EN). Underlying causes or precipitating events associated with EN were identified in nine of 47 patients (19%) with histologically proven EN. The most common underlying cause was URI (n = 4), followed by drug intake (n = 3), tuberculosis (n = 1) and pregnancy (n = 1). The male:female ratio was 12:26 for the first group, however all patients in the second group were female. The highest frequency was observed in the fourth decade, followed by the fifth decade of life in the first group. In contrast, the age at diagnosis in the second group was regularly distributed from the second to the fifth decade (three patients in their 10s, two patients in their 20s, two patients in their 30s, and two patients in their 40s). No significant differences in the distribution of lesions were observed between groups. The mean age (± SD) was 36.0 years (± 13.3) and 27.3 years (± 11.2), respectively. The mean age tended to be younger in the second group, but no significant difference was found. All patients had multiple lesions in both groups (Fig. 1A). Involvement of the shins and calves was observed in 17 of 38 patients (45%) in the first group and in three of nine patients (33%) in the second group. Both shins and calves were frequently involved in both groups. Nine of 38 patients in the first group had a previous history of recurrent oral aphthous ulcers. Of this group, a previous history of epididymitis was noted in one patient, and another patient had already been treated for an incomplete type of BD.
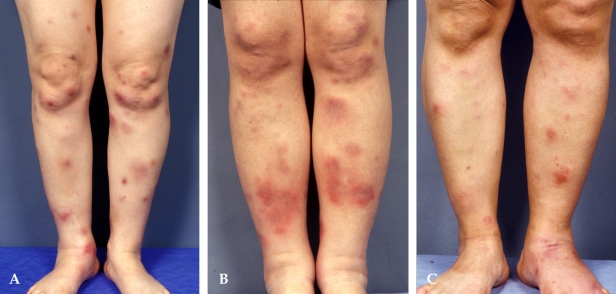
Fig. 1.
Clinical photographs of (A) EN, (B) EN-like lesion in BD, and (C) NV. Multiple, bilateral inflammatory subcutaneous nodules and plaques on the legs were observed. No ulceration was observed in these three diseases. No prominent differences were noted clinically.
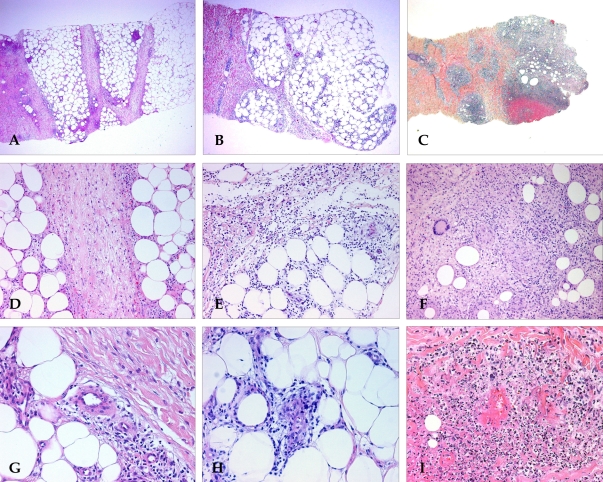
Laboratory data for both groups is described in Table 3. No statistically significant differences were found between the two groups. Additionally, no significant differences in histologic features were observed between the two groups. Septal panniculitis (Fig. 2A) was predominantly observed in 27 of 38 patients (71%) in the first group and seven of nine patients (78%) in the second group. Variable degrees of lymphohistiocytic infiltration (Fig. 2D) were present in 35 of 38 patients (92%) in the first group and seven of nine patients (78%) in the second group; and the frequency of granulomatous inflammation was 39% and 44%, respectively.
Fig. 2.
Histopathologic findings of EN, EN-like lesion in BD, and NV. Pattern of panniculitis: Mostly septal panniculitis of EN (A, H & E stain, × 40). Inflammation presents mainly in and around the septa. Mixed septal and lobular panniculitis of EN-like lesion in BD (B, H & E stain, × 40). Inflammatory cells diffusely infiltrated in septa and lobules without clear compartmentalization. Mostly lobular panniculitis of NV (C, H & E stain, × 40). A region of caseation necrosis and surrounding inflammation replacing the septa and lobules was observed. Inflammation: There is an edema of the septa with paraseptal inflammation composed of lymphocytes mixed with histiocytes in EN (D, H & E stain, × 200). A dense inflammatory infiltrate of neutrophils mixed with lymphocytes was observed in the EN-like lesion (E, H & E stain, × 200). Severe lobular inflammation in NV (F, H & E stain, × 200), with extensive necrosis of adipocytes and granulomatous inflammation was observed. Vasculitis: No vasculitis was observed in EN (G, H & E stain, × 400). Lymphocytic vasculitis in EN-like lesion (H, H & E stain, × 400). Lymphocytic infiltrate around and within vessel wall was observed. Neutrophilic vasculitis in NV (I, H & E stain, × 400). Destruction of the vascular wall, surrounding neutrophils and nuclear dusts was observed.
The major differential diagnoses of EN were EN-like lesions in BD (Fig. 1B) and NV (Fig. 1C). There was no significant difference in clinical findings among these three diagnoses, though their histologic features did differ (Table 4). In contrast to EN, mixed septal and lobular panniculitis (52%) and mostly lobular panniculitis (36%) were frequently observed in patients with EN-like lesions in BD on histologic examination (Fig. 2B and E). Vasculitis was present in 22 of 25 patients with EN-like lesions. Lymphocytic vasculitis (Fig. 2H) was observed in 15 patients, and neutrophilic vasculitis was observed in 7 patients. Conversely, the most common type of panniculitis in patients with NV was mostly lobular panniculitis (63%) (Fig. 2C). Neutrophilic vasculitis (Fig. 2I) was also commonly observed in patients with NV, and granulomatous inflammation and extensive fat necrosis (Fig. 2F) were more frequently observed in NV than EN and EN-like lesions in BD.
Table 4.
Comparison of Clinical and Histologic Findings among EN, EN-Like Lesion, and NV
EN, erythema nodosum; NV, nodular vasculitis.
Excluding EN-like lesions and NV, eight disorders (polyarteritis nodosa, sclerosing panniculitis, thrombophlebitis, Sweet's syndrome, leukemia cutis, Henoch-Schonlein purpura, paraffinoma, and traumatic fat necrosis) were included in this study (Table 1). In this minority group, three patients were male and eight patients were female, with a mean age (± SD) of 30.4 years (± 17.4). The shin was involved in all cases, and seven patients had skin lesions which affected both shins and calves. In two patients skin lesions were located on both the upper and lower limbs. None of the patients demonstrated ulceration, and clinical features were similar to those of EN. Diagnoses in these patients were confirmed by histopathologic examination.
DISCUSSION
Our findings indicate that a clinical diagnosis of EN should be made with caution. The diagnosis of EN is typically clinical,3 even though in this study the frequency of histologically proven EN following clinical diagnosis was less than 50%. No significant differences in demographics or clinical characteristics were observed between the EN group and the group with other diseases. Both groups demonstrated similarities in the male: female ratio, mean age at diagnosis, age distribution, and location of lesions.
The rate of secondary EN was reported in range between 28% and 68% in previous studies,2,10,11 but was less than 20% in this study. This lower rate is probably due to an inability to identify underlying causes; EN usually subsides in 3 to 6 weeks without any treatment. In contrast to previous European studies,11-13 none of our patients had underlying sarcoidosis or inflammatory bowel disease. URI was the most frequent underlying cause associated with EN in this study, corresponding with previous reports.2,12,13 Although tuberculosis is still a predominant cause of EN in some countries,14 it was a rare cause of EN in our study. It is postulated that our results reflect a decreased incidence of tuberculosis in Korea.15
It is challenging to find differing characteristics between idiopathic and secondary EN. The mean age of patients with secondary EN tended to be younger than patients with idiopathic EN. However, in contrast to a previous report,12 no significant difference was observed between the groups. Overall, no significant characteristics between groups were noted in this study. Because this was a retrospective study, we were limited as to obtaining useful information to distinguish between idiopathic and secondary EN. To determine characteristics that can differentiate idiopathic from secondary EN, a larger patient population and prospectively designed study would be necessary.
Nine of 38 patients with idiopathic EN had a previous history of recurrent oral aphthous ulcer. Most of these patients might be diagnosed with a suspected type of BD by the Research Committee of Behçet's Disease of Japan.16 However, it was difficult to rule out the possibility that EN merely coexisted with recurrent oral ulcers. One patient also had a previous history of epididymitis, a relatively characteristic finding in BD. One patient suffered from an incomplete type of BD and was already treated for BD. Due to medication or disease activity, EN, rather than EN-like lesions, may have manifested in these two patients. When EN develops in a patient with recurrent oral ulcer other symptoms of BD can develop in the future; therefore, long-term follow up is required.
Similar to EN, EN-like lesions and NV were also common diseases affecting the lower legs in this study. We could not clinically identify features that would distinguish these three diseases. Typically, lesions of NV appear on the posterior aspects of the lower legs and often ulcerate. However, our clinical findings of NV showed some different features compared with the descriptions in textbook. Lesions of NV involved the shins more frequently than the calves. About half of the patients with EN and NV had nodular lesions on both shins and calves. Moreover, ulceration, considered a differentiating feature of NV from EN, was very rare (12.5%). Similar results were described in other Korean articles.17,18 Thus, we feel the presence of ulceration may not be useful in differentiating NV from EN in Korea.
It is easy to distinguish between EN and NV after histologic examination. Lobular panniculitis and neutrophilic vasculitis were the most common findings in NV. EN-like lesions in BD more frequently showed mixed septal and lobular or lobular panniculitis than true EN. We also observed vasculitis in 22 of 25 patients. Lymphocytic vasculitis was observed more frequently than neutrophilic vasculitis, which is in close agreement with previous reports.19 However, it is hypothesized that EN-like lesions in BD cannot be differentiated from EN secondary to other systemic disorders.20 Although mixed or lobular panniculitis and lymphocytic vasculitis are seen more frequently, and granulomatous inflammation is observed less frequently in EN-like lesions than EN; a definite histologic diagnosis of an EN-like lesion may be difficult if the intensity of inflammation is mild and no evidence of vasculitis is present. Cardinal manifestations, such as recurrent oral aphthous ulcer, recurrent genital ulcer, and uveitis are helpful aids in the diagnosis of BD.
Other disorders, which were a minority in this study, were similar to EN clinically. It was difficult to differentiate these disorders from EN based on clinical features alone, and thus histopathologic examination was required. Detailed medical history also aided in a correct diagnosis. Because these disorders were in the minority, detailed analyses were difficult compared to an EN-like lesion or NV.
In conclusion, we believe a diagnosis of EN based on clinical features alone is very difficult. Besides EN, EN-like lesions in BD and NV should be considered in every differential diagnosis for erythematous nodular lesions affecting the lower limbs. We feel that skin biopsy is mandatory for an accurate diagnosis of lower extremity erythematous nodular lesions.
References
- 1.Camilleri MJ, Su WPD. Panniculitis. In: Freedberg IM, Eisen AZ, Wolff K, Austen KF, Goldsmith LA, Katz SI, editors. Fitzpatrick's dermatology in general medicine. 6th ed. New York: McGraw-Hill; 2003. pp. 1047–1063. [Google Scholar]
- 2.Tay YK. Erythema nodosum in Singapore. Clin Exp Dermatol. 2000;25:377–380. doi: 10.1046/j.1365-2230.2000.00667.x. [DOI] [PubMed] [Google Scholar]
- 3.Barham KL, Jorizzo JL, Grattan B, Cox NH. Vasculitis and neutrophilic vascular reactions. In: Burns T, JBreathnach S, Cox N, Griffiths C, editors. Rook's textbook of dermatology. 7th ed. Oxford: Blackwell publishing; 2004. pp. 49.1–46. [Google Scholar]
- 4.Requena L, Yus ES. Panniculitis. Part I. Mostly septal panniculitis. J Am Acad Dermatol. 2001;45:163–183. quiz 184–quiz 186. doi: 10.1067/mjd.2001.114736. [DOI] [PubMed] [Google Scholar]
- 5.Requena L, Sánchez Yus E. Panniculitis. Part II. Mostly lobular panniculitis. J Am Acad Dermatol. 2001;45:325–361. quiz 362–quiz 364. doi: 10.1067/mjd.2001.114735. [DOI] [PubMed] [Google Scholar]
- 6.Bak H, Lee JW, Ahn HJ, Hwang SM, Choi EH, Lee SH, et al. An unusual case with membranous lipodystrophy in a hypertensive patient with transepidermal elimination. Yonsei Med J. 2006;47:428–431. doi: 10.3349/ymj.2006.47.3.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barnhill RL, Busam KJ, Nousari CH, Xu X, Barksdale SK. Vascular diseases. In: Elder DE, Elenitsas R, Johnson BL Jr, Murphy GF, editors. Lever's histopathology of the skin. 9th ed. Philadelphia: Lippincott Williams & Wilkins; 2005. pp. 215–242. [Google Scholar]
- 8.Carlson JA, Mihm MC, LeBoit PE. Cutaneous lympho-cytic vasculitis: a definition, a review, and a proposed classification. Semin Diagn Pathol. 1996;13:72–90. [PubMed] [Google Scholar]
- 9.International study group for Behçet's disease (ISGBD) Criteria for diagnosis of Behçet disease. Lancet. 1990;335:1078–1080. [PubMed] [Google Scholar]
- 10.Mert A, Ozaras R, Tabak F, Pekmezci S, Demirkesen C, Ozturk R. Erythema nodosum: an experience of 10 years. Scand J Infect Dis. 2004;36:424–427. doi: 10.1080/00365540410027184. [DOI] [PubMed] [Google Scholar]
- 11.Psychos DN, Voulgari PV, Skopouli FN, Drosos AA, Moutsopoulos HM. Erythema nodosum: the underlying conditions. Clin Rheumatol. 2000;19:212–216. doi: 10.1007/s100670050159. [DOI] [PubMed] [Google Scholar]
- 12.García-Porrúa C, González-Gay MA, Vázquez-Caruncho M, López-Lazaro L, Luerio M, Fernández ML, et al. Erythema nodosum: etiologic and predictive factors in a defined population. Arthritis Rheum. 2000;43:584–592. doi: 10.1002/1529-0131(200003)43:3<584::AID-ANR15>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 13.Cribier B, Caille A, Heid E, Grosshans E. Erythema nodosum and associated diseases. A study of 129 cases. Int J Dermatol. 1998;37:667–672. doi: 10.1046/j.1365-4362.1998.00316.x. [DOI] [PubMed] [Google Scholar]
- 14.Tantisirin O, Puavilai S. Long-term follow-up of erythema nodosum. J Med Assoc Thai. 2003;86:1095–1100. [PubMed] [Google Scholar]
- 15.Global TB database. World Health Organization (WHO); Available from: http://www.who.int/tb/country/global_tb_database/en/index.html. [Google Scholar]
- 16.Mizushima Y, Inaba G, Mimura Y, Ono S. Diagnostic criteria for Behçet's disease in 1987, and guideline for treating Behçet's disease. Saishin Igaku. 1988;43:391–393. [Google Scholar]
- 17.Kim YS, Kim SN. A clinical study on erythema nodosum and erythema induratum. Korean J Dermatol. 1984;22:475–482. [Google Scholar]
- 18.Choi US, Bang D, Lee KG, Chun SI. The clinical and histopathological study of erythema induratum and erythema nodosum. Korean J Dermatol. 1991;29:304–312. [Google Scholar]
- 19.Kim B, LeBoit PE. Histopathologic features of erythema nodosum-like lesions in Behçet's disease: a comparison with erythema nodosum focusing on the role of vasculitis. Am J Dermatopathol. 2000;22:379–390. doi: 10.1097/00000372-200010000-00001. [DOI] [PubMed] [Google Scholar]
- 20.Chun SI, Su WP, Lee S, Rogers RS. Erythema nodosum- like lesions in Behçet's syndrome: a histopathologic study of 30 cases. J Cutan Pathol. 1989;16:259–265. doi: 10.1111/j.1600-0560.1989.tb00049.x. [DOI] [PubMed] [Google Scholar]