Abstract
Purpose
To determine the effects of new breast cancer treatments and to provide a baseline for monitoring the development of breast cancer in Korean women, we conducted an analysis at our institution to determine long-term clinicopathological features, survival rates, and prognostic factors.
Materials and Methods
This study retrospectively analyzed 2,403 patients between Sep 1994 and Dec 2002, who underwent breast cancer surgery at Samsung Medical Center in Korea. Demographic data, pathologic records and surgical records were collected.
Results
After a median follow-up duration of 121.9 (range: 2-158.1) months, the 5-year disease free survival (DFS) was 82.8% and the 10-year DFS was 74.7%. The 5-year and 10-year overall survival (OS) rates were 89.4% and 82.9%, respectively. Using multivariate analyses, we determined that the nodal status (p < 0.001), angioinvasion (p < 0.001), positive PR (p < 0.001), and C-erb-B2 (p < 0.001) were independent prognostic factors for OS. The frequency of breast conserving surgery was 33.9% before Dec 1999, and increased up to 44.1% by year Dec 2002.
Conclusion
Most of the prognostic variables and clinical characteristics of the Korean breast cancer patients were similar to those reported for Western populations. However, the age distribution in Korean patients seemed to be different from that in patients from Western countries.
Keywords: Breast cancer, survival, disease free survival, breast conserving surgery
INTRODUCTION
Breast cancer is one of the leading causes of cancer death in Korean females.1 In 2002, more than 7,300 patients were diagnosed with breast cancer, accounting for about one quarter of all new cancers in Korean women.2 Western European and North American populations are at highest risk, with a lifetime risks up to age 74 years around 8-10%, while the lowest risk, generally around 1%, occurs in Asian populations.3 Although the incidence of breast cancer in Korea is still lower than in other countries, it has been steadily increasing from 16.7 per 100,000 women in 1996 to 36.5 per 100,000 women in 2001.4 The mortality rate from breast cancer in Korea has also increased up to 5.6 per 100,000 women.1
In the past decade, innovative treatment approaches have been developed for breast cancer patients in an attempt to improve treatment outcomes. There has been widespread acceptance of using breast conserving therapy over radical mastectomy in selected patients and the practice of sentinel lymph node biopsy in early breast cancer is increasing. Another major change in the past decade is the more widespread use of systemic adjuvant therapy. According to historical clinical trials, these treatment methods have been shown to be beneficial for breast cancer cases regardless of race or ethnicity.
This single institution analysis was conducted to determine the long-term clinicopathological features, survival rate, and prognostic factors in Korean breast cancer patients. We also provide a baseline for monitoring the progression of breast among Korean patients.
MATERIALS AND METHODS
In this study, we retrospectively analyzed 2,403 patients who underwent breast cancer surgery at Samsung Medical Center in Korea between Sep 1994 and Dec 2002. Demographic data, pathologic records and surgical records were collected. Pathologic records were reviewed to determine the tumor size and nodal status. The staging process was based on the TNM classification proposed by the American Joint Committee of Cancer, 6th edition.5 In cases of multicentric or multifocal cancers with different stages, the most advanced stage was considered in the analysis.
Histology was grouped into ductal carcinoma in situ (DCIS), infiltrating ductal carcinoma, tubular carcinoma, mucinous carcinoma, medullary carcinoma, papillary carcinoma, infiltrating lobular carcinoma, lobular carcinoma in situ, malignant phyllodes tumor, non-epithelial carcinoma, and metastatic carcinoma. The histological grading was grouped into three categories according to the Nottingham modification of the Bloom and Richardson grading system6 (well differentiated, moderately differentiated, poorly differentiated). For pathologic diagnosis, ER, PgR, HER-2, and P53 status were assessed before the start of chemotherapy and after surgery. Tissues were routinely fixed in 10% buffered formalin and then paraffin-embedded. Reagents used for immunohistochemical studies were as follows: ER (1:50 dilution, DAKO, Glostrup, Denmark), PgR (1:50, DAKO, Glostrup, Denmark), HER-2 (1:30, Zymed, San Francisco, CA, USA), and p53 (1:80, Zymed, San Francisco, CA, USA). All staining was performed on paraffin sections as previously described.7 Briefly, paraffin-embedded tissue sections (4µm in thickness) were placed on silane-coated glass slides, deparaffinated in xylene, rehydrated in ethanol and washed in Tris-buffered saline. Immunostaining was performed using the avidin-biotin-peroxidase complex method. Negative and positive controls were included with each assay. Tumors were considered ER- or PgR-positive if ≥ 10% of the tumor cells were positive. A semi-quantitative scoring system (the Allred score) was used to evaluate the proportion and intensity of stained cells.8 A total score of 3 or more defined a positive staining. HER-2 status was scored on a scale of 0-3+ according to the Dako scoring system, where ≥ 1+ was considered as overexpression. C-erb-B2 positivity was defined as greater than one positive in this study. Other factors from the pathologic report that were analyzed included: multicentricity, extensive intraductal component (EIC), lymphatic invasion and angioinvasion. Synchronous cancers were defined as cancers that occur within a 12-month interval. Menopause was defined as the lack of menstrual periods for 12 consecutive months with no other identifiable biological or physiological cause.
Neoadjuvant chemotherapy was given to patients with large primary tumors (> 5cm, T3) or with skin/chest wall involvement (T4) and/or fixed axillary (N2) or ipsilateral internal mammary (N3) lymph node involvement. Adjuvant chemotherapy was recommended to patients having a tumor size greater than 1cm or a positive lymph node. Adjuvant hormonal therapy was recommended for estrogen receptor-positive or progesterone receptor-positive cancers and treatment was continued for 5-years.
Overall survival (OS) was measured from the time of surgery until death or the time to the last follow-up. Disease-free survival was defined as the interval from the date of operation to the date of first disease recurrence. OS and disease free survival (DFS) was estimated using the Kaplan-Meier method. The two-sided log-rank test was used to test the association between patient variables and survival. Multivariate analysis was done using a Cox proportional hazard regression. A p value less than 0.05 was considered statistically significant.
RESULTS
Patients
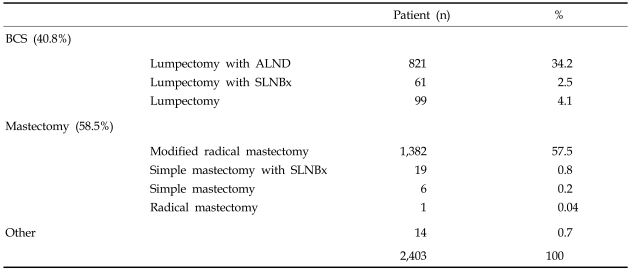
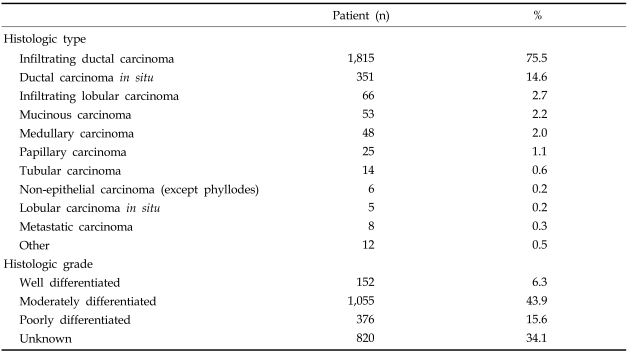
In total, 2,403 patients were included in our analysis. The mean age was 46.6 years (range: 20-82 years), proportion of patients younger than 45 was 46.7%. The incidences of synchronous and metachronous bilateral breast cancers were 2% and 0.4%, respectively. The methods of surgical treatment are shown in Table 1. Breast conserving therapy was performed in 40.8%, mastectomy was performed in 58.5%, and other surgeries such as simple excision or mass excision with or without lymph node biopsy were done in 0.7% of patients.Infiltrating ductal carcinoma accounted for more than two thirds (75.5%) of all patients followed by DCIS (14.6%), mucinous carcinoma (2.2%), medullary adenocarcinoma (2.0%), and papillary carcinoma (1.1%). The most common histologic grade was the moderately differentiated group (43.9%) (Table 2). Estrogen receptor (ER) was positive in 1,345 (63.6%) and negative in 770 (36.4%) patients. Progesterone receptor was positive in 1,018 (48.4%) and negative in 1,086 (51.6%) patients. The C-erb-B2 expression was positive in 981 (48.3%) and negative in 1,048 patients (51.7%). The p53 expression was positive in 1,018 (57.1%) and negative in 793 patients (42.8%).
Table 1.
Methods of Surgical Treatment
ALND, axillary lymph node dissection; SLNBx, sentinel lymph node biospy.
Table 2.
Frequency and Percentage of the Histologic Type and Grade of Breast Cancer
The mean tumor size was 2.6cm (range: 0.1-14.5cm). There were 167 patients (7.3%) with tumors greater than 5cm and 357 patients (14.6%) with carcinoma in situ. Of the 2,403 patients, 1,422 patients (59.2%) showed negative nodal status. Among the 882 patients who had positive lymph nodes, the number of metastatic lymph nodes was 1 to 3 in 498 patients (20.7%), 4 to 5 in 222 patients (9.2%) and more than 5 in 162 patients (6.7%).
Survival
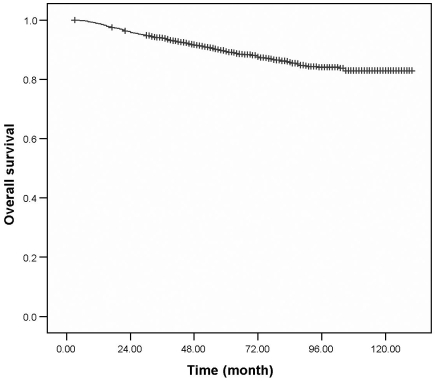
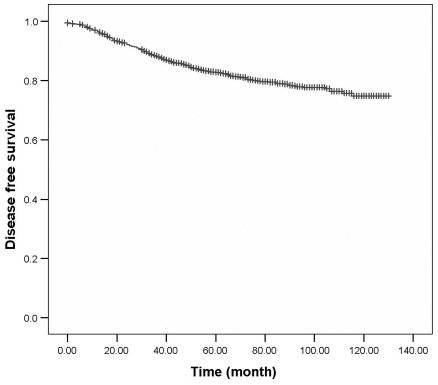
After a median follow-up duration of 121.9 (range: 2-158.1) months, the 5-year DFS was 82.8% and 10-year DFS was 74.7%. The 5-year OS was 89.4% and 10-year OS was 82.9% (Fig. 1,2). For the patients who underwent breast surgery before 1999, the 5-year OS rate was 86.5 %, but the OS rate increased to 91.5% for patients who underwent breast surgery after 1999. There were 414 (17.2%) patients who developed recurrences during the follow-up period and among these, 165 (2.5%) experienced local recurrence in the ipsilateral breast.
Fig. 1.
Overall survival from breast cancer at Samsung Medical Center.
Fig. 2.
Disease-free survival from breast cancer at Samsung Medical Center.
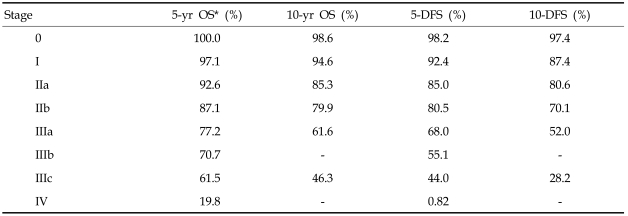
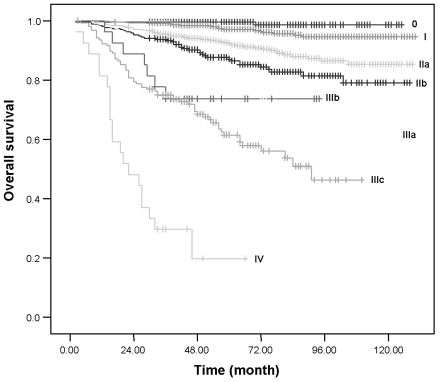
The 5-year and 10-year OS and DFS rates according to TNM stage are presented in Table 3 and Fig. 3. The 10-year OS for stage IIIb could not be estimated due to the small number of patients (n = 28).
Table 3.
Survival according to Stage
OS, overall survival; DFS, disease free survival.
Fig. 3.
Overall survival according to TNM stage at Samsung Medical Center.
The 5-year OS according to the surgical method (BCS vs. MRM) was not significantly different in stage I (96.8% vs. 96.5%, p = 0.89), stage IIa (92.9% vs. 88.1% p = 0.26) or stage IIb (90.0% vs. 84.4%, p = 0.089). The DFS according to the surgical method also showed no significant difference in stage I (90.5% vs. 92.2%, p = 0.27) or stage IIa (78.5% vs. 79.2%, p = 0.37). However, the DFS was higher in patients treated with BCS in stage IIb patients (83.8% vs. 74.5%, p = 0.043).
Prognostic factors
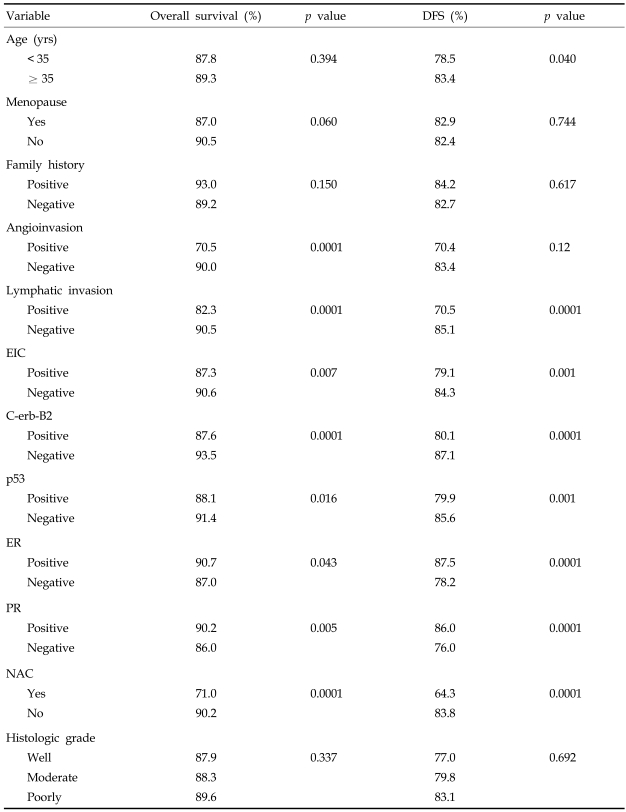
Using univariate analysis, we found that age (< 35 years) was not associated with OS (p = 0.394) but did significantly affect DFS (p = 0.040). Other significant prognostic factors for survival were angioinvasion (p < 0.001), lymphatic invasion (p < 0.001), ER (p = 0.043), PR (p = 0.005), p53 (p = 0.016), EIC (p = 0.007) and C-erb-B2 (< 0.001) (Table 4).
Table 4.
Univariate Analysis of Prognostic Factors
EIC, extensive intraductal component; ER, estrogen receptor; PR, progesterone receptor; NAC, neoadjuvant chemotherapy.
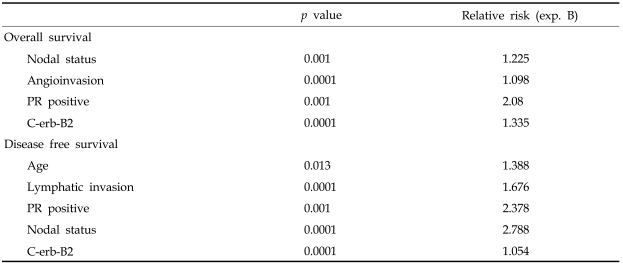
In multivariate analyses, nodal status (p < 0.001), angioinvasion (p < 0.001), positive PR (p < 0.001), and C-erb-B2 (p < 0.001) were independent prognostic factors for OS. For DFS, age (p = 0.021), lymphatic invasion (p < 0.001), nodal status (p < 0.001), positive PR (p < 0.001), and C-erb-B2 (p < 0.001) were identified as independent prognostic factors (Table 5).
Table 5.
Multivariate Analysis for Overall and Disease Free Survival
PR, progesterone receptor.
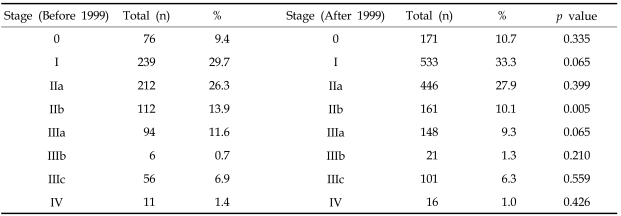
Table 6 shows the stage distribution according to the year of breast surgery, before or after 1999, at Samsung Medical Center. Proportions of stages 0, I and IIa increased after 1999 compared with those before 1999 (10.7%, 33.3%, 27.9% vs. 9.4%, 29.7% and 26.3%, respectively).
Table 6.
Percentage of Patients according Stage before and after Year 1999
DISCUSSION
The incidence of breast cancer is low in Korea compared to in Western countries, but it is gradually increasing. Many epidemiological studies have been published on the differences in incidence rate, survival rate, ethnicity, race and among Westernized countries and non-Westernized countries.9-11
The age-specific incidence of Korean breast cancer shows a parabolic curve with its peak incidence around 45 years. Interestingly, patients younger than 50 years included 61.9% of total breast cancer patients. According to the Korean Breast Cancer Society, the age of peak incidence is in the fifth decade.12 In contrast, the American Cancer Society reported that the increase in breast cancer incidence occurs primarily in elderly women, i.e. age 50 years or older (76.8%).13 According to their data, 48% of new cases appear in patients older than 65 years. The SEER database noted the following incidences: 60 cases per 100,000 for patients younger than the age of 65, 322 cases per 100,000 for patients older than the age of 65, and 375 cases per 100,000 population for persons older than the age of 85.14 Harris et al. also reported that in the United States, the incidence of breast cancer increases after menopause where 230 and 300 breast cancer per 100,000 occurs, for patients in their 60s and 70s, respectively.15 Although elderly patients have been routinely excluded from the most of the large clinical trials in the past and the accurate data on staging, treatment and outcomes are still lacking in Western countries,16 the above findings suggest the peak incidence age of breast cancer in Korea is at least 10 to 20 years younger than in Caucasians.
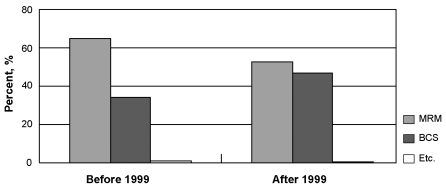
The mainstream surgical modality for early breast cancer at our institution was BCS rather than MRM at the time of data collection. Also, the number of partial mastectomy with sentinel lymph node biopsy with or without axillary dissection has been rapidlyincreasing in recent years at our institution. Sentinel lymph node biopsy was only performed in 80 patients during Sep 1994 and Dec 2002, but the number of patients in which it was performed increased to 751 by Dec 2004. The Korean Breast Cancer Society reported a gradual increase in the frequency of BCS with 17.1% in 1996, 25.9% in 2000 and up to 45.1% by 2005 from the nationwide survey. At the Samsung Medical Center, BCS was only performed in 33.9% of cases before Dec 1999, but increased up to 44.1% by year Dec 2002 (Fig. 4). As concluded by the NSABP Protocol B-06 for a twenty year follow-up of a randomized trial comparing invasive breast cancer according to surgical methods,17 there were no difference in OS for mastectomy and BCS in early stages. Although the National Cancer Institute Consensus Conference in 1990 concluded that there was no difference in DFS for early breast cancer (stages I and II) after breast conserving therapy compared to mastectomy,18 our results demonstrate that DFS was prolonged in patients with stage IIb who underwent BCS as compared to those who underwent mastectomy. The additional radiation therapy after BCS might have contributed to this finding.
Fig. 4.
Operative pattern changes at Samsung Medical Center.
The percentage of early stage cancer from stage 0 through I is 40.2% in our database, which is higher than in other studies in Korea (range: 13.3-25.2%)19 and lower than in a report from the United States (56.2%).20 Thus, more effort is necessary to increase the early detection of breast cancer through regular screening. Kim et al. reported that the 5-year overall survival, local-relapse-free survival, distant-metastasis-free survival and disease-free survival rates for early breast cancer were 95.3%, 97.2%, 91.3% and 88.5%, respectively.21 The survival rates in this analysis were comparable to those reported by other large studies.20
Notably, the number of dissected regional lymph nodes was strongly correlated with survival. The 5-year and 10-year survival rates for the lymph node positive group were 77.8% and 65.9%, respectively, while those in the lymph node negative group were 96.6% and 93.8%, respectively. Cheng et al.22 reported a 5-year survival of 93% for node negative patients, 85.3% for nodes between 1 to 3, 76.9% for nodes between 4 to 9 and 50.7% for nodes greater than 10. Fisher et al.23 published a 10-year 75% overall survival for node negative patients, 62% for nodes between 1 to 3, 42% for nodes between 4 to 9 and 20% for nodes greater than 10. The results from our study show equivalent or slightly better survival; 5-year OS was 92.0% for node negative, 91.7% for nodes between 1 to 3, 91.3% for nodes between 4 to 9 and 83% for nodes greater than 10. The 10-year OS was 84.7% for node negative, 83.5% for nodes between 1 to 3 was, 81.8% for nodes between 4 to 9 and 66.9% for nodes greater than 10. Although the therapeutic and diagnostic benefits of axillary lymph node dissection are widely accepted, sentinel lymph node biopsy should be considered as an alternative approach. Interestingly, none of the sentinel node negative patients had recurred systemically or locally at the time of analysis.
In the current data set, we evaluated whether the time period (before or after 1999) in which the breast cancer was detected had an effect on the overall survival. Patients who developed breast cancer in more recent years (after 1999) had an improved survival. The improved 5-year OS rates since 1999 as compared to the survival rates before 1999 according to the time of surgery (91.5% vs. 86.5%, respectively) may be due to an increase in early detection of breast cancer and improvement of the postoperative chemotherapeutic agents and radiation techniques.
In Korea, the number of patients undergoing BCS is rapidly increasing and this method is widely accepted as the first choice for treatment of early stage breast cancer. Most of the prognostic variables and clinical characteristics of Korean breast cancer patients are similar to those reported by Western groups. However, the age distribution seems to differ with that reported in Western countries. The results of our current analysis also suggest that the survival of women with breast cancer has improved over the past decade.
References
- 1.National Statistical Office. Annual Report on the Causes Death Statistics. Seoul, Republic of Korea: 2003. [Google Scholar]
- 2.National Cancer Center. Annual Report of Cancer Registry. Seoul, Republic of Korea: 2002. [Google Scholar]
- 3.Parkin DM, Whelan SL, Ferlay J, Teppo L, Thomas DB. Cancer Incidence in Five Continents. Lyon, France: International Agency for Research on Cancer; 2002. [Google Scholar]
- 4.Korean Breast Cancer Society. Korean breast cancer data of 1996. J Korean Surg Soc. 1998;55:621–635. [Google Scholar]
- 5.Greene FL, Page DL, Fleming ID, Fritz AG, Balch CM, Haller DG, et al. AJCC cancer staging manual. New York: Springer-Valag; 2002. pp. 255–282. [Google Scholar]
- 6.Elston CW, Ellis IO. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long- term follow-up. Histopathology. 1991;19:403–410. doi: 10.1111/j.1365-2559.1991.tb00229.x. [DOI] [PubMed] [Google Scholar]
- 7.Oh YL, Choi JS, Song SY, Ko YH, Han BK, Nam SJ, et al. Expression of p21Waf1, p27Kip1 and cyclin D1 proteins in breast ductal carcinoma in situ: Relation with clinicopathologic characteristics and with p53 expression and estrogen receptor status. Pathol Int. 2001;51:94–99. doi: 10.1046/j.1440-1827.2001.01173.x. [DOI] [PubMed] [Google Scholar]
- 8.Allred DC, Bustamante MA, Daniel CO, Gaskill HV, Cruz AB., Jr Immunocytochemical analysis of estrogen receptors in human breast carcinomas. Evaluation of 130 cases and review of the literature regarding concordance with biochemical assay and clinical relevance. Arch Surg. 1990;125:107–113. doi: 10.1001/archsurg.1990.01410130113018. [DOI] [PubMed] [Google Scholar]
- 9.Campbell JB. Breast cancer-race, ethnicity, and survival: a literature review. Breast Cancer Res Treat. 2002;74:187–192. doi: 10.1023/a:1016178415129. [DOI] [PubMed] [Google Scholar]
- 10.Hsu JL, Glaser SL, West DW. Racial/ethnic differences in breast cancer survival among San Francisco Bay Area women. J Natl Cancer Inst. 1997;89:1311–1312. doi: 10.1093/jnci/89.17.1311. [DOI] [PubMed] [Google Scholar]
- 11.Eley JW, Hill HA, Chen VW, Austin DF, Wesley MN, Muss HB, et al. Racial differences in survival from breast cancer. Results of the National Cancer Institute Black/White Cancer Survival Study. JAMA. 1994;272:947–954. doi: 10.1001/jama.272.12.947. [DOI] [PubMed] [Google Scholar]
- 12.The Korean Breast Cancer Society. Clinical characteristics of Korean breast cancer patients in 1998. J Korean Med Sci. 2000;15:569–579. doi: 10.3346/jkms.2000.15.5.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parker SL, Tong T, Bolden S, Wingo PA. Cancer statistics, 1997. CA Cancer J Clin. 1997;47:5–27. doi: 10.3322/canjclin.47.1.5. [DOI] [PubMed] [Google Scholar]
- 14.Yancik R, Ries LG, Yates JW. Breast cancer in aging women. A population-based study of contrasts in stage, surgery, and survival. Cancer. 1989;63:976–981. doi: 10.1002/1097-0142(19890301)63:5<976::aid-cncr2820630532>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 15.Harris JR, Lippman ME, Morrow M, Hellman S. Disease of the Breast. Philadelphia: Lippincott-Raven; 1996. p. 160. [Google Scholar]
- 16.Wyld L, Reed MW. The need for targeted research into breast cancer in the elderly. Br J Surg. 2003;90:388–399. doi: 10.1002/bjs.4124. [DOI] [PubMed] [Google Scholar]
- 17.Fisher B, Anderson S, Bryant J, Margolese RG, Deutsch M, Fisher ER, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med. 2002;347:1233–1241. doi: 10.1056/NEJMoa022152. [DOI] [PubMed] [Google Scholar]
- 18.NIH consensus conference. Treatment of early-stage breast cancer. JAMA. 1991;265:391–395. [PubMed] [Google Scholar]
- 19.Son BH, Yoon HS, Kwak HS, Lee PC, Ko KB, Kim JS, et al. Clinical analysis of breast cancer surgeries in Korea. J Korean Surg Soc. 2001;60:470–476. [Google Scholar]
- 20.Bland KI, Menck HR, Scott-Conner CE, Morrow M, Winchester DJ, Winchester DP. The National Cancer Data Base 10-year survey of breast carcinoma treatment at hospitals in the United States. Cancer. 1998;83:1262–1273. doi: 10.1002/(sici)1097-0142(19980915)83:6<1262::aid-cncr28>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 21.Kim KJ, Huh SJ, Yang JH, Park W, Nam SJ, Kim JH, et al. Treatment results and prognostic factors of early breast cancer treated with a breast conserving operation and radiotherapy. Jpn J Clin Oncol. 2005;35:126–133. doi: 10.1093/jjco/hyi039. [DOI] [PubMed] [Google Scholar]
- 22.Cheng SH, Tsou MH, Liu MC, Jian JJ, Cheng JC, Leu SY, et al. Unique features of breast cancer in Taiwan. Breast Cancer Res Treat. 2000;63:213–223. doi: 10.1023/a:1006468514396. [DOI] [PubMed] [Google Scholar]
- 23.Fisher ER, Anderson S, Redmond C, Fisher B. Pathologic findings from the National Surgical Adjuvant Breast Project protocol B-06. 10-year pathologic and clinical prognostic discriminants. Cancer. 1993;71:2507–2514. doi: 10.1002/1097-0142(19930415)71:8<2507::aid-cncr2820710813>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]