Abstract
Purpose
We have experienced 23 patients who had underwent cervical disc replacement with Mobi-C disc prosthesis and analyzed their radiological results to evaluate its efficacy.
Patients and Methods
This study was performed on 23 patients with degenerative cervical disc disease who underwent CDR with Mobi-C disc prosthesis from March 2006 to June 2006.
Results
The age of the study population ranged from 31 to 62 years with mean of 43 years, and 16 male and 7 female cases. Regarding axial pain, the average preoperative VAS score was 6.47 ± 1.4, while at final follow-up it was 1.4 ± 0.7 (p < 0.001). The preoperatively VAS score for radiculopathy was 6.7 ± 0.7 compared with an average score of 0 ± 0 at the final follow-up (p < 0.001). At postoperative 6th month, Odom's criteria were excellent, good, or fair for all 23 patients (100%). 7 patients (30.4%) were classified as excellent, 15 patients (65.2%) as good, and 1 patients (4.4%) as fair. Prolo economic and functional rating scale was average 8.9 ± 0.7 at postoperative 6th month. ROM in C2-7, ROM of FSU, and ROM in upper adjacent level were well preserved after CDR.
Conclusion
This report would be the first document about the CDR with Mobi-C disc prosthesis in the treatment of degenerative cervical disc disease. CDR with Mobi-C disc prosthesis provided a favorable clinical and radiological outcome in this study. However, Long-term follow-up studies are required to prove its efficacy and ability to prevent adjacent segment disease.
Keywords: Cervical, arthroplasty, Mobi-C disc prosthesis
INTRODUCTION
Anterior cervical interbody fusion has been widely performed for the treatment of degenerative cervical disc disease. However, anterior cervical interbody fusion has many adverse effects and limitations.1-5 Cervical disc replacement (CDR) is a popular alternative to anterior cervical interbody fusion.6-11 The main advantage of CDR is the maintenance of mobility and function in involved cervical spinal segments, leading to a decrease in adjacent segment degeneration.7,12 Many authors report that CDR may reduce the spread of degenerative cervical disc disease to adjacent discs to a greater extent than traditional surgical methods. The other advantages of CDR are immediate implant stability, a lack of complications due to nonunion, and the avoidance of graft harvesting. As compared to anterior cervical interbody fusion, use of CDR may achieve low morbidity and excellent postoperative outcomes. CDR may also avoid problems associated with anterior cervical interbody fusion. However, disc prosthesis may cause new and unexpected adverse side effects.1,13,14
Many types of CDR implants are widely used, such as ProDisc-C (Synthes Spine, Paoli, PA, USA), Bryan Cervical Disc (Medtronic Sofamor Danek, Memphis, TN, USA), Prestige I (Medtronic Sofamor Danek, Memphis, TN, USA), and the Bristol Disc (Medtronic Sofamor Danek, Memphis, TN, USA).7,14 Surgical results and problems associated with CDR differ by prosthesis. Thus, this study was designed to evaluate the clinical efficacy of CDR using the Mobi-C disc prosthesis. We evaluated the clinical results and dynamics of 23 patients who underwent CDR using the Mobi-C disc prosthesis (LDR medical, Troyes, France).
PATIENTS AND METHODS
Device
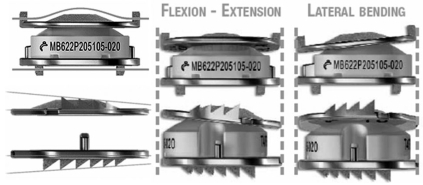
Mobi-C disc prostheses (LDR medical, Troyes, France) are composed of two titanium shells with an intervening polyethylene insert (Fig. 1). The implant is a metal-on-polyethylene articulating device similar in design to the Mobidisc lumbar disc prosthesis. With lateral self-retaining teeth, the implant is designed for optimal anchorage and stability. The inclined shape of the teeth favors the introduction of the implant and ensures a reliable anchorage on the solid parts of the vertebral plates. The mobility is self-controlled by the compression of the implant. The self-centering mobile insert favors the instantaneous rotation centers and allows natural physiological movement back to the treated intervertebral segment with respect to the cervical lordosis. The mobility of the insert decreases the transmission of the constraints on the bone-implant interface and reduces the constraints of the posterior facet joints. The design provides a normal range of motion (ROM) in flexion/extension, lateral bending, rotation, translation, and coupled motions.
Fig. 1.
Mobi-C disc prosthesis.
Surgical technique
The approach is identical to a classical cervical anterior interbody fusion. The design of the prosthetic plate adapts itself to the anatomy of the vertebral plates and requires no specific shaping of the vertebral body. When the discectomy is completed, a width gauge is placed in contact with the uncus base. The instrument incorporates a central reference line that was aligned with the midline of the vertebra. The depth measurement was completed by placing the hook of the depth gauge behind the posterior wall of the vertebral plates. The depth and width measures allowed us to determine the appropriate template size. The trial implant was inserted under C-arm guidance until it was 1mm from the posterior wall of the vertebral body. The entire implant was gripped with an adapted implant holder, which must be located in the disc axis and maintain contact with the anterior face of the vertebral body. The prosthesis was then slowly inserted with a mallet. The anterior posterior positioning of the implant was adjusted intraoperatively under C-arm guidance. The implant holder allowed us to control the position and rotation of the implant during insertion.
Patients and analysis
This study was performed on 23 patients with degenerative cervical disc disease who underwent CDR using the Mobi-C disc prosthesis between March and June 2006. Age, gender, and the severity of symptoms were analyzed based on patient's medical records.
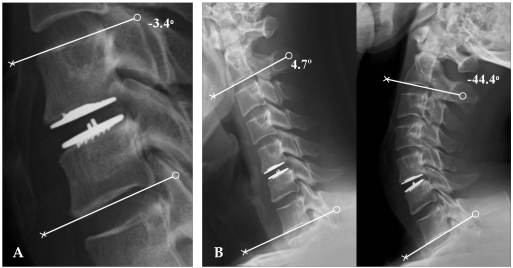
All patients were assessed before surgery and underwent radiographic analysis to evaluate the dynamics. A static cervical spine lateral radiograph was taken in the neutral position. Dynamic cervical spine lateral radiographs were taken with full flexion and full extension. Static and dynamic cervical spine radiographs were used to evaluate ROM at surgery and in cervical vertebrae 2-7 (C2-7) to compare cervical-sagittal balance. Dynamic cervical spine radiographs were used to compare motion during the procedure. Motion of adjacent vertebrae and of the whole cervical spine was also evaluated. All patients underwent Magnetic Resonance Imaging (MRI) to provide an anatomic definition of disks, neural foramina, compression of exiting nerve roots, and high signal intensity within the spinal cord. Computed Tomography (CT) clearly visualized disk-osteophyte complexes and calcified ridges. ROM for the cervical spine was defined as the difference in the Cobb's angle between full flexion and extension as shown in lateral radiographs. To analyze movement for the proposed arthroplasty, we examined the functional spinal unit (FSU) angle. FSU angle was formed by lines drawn during surgery at the inferior margin of the upper vertebral body and at the inferior margin of lower vertebral body. ROM of the FSU was defined as the segmental angle between full flexion and extension. This was calculated with the PACS workstation (Centricity 2.0, General electrics medical systems, Milwaukee, WI, USA), which uses extrapolative algorithms to calculate the intersecting angle between two lines that are drawn by the investigator (Fig. 2). A positive angle reflected lordotic angulation, whereas a negative measurement denoted kyphosis.15,16 ROM in upper adjacent vertebrae was defined as the likely difference of angle between the full flexion and extension. We then determined the postoperative angle as a percentage of the preoperative value because surgical levels were different.
Fig. 2.
ROM of the functional spinal unit (FSU) and C2-7. (A) ROM of the FSU was calculated using dynamic lateral radiographs and quantitative measurement software. (B) The ROM of the cervical spine was defined as the difference in the Cobb's angle between full flexion and extension as shown in lateral radiographs.
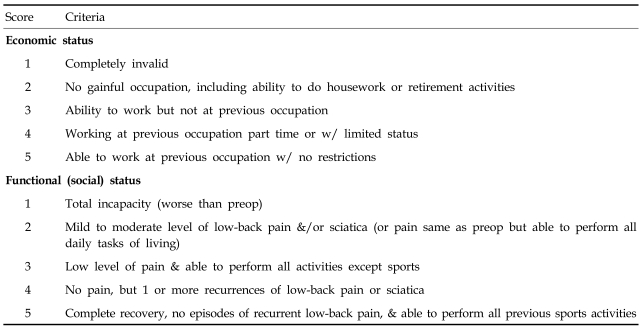
Additionally, we evaluated clinical outcomes and postoperative complications. Preoperative and final follow-up Visual Analog Scales (VAS) for axial pain and radiculopathy were prospectively collected and used in this analysis. The measurement was assessed using a 10-point VAS with endpoint anchors of no pain (0 point) and severe pain (10 points). A modified Japanese Orthopaedic Association (JOA) scoring system was used to pre- and postoperatively evaluate the severity of myelopathy.17 The modified JOA scoring system consists of seven categories: motor function of fingers, shoulder and elbow, and lower extremity; sensory function of the upper extremity, trunk, and lower extremity; and bladder function. The total score for a healthy patient is 17. Clinical outcomes were evaluated using the Prolo economic and functional rating scale (Table 1), based on the results of the follow-up physical examinations and interview.18 Results were scored according to modified Odom's criteria6 and categorized as excellent, good, fair, or poor.
Table 1.
Prolo Functional Economic Outcome Rating Scale
Patients with an excellent rating showed improvement in at least 80% of the preoperative signs and symptoms, with not more than 10% deterioration. Those patients scored as good improved in at least 70% of the preoperative signs and symptoms and had no more than 15% deterioration. Patients with a rating of fair improved in at least 50% of the preoperative signs and symptoms and had no more than 20% deterioration. Finally, patients categorized as poor, improved in less than 50% of the preoperative signs and symptoms, or had more than 20% deterioration. Pre- and postoperative VAS scores for axial pain and radiculopathy were compared using two-sample t tests paired for means. The pre- and postoperative Prolo economic and functional rating scale score was also compared using two-sample t tests paired for means. A p value of < 0.05 was regarded as significant.
RESULTS
The twenty-three patients in this study underwent 40 CDRs with Mobi-C disc prostheses in 4 months. The age of the study population ranged from 31 to 62 years with a mean age of 43 years. 16 of the patients were male, and 7 were female. Symptom duration ranged from 2 weeks to 36 months (mean 7.5 months), and the mean follow-up period was 6 months. Two discs were replaced at C3-4, four at C4-5, eleven at C5-6, and six at C6-7. Six patients underwent both anterior cervical interbody fusion and CDR with Mobi-C disc prostheses together in different levels. One patient underwent CDR with Mobi-C disc prostheses in two levels. Two patients underwent anterior cervical fusion previously.
Clinical symptoms and surgical outcome
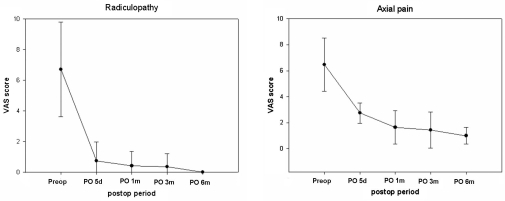
The clinical symptoms of the patients were grouped into axial pain, radiculopathy, and myelopathy. There were no statistically significant improvements in mean VAS scores from prior to surgery to the late postoperative follow-up evaluations (Fig. 3). The average preoperative and final follow-up VAS scores for axial pain were 6.47 ± 1.4 and 1.4 ± 0.7, respectively (p < 0.001). The preoperative VAS score for radiculopathy was 6.7 ± 0.7, compared with an average score of 0 ± 0 at the final follow-up (p < 0.001). Radiculopathy decreased to normal in 6 months. Axial pain decreased, but mild axial pain remained at 6 months. The preoperative JOA score for myelopathy was 16.4 ± 1.0, compared with an average score of 16.8 ± 0.7 at the final follow-up (p < 0.001). Eight patients (20.5%) had myelopathy preoperatively. There was no statistical significance because the 8 cases of preoperative myelopathy were not serious. All patients could return to work 1 month after surgery, and most patients needed no pain medication by 2 months after surgery.
Fig. 3.
Radiculopathy decreased to normal in 6 months. Axial pain decreased, but mild axial pain remained.
After CDR with Mobi-C disc prostheses, there were no complications or neurological deteriorations, including postoperative dysphasia, dysphonia, and hoarseness. Six months after surgery, Odom's criteria were excellent, good, or fair for all 23 patients (100%). 7 patients (30.4%) were classified as excellent, 15 patients (65.2%) were classified as good, and 1 patient (4.4%) was classified as fair. The Average Prolo economic and functional rating was 8.9 ± 0.7 six months after surgery. This result is greater than the targeted success rate. Both Odom's criteria and Prolo economic and function rating scale were good In 1 patient who underwent CDR with Mobi-C disc prostheses in two levels and in the 2 patients who previously underwent anterior cervical fusion.
Functional outcome
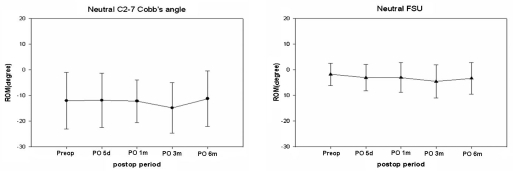
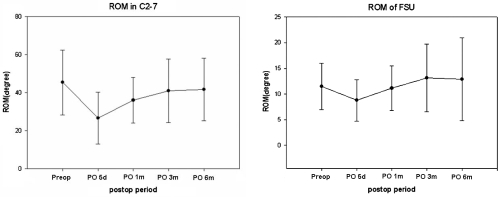
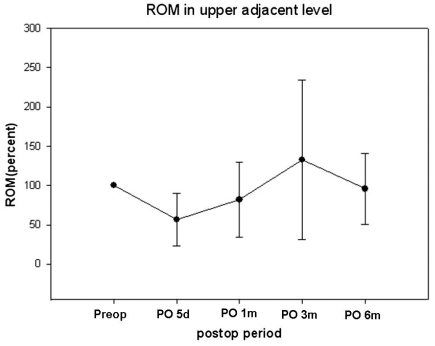
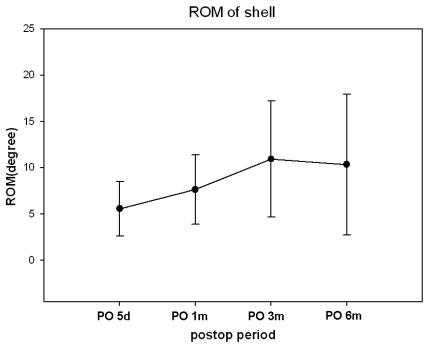
Preoperative neutral C2-7 Cobb's angle was lordotic in most patients. Only 3 patients (13.1%) had kyphotic preoperative neutral C2-7 Cobb's angle, but neutral FSU angle was kyphotic in 11 patients (47.8%). The mean neutral FSU angle was 1.25 degrees preoperatively and -4.2 degrees 6 months after surgery (Fig. 4). Neutral C2-7 Cobbs and Neutral FSU angles were well preserved during the 6-month postoperative period. C2-7 ROM ranged from 20.6 degrees to 77.7 degrees (mean 43.4 degree). The mean C2-7 ROM was 56.25 degrees preoperatively and 52.56 degrees at the 6-month postoperative follow-up (Fig. 5). The mean FSU ROM was 10.61 degrees preoperatively and 14.55 degrees at the 6-month postoperative follow-up. C2-7 ROM decreased abruptly right after CDR but returned to preoperative ROM in 6 months. FSU ROM decreased right after CDR and returned to preoperative ROM in 1 month. This restoration persisted for the duration of the follow-up period. ROM did not increase beyond preoperative FSU ROM. Percent ROM in upper adjacent vertebrae decreased to 73% right after CDR, increased to 133% 3 months after surgery and decreased to 97% 6 months after surgery (Fig. 6). Thus, ROM of adjacent segments showed hypermobility at 3 months and then returned to preoperative ROM at 6 months. It did not increase perative period. The mean shell ROM was 4.72 degrees preoperatively and 10.31 degrees 6 months after surgery (Fig. 7). Shell ROM gradually increased to normal ROM. Even though Mobi-C has different dynamic motions, compared to other cervical prostheses, cervical mobility was well preserved in both surgical level and the entire cervical spine after CDR with Mobi-C disc prosthesis.
Fig. 4.
Neutral C2-7 Cobb's and Neutral FSU angles were well preserved during the 6 months of follow-up after CDR with Mobi-C disc prostheses.
Fig. 5.
ROM in C2-7 decreased abruptly after CDR with Mobi-C disc prostheses but returned to preoperative ROM at 6 months. FSU ROM decreased after CDR with Mobi-C disc prostheses but returned to preoperative ROM in 1 month. The restored ROM persisted to the 6th month follow-up.
Fig. 6.
ROM of adjacent segments decreased right after CDR. These vertebrae show hypermobility at 3 months and preoperative ROM at 6 months. ROM did not increase beyond preoperative ROM.
Fig. 7.
The ROM of shell gradually increased and reached to normal ROM 6 months after surgery.
DISCUSSION
Anterior interbody fusion was a widely performed treatment for degenerative cervical disc disease. Long-term radiographic follow-up of patients who have undergone anterior cervical fusion has demonstrated hypermobility and degenerative changes in the non-fused segments of the spine, including disc space narrowing, endplate sclerosis, and osteophyte formation.19-21 Hilibrand et al. found that symptomatic adjacent segment disease occurred at an average rate of 2.9% per year during the first 10 postoperative years in patients who underwent cervical anterior interbody fusion.33
CDR with an artificial disc is widely performed to prevent adjacent segment disease and maintain normal ROM after surgery. CDR also decreases complications related to anterior interbody fusion, including donor site pain and pseudoarthrosis. There are many other implant systems currently in use, and we have performed CDR with some of them. Mobi-C disc prostheses are recently designed, metal-on-polyethylene articulating devices similar to the Mobidisc lumbar disc prosthesis.
Surgical insertion of the Mobi-C disc prosthesis is very simple, safe, and reproducible, because the implant holder easily allows the adjustment of position, axis, and depth. CDR with Mobi-C disc prostheses is applicable to broad indication. For these reasons, we have included patients who suffer degenerative cervical disc disease in one or two levels, underwent either radiculopathy or myelopathy, and are not responding to conservative treatment. Relative inclusion criteria are adjacent segment disease after cervical anterior interbody fusion, multilevel disease, and invisible surgical level under C-arm guidance.
Early clinical results are excellent. The potential role of cervical arthroplasty in patients who have undergone previous cervical surgery, however, is unknown. Insertion of the Mobi-C disc prosthetic disc in patients who have previously undergone cervical anterior interbody fusion, in general, appears to be safe. The results show that properly placed devices do not migrate, and the devices allow for segmental motion. The implantation of the device alleviates pain and improves function, according to neurologic signs and symptoms, to a level at least equivalent to that of other implant systems.
This study showed that changes in C2-7 ROM were minimal during the 6-month follow-up. Upper adjacent segments were hypermobile during this period. CDR preserved C2-7 and FSU ROM and thus might play an important role in the restoration of ROM in upper adjacent segments.
Although the short-term follow-up results of the Mobi-C disc prosthesis are excellent, at least a 5-year follow-up will be needed to assess the long-term functionality of the prosthesis and the protective influence on adjacent levels, compared with other implants.
This is the first report to document CDR using Mobi-C disc prostheses in the treatment of degenerative cervical disc disease. CDR with Mobi-C disc prostheses appears to be safe. The surgical technique is simple and reproducible. Furthermore, CDR with Mobi-C disc prosthesis provided a favorable clinical and radiological outcome in this study. Symptoms improved throughout the 6-month follow-up, at which point the Mobi-C disc prosthesis demonstrated great success. However, Long-term follow-up studies are required to prove its efficacy and ability to prevent adjacent segment disease.
References
- 1.Yoon DH, Yi S, Shin HC, Kim KN, Kim SH. Clinical and radiological results following cervical arthroplasty. Acta Neurochir (Wien) 2006;148:943–950. doi: 10.1007/s00701-006-0805-6. [DOI] [PubMed] [Google Scholar]
- 2.Hilibrand AS, Robbins M. Adjacent segment degeneration and adjacent segment disease: the consequences of spinal fusion? Spine J. 2004;4(6 Suppl):190S–194S. doi: 10.1016/j.spinee.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 3.Hilibrand AS, Carlson GD, Palumbo MA, Jones PK, Bohlman HH. Radiculopathy and myelopathy at segments adjacent to the site of a previous anterior cervical arthrodesis. J Bone Joint Surg Am. 1999;81:519–528. doi: 10.2106/00004623-199904000-00009. [DOI] [PubMed] [Google Scholar]
- 4.Robertson JT, Papadopoulos SM, Traynelis VC. Assessment of adjacent-segment disease in patients treated with cervical fusion or arthroplasty: a prospective 2-year study. J Neurosurg Spine. 2005;3:417–423. doi: 10.3171/spi.2005.3.6.0417. [DOI] [PubMed] [Google Scholar]
- 5.Hilibrand AS, Yoo JU, Carlson GD, Bohlman HH. The success of anterior cervical arthrodesis adjacent to a previous fusion. Spine. 1997;22:1574–1579. doi: 10.1097/00007632-199707150-00009. [DOI] [PubMed] [Google Scholar]
- 6.Goffin J, Casey A, Kehr P, Liebig K, Lind B, Logroscino C, et al. Preliminary clinical experience with the Bryan Cervical Disc Prosthesis. Neurosurgery. 2002;51:840–847. doi: 10.1227/00006123-200209000-00048. [DOI] [PubMed] [Google Scholar]
- 7.Durbhakula MM, Ghiselli G. Cervical total disc replacement, part I: rationale, biomechanics, and implant types. Orthop Clin North Am. 2005;36:349–354. doi: 10.1016/j.ocl.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 8.Duggal N, Pickett GE, Mitsis DK, Keller JL. Early clinical and biomechanical results following cervical arthroplasty. Neurosurg Focus. 2004;17:E9. doi: 10.3171/foc.2004.17.3.9. [DOI] [PubMed] [Google Scholar]
- 9.Wang Y, Xiao SH, Lu N, Zhang XS. Clinical report of cervical arthroplasty in management of spondylotic myelopathy. Zhonghua Wai Ke Za Zhi. 2004;42:1333–1337. [PubMed] [Google Scholar]
- 10.Tian W, Liu B, Li Q, Hu L, Li ZY, Yuan Q, et al. Early clinical outcome of cervical artificial disc replacement. Zhonghua Yi Xue Za Zhi. 2005;85:37–40. [PubMed] [Google Scholar]
- 11.Sekhon LH. Cervical arthroplasty in the management of spondylotic myelopathy: 18-month results. Neurosurg Focus. 2004;17:E8. doi: 10.3171/foc.2004.17.3.8. [DOI] [PubMed] [Google Scholar]
- 12.Dmitriev AE, Cunningham BW, Hu N, Sell G, Vigna F, McAfee PC. Adjacent level intradiscal pressure and segmental kinematics following a cervical total disc arthroplasty: an in vitro human cadaveric model. Spine. 2005;30:1165–1172. doi: 10.1097/01.brs.0000162441.23824.95. [DOI] [PubMed] [Google Scholar]
- 13.Anderson PA, Sasso RC, Rouleau JP, Carlson CS, Goffin J. The Bryan Cervical Disc: wear properties and early clinical results. Spine J. 2004;4(6 Suppl):303S–309S. doi: 10.1016/j.spinee.2004.07.026. [DOI] [PubMed] [Google Scholar]
- 14.Bertagnoli R, Duggal N, Pickett GE, Wigfield CC, Gill SS, Karg A, et al. Cervical total disc replacement, part two: clinical results. Orthop Clin North Am. 2005;36:355–362. doi: 10.1016/j.ocl.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 15.Pickett GE, Mitsis DK, Sekhon LH, Sears WR, Duggal N. Effects of a cervical disc prosthesis on segmental and cervical spine alignment. Neurosurg Focus. 2004;17:E5. doi: 10.3171/foc.2004.17.3.5. [DOI] [PubMed] [Google Scholar]
- 16.Johnson JP, Lauryssen C, Cambron HO, Pashman R, Regan JJ, Anand N, et al. Sagittal alignment and the Bryan cervical artificial disc. Neurosurg Focus. 2004;17:E14. doi: 10.3171/foc.2004.17.6.14. [DOI] [PubMed] [Google Scholar]
- 17.Vernon H, Mior S. The Neck Disability Index: a study of reliability and validity. J Manipulative Physiol Ther. 1991;14:409–415. [PubMed] [Google Scholar]
- 18.Prolo DJ, Oklund SA, Butcher M. Toward uniformity in evaluating results of lumbar spine operations. A paradigm applied to posterior lumbar interbody fusions. Spine. 1986;11:601–606. doi: 10.1097/00007632-198607000-00012. [DOI] [PubMed] [Google Scholar]
- 19.Goffin J, van Loon J, Van Calenbergh F, Plets C. Long- term results after anterior cervical fusion and osteosynthetic stabilization for fractures and/or dislocations of the cervical spine. J Spinal Disord. 1995;8:499–508. [PubMed] [Google Scholar]
- 20.Gore DR, Gardner GM, Sepic SB. Roentgenographic findings following anterior cervical fusion. Skeletal Radiol. 1986;15:556–559. doi: 10.1007/BF00361055. [DOI] [PubMed] [Google Scholar]
- 21.Baba H, Furusawa N, Imura S, Kawahara N, Tsuchiya H, Tomita K. Late radiographic findings after anterior cervical fusion for spondylotic myeloradiculopathy. Spine. 1993;18:2167–2173. doi: 10.1097/00007632-199311000-00004. [DOI] [PubMed] [Google Scholar]