Abstract
Purpose
The primary objective of this study was to examine the changes of basal cortisol and DHEA levels present in saliva and serum with age, and to determine the correlation coefficients of steroid concentrations between saliva and serum. The secondary objective was to obtain a standard diurnal rhythm of salivary cortisol and DHEA in the Korean population.
Materials and Methods
For the first objective, saliva and blood samples were collected between 10 and 11 AM from 359 volunteers ranging from 21 to 69 years old (167 men and 192 women). For the second objective, four saliva samples (post-awakening, 11AM, 4PM, and bedtime) were collected throughout a day from 78 volunteers (42 women and 36 men) ranging from 20 to 40 years old. Cortisol and DHEA levels were measured using a radioimmunoassay (RIA).
Results
The morning cortisol and DHEA levels, and the age-related steroid decline patterns were similar in both genders. Serum cortisol levels significantly decreased around forty years of age (p < 0.001, when compared with people in their 20s), and linear regression analysis with age showed a significant declining pattern (slope = -2.29, t = -4.297, p < 0.001). However, salivary cortisol levels did not change significantly with age, but showed a tendency towards decline (slope = -0.0078, t = -0.389, p = 0.697). The relative cortisol ratio of serum to saliva was 3.4-4.5% and the ratio increased with age (slope = 0.051, t = 3.61, p < 0.001). DHEA levels also declined with age in saliva (slope = -0.007, t = -3.76, p < 0.001) and serum (slope = -0.197 t = -4.88, p < 0.001). In particular, DHEA levels in saliva and serum did not start to significantly decrease until ages in the 40s, but then decreased significantly further at ages in the 50s (p < 0.001, when compared with the 40s age group) and 60s (p < 0.001, when compared with the 50 age group). The relative DHEA ratio of serum to saliva was similar throughout the ages examined (slop = 0.0016, t = 0.344, p = 0.73). On the other hand, cortisol and DHEA levels in saliva reflected well those in serum (r = 0.59 and 0.86, respectively, p < 0.001). The highest salivary cortisol levels appeared just after awakening (about two fold higher than the 11 AM level), decreased throughout the day, and reached the lowest levels at bedtime (p < 0.001, when compared with PM cortisol levels). The highest salivary DHEA levels also appeared after awakening (about 1.5 fold higher than the 11AM level) and decreased by 11AM (p < 0.001). DHEA levels did not decrease further until bedtime (p = 0.11, when compared with PM DHEA levels).
Conclusion
This study showed that cortisol and DHEA levels change with age and that the negative slope of DHEA was steeper than that of cortisol in saliva and serum. As the cortisol and DHEA levels in saliva reflected those in serum, the measurement of steroid levels in saliva provide a useful and practical tool to evaluate adrenal functions, which are essential for clinical diagnosis.
Keywords: Saliva cortisol, saliva DHEA, correlation, age-related changes, diurnal rhythm
INTRODUCTION
Cortisol and dehydroepiandrosterone (DHEA) are major steroids of the adrenal gland. Cortisol is secreted as a result of steroidogenic activity of the zona fasciculata and DHEA is the main steroid product of the zona reticularis. These steroids are produced in response to stimulation by adrenocorticotropic hormone (ACTH) in the pituitary.1 Cortisol is the major glucocorticoid in humans and has a wide range of influences on metabolism, immunoregulation, vascular responsiveness, cognition, and behavior.1,2 Cortisol also has an impact on numerous pathological conditions including inflammatory autoimmune disorders,3 atopic conditions,4 metabolic syndrome,5 and depression.6
There are some distinctive characteristics of hormone secretion by the hypothalamus-pituitary-adrenal gland (HPA) axis such as: age-related changes, response to physical or psychological stress, and profound circadian rhythm. Some investigators have shown that basal cortisol levels are similar in young and elderly persons,7 while others observed increases8 or decreases with age.9 Thus, the age-related basal cortisol production in humans is controversial. However, it is generally accepted that basal levels of DHEA and its sulfate ester, DHEA-S, decline progressively with age.10 Therefore, the increased ratio of cortisol to DHEA or DHEA-S are used as aging bio-markers.11 Changes in these steroid secretion patterns are considered to aggravate some age-related diseases, although their exact biological significance is not completely understood.12
The HPA axis has a profound circadian rhythm and follows the wake-sleep cycle.13 The highest cortisol levels are present after awakening, and thereafter decline to reach their lowest levels at bedtime. Studies with steroids like cortisol that have diurnal rhythms in plasma are very limited because of methodological restrictions on repeated plasma sampling. Only a few research projects have used plasma samples, and have demonstrated that no significant modification occurred in the diurnal patterns of cortisol or DHEA levels between elder and younger age groups.12,14, 15 As more sophisticated hormone assay techniques for saliva were developed, more researchers became involved in the study of age and stress-related adrenal steroid circadian rhythm changes.16 Saliva provides a useful sample for cortisol measurement in many cases because the level of cortisol in saliva reflects that in blood. Also, saliva is easier than blood for repeated collection.17,18 Therefore, detailed changes in adrenal diurnal rhythm could be established with saliva analysis. Recently, the analysis of salivary steroids is becoming a widely accepted screening tool for adrenal or gonadal function. Individual circadian rhythm has become more important than the absolute hormonal concentrations in disease diagnosis.19,20 This study examines whether basal adrenal steroid (cortisol and DHEA) levels change with age by using matched serum and saliva samples, and evaluates the availability of saliva for adrenal function studies. We also examined the salivary diurnal rhythm of cortisol and DHEA in the Korean population, and confirmed that salivary steroid levels reflect that of serum levels.
MATERIALS AND METHODS
Participants
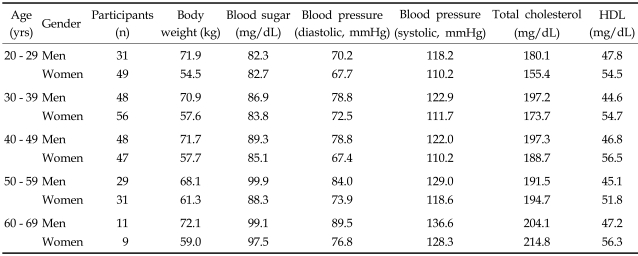
Among the applicants for medical examination in a CHA health-promoting center, 359 city dwelling volunteers (192 women and 167 men) were recruited from July to September, 2004. Their saliva and blood samples were collected between 10 and 11 AM. Four saliva samples throughout the day (20-30 minutes after awakening, 11 AM, 4 PM, and bedtime) were collected from 78 participants (42 women and 36 men; 20 to 40 years of age) who had a normal sleep and wake-up cycle. None of the participants selected in both groups presented with diabetes, hypo- or hypertension. Those who were undergoing hormone replacement therapy, taking hormones for birth control, or using sleeping pills were excluded. General information about volunteer health is summarized in Table. 1
Table 1.
Summary of Basic Health Status of Volunteers and Number of Participants in Each Age Group
Saliva and blood collection
Participants were asked to rinse their mouth with water before collecting saliva. A minimum volume of 1 mL saliva was obtained directly by expectorating into a collecting tube. Food and beverages such as tea, soft drinks, and coffee were not permitted 30 minutes prior to any sample collection. Because salivary cortisol concentration is independent of flow rate and sugarless gum does not interfere with the salivary assay, 21 participants were permitted to chew sugarless gum if needed to stimulate saliva flow. Participants were also instructed not to brush their teeth 30 minutes before saliva collection and to refrain from wearing lipstick on the day of saliva collection. When saliva and blood were simultaneously collected from a volunteer, saliva was always collected first. Saliva samples which were contaminated with blood were excluded from this study. The collected saliva was subjected to two freezing-thawing cycles in order to precipitate mucins, and then centrifuged (10,000 × g, 15 min, 4 ℃).22 The supernatant was collected and stored at -70 ℃ until the day of assay. Blood samples collected between 10 to 11 AM were kept at room temperature and allowed to clot. The samples were then centrifuged to obtain serum, which was stored at -70 ℃ until the day of assay.
Measurement of cortisol and DHEA in serum
Total cortisol and DHEA levels in serum were measured with a commercial RIA kit (Diagnostic Products Cooperation, DPC). The intra-assay variation coefficient for cortisol was 3.5% (n = 10) and 3.7% for that of DHEA (n = 10). The inter-assay coefficient of variation for cortisol was 4.5% (n = 6) and that for DHEA was 4.3% (n = 6)
Measurement of cortisol and DHEA in saliva
Commercially supplied kits were not adequate for the measurement of cortisol levels in saliva.23 Therefore, sophisticated assay techniques were required for the analysis of salivary steroids. We developed a hormone assay system based on a liquid phased-double antibody method. For standards, pure cortisol and DHEA were obtained from Sigma-Aldrich Chemical Co. (St. Louis, MO, USA). These steroids were dissolved in methanol (1.0mg/mL) and further diluted with gelatin containing 0.1M phosphate buffered saline (GPBS, pH = 7.0, containing 0.015M NaN3 and 0.1% gelatin). Iodine-125, labeled cortisol [cortisol-3-(O-caboxymethyl oximino)-2-125I iodohistamine], was obtained from Amersham Biosciences (Buckinghamshire, UK) and 125I-labeled DHEA, a component of the DHEA RIA kit obtained from DPC, was used for the salivary DHEA assay. The cortisol and DHEA antiserums were purchased from Biogenesis (Oxford, UK). The cortisol antiserum cross-reacts 0.001% with aldosterone, 4.1% with 11-deoxycorticosterone, 5.7% with 11-deoxycortisol, 0.5% with 21-deoxycortisol, 1.2% with corticosterone, and less than 0.01% with other steroids. The DHEA antiserum cross-reacts 6.3% with 5α-andorstane-3β, 17β-diol, 1.3% with androstenedione, 0.1% with testosterone, and less than 0.05% with other related compounds. General assay procedures were adapted from those described by our previous reports.24,25 The assay procedure is briefly described below. The volume of standards added to assay tubes was 0.1mL. A standard with no added cortisol or DHEA was also included. Tubes in which GPBS was substituted for the antiserum were prepared to estimate nonspecific binding (NSB). An equal volume of charcoal-stripped saliva was added to the standard tubes as in sample tubes. The total radioactivity of 125I-cortisol or 125I-DHEA per tube was approximately 20,000 cpm. The antiserum was diluted to give approximately 30-35% binding. The total reaction volume was 0.4 mL/tube. Routinely, two sets of standards were included in each assay. Standards and samples were incubated overnight at 4℃. Then 0.5 mL of an antirabbit IgG coated (developed in goat) cellulose bead solution was applied to the assay tubes. The tubes were incubated at room temperature for 30 minutes, and then centrifuged (1,500 × g, 20 minutes). The supernatant was aspirated, and the radioactivity in the pellet was counted using a gamma counter (Cobra 5005, Perkin Elmer Life and Analytical Sciences). The steroid levels for individual assay were calculated by interpolation from the standard curves using RiaSmart software (PerkinElmer Life and Analytical Sciences). Multiple tubes of low and high level quality control (QC) samples were prepared and kept frozen for use in each assay. The intra-assay coefficient of variation for cortisol was 5.4% (n = 10) and 5.1% for DHEA (n = 10). The inter-assay coefficient of variation for cortisol was 6.5% (n = 6) and 6.5% for DHEA (n = 6). The analytical sensitivity for cortisol was 1 pg/mL and that for DHEA was 0.1pg/mL.
Preparation of steroid-free saliva and determination of RIA accuracy
Steroid-free saliva was prepared as follows. Approximately 50,000 cpm of tritium labeled cortisol (1,2,6,7-3H-hydrocortisone, PerkinElmer Life and Analytical Sciences) or DHEA (1,2,6,7-3H-DHEA, PerkinElmer Life and Analytical Sciences) was added to 20mL of saliva sample. Twenty percent (w/v) of activated charcoal powder (Sigma-Aldrich Chemical Co.) was added to each tube and stirred at 4℃ for overnight. The tubes were then centrifuged (15,000 × g, 30 minutes, 4℃) and the supernatant was collected. The radioactivity remaining in the supernatant was determined using a liquid scintillation analyzer (Tricarb 2900, PerkinElmer Life and Analytical Sciences). The charcoal stripping procedures were repeated until there were not more than 100 cpm remaining in a 1 mL sample. The charcoal stripped sample was filtered through a 0.45µm filter and stored at -70 ℃ until use. To determine the RIA accuracy, unlabeled cortisol or DHEA was added to the charcoal stripped saliva, and the steroid level was determined using RIA as described above. Non-detectable levels of cortisol or DHEA were observed in the charcoal stripped saliva. Exogenously added cortisol (250pg/0.1mL) was detected with a range from 236 to 277pg/0.1mL (255.2 ± 14.7 pg/0.1mL, n = 10), while exogenous DHEA (50 pg/0.1mL) was detected with a range from 48 to 61pg/0.1mL (53.9 ± 3.5pg/0.1mL, n = 10).
Statistical analyses
Conventional methods (mean and SD, Student's t-test, and ANOVA) were used to analyze the diurnal variation in cortisol and DHEA levels with the level of significance set at p < 0.01. Linear regression analysis was carried out to determine the relationship between hormone levels and age by using STATISTICA version 5.1 for Windows (Tulsa, OK, USA). The Pearson's correlation (r, P) was calculated for correlations of hormone levels between saliva and serum with GraphPad Prism version 4 for Windows (San Diego, CA, USA)
RESULTS
Changes in salivary and serum cortisol levels with age
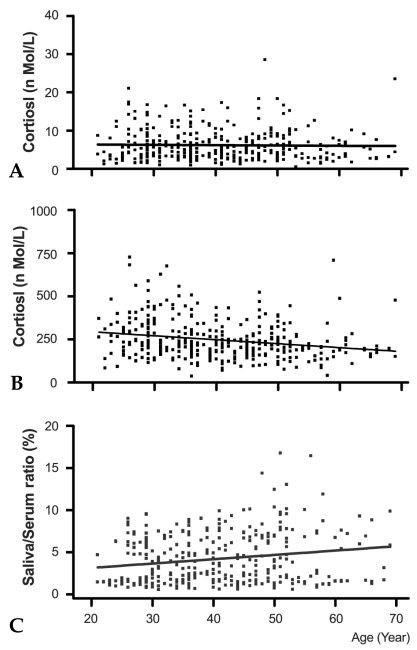
Fig. 1 shows the relationship of salivary (A) and serum (B) cortisol levels (sample collection, 10-11 AM) with age. The age-related decline pattern and concentration of cortisol appeared to be very similar in both genders. Therefore, cortisol levels of men and women were combined together in the plot. Salivary cortisol levels were similar at all ages examined (9.6 ± 0.68, p = 0.62 by ANOVA) and the results of linear regression analysis showed that the salivary cortisol levels (Fig. 1A) did not significantly change with age (slope = -0.078, t = -0.389, p = 0.697). However, serum cortisol levels (Fig. 1B) declined significantly with age (slope = -2.29, t = -4.43, p < 0.001). The serum cortisol levels in the 20s age group were relatively higher (288.2 ± 14.5nmol/L) than those in other age groups (30s; 258.1 ± 11.7, 40s; 228.4 ± 9.3, 50s; 216.0 ± 13.6, and 60s; 221.4 ± 21.7nmol/L). In addition, serum cortisol levels were significantly lower in the 40s age group when compared with the 20s age group (p < 0.01, by Duncan's multiple range test). Saliva represented 3.4% of cortisol levels found in serum for the 20s age group, but this ratio increased with age (3.8%, 4.5%, 4.9%, and 4.9%; 30s, 40s, 50s, and 60s, respectively). The results of linear regression analysis also showed that the saliva: serum cortisol ratio increased with age (Fig. 1C, slope = 0.051, t = 3.61, p < 0.001).
Fig. 1.
Age-related AM cortisol level changes in saliva (A) and serum (B). Samples were collected at 10 to 11AM and cortisol levels in 358 paired saliva and serum samples were determined with RIA. Each point on the figure represents cortisol levels of a participant. The relative cortisol ratio of serum to saliva is depicted in (C). The indicated line represents the best fit linear regression with age. Predicted salivary cortisol level (nmol/L) = 6.4-0.078x, beta = -0.022, p = 0.697, n = 356; predicted serum cortisol level (nmol/L) = 339-2.29x, beta = -0.22, p #x003C; 0.001, n = 359; predicted saliva/serum ratio = 2.69+0.051x, beta = 0.189, p = 0.0035, n = 359.
Changes in salivary and serum DHEA levels with age
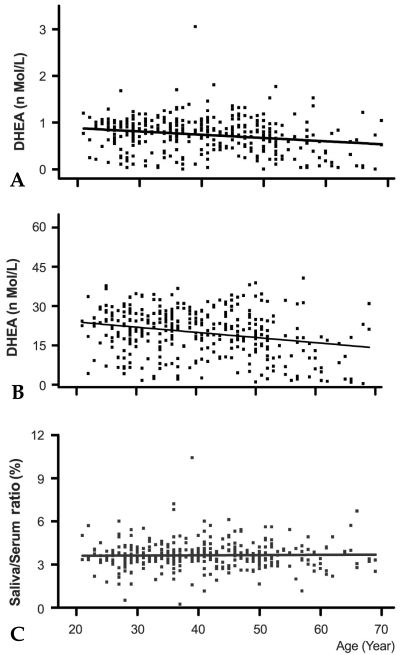
Fig. 2 shows the relationship of AM salivary (A) and serum (B) DHEA levels with age. Similar levels of DHEA were detected in saliva collected from the 20s, 30s, and 40s age groups (0.79 ± 0.03 nmol/L, p = 0.9 by ANOVA), but DHEA levels decreased substantially in the 50s age group (0.62 ± 0.0.5nmol/L, p < 0.01, when compared with the 40s age group), and decreased further in the 60s age group (0.42 ± 0.07nmol/L, p < 0.05, when compared with the 50s age group). Results of linear regression analysis also showed that salivary DHEA levels declined significantly with age (Fig. 2A, slope = -0.007, t = -3.761, p < 0.001). The declining pattern of serum DHEA levels with age was similar to that observed in saliva. Serum DHEA levels were similar between the 20s, 30s, and 40s age groups (21.2 ± 0.2nmol/L), but the levels were significantly less in the 50s age group (16.9 ± 1.3nmol/L, p < 0.01), and decreased further in the 60s age group (10.9 ± 1.8 nmol/L, p < 0.01). Results of linear regression analysis also showed that serum DHEA levels declined significantly with age (Fig. 2B, slope = -0.197, t = -4.9, p < 0.001). Saliva represented 3.5-3.7% of the DHEA concentrations found in serum, and this ratio did not change significantly with age (Fig. 2C, slope = 0.0016, t = 0.344, p = 0.73).
Fig. 2.
Age-related basal DHEA level changes in saliva (A) and serum (B). Samples were collected at 10 to 11AM. Cortisol levels in 358 paired saliva and serum samples were determined with RIA. Each point on the figure represents cortisol levels of a participant. The relative DHEA ratio of serum to saliva is depicted in (C). The indicated line represents the best fit linear regression with age. Predicted salivary DHEA level (nmol/L) = 1.009-0.007x, beta = -0.21, p #x003C; 0.001, n = 356; predicted serum DHEA level (nmol/L) = 27.9-0.197x, beta = -0.25, p #x003C; 0.001, n = 359; predicted saliva/serum ratio = 3.58+0.0016x, beta = 0.18, p = 0.73, n = 359.
Correlation of steroid concentration between saliva and serum
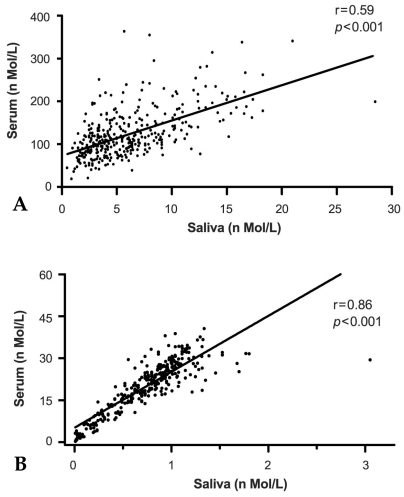
Fig. 3 shows the correlation of cortisol (Fig. 3A) and DHEA (Fig. 3B) concentrations between saliva and serum. Data obtained from 358 saliva and serum samples were used to determine the correlation coefficients. The data presented here was obtained from only paired saliva and serum samples; the results show a direct comparison of hormone levels in saliva with simultaneously obtained serum samples in the absence of hormonal stimulation or suppression. A Pearson's correlation (r, P) was calculated for the comparison of hormone levels between saliva and serum samples. Cortisol levels in saliva were well correlated with those in serum (Fig. 3A, r = 0.59, p < 0.0001). Furthermore, DHEA levels in saliva were also highly correlated with those in serum (Fig. 3B, r = 0.864, p < 0.001).
Fig. 3.
Correlation of steroid concentration in saliva and serum. Data was collected from 358 paired saliva and serum samples, and correlation coefficients (Pearson r, P) were evaluated. Regression statistics cortisol (A) slope = 8.15 (SE, 0.58), Y intercept t = 73.33 (SE, 4.8) nmol/L, R2 = 0.35, Sy.x = 45.83. Pearson r = 0.59, 95%CI = 0.52-0.66, p #x003C; 0.0001. Regression statistics DHEA (B) slope = 19.98 (SE, 0.6), Y intercept = 5.17 (SE, 0.51) nmol/L, R2 = 0.74, Sy.x = 4.42. Pearson r = 0.86, 95%CI = 0.83-0.88, p #x003C; 0.0001.
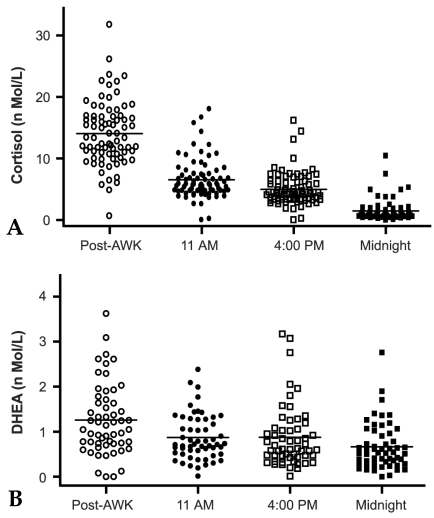
Diurnal rhythm of salivary cortisol and DHEA
Fig. 4 shows diurnal changes in salivary cortisol and DHEA levels. Saliva samples were collected at four designated time points throughout a day from 74 volunteers (ages ranging from 20 to 40 years). Cortisol and DHEA levels were measured and are depicted in Fig. 4A and B, respectively. Salivary cortisol diurnal rhythm peak levels occurred first just after awakening (14.1 ± 0.58 nmol/L), followed by a marked decline thereafter, and finally reaching its lowest level at midnight (11 AM; 6.5 ± 0.37, 4 PM; 4.9 ± 0.3, midnight; 1.5 ± 0.18nmol/L)(Fig. 4A). The diurnal pattern of salivary DHEA followed that of cortisol, with the highest levels observed following awakening (1.3 ± 0.2nmol/L) and gradually decreasing thereafter (0.9 ± 0.06, 0.9 ± 0.09, and 0.7 ± 0.06nmol/L, 11AM, 4PM, and midnight, respectively). There was a significant decrease in DHEA concentration between post- awakening and 11AM (p < 0.001, by t-test), but a slight decrease from 11AM to midnight was observed (p = 0.11 by ANOVA) (Fig. 4B).
Fig. 4.
Diurnal pattern of salivary cortisol (A) and DHEA (B). Four samples were collected throughout the day (after awakening, 11AM, 4PM, and before bedtime) from 78 volunteers who were 30 to 40 years of age. Steroid levels were determined with RIA. Each point represents cortisol (A) or DHEA (B) levels of a participant. The bar represents the mean values of each sample time.
DISCUSSION
The present study showed that age-related adrenal steroids level changes, serum cortisol levels slightly declined with age, but DHEA levels significantly decreased in the 50s age group and thereafter. This study also showed that saliva can be used as an alternative body fluid for studying adrenal functions, because the steroid concentrations in saliva reflect well those in serum and exhibited an evident diurnal rhythm. Thus, steroid analysis in saliva can be applied for the study of real life situations, such as stress-related diurnal pattern changes.
It has been well established that major gonadal steroid production patterns change with age in men and women. Total testosterone levels in men were known to decrease 1.6% annually throughout their lifespan.11,26 Estradiol or progesterone levels also decrease with age, especially at the second late stage of menopause.27,28 Relative hormonal ratios of gonadal steroid and other hormones, such as LH, FSH, or inhibins, are used as a biomarkers for andropause for men29-31 or menopause for women.32 Many studies have observed age-related adrenal DHEA or DHEA-S secretion pattern changes,33,34 but such studies on the age-related basal cortisol secretion changes in serum are controversial.7-9 The present study demonstrates that salivary cortisol levels tended to decline, but not significantly, until the 60s age level (Fig. 1A), while serum cortisol levels declined significantly with age (Fig. 1B). The AM cortisol levels in serum were predicted by the equation 339-2.29x, which implied that cortisol levels will decrease annually. The age-related negative slope of AM cortisol was less steep than that of serum or salivary testosterone,26,31 but the estimated decline rate of AM cortisol was similar to another report.31
It is generally accepted that aging in healthy people is accompanied with little change in the cortisol and aldosterone ratio, but the ratio of cortisol and DHEA changed sharply.35 An imbalance of cortisol and DHEA with age is called an adrenopause.11 An adrenal steroid imbalance, or adrenopause, is likely to occur in the 50s age group among Koreans. Salivary or serum DHEA levels did not change significantly in the 40s age group, but DHEA levels significantly decreased at the 50s age group (Fig. 2A and 2B). The declining slope was steeper (slope = -0.016) in the 40s and 50s age groups than in the 20s to 40s age groups (slope = 0.001). On the basis of the data shown in Fig. 1 and 2, we also found that the ratio of cortisol to DHEA in saliva or serum (F/D ratio) changed with age. The F/D ratio was similar in the 20s to 40s age group in saliva (11.1 ± 1.7) and serum (12.4 ± 0.3), but the ratio gradually increased (15.3 and 14.0, in saliva and serum, respectively) in the 50s age group. This pattern was maintained (23.9 and 18.6, in saliva and serum, respectively) in the 60s age group (data not depicted on the graph). It is well known that the age-related DHEA or DHEA-S decline occurs in both genders,36 but the causes of adrenopause remain unclear. Some studies have shown a decrease of the number of functional reticularis cells37 and a reduction of reticularis mass followed with age.38 Other immunohistochemical analyses showed that immunoreacticities of adrenal steroidogenic enzymes did not change with age,39 but little information is available on the changes in steroidogenic enzyme activity with age. The functional and morphological changes of the reticularis could be responsible for the diminished production of DHEA and changes in the steroid secretion pattern with age.
Saliva can be utilized as an alternative body fluid for research in real life situations, such as stress-related diurnal rhythm changes. More importantly, the steroid concentration in saliva was found to reflect the free-form concentration in serum with correlation coefficients of 0.54 to 0.97.40,41 We also showed that the concentrations of cortisol and DHEA in saliva are well correlated with those in serum (r=0.59 and 0.86, respectively) (Fig. 3). Correlation coefficients of cortisol were lower than those of DHEA because the age-related decline patterns of cortisol in saliva (Fig. 1A) and serum (Fig. 1B) were different from those of DHEA (Fig. 2). Interestingly, the correlation coefficiency of cortisol was stable until 40 years of age (r = 0.63, p < 0.001), but then decreased with age (0.38 and 0.43, for 50s and 60s, respectively, data not shown). However, the correlation coefficiency of DHEA did not change with age. The observed decrease in cortisol correlation coefficiency is reasonable because the cortisol concentration ratio of saliva to serum increased with age (Fig. 1C), and the negative slope was different between saliva and serum (Fig. 1A and B). It is likely that a varying concentration,42 affinity, or binding capacity of cortisol-binding globulin (CBG) are other factor(s) involved in the correlation coefficiency differences and declining slope with age.
In the present study, the diurnal rhythms of salivary cortisol and DHEA were observed in the Korean population (Fig. 4). The post-awakening cortisol levels (14.1nmol/L) were close to the total levels of three secretory episodes (13.0nmol/L) and approximately twice as high as those of 11AM (6.5nmol/L, Fig. 4A). The post-awakening salivary DHEA levels were higher (0.39nmol/L) than those of 11AM and were approximately 55% of the total levels from the other three measures (2.4nmol/L, Fig. 4B). These results are in good agreement with other reports.17,22 The measurement of cortisol and DHEA in saliva is becoming more widely accepted as a screening test for diagnosis of the HPA axis because they provide information about the absolute hormonal concentration and an individual's diurnal rhythm.20 This combined data can be applied to the various diagnostic examination fields, such as chronic fatigue syndrome,43 depression,6 and Cushing's disease.44 In particular, assessment of the post-awakening rise in levels provides a useful diagnostic tool for stress-related disease, age-related disease, and pre-clinical states.20,43,44
In conclusion, the data presented here show various age-related cortisol and DHEA level changes, and that DHEA levels decreased significantly around 50 years of age. Decreases of DHEA levels are responsible for the F/D ratio changes with age. Saliva is an appropriate surrogate for serum in the research or diagnosis of adrenal function because it reflects the concentrations of steroids in serum and provides valuable information of an individual's diurnal rhythm.
References
- 1.DeGroot LJ, Jameson JL. Endocrinology. 5th ed. Philadelphia: Elsevier Saunders; 2005. pp. 2287–2297. [Google Scholar]
- 2.Johnson KL, Rn CR. The hypothalamic-pituitary-adrenal axis in critical illness. AACN Clin Issues. 2006;17:39–49. doi: 10.1097/00044067-200601000-00006. [DOI] [PubMed] [Google Scholar]
- 3.Jefferies WM. Cortisol and immunity. Med Hypotheses. 1991;34:198–208. doi: 10.1016/0306-9877(91)90212-h. [DOI] [PubMed] [Google Scholar]
- 4.Schleimer RP. Interactions between the hypothalamic- pituitary-adrenal axis and allergic inflammation. J Allergy Clin Immunol. 2000;106(5 Suppl):270–274. doi: 10.1067/mai.2000.110162. [DOI] [PubMed] [Google Scholar]
- 5.Rosmond R, Dallman MF, Bjornatorp P. Stress-related cortisol secretion in men: relationships with abdominal obesity and endocrine, metabolic and hemodynamic abnormalities. J Clin Endocrinol Metab. 1998;83:1853–1859. doi: 10.1210/jcem.83.6.4843. [DOI] [PubMed] [Google Scholar]
- 6.de Kloet ER. Hormones, brain and stress. Endocr Regul. 2003;37:51–68. [PubMed] [Google Scholar]
- 7.Born J, Ditschuneit I, Schreiber M, Dodt C, Fehm HL. Effects of age and gender on pituitary-adrenocortical responsiveness in humans. Eur J Endocrinol. 1995;132:705–711. doi: 10.1530/eje.0.1320705. [DOI] [PubMed] [Google Scholar]
- 8.Gotthardt U, Schweiger U, Fahrenberg J, Lauer CJ, Holsboer F, Heuser I. Cortisol, ACTH, and cardiovascular response to a cognitive challenge paradigm in aging and depression. Am J Physiol. 1995;268:R865–R873. doi: 10.1152/ajpregu.1995.268.4.R865. [DOI] [PubMed] [Google Scholar]
- 9.Lupien S, Lecours AR, Lussier I, Schwartz G, Nair NP, Meaney MJ. Basal cortisol levels and cognitive deficits in human aging. J Neurosci. 1994;14:2893–2903. doi: 10.1523/JNEUROSCI.14-05-02893.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferrari E, Magri F, Dori D, Migliorati G, Nescis T, Molla G, et al. Neuroendocrine correlates of the aging brain in humans. Neuroendocrinology. 1995;61:464–470. doi: 10.1159/000126869. [DOI] [PubMed] [Google Scholar]
- 11.Maes M, Calabrese J, Lee M, Meltzer HY. Effects of age on spontaneous cortisolaemia of normal volunteers and depressed patients. Psychoneuroendocrinology. 1994;19:79–84. doi: 10.1016/0306-4530(94)90061-2. [DOI] [PubMed] [Google Scholar]
- 12.Sherman B, Wysham C, Pfohl B. Age-related changes in the circadian rhythm of plasma cortisol in man. J Clin Endocrinol Metab. 1985;61:439–443. doi: 10.1210/jcem-61-3-439. [DOI] [PubMed] [Google Scholar]
- 13.Buckley TM, Schatzberg AF. On the interactions of the hypothalamic-pituitary-adrenal (HPA) axis and sleep: normal HPA axis activity and circadian rhythm, exemplary sleep disorders. J Clin Endocrinol Metab. 2005;90:3106–3114. doi: 10.1210/jc.2004-1056. [DOI] [PubMed] [Google Scholar]
- 14.Van Cauter E, Leproult R, Kupfer DJ. Effects of gender and age on the levels and circadian rhythmicity of plasma cortisol. J Clin Endocrinol Metab. 1996;81:2468–2473. doi: 10.1210/jcem.81.7.8675562. [DOI] [PubMed] [Google Scholar]
- 15.Zhao ZY, Xie Y, Fu YR, Li YY, Bogdan A, Touitou Y. Circadian rhythm characteristics of serum cortisol and dehydroepiandrosterone sulfate in healthy Chinese men aged 30 to 60 years. A cross-sectional study. Steroids. 2003;68:133–138. doi: 10.1016/s0039-128x(02)00167-8. [DOI] [PubMed] [Google Scholar]
- 16.Castro M, Elias PC, Martinelli CE Jr, Antonini SR, Santiago L, Moreira AC. Salivary cortisol as a tool for physiological studies and diagnostic strategies. Braz J Med Biol Res. 2000;33:1171–1175. doi: 10.1590/s0100-879x2000001000006. [DOI] [PubMed] [Google Scholar]
- 17.Vining RF, McGinley RA, Maksvytis JJ, Ho KY. Salivary cortisol: a better measure of adrenal cortical function than serum cortisol. Ann Clin Biochem. 1983;20:329–335. doi: 10.1177/000456328302000601. [DOI] [PubMed] [Google Scholar]
- 18.Kirschbaum C, Hellhammer DH. Salivary cortisol in psychoneuroendocrine research: recent developments and applications. Psychoneuroendocrinology. 1994;19:313–333. doi: 10.1016/0306-4530(94)90013-2. [DOI] [PubMed] [Google Scholar]
- 19.Clow A, Thorn L, Evans P, Hucklebridge F. The awakening cortisol response: methodological issues and significance. Stress. 2004;7:29–37. doi: 10.1080/10253890410001667205. [DOI] [PubMed] [Google Scholar]
- 20.Trilck M, Flitsch J, Ludecke DK, Jung R, Petersenn S. Salivary cortisol measurement-a reliable method for the diagnosis of Cushing's syndrome. Exp Clin Endocrinol Diabetes. 2005;113:225–230. doi: 10.1055/s-2005-837667. [DOI] [PubMed] [Google Scholar]
- 21.Granger DA, Schwartz EB, Booth A, Curran M, Zakaria D. Assessing dehydroepiandrosterone in saliva: a simple radioimmunoassay for use in studies of children, adolescents and adults. Psychoneuroendocrinology. 1999;24:567–579. doi: 10.1016/s0306-4530(99)00013-x. [DOI] [PubMed] [Google Scholar]
- 22.Groschl M, Wagner R, Rauh M, Dorr HG. Stability of salivary steroids: the influences of storage, food and dental care. Steroids. 2001;66:737–741. doi: 10.1016/s0039-128x(01)00111-8. [DOI] [PubMed] [Google Scholar]
- 23.Nahoul K, Patricot MC, Bressot N, Penes MC, Revol A. Measurement of salivary cortisol with four commercial kits. Ann Biol Clin (Paris) 1996;54:75–82. [PubMed] [Google Scholar]
- 24.Kwon HB, Ahn RS. Relative roles of theca and granulosa cells in ovarian follicular steroidogenesis in the amphibian, Rana nigromaculata. Gen Comp Endocrinol. 1994;94:207–214. doi: 10.1006/gcen.1994.1077. [DOI] [PubMed] [Google Scholar]
- 25.Hong CY, Park JH, Ahn RS, Im SY, Choi HS, Soh J, et al. Molecular mechanism of suppression of testicular steroidogenesis by proinflammatory cytokine tumor necrosis factor alpha. Mol Cell Biol. 2004;24:2593–2604. doi: 10.1128/MCB.24.7.2593-2604.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Feldman HA, Longcope C, Derby CA, Johannes CB, Araujo AB, Coviello AD, et al. Age trends in the level of serum testosterone and other hormones in middle- aged men: longitudinal results from the Massachusetts male aging study. J Clin Endocrinol Metab. 2002;87:589–598. doi: 10.1210/jcem.87.2.8201. [DOI] [PubMed] [Google Scholar]
- 27.MacNaughton J, Banah M, McCloud P, Hee J, Burger H. Age related changes in follicle stimulating hormone, luteinizing hormone, oestradiol and immunoreactive inhibin in women of reproductive age. Clin Endocrinol (Oxf) 1992;36:339–345. doi: 10.1111/j.1365-2265.1992.tb01457.x. [DOI] [PubMed] [Google Scholar]
- 28.Burger HG. The endocrinology of the menopause. J Steroid Biochem Mol Biol. 1999;69:31–35. doi: 10.1016/s0960-0760(98)00145-9. [DOI] [PubMed] [Google Scholar]
- 29.Elmlinger MW, Kuhnel W, Ranke MB. Reference ranges for serum concentrations of lutropin (LH), follitropin (FSH), estradiol (E2), prolactin, progesterone, sex hormone-binding globulin (SHBG), dehydroepiandrosterone sulfate (DHEAS), cortisol and ferritin in neonates, children and young adults. Clin Chem Lab Med. 2002;40:1151–1160. doi: 10.1515/CCLM.2002.202. [DOI] [PubMed] [Google Scholar]
- 30.Muller M, den Tonkelaar I, Thijssen JH, Grobbee DE, van der Schouw YT. Endogenous sex hormones in men aged 40-80 years. Eur J Endocrinol. 2003;149:583–589. doi: 10.1530/eje.0.1490583. [DOI] [PubMed] [Google Scholar]
- 31.Lukas WD, Campbell BC, Ellison PT. Testosterone, aging, and body composition in men from Harare, Zimbabwe. Am J Hum Biol. 2004;16:704–712. doi: 10.1002/ajhb.20083. [DOI] [PubMed] [Google Scholar]
- 32.Robertson DM, Burger HG. Reproductive hormones: ageing and the perimenopause. Acta Obstet Gynecol Scand. 2002;81:612–616. doi: 10.1034/j.1600-0412.2002.810706.x. [DOI] [PubMed] [Google Scholar]
- 33.Labrie F, Belanger A, Cusan L, Gomez JL, Candas B. Marked decline in serum concentrations of adrenal C19 sex steroid precursors and conjugated androgen metabolites during aging. J Clin Endocrinol Metab. 1997;82:2396–2402. doi: 10.1210/jcem.82.8.4160. [DOI] [PubMed] [Google Scholar]
- 34.Alesci S, Koch CA, Bornstein SR, Pacak K. Adrenal androgens regulation and adrenopause. Endocr Regul. 2001;35:95–100. [PubMed] [Google Scholar]
- 35.Valenti G. Adrenopause: an imbalance between dehydroepiandrosterone (DHEA) and cortisol secretion. J Endocrinol Invest. 2002;25(10 Suppl):29–35. [PubMed] [Google Scholar]
- 36.Rehman KS, Carr BR. Sex differences in adrenal androgens. Semin Reprod Med. 2004;22:349–360. doi: 10.1055/s-2004-861551. [DOI] [PubMed] [Google Scholar]
- 37.Parker CR, Jr, Mixon RL, Brissie RM, Grizzle WE. Aging alters zonation in the adrenal cortex of men. J Clin Endocrinol Metab. 1997;82:3898–3901. doi: 10.1210/jcem.82.11.4507. [DOI] [PubMed] [Google Scholar]
- 38.Staton BA, Mixon RL, Dharia S, Brissie RM, Parker CR., Jr Is reduced cell size the mechanism for shrinkage of the adrenal zona reticularis in aging? Endocr Res. 2004;30:529–534. doi: 10.1081/erc-200043617. [DOI] [PubMed] [Google Scholar]
- 39.Suzuki T, Sasano H, Takeyama J, Kaneko C, Freije WA, Carr BR, et al. Developmental changes in steroidogenic enzymes in human postnatal adrenal cortex: immunohistochemical studies. Clin Endocrinol (Oxf) 2000;53:739–747. doi: 10.1046/j.1365-2265.2000.01144.x. [DOI] [PubMed] [Google Scholar]
- 40.Wedekind D, Bandelow B, Broocks A, Hajak G, Ruther E. Salivary, total plasma and plasma free cortisol in panic disorder. J Neural Transm. 2000;107:831–837. doi: 10.1007/s007020070062. [DOI] [PubMed] [Google Scholar]
- 41.Kirschbaum C, Hellhammer DH. Salivary cortisol in psychobiological research: an overview. Neuropsychobiology. 1989;22:150–169. doi: 10.1159/000118611. [DOI] [PubMed] [Google Scholar]
- 42.Kudielka BM, Buske-Kirschbaum A, Hellhammer DH, Kirschbaum C. HPA axis responses to laboratory psychosocial stress in healthy elderly adults, younger adults, and children: impact of age and gender. Psychoneuroendocrinology. 2004;29:83–98. doi: 10.1016/s0306-4530(02)00146-4. [DOI] [PubMed] [Google Scholar]
- 43.Jerjes WK, Cleare AJ, Wessely S, Wood PJ, Taylor NF. Diurnal patterns of salivary cortisol and cortisone output in chronic fatigue syndrome. J Affect Disord. 2005;87:299–304. doi: 10.1016/j.jad.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 44.Castro M, Elias PC, Martinelli CE, Jr, Antonini SR, Santiago L, Moreira AC. Salivary cortisol as a tool for physiological studies and diagnostic strategies. Braz J Med Biol Res. 2000;33:1171–1175. doi: 10.1590/s0100-879x2000001000006. [DOI] [PubMed] [Google Scholar]