Abstract
Purpose
Pulse wave velocity (PWV) is at least partially controlled by vascular tone. Vascular tone and underlying physiological processes such as sympathetic activity, plasma catecholamin, and cortisol levels have been shown to follow diurnal variations.
Materials and Methods
Carotid-to-radial PWV was non-invasively assessed by applanation tonometry in 21 young (26.5 ± 2.3 years) healthy men at three different time points (8:00hr, 12:00hr, 17:00hr) during a day. Additionally, heart rate, systolic, diastolic and mean blood pressure, and radial pulse pressure were assessed at the same time points.
Results
The mean PWV was significantly higher at 8:00hr compared with the mean PWV assessed at later time points. No significant differences were found between mean PWV at 12:00hr and at 17:00hr. When PWV was corrected for blood pressure, the difference between values at 8:00hr and 12:00hr was no longer significant. Systolic, diastolic and mean blood pressure were significantly lower at 17:00hr compared with those at 8:00hr.
Conclusion
A small but significant diurnal variation of PWV was observed in young healthy men, which might have been caused at least partly by variations of blood pressure. This finding could be of value, when PWV is used in human research. Thus, in longitudinal investigations the measurements should be performed at similar time points in the course of a day, in order to obtain comparable data. Additionally, our observations ought to be of assistance to studies in which novel pharmacological compounds with activity on the vasculature are investigated.
Keywords: Applanation tonometry, arterial stiffness, diurnal rhythm, pulse wave velocity
INTRODUCTION
Arterial stiffness has been recognized as an important component of cardiovascular physiology.1 Pulse wave velocity (PWV) is a measure of arterial stiffness and can be assessed non-invasively by applanation tonometry, a simple and reproducible method. PWV provides information on the time period within which a pressure or flow wave travels a known distance. The principle of measurement is based on the recording of peripheral arterial pulse pressure waveforms at two different sites.2
PWV is related to the square root of the elasticity modulus and rises in stiffer arteries according to the Moens-Korteweg equation.3 Hence, the stiffer the artery, the faster the speed of the pressure wave. Furthermore, an increase in PWV is accompanied by a reduced time period within which pressure waves reflected from the periphery of the circulation return to the aorta and vice versa. At the optimal timing of such a ventricular-vascular coupling, the reflected waves return to the ascending aorta during diastole, enhance coronary blood flow, and do not affect aortic systolic pressure.4 With vascular stiffening, PWV and the amplitude of the reflected waves both increase so that the reflected wave arrives earlier, in systole rather than in diastole, and thereby augments central systolic pressure. This early wave reflection increases systolic and pulse pressures and may decrease coronary perfusion.5
Arterial stiffness is at least partially controlled by vascular tone.6,7 Vascular tone and underlying physiological factors, such as sympathetic nerve activity and plasma catecholamine and cortisol levels, have been shown to follow diurnal variations.6,8,9 In the present study, we investigated whether under physiological conditions PWV shows diurnal variations. Thus, in the course of a day PWV was assessed in young healthy men by applanation tonometry via the carotid-to-radial method.
MATERIALS AND METHODS
Subjects
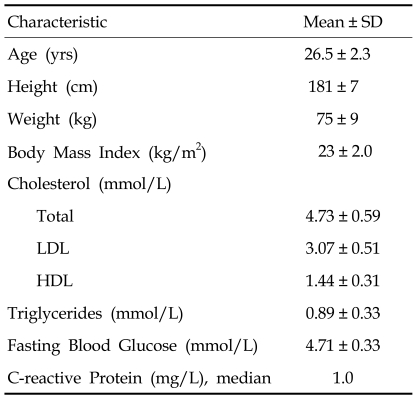
Twenty-one healthy, adult male health professionals, including medical students, aged 21 to 30 years (26.5 ± 2.3 years, mean ± standard deviation (SD)) were studied. The healthy subjects were selected on the basis that they were normotensive, non-diabetic, non-smokers, non-hypercholesterolemic, and without a history of premature vascular disease. In addition, all subjects were not on any medication, including vitamins or antioxidant supplements, at least 6 months prior to and during the study period. All subjects were also not on-call the three nights prior to the measurements. The study was carried out according to the principles of the Declaration of Helsinki, and all subjects gave informed consent. The study protocol was approved by the local ethics committee.
Assessment of carotid-to-radial PWV
Applanation tonometry was performed with the Sphygmocor instrument (Sphygmocor AtCor Medical, version 6.31). A high-fidelity micromanometer (SPC-301, Millar Instruments) was administered to flatten the right carotid artery and the left radial artery, and the pulse was continuously recorded. The timing of the waveforms was compared with that of the R wave on a simultaneously recorded ECG. The transit distance was assessed by subtracting the distance between the supra-sternal notch and the carotid applanation point from the distance between the supra-sternal notch and the radial applanation point, which then allowed PWV to be determined by the system software of the Sphygmocor instrument. Two consecutive measurements within each subject at each time point were performed, and the mean thereof was used. The repeatability of the method was assessed by calculating the standard deviations of those two consecutive measurements. This within-subject standard deviation was used as a measure for repeatability according to Bland and Altman.10 The overall within-subject standard deviation was found to be 0.27m/s, respectively. The range of intrasubject standard deviations was from 0.0 to 0.42m/s.
Just prior to applanation tonometry the subject's blood pressure (BP) was measured in the right brachial artery by using an automated oscillometric method (boso medicus, Bosch+Sohn GMBH, Jungingen, Germany). The mean BP was calculated according to the equation: mean BP = diastolic BP+pulse pressure/3.11
Study protocol
All subjects had at least three nights of sleep before measurements were taken. Subjects were studied in a quiet temperature-controlled laboratory (24+1℃) after 20 minutes of lying supine. Measurements of each subject were obtained at three time points (8:00hr, 12:00hr, and 17:00hr). All measurements were performed prior to any meals. During the study, heavy meals, including high-fat foods, coffee, tea, and alcoholic beverages, were not permitted at least 12hr prior to and during the study. Also, subjects were instructed not to physically exercise during the course of the study. All assessments were performed by the same operator within a twenty-four hour period. Seven subjects started at 8:00hr, seven subjects at 12:00hr, and the remainder at 17:00hr hours to avoid a possible time bias.
Statistical analysis
Statistical analysis was performed using the Statistical Package for Social Sciences (version 10, SPSS Inc., Chicago, IL, USA). Values are expressed as mean ± standard deviation or as median (range), if not stated otherwise. The changes of PWV and other hemodynamic parameters over time were tested by Wilcoxon and Friedman tests. Significance tests were 2-sided, and p < 0.05 were considered significant.
RESULTS
The study population (n = 21) exhibited normal baseline characteristics for young, healthy men (Table 1). As shown in Table 2, the mean PWV showed a significant diurnal variation with the highest values of 7.4 ± 0.6m/s at 8:00hr decreasing to 7.1 ± 0.6m/s at 12:00hr (p < 0.05 vs 8:00 hr) and 7.0 ± 0.6m/s at 17:00hr (p < 0.05 vs 8:00hr). No significant difference was observed between the mean PWV at 12:00hr and that at 17:00hr. When PWV was corrected for BP, the difference between values at 8:00hr and 12:00hr was no longer significant. The mean values of systolic BP, diastolic BP, and mean BP were significantly lower at 17:00hr compared with those at 8:00hr. No significant difference was detectable between 8:00hr and 12:00hr and between 12:00hr and 17:00hr. The mean radial pulse pressure showed no significant variation among the three time points (Table 2).
Table 1.
Baseline Characteristics of Study Participants
Table 2.
Diurnal Variation of Hemodynamic Parameters
Pulse Wave Velocity BP, Pulse Wave Velocity corrected for Blood Pressure.
Wilcoxon test: *p < 0.05 vs 8:00.
†Friedman test.
DISCUSSION
The present study demonstrates that PWV, a measure of large muscular artery stiffness, exhibits a small but significant diurnal variation in young, healthy men. This suggests that PWV is physiologically increased in the morning and decreases later in the day. A similar diurnal variation has been reported in vascular tone when measured in normal subjects at various time points during the day. So, in the morning hours an increased α-mediated sympathetic vasoconstriction has been observed in normal subjects, which may indicate a morning surge in sympathetic nerve activity possibly also affecting PWV.6 This increase could be due to either an endogeneous rhythm or possibly increased physical activity in the morning. To minimize the influence of physical activity in our study, the participants were instructed not to exercise during the entire study period. Also, the participants rested in a supine position for 20 minutes prior to the initiation of measurements. Thus, the higher PWV observed at the morning hour is more likely due to an endogeneous rhythm than to physical activity. A significant difference in PWV was not observed between the measurements at 12:00hr and 17:00hr. However, we cannot exclude that such a difference might exist and could turn out to be significant in a study using a larger sample.
Recently, we have shown a significant diurnal variation in the aortic augmentation index in young, healthy men.12 Like PVW, the aortic augmentation index is a measure of arterial stiffness, but in contrast to PWV, the aortic augmentation index depends additionally on small artery stiffness and the magnitude of wave reflection from the periphery.5 Furthermore, Kelly and colleagues found that the aortic augmentation index varied independently from PWV after the administration of vasoactive compounds.13
In a study by Lantelme and colleagues, PWV and heart rate have been shown to be related to each other when assessed in a gender mixed population of patients with arrhythmia. In these patients, heart rates were controlled by pacemakers applying rates from 60 to 100 bpm in 10 bpm increments. This resulted in a clear dose response in PWV, which gradually increased with heart rate. However, the pulse wave velocities obtained from consecutive measurements at 10 bpm increments were not found to be significantly different.14 In our study population mean PWV varied according to mean heart rate. However, the maximum mean difference in heart rate was only 4 bpm. Thus, it is unlikely that the even smaller but significant variation of the mean heart rate in our study was responsible for the significant variation in mean PWV. However, from our data we cannot entirely exclude that such a possibility may exist.
In our study, carotid-to-radial PWV, which is a measurement in a mainly muscular segment, was assessed. Another possibility for assessing PWV is carotid-to-femoral PWV, which is a measurement in a predominantly elastic segment.2 Muscular and elastic segments of the arterial tree differ in function from the muscular segment representing a predominantly conduit function and the elastic segment representing a predominantly cushioning function on the BP. Those differing functions are also reflected in their response to controlling vasoactive stimuli, e.g. arterial stiffness and PWV.15 In order to detect possible small variations of PWV, we decided to use carotid-to-radial PWV because of the higher reproducibility of this method since good quality recordings are more difficult to obtain from the femoral artery than from the radial artery.16 Thus, our study is limited to carotid-to-radial PWV. In many other studies, carotid-to-femoral PWV has been assessed, and it remains unclear whether diurnal variations of PWV would occur with the carotid-to-femoral PWV.
In our subjects, systolic, diastolic and mean BP showed a similar variation to that of PWV. However, the difference between BP values obtained at 8:00hr and 12:00hr failed to reach significance, perhaps due to the limited number of subjects studied. When PWV was corrected for BP, the difference between 8:00hr and 12:00hr was no longer significant. Thus, the observed diurnal variation of PWV might be caused at least partly by variations of BP since BP has been reported to be an important factor influencing PWV.1 However, the absence of a correlation between BP and PWV suggests that a clear linear relationship may not exist. This view is supported by the study of Oren et al., who observed that BP does not necessarily predict aortic stiffness in healthy, young adults.17
A literature review revealed that PWV values differ markedly among the various studies.13,18,19 This can be attributed to differences of the study populations, i.e. health states, gender, age, and smoking behavior or to day-to-day and seasonal variations or technical related variations.2,20-25 Because time points of the measurements were usually not reported in the studies, the differences could to some degree also be ascribed to diurnal variations of PWV, which we have observed.
In a previous study, ter Avest et al. assessed PWV in 19 healthy volunteers at 9:00hr and 14:00hr and found no significant difference between the two time points.26 Our differing results can be explained by the differences in the selection of the study groups. They selected a gender mixed study group of 19 individuals with ages ranging from 25 to 63 years, whereas we selected young healthy males only. Also, ter Avest and colleagues used carotid-to-femoral PWV assessment, whereas we used carotid-to-radial PWV, because this method has been shown to exhibit a higher degree of reproducibility.16 In addition, changes in the stiffness of elastic arteries are passive secondary events, whereas changes in the stiffness of muscular arteries are actively performed. We believe that all of those differences including the different time points of assessments may well explain our contrasting findings.
Myocardial infarction, sudden cardiac death, transient myocardial ischemia, acute aortic dissection, and stroke have been shown to occur more frequently in the morning than at other times of the day.27-31 Arterial stiffness has been recognized as an important risk factor of cardiovascular events.32 Further investigations are required to find out whether our observed increased PWV in young healthy men at morning hours may also occur in patients with atherosclerotic diseases and, should this be the case, whether our observation would be of clinical relevance.
In conclusion, our study shows that arterial stiffness assessed as carotid-to-radial PWV exhibits a small but significant diurnal variation. This observation could be of value when applanation tonometry is applied to human research. Thus, in order to arrive at comparable measurements especially in longitudinal studies, the measurements should be performed at similar time points in the course of a day. Additionally, our observation should be helpful to studies in which pharmacological compounds with activity on the vasculature are investigated in healthy subjects.
References
- 1.O'Rourke M, Kelly RP. Wave reflection in the systemic circulation and its implications in ventricular function. J Hypertens. 1993;11:327–337. doi: 10.1097/00004872-199304000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Cameron JD, Bulpitt CJ, Pinto ES, Rajkumar C. The aging of elastic and muscular arteries: a comparison of diabetic and nondiabetic subjects. Diabetes Care. 2003;26:2133–2138. doi: 10.2337/diacare.26.7.2133. [DOI] [PubMed] [Google Scholar]
- 3.Oliver JJ, Webb DJ. Noninvasive assessment of arterial stiffness and risk of atherosclerotic events. Arterioscler Thromb Vasc Biol. 2003;23:554–566. doi: 10.1161/01.ATV.0000060460.52916.D6. [DOI] [PubMed] [Google Scholar]
- 4.Nichols WW. Clinical measurement of arterial stiffness obtained from noninvasive pressure waveforms. Am J Hypertens. 2005;18:3S–10S. doi: 10.1016/j.amjhyper.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 5.London GM, Marchais SJ, Guerin AP, Pannier B. Arterial stiffness: pathophysiology and clinical impact. Clin Exp Hypertens. 2004;26:689–699. doi: 10.1081/ceh-200031982. [DOI] [PubMed] [Google Scholar]
- 6.Panza JA, Epstein SE, Quyyumi AA. Circadian variation in vascular tone and its relation to alpha-sympathetic vasoconstrictor activity. N Engl J Med. 1991;325:986–990. doi: 10.1056/NEJM199110033251402. [DOI] [PubMed] [Google Scholar]
- 7.Wilkinson IB, MacCallum H, Hupperetz PC, van Thoor CJ, Cockcroft JR, Webb DJ. Changes in the derived central pressure waveform and pulse pressure in response to angiotensin II and noradrenaline in man. J Physiol. 2001;530:541–550. doi: 10.1111/j.1469-7793.2001.0541k.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weitzman ED, Fukushima D, Nogeire C, Roffwarg H, Gallagher TF, Hellman L. Twenty-four hour pattern of the episodic secretion of cortisol in normal subjects. J Clin Endocrinol Metab. 1971;33:14–22. doi: 10.1210/jcem-33-1-14. [DOI] [PubMed] [Google Scholar]
- 9.Muller JE, Tofler GH, Stone PH. Circadian variation and triggers of onset of acute cardiovascular disease. Circulation. 1989;79:733–743. doi: 10.1161/01.cir.79.4.733. [DOI] [PubMed] [Google Scholar]
- 10.Bland JM, Altman DG. Measurement error. BMJ. 1996;313:744. doi: 10.1136/bmj.313.7059.744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O'Callaghan CJ, Straznicky NE, Komersova K, Louis WJ. Systematic errors in estimating mean blood pressure from finger blood pressure measurements. Blood Press. 1998;7:277–281. doi: 10.1080/080370598437123. [DOI] [PubMed] [Google Scholar]
- 12.Bodlaj G, Berg J, Biesenbach G. Diurnal variation of arterial stiffness and subendocardial perfusion noninvasively assessed using applanation tonometry in healthy young men. Wien Klin Wochenschr. 2005;117:348–352. doi: 10.1007/s00508-005-0357-4. [DOI] [PubMed] [Google Scholar]
- 13.Kelly RP, Millasseau SC, Ritter JM, Chowienczyk PJ. Vasoactive drugs influence aortic augmentation index independently of pulse-wave velocity in healthy men. Hypertension. 2001;37:1429–1433. doi: 10.1161/01.hyp.37.6.1429. [DOI] [PubMed] [Google Scholar]
- 14.Lantelme P, Mestre C, Lievre M, Gressard A, Milon H. Heart rate: an important confounder of pulse wave velocity assessment. Hypertension. 2002;39:1083–1087. doi: 10.1161/01.hyp.0000019132.41066.95. [DOI] [PubMed] [Google Scholar]
- 15.Safar ME, Levy BI, Struijker-Boudier H. Current perspectives on arterial stiffness and pulse pressure in hypertension and cardiovascular disease. Circulation. 2003;107:2864–2869. doi: 10.1161/01.CIR.0000069826.36125.B4. [DOI] [PubMed] [Google Scholar]
- 16.Wilkinson IB, Fuchs SA, Jansen IM, Spratt JC, Murray GD, Cockcroft JR, et al. Reproducibility of pulse wave velocity and augmentation index measured by pulse wave analysis. J Hypertens. 1998;16:2079–2084. doi: 10.1097/00004872-199816121-00033. [DOI] [PubMed] [Google Scholar]
- 17.Oren A, Vos LE, Uiterwaal CS, Gorissen WH, Grobbee DE, Bots ML. Adolescent blood pressure does not predict aortic stiffness in healthy young adults. The Atherosclerosis Risk in Young Adults (ARYA) study. J Hypertens. 2003;21:321–326. doi: 10.1097/00004872-200302000-00023. [DOI] [PubMed] [Google Scholar]
- 18.Yasmin, Brown MJ. Similarities and differences between augmentation index and pulse wave velocity in the assessment of arterial stiffness. QJM. 1999;92:595–600. doi: 10.1093/qjmed/92.10.595. [DOI] [PubMed] [Google Scholar]
- 19.Millasseau SC, Kelly RP, Ritter JM, Chowienczyk PJ. Determination of age-related increases in large artery stiffness by digital pulse contour analysis. Clin Sci. 2002;103:371–377. doi: 10.1042/cs1030371. [DOI] [PubMed] [Google Scholar]
- 20.Blacher J, Asmar R, Djane S, London GM, Safar ME. Aortic pulse wave velocity as a marker of cardiovascular risk in hypertensive patients. Hypertension. 1999;33:1111–1117. doi: 10.1161/01.hyp.33.5.1111. [DOI] [PubMed] [Google Scholar]
- 21.Blacher J, Guerin AP, Pannier B, Marchais SJ, Safar ME, London GM. Impact of aortic stiffness on suvival in end-stage renal disease. Circulation. 1999;99:2434–2439. doi: 10.1161/01.cir.99.18.2434. [DOI] [PubMed] [Google Scholar]
- 22.Hayward CS, Kelly RP. Gender-related differences in the central arterial pressure waveform. J Am Coll Cardiol. 1997;30:1863–1871. doi: 10.1016/s0735-1097(97)00378-1. [DOI] [PubMed] [Google Scholar]
- 23.Kawasaki T, Sasayama S, Yagi S, Asakawa T, Hirai T. Non-invasive assessment of the age related changes in stiffness of major branches of the human arteries. Cardiovasc Res. 1987;21:678–687. doi: 10.1093/cvr/21.9.678. [DOI] [PubMed] [Google Scholar]
- 24.Mahmud A, Feely J. Effect of smoking on arterial stiffness and pulse pressure amplification. Hypertension. 2003;41:183–187. doi: 10.1161/01.hyp.0000047464.66901.60. [DOI] [PubMed] [Google Scholar]
- 25.Chiu YC, Arand PW, Shroff SG, Feldman T, Carroll JD. Determination of pulse wave velocities with computerized algorithms. Am Heart J. 1991;121:1460–1470. doi: 10.1016/0002-8703(91)90153-9. [DOI] [PubMed] [Google Scholar]
- 26.ter Avest E, Holewijn S, Stalenhoef AF, de Graaf J. Variation in non-invasive measurements of vascular function in healthy volunteers during daytime. Clin Sci. 2005;108:425–431. doi: 10.1042/CS20040300. [DOI] [PubMed] [Google Scholar]
- 27.Ridker PM, Manson JE, Buring JE, Muller JE, Hennekens CH. Circadian variation of acute myocardial infarction and the effect of low-dose aspirin in a randomized trial of physicians. Circulation. 1990;82:897–902. doi: 10.1161/01.cir.82.3.897. [DOI] [PubMed] [Google Scholar]
- 28.Willich SN, Levy D, Rocco MB, Tofler GH, Stone PH, Muller JE. Circadian variation in the incidence of sudden cardiac death in Framingham Heart Study population. Am J Cardiol. 1987;60:801–806. doi: 10.1016/0002-9149(87)91027-7. [DOI] [PubMed] [Google Scholar]
- 29.Rocco MB, Barry J, Campbell S, Nabel E, Cook EF, Goldman L, et al. Circadian variation of transient myocardial ischemia in patients with coronary artery disease. Circulation. 1987;75:395–400. doi: 10.1161/01.cir.75.2.395. [DOI] [PubMed] [Google Scholar]
- 30.Mehta RH, Manfredini R, Hassan F, Sechtem U, Bossone E, Oh JK, et al. Chronobiological patterns of acute aortic dissection. Circulation. 2002;106:1110–1115. doi: 10.1161/01.cir.0000027568.39540.4b. [DOI] [PubMed] [Google Scholar]
- 31.Manfredini R, Gallerani M, Portaluppi F, Salmi R, Fersini C. Chronobiological patterns of onset of acute cerebrovascular diseases. Thromb Res. 1997;88:451–463. doi: 10.1016/s0049-3848(97)00286-7. [DOI] [PubMed] [Google Scholar]
- 32.Boutouyrie P, Tropeano AI, Asmar R, Gautier I, Benetos A, Lacolley P, et al. Aortic stiffness is an independent predictor of primary coronary events in hypertensive patients: a longitudinal study. Hypertension. 2002;39:10–15. doi: 10.1161/hy0102.099031. [DOI] [PubMed] [Google Scholar]