Abstract
Purpose
Erythromycin-resistant β-hemolytic streptococci (BHS) has recently emerged and quickly spread between and within countries throughout the world. In this study, we evaluate the antimicrobial susceptibility patterns and erythromycin resistance mechanisms of BHS during 2003-2004.
Materials and Methods
The MICs of seven antimicrobials were determined for 204 clinical isolates of BHS from 2003 to 2004. Resistance mechanisms of erythromycin-resistant BHS were studied by the double disk test as well as by polymerase chain reaction (PCR).
Results
Compared with our previous study, resistance among Streptococcus pyogenes isolates to a variety of drugs decreased strikingly: from 25.7% to 4.8% in erythromycin; 15.8% to 0% in clindamycin; and 47.1% to 19.0% in tetracycline. The prevalent phenotypes and genotypes of macrolide-lincosamide-streptograminB (MLSB) resistance in Streptococcus pyogenes isolates have been changed from the constitutive MLSB phenotype carrying erm(B) to the M phenotype with mef(A) gene. In contrast with Streptococcus pyogenes, resistance rates to erythromycin (36.7%), clindamycin (43.1%), and tetracycline (95.4%) in Streptococcus agalactiae isolates did not show decreasing trends. Among the Streptococcus dysgalactiae subsp. equisimilis isolates (Lancefield group C, G), resistance rates to erythromycin, clindamycin, tetracycline and chloramphenicol were observed to be 9.4%, 3.1%, 68.8%, and 9.4%, respectively.
Conclusion
Continual monitoring of antimicrobial resistance among large-colony-forming BHS is needed to provide the medical community with current data regarding the resistance mechanisms that are most common to their local or regional environments.
Keywords: β-hemolytic streptococci, antibiotic resistance, macrolides, erythromycin, Streptococcus agalactiae, Streptococcus pyogenes
INTRODUCTION
β-hemolytic streptococcal (BHS) isolates from humans can be subdivided into large-colony and small-colony (< 0.5 mm in diameter) formers. Streptococcus pyogenes (Lancefield group A), Streptococcus agalactiae (group B), and Streptococcus dysgalactiae subsp. equisimilis (group C, G) belong to large-colony formers.1 Although large-colony-forming β-hemolytic streptococci (LCF-BHS) are still susceptible to β-lactams, macrolides or lincosamides are recommended as alternative choices when indicated.1-3 However, recent studies have shown that changes in the susceptibility of LCF-BHS to erythromycin and clindamycin have been substantial, although differences in resistance rates to these agents exist according to geographical variation and investigators.4-8 The high transmissibility of LCF-BHS, including resistant clones and the association of increased macrolide usage, may play a significant role in the variable resistance rates that have been reported during the last decade.9-11
In Korea, resistant bacteria are more prevalent than in other industrialized countries, and their presence suggests a high level of antimicrobial selective pressure as well as the nosocomial spread of resistant bacteria.12 In response to this public health problem, the Korean government instituted a new health policy, 'the separation of prescribing and dispensing (SPD) of medications', on July 1, 2000. The purpose of this policy was to provide greater differentiation between the roles of physicians and pharmacists than had historically existed in South Korea. In our previous study,13 however, the resistance rates to erythromycin and clindamycin among Streptococcus pyogenes, Streptococcus agalactiae, and group C streptococci isolates were still high during the period of 2001-2002.
Two major mechanisms account for erythromycin resistance in many gram-positive bacteria: target site modification and active efflux. Target site modification is mediated by erythromycin resistance methylase that is encoded by erm class genes. Methylases cause a conformational change in the prokaryocytic ribosome, leading to reduced binding of macrolide-lincosamide-streptograminB (MLSB) antibiotics to the target site in the 50S ribosomal subunit. The phenotype expression of MLSB resistance in streptococci can be either constitutive or inducible. Macrolide efflux, which is effected by a membrane protein encoded by the mef class genes, has recently emerged among Streptococcus pyogenes and Streptococcus pneumoniae in many countries.14 It has been well documented that the frequency of MLSB resistance phenotypes among streptococci varies considerably between countries.14
The objectives of the present study were to investigate the incidence and trend in susceptibility among the LCF-BHS isolated from clinical specimens in a Korean hospital and to clarify the phenotypes and genotypes of erythromycin-resistant LCF-BHS. We also explored the correlation between serotypes and genotypes of erythromycin-resistant Streptococcus agalactiae.
MATERIALS AND METHODS
A total of 204 strains of LCF-BHS were obtained from various clinical specimens between January 2003 and December 2004 at Wonju Christian Hospital in Korea. Multiple isolates from the same patient were avoided. The isolates were identified by standard criteria on the basis of hemolytic patterns on 5% sheep blood agar, colony morphology, Gram stain, catalase reaction, Streptex latex agglutination assay (Murex Biotech Limited, Dartford, England), and API Rapid ID32 STREP system (bioMérieux, Marcy l'Etoile, France).
The strains were stored in thioglycollate broth with 20% glycerol at -70℃ until analyzed. The frozen isolates of LCF-BHS were thawed, inoculated onto a 5% sheep blood agar plate and incubated at 35℃ overnight. Pure isolates of LCF-BHS obtained from three consecutive subcultures were tested for susceptibility and polymerase chain reaction (PCR).
Susceptibility to penicillin G, erythromycin, clindamycin, tetracycline, ceftriaxone, chloramphenicol (Sigma Chemical Co, St. Louis, MO, USA) and vancomycin (Daewoong Lilly, Seoul, Korea) was tested by the agar dilution method according to the recommendations of the Clinical and Laboratory Standards Institute.15 The Streptococcus pneumoniae ATCC 49619 strain was simultaneously tested to monitor the accuracy of minimal inhibitory concentrations of LCF-BHS. The resistance phenotypes of erythromycin-resistant isolates were determined by the doubledisc test with erythromycin (15 µg) and clindamycin (2 µg) disks.13
The genomic DNA extractions were carried out with the Easy-DNA kit (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's instructions. The presence of erm and mef class genes was determined by PCR amplification using previously described primers specific for erm(A), erm(B), erm(C), erm(TR), and mef(A).13
GBS serotypes Ia, Ib, and II~VIII were determined by use of a coagglutination test (ESSUM Group B Streptococcus Serotyping Test; Bacterum AB, Umeå, Sweden).13
RESULTS
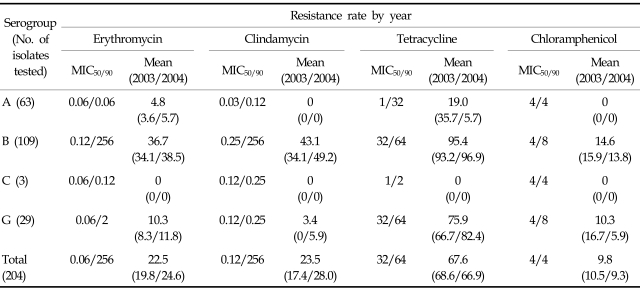
The overall non-susceptible (intermediate and resistance) rates of LCF-BHS were 67.6% to tetracycline, 23.5% to clindamycin, 22.5% to erythromycin and 9.8% to chloramphenicol, whereas all isolates were susceptible to penicillin G, ceftriaxone, and vancomycin. Resistant rates to tetracycline, erythromycin, and clindamycin of Streptococcus agalactiae and Streptococcus pyogenes isolates were 95.4% versus 19.0%, 36.7% versus 4.8%, and 43.1% versus 0%, respectively. Three isolates of group C LCF-BHS were susceptible to all tested antimicrobial agents. Resistant rates to chloramphenicol, erythromycin, and clindamycin of group G LCF-BHS were higher than those of Streptococcus pyogenes (Table 1).
Table 1.
Antimicrobial Susceptibilities of Large-Colony-Forming β-Hemolytic Streptococci
MIC, minimal inhibitory concentration.
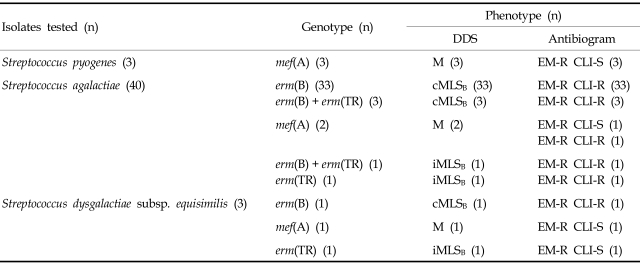
Of the 46 erythromycin-resistant LCF-BHS isolates (Table 2), 37 isolates (80.4%) had the constitutive macrolide-lincosamide-streptograminB (cMLSB)phenotype, six isolates (13.0%) had the M phenotype, and three (6.5%) isolates had the inducible MLSB (iMLSB) phenotype. Of the 40 erythromycin-resistant Streptococcus agalactiae strains, the most prevalent gene was erm(B) (92.5%). All three erythromycin-resistant Streptococcus pyogenes isolates had mef(A) gene. Four isolates of Streptococcus agalactiae had both of erm(B) and erm(TR) genes. Three isolates of Streptococcus dysgalactiae subsp. equisimilis had different resistance genes.
Table 2.
Distributions of Phenotype and Genotype of MLSB Resistance among 46 Isolates of Erythromycin-Resistant Large-Colony-Forming β-Hemolytic Streptococci
DDS, erythromycin and clindamycin double disk synergy test; MLSB, macrolide-lincosamide-streptograminB; cMLSB, constitutive resistance to MLSB; M, M phenotype; iMLSB, inducible resistance to MLSB; EM-R, erythromycin-resistant; CLI-S, clindamycin-susceptible; CLI-R, clindamycin-resistant.
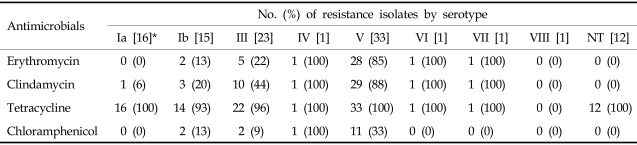
The serotype frequency of 103 Streptococcus agalactiae isolates was V (32.0%), III (22.3%), Ia (15.5%), and Ib (14.6%). The resistance rates to erythromycin by serotypes were 85% (V), 22% (III), 13% (Ib), and 0% (Ia) (Table 3).
Table 3.
Rates of Antimicrobial Resistance of Streptococcus agalactiae by Serotypes
NT, not-typeable.
*The numbers in brackets mean the total No. of Streptococcus agalactiae isolates by serotype.
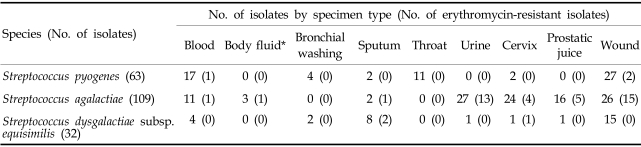
Among the LCF-BHS isolates, 72 (35.3%) were from genitourinary specimens, 68 (33.3%) from wounds, 32 (15.7%) from blood, 18 (8.8%) from lower respiratory tract specimens, 11 (5.3%) from throat and 3 (1.5%) from other body fluids. Streptococcus pyogenes were frequently isolated from the throat, blood, and wounds, whereas Streptococcus agalactiae and Streptococcus dysgalactiae subsp. equisimilis were prevalent in genitourinary tract specimens and lower respiratory tract specimens, respectively (Table 4).
Table 4.
Distribution of Erythromycin-Resistant Large-Colony-Forming -Hemolytic Streptococci β according to Specimen Type
*Cerebrospinal fluid (2), amniotic fluid (1).
DISCUSSION
Until the 1980s, LCF-BHS were generally considered uniformly susceptible to erythromycin and clindamycin, but resistance spread rapidly in the 1990s. The prevalence of erythromycin-resistant LCF-BHS has been reported to be variable and depends on the country, selective pressure, serogroup, serotype, age, and season. Compared with our previous study,13 we observed that resistance among Streptococcus pyogenes isolates decreased from 25.7% to 4.8% in erythromycin, 15.8% to 0% in clindamycin, and 47.1% to 19.0% in tetracycline. In addition, the prevalent phenotypes and genotypes of MLSB resistance in Streptococcus pyogenes isolates have changed from the cMLSB phenotype carrying erm (B) to the M phenotype with the mef(A) gene. The determination of antibiotic prescriptions in outpatient clinics is an important factor to consider when decreasing resistance rates to commonly used antimicrobial agents, especially in skin and upper respiratory infections, are observed.
The isolation rate of Streptococcus pyogenes from throat specimens was 2.0% (2/102) in our hospital during the period of 1997-2000.16 These results suggested that resistance rates to commonly-used antimicrobial agents in outpatient clinics and the distribution of MLSB resistance phenotypes were partly influenced by selective pressure.
In contrast with Streptococcus pyogenes, resistance rates to erythromycin, clindamycin, and tetracycline in Streptococcus agalactiae isolates did not show decreasing trends in this study. The continued high resistance rates to erythromycin, clindamycin, and tetracycline are considered related to the clonal spread of serotype V with a multi-drug resistance phenotype.17 The resistance rates to clindamycin of our serotypes Ib and III isolates were higher than that of erythromycin, while the other serotypes were nearly equal in susceptible rates to erythromycin and clindamycin. Our results show resistance to clindamycin to be more common than resistance to erythromycin, and similar results have been reported in Taiwan and New Zealand.18,19 The distribution of MLSB resistant genes and the isolation frequency of serotypes of GBS may be major factors contributing to the difference between erythromycin and clindamycin resistance in different countries.
Malbruny et al. have reported that a new LSA (lincosamide-streptogramin A) phenotype was noted in erythromycin-susceptible, clindamycinresistant Streptococcus agalactiae isolates from New Zealand, and that III (13/19) and I (5/19) were the main serotypes of GBS with LSA phenotype.19 However, in spite of their extensive molecular studies, the resistance mechanism of LSA in Streptococcus agalactiae was not elucidated.
The overall resistance rates to erythromycin and clindamycin in group C and G BHS seemed to be somewhat lower than those of our previous results.13 Streptococcus dysgalactiae subsp. equisimilis colonizes and causes various infections in humans.20,21 Zaoutis et al. reported that three isolates (group G; 2, group C; 1) of 23 Streptococcus dysgalactiae subsp. equisimilis were resistant to erythromycin.21 Hashikawa et al. documented that all eleven of the Streptococcus dysgalactiae subsp. equisimilis strains were sensitive to β-lactam antibiotics, vancomycin, and chloramphenicol, whereas about half of the strains were tetracycline resistant, and one strain was resistant to erythromycin and clindamycin harbored erm(B).22 Our findings were similar to those of the aforementioned investigators' reports.
Continual monitoring of antimicrobial resistance among LCF-BHS is needed to provide the medical community with current data regarding the resistance mechanisms that are most common to their local or regional environments. Additionally, further epidemiologic studies are needed to confirm whether or not our susceptibility data on LCF-BHS are restricted to our geographic area.
References
- 1.Ruoff KL, Whiley RA, Beighton D. Streptococcus. In: Murray PR, Baron EJ, Jorgensen JH, Pfaller MA, Yolken RH, editors. Manual of clinical microbiology. 8th ed. Washington DC: American Society for Microbiology; 2003. pp. 405–421. [Google Scholar]
- 2.Bisno AL, Gerber MA, Gwaltney JM, Jr, Kaplan EL, Schwartz RH. Practice guidelines for the diagnosis and management of group A streptococcal pharyngitis. Clin Infect Dis. 2002;35:113–125. doi: 10.1086/340949. [DOI] [PubMed] [Google Scholar]
- 3.Schuchat A. Group B streptococcal disease: from trials and tribulations to triumph and trepidation. Clin Infect Dis. 2001;33:751–756. doi: 10.1086/322697. [DOI] [PubMed] [Google Scholar]
- 4.Biedenbach DJ, Stephen JM, Jones RN. Antimicrobial susceptibility profile among β-haemolytic Streptococcus spp. collected in the SENTRY Antimicrobial Surveillance Program-North America, 2001. Diagn Microbiol Infect Dis. 2003;46:291–294. doi: 10.1016/s0732-8893(03)00065-8. [DOI] [PubMed] [Google Scholar]
- 5.Carroll KC, Monroe P, Cohen S, Hoffman M, Hamilton L, Korgenski K, et al. Susceptibility of β-hemolytic streptococci to nine antimicrobial agents among four medical centers in Salt Lake City, Utah, USA. Diagn Microbiol Infect Dis. 1997;27:123–128. doi: 10.1016/s0732-8893(97)00025-4. [DOI] [PubMed] [Google Scholar]
- 6.Gordon KA, Beach ML, Biedenbach DJ, Jones RN, Rhomberg PR, Mutnick AH. Antimicrobial susceptibility patterns of β-hemolytic and viridans group streptococci: report from the SENTRY Antimicrobial Surveillance Program (1997-2000) Diagn Microbiol Infect Dis. 2002;43:157–162. doi: 10.1016/s0732-8893(02)00374-7. [DOI] [PubMed] [Google Scholar]
- 7.Traub WH, Leonhard B. Comparative susceptibility of clinical group A, B, C, F, and G β-hemolytic streptococcal isolates to 24 antimicrobial drugs. Chemotherapy. 1997;43:10–20. doi: 10.1159/000239529. [DOI] [PubMed] [Google Scholar]
- 8.Wu JJ, Lin KY, Hsueh PR, Liu JW, Pan HI, Sheu SM. High incidence of erythromycin-resistant streptococci in Taiwan. Antimicrob Agents Chemother. 1997;41:844–846. doi: 10.1128/aac.41.4.844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Avanzini C, Bosio K, Volpe G, Dotti G, Savoia D. Streptococcus pyogenes collected in Torino (northwest Italy) between 1983 and 1998: survey of macrolide resistance and trend of genotype by RAPD. Microb Drug Resist. 2000;6:289–295. doi: 10.1089/mdr.2000.6.289. [DOI] [PubMed] [Google Scholar]
- 10.Kataja J, Huovinen P, Efstratiou A, Pérez-Trallero E, Seppälä H. Macrolide resistance study group. Clonal relationships among isolates of erythromycin-resistant Streptococcus pyogenes of different geographical origin. Eur J Clin Microbiol Infect Dis. 2002;21:589–595. doi: 10.1007/s10096-002-0771-8. [DOI] [PubMed] [Google Scholar]
- 11.Seppälä H, Klaukka T, Vuopio-Varkila J, Muotiala A, Helenius H, Lager K, et al. The effect of changes in the consumption of macrolide antibiotics on erythromycin resistance in group A streptococci in Finland. N Engl J Med. 1997;337:441–446. doi: 10.1056/NEJM199708143370701. [DOI] [PubMed] [Google Scholar]
- 12.Chong Y, Lee K. Present situation of antimicrobial resistance in Korea. J Infect Chemother. 2000;6:189–195. doi: 10.1007/s101560070001. [DOI] [PubMed] [Google Scholar]
- 13.Kim HY, Uh Y. Macrolide resistance in β-hemolytic streptococci: changes after the implementation of the separation of prescribing and dispensing of medications policy in Korea. Yonsei Med J. 2004;45:591–597. doi: 10.3349/ymj.2004.45.4.591. [DOI] [PubMed] [Google Scholar]
- 14.Uh Y, Jang IH, Hwang GY, Lee MK, Yoon KJ, Kim HY. Antimicrobial susceptibility patterns and macrolide resistance genes of β-hemolytic streptococci in Korea. Antimicrob Agents Chemother. 2004;48:2716–2718. doi: 10.1128/AAC.48.7.2716-2718.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing; sixteenth informational supplement. Wayne (PA): Clinical and Laboratory Standards Institute; 2006. CLSI document M100-S16. [Google Scholar]
- 16.Uh Y, Jang IH, Yoon KJ, Kim HY. Serogroup distribution of beta-hemolytic streptococci isolated from a tertiary care hospital at Wonju area during the recent 4 years (1997-2000) Korean J Infect Dis. 2001;33:173–180. [Google Scholar]
- 17.Uh Y, Yong D, Lee K, Kwon O, Yoon KJ. Emergence of erythromycin-resistant Streptococcus agalactiae serotype V is due to clonal spread. Korean J Lab Med. 2005;25:S564. [Google Scholar]
- 18.Ko WC, Lee HC, Wang LR, Lee CT, Liu AJ, Wu JJ. Serotyping and antimicrobial susceptibility of group B Streptococcus over an eight-year period in southern Taiwan. Eur J Clin Microbiol Infect Dis. 2001;20:334–339. doi: 10.1007/s100960100505. [DOI] [PubMed] [Google Scholar]
- 19.Malbruny B, Werno AM, Anderson TP, Murdoch DR, Leclercq R. A new phenotype of resistance to lincosamide and streptogramin A-type antibiotics in Streptococcus agalactiae in New Zealand. J Antimicrob Chemother. 2004;54:1040–1044. doi: 10.1093/jac/dkh493. [DOI] [PubMed] [Google Scholar]
- 20.Woo PC, Fung AM, Lau SK, Wong SS, Yuen KY. Group G beta-hemolytic streptococcal bacteremia characterized by 16S ribosomal RNA gene sequencing. J Clin Microbiol. 2001;39:3147–3155. doi: 10.1128/JCM.39.9.3147-3155.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zaoutis T, Schneider B, Steele Moore L, Klein JD. Antibiotic susceptibilities of group C and group G streptococci isolated from patients with invasive infections: evidence of vancomycin tolerance among group G serotypes. J Clin Microbiol. 1999;37:3380–3383. doi: 10.1128/jcm.37.10.3380-3383.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hashikawa S, Iinuma Y, Furushita M, Ohkura T, Nada T, Torii K, et al. Characterization of group C and G streptococcal strains that cause streptococcal toxic shock syndrome. J Clin Microbiol. 2004;42:186–192. doi: 10.1128/JCM.42.1.186-192.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]