Abstract
Purpose
The arterial pulsatility index (PI) is measured by transcranial Doppler ultrasonography (TCD) and is postulated to reflect the vascular resistance distal to the artery being examined. An increased PI of the intracranial artery is often reported with diabetes mellitus (DM), old age, hypertension, intracranial hypertension, vascular dementia, and small artery disease. Microvascular complication of DM, which may contribute to cerebral infarction, involves the small perforating artery and may influence the PI of the proximal artery.
Materials and Methods
We performed a TCD examination in patients with type 2 DM with acute lacunar infarction (DML, n = 35), type 2 DM without cerebral infarction (DMO, n = 69), and in control cases with no DM or cerebral infarction (control group, n = 41). We then compared the TCD findings among these groups.
Results
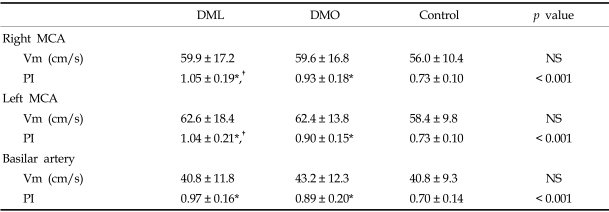
The PI was significantly higher in the DML and DMO groups than in the control group (1.05, 0.93, 0.73, respectively, for the right middle cerebral artery; 1.04, 0.90, 0.73, respectively, for the left middle cerebral artery; 0.97, 0.89, 0.70, respectively, for the basilar artery). The PI was also significantly higher in the DML group than in the DMO group for both middle cerebral arteries. The flow velocity was comparable among the three groups.
Conclusion
The elevated PI of the intracranial arteries may reflect diabetic cerebral microvascular complications. The PI measurement using TCD may be a useful predictor of lacunar infarction in type 2 DM patients.
Keywords: Diabetes mellitus, lacunar infarction, transcranial Doppler, pulsatility index
INTRODUCTION
Diabetes mellitus (DM) is known as one of the major risk factors for ischemic stroke, with a relative risk for ischemic stroke of 2.5 in males and 3.6 in females.1 In addition, DM or hyperglycemic patients have a worse prognosis and an increased mortality after stroke.2,3 DM is reported to be associated with an increased risk of stroke subtypes, especially large artery disease and small vessel disease.4-6 Cerebral macroangiopathy in DM may be related to large artery disease and can be assessed by methods such as carotid ultrasonography, cerebral angiography, MR angiography,and CT angiography. Carotid intima-media thickness as measured by B-mode carotid ultrasonography is known to be associated with the risk of coronary artery disease and stroke.7,8 In ischemic stroke, increased carotid intima-media thickness is more related to non-lacunar infarction than to lacunar infarction.9 Therefore, carotid intima-media thickness may be a useful marker for diabetic cerebral macroangiopathy.10 In contrast to macroangiopathy, diabetic cerebral microangiopathy, which may contribute to the development of lacunar infarction involving the small perforating artery, has no specific diagnostic tool. Transcranial Doppler ultrasonography (TCD) is a noninvasive and easily applicable method for detecting an intracranial arterial abnormality, especially a steno-occlusive lesion. The pulsatility index (PI), which is calculated from the measured flow velocity by TCD, has been reported to increase with DM, old age, hypertension, intracranial hypertension, vascular dementia, and small artery disease.11-15 The PI is postulated to reflect the vascular resistance distal to the examined artery. Therefore, the pathologies of small intracranial perforating arteries may affect the PI of the proximal artery. The increment of PI in normotensive DM patients without cerebral infarction has previously been reported.14 This increment is especially pronounced if it is combined with a microvascular complication such as retinopathy, neuropathy, or nephropathy.14,16 While there have been reports comparing the PI increment in ischemic stroke patients with and without DM, there have been none on the influence of cerebral infarction on the PI in DM patients.17,18 In this study we evaluated the differences in the PI between DM patients with and without lacunar infarction to determine if PI can be used as a risk predictor for developing lacunar infarction in DM patients.
MATERIALS AND METHODS
We retrospectively reviewed the stroke database to find acute cerebral infarction patients who were admitted within a week after the onset of symptoms. From March 2003 to December 2004, we gathered a group of DM patients with lacunar infarction (DML) according to our criteria. During that period, a group of DM patients without a history of cerebral infarction (DMO) and a control group of patients with no history of DM or cerebral infarction were referred from the diabetes clinic in our hospital for TCD, and these patients were enrolled in this study. The diagnosis of DM was made when the fasting glucose level was more than 7.0 mmol/L (126 mg/dL) or if the patient had taken oral hypoglycemic agents or insulin to control blood glucose levels. The DML group was selected according to the following criteria: 1) a brain MRI, TCD and MR angiography or digital subtraction angiography study had been performed, 2) an infarction size less than 2 cm as measured by diffusion-weighted MRI involving the perforating arterial territory, 3) no significant arterial stenosis or occlusion on MR angiography or digital subtraction angiography, 4) no defined cardioembolic source, and 5) diagnosis of DM.
All TCD examinations were performed by one neurologist using the Companion Micro (EME, Germany). The intracranial and extracranial arteries were examined using a 2 MHz probe. Of the examined arteries, we analyzed the data from the middle cerebral artery (MCA) at a depth of 56 - 64 mm and from the basilar artery (BA) at a depth of 78 - 86 mm. The data with the highest mean flow velocity were selected from several measurements in this depth range. The PI value is automatically calculated with the equation, PI = (systolic flow velocity-diastolic flow velocity) / mean flow velocity. As we did not perform an imaging study such as MR angiography or digital subtraction angiography in the DMO or control group, we excluded patients with a high mean flow velocity, which is suggestive of significant arterial stenosis (> 100 cm/s for MCA; > 70 cm/s for BA).19,20
Clinical and laboratory data were obtained from a review of the stroke registry and the hospital's medical records. For the DMO and control group, diabetic microvascular complications such as retinopathy, nephropathy, and peripheral neuropathy were evaluated as part of a baseline check-up program at the Diabetes clinic. In the DML group, microvascular complications were evaluated by reviewing the past clinical history and laboratory tests at the time of admission.
All statistical tests were done with SPSS 14.0 software. We compared the demographic, clinical, and biochemical variables among the three groups using the Chi-square test, t-test, and ANOVA test. Differences in TCD variables were tested using the ANOVA test and when differences were significant, we performed a Bonferroni post-hoc test. The correlations between PIs of the different arteries were tested using a Pearson correlation test. A p value of < 0.05 was considered significant.
RESULTS
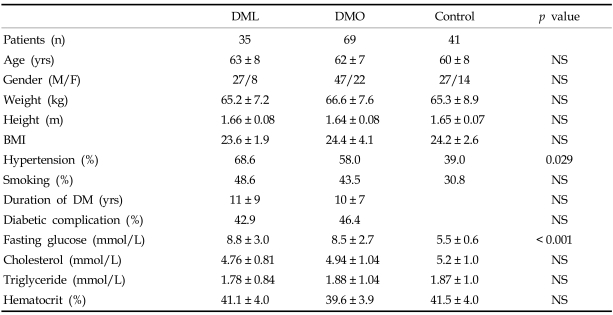
A total of 145 subjects (35 DML, 69 DMO and 41 controls) were analyzed. In the DML group, the number of patients with pure motor, pure sensory, sensorimotor, ataxic hemiparesis, dysarthria-clumsy hand, and atypical lacunar syndrome were 17, 3, 9, 3, 2, and 1, respectively. There were no differences in demographic, clinical, or biochemical variables among the three groups, except for in the proportion of patients with hypertension and in fasting glucose levels (Table 1). The DML and DMO groups had more hypertension and a higher fasting glucose level than did the control group, but there were no differences between the DML and DMO groups. A TCD examination showed a poor temporal window in 28 patients (right in 9, left in 5, both in 14), in whom we could not detect the MCA flow. Meanwhile the BA flow was detected in all the subjects. Both the MCA and BA mean arterial blood flow velocities were comparable among the three groups, but the PI was significantly different among the three groups (Table 2). The DML and DMO groups showed a significantly higher PI than the control group (1.05, 0.93, 0.73, respectively, for right MCA; 1.04, 0.90, 0.73, respectively, for left MCA; 0.97, 0.89, 0.70, respectively, for BA). When we compared the DML and DMO groups with the Bonferroni posthoc test, the PI of both MCAs was significantly elevated in the DML group. The PI of the BA was also elevated in the DML group in comparison to the DMO group, but the difference was not significant (p = 0.095). When we tested the correlations among the PIs of these three arteries, we found a significant correlation; the correlation coefficient was 0.789 between the right MCA and BA, 0.805 between the left MCA and BA, and 0.914 between the right and left MCA.
Table 1.
Demographic, Clinical, and Biochemical Profiles of the Three Groups
BMI, body mass index; DML, DM patients with acute lacunar infarction; DMO, DM patients without cerebral infarction; NS, non significant.
Diabetic complication includes retinopathy, nephropathy and peripheral neuropathy.
Values are mean ± SD or literal value.
Table 2.
Mean Flow Velocity and Pulsatility Index Measurements in Three Groups
DML, DM patients with acute lacunar infarction; DMO, DM patients without cerebral infarction; MCA, middle cerebral artery; NS, non significant; PI, pulsatility index; Vm, mean flow velocity.
*p < 0.001 compared to control group.
†p < 0.05 compared to DMO group; values are mean ± SD.
DISCUSSION
An increase in the PI of the intracranial artery has been reported for several diseases and conditions. In this study we confirmed that DM is associated with an elevated PI and that the presence of lacunar infarction in type 2 DM patients is associated with a greater PI. There were no differences between the DML and DMO groups for any other variables that could influence PI, such as the duration of DM, presence of hypertension, associated microvascular complications, or age. Therefore, lacunar infarction is the only factor elevating the PI in this study.
In subgroup analysis, the PI of DM patients with microvascular complications was elevated compared with that of patients without microvascular complications in both the DML and DMO groups, but the difference was not significant. This finding differs from a previous report that shows that DM patients with microvascular complications have higher PIs than those without complications.14 We postulate that the higher age and longer mean duration of DM within the complication group of the previous study made the PI difference between the two groups larger than that of this study. We also think that if we gathered more DM patients in this study, the PI might show a significant difference with respect to combined microvascular complications.
Hypertension is reported to increase the PI of the intracranial artery.12,21 In this study, the high prevalence of hypertension in the DM groups can exaggerate the PI differences between the DM groups and control. However, there were no statistically significant differences in PI associated with the presence of combined hypertension in each subgroup, although there was a trend of a higher PI when the patient had hypertension. In addition, there were no significant differences in the proportion with combined hypertension between the DML (69% in right MCA tested, 72% in left MCA tested) and DMO (60% in right MCA tested, 55% in left MCA tested) groups. Therefore, we can exclude the possibility that the PI increased because of hypertension in this study, especially in the DM group.
TCD examination is reported to be unsuccessful due to the poor temporal window in a substantial portion of subjects, especially in Asians and Africans.22-24 The high correlation between the PI of the MCA and BA in this study suggests that DM causes diffuse vascular changes in the entire intracranial arterial system. It also suggests that the PI of the BA can be used as an indicator of cerebral microangiopathy in cases with an unobtainable MCA flow by TCD examination due to a poor temporal window.
This study had several limitations. We did not perform an imaging study to exclude the possibility of significant arterial stenosis in the DMO and control groups. Instead we used mean flow velocity criteria to detect significant stenosis. The sensitivity and specificity of TCD velocity criteria for significant stenosis are not so high in the vertebrobasilar system that there is a chance of sampling error in this study.20 Another limitation is the TCD examination time after onset of stroke in the DML group. Although we assumed that there is less hemodynamic change in lacunar infarction than in large artery disease, we cannot exclude the possibility of PI variation during the acute stage of cerebral infarction due to intracranial hemodynamic instability.25,26 However, since we performed most of the TCD examinations after stabilization of neurologic symptoms, usually at end of the first week after the onset of stroke, we could minimize the effect of hemodynamic instability during the acute stage.
In conclusion, the PI of the intracranial arteries is increased in DM patients, especially in DM patients with lacunar infarction. Development of lacunar infarction suggests that advanced diabetic microvascular changes occurred in the small arteries of the brain, and these microvascular changes are reflected in the intracranial PI as measured by TCD. Further study using long term serial follow-up TCD may clarify the temporal changes in the PI of DM patients and verify the clinical usefulness of PI as a surrogate marker for developing lacunar infarction in DM patients.
References
- 1.Kannel WB, McGee DL. Diabetes and cardiovascular disease. The Framingham study. JAMA. 1979;241:2035–2038. doi: 10.1001/jama.241.19.2035. [DOI] [PubMed] [Google Scholar]
- 2.Arboix A, Rivas A, Garcia-Eroles L, de Marcos L, Massons J, Oliveres M. Cerebral infarction in diabetes: clinical pattern, stroke subtypes, and predictors of in-hospital mortality. BMC Neurol. 2005;5:9. doi: 10.1186/1471-2377-5-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weih M, Amberger N, Wegener S, Dirnagl U, Reuter T, Einhäupl K. Sulfonylurea drugs do not influence initial stroke severity and in-hospital outcome in stroke patients with diabetes. Stroke. 2001;32:2029–2032. [PubMed] [Google Scholar]
- 4.Karapanayiotides T, Piechowski-Jozwiak B, van Melle G, Bogousslavsky J, Devuyst G. Stroke patterns, etiology, and prognosis in patients with diabetes mellitus. Neurology. 2004;62:1558–1562. doi: 10.1212/01.wnl.0000123252.55688.05. [DOI] [PubMed] [Google Scholar]
- 5.Arboix A, Morcillo C, Garcìa-Eroles L, Oliveres M, Massons J, Targa C. Different vascular risk factor profiles in ischemic stroke subtypes: a study from the "Sagrat Cor Hospital of Barcelona Stroke Registry". Acta Neurol Scand. 2000;102:264–270. doi: 10.1034/j.1600-0404.2000.102004264.x. [DOI] [PubMed] [Google Scholar]
- 6.Megherbi SE, Milan C, Minier D, Couvreur G, Osseby GV, Tilling K, et al. Association between diabetes and stroke subtype on survival and functional outcome 3 months after stroke: data from the European BIOMED Stroke Project. Stroke. 2003;34:688–694. doi: 10.1161/01.STR.0000057975.15221.40. [DOI] [PubMed] [Google Scholar]
- 7.Staub D, Meyerhans A, Bundi B, Schmid HP, Frauchiger B. Prediction of cardiovascular morbidity and mortality: comparison of the internal carotid artery resistive index with the common carotid artery intima-media thickness. Stroke. 2006;37:800–805. doi: 10.1161/01.STR.0000202589.47401.c6. [DOI] [PubMed] [Google Scholar]
- 8.O'Leary DH, Polak JF, Kronmal RA, Manolio TA, Burke GL, Wolfson SK., Jr Carotid-artery intima and media thickness as a risk factor for myocardial infarction and stroke in older adults. Cardiovascular Health Study Collaborative Research Group. N Engl J Med. 1999;340:14–22. doi: 10.1056/NEJM199901073400103. [DOI] [PubMed] [Google Scholar]
- 9.Cupini LM, Pasqualetti P, Diomedi M, Vernieri F, Silvestrini M, Rizzato B, et al. Carotid artery intima-media thickness and lacunar versus nonlacunar infarcts. Stroke. 2002;33:689–694. doi: 10.1161/hs0302.103661. [DOI] [PubMed] [Google Scholar]
- 10.Fukuhara T, Hida K. Pulsatility index at the cervical internal carotid artery as a parameter of microangiopathy in patients with type 2 diabetes. J Ultrasound Med. 2006;25:599–605. doi: 10.7863/jum.2006.25.5.599. [DOI] [PubMed] [Google Scholar]
- 11.Hassler W, Steinmetz H, Gawlowski J. Transcranial Doppler ultrasonography in raised intracranial pressure and in intracranial circulatory arrest. J Neurosurg. 1988;68:745–751. [PubMed] [Google Scholar]
- 12.Cho SJ, Sohn YH, Kim GW, Kim JS. Blood flow velocity changes in the middle cerebral artery as an index of the chronicity of hypertension. J Neurol Sci. 1997;150:77–80. doi: 10.1016/s0022-510x(97)05391-4. [DOI] [PubMed] [Google Scholar]
- 13.Foerstl H, Biedert S, Hewer W. Multiinfarct and Alzheimer-type dementia investigated by transcranial Doppler sonography. Biol Psychiatry. 1989;26:590–594. doi: 10.1016/0006-3223(89)90084-x. [DOI] [PubMed] [Google Scholar]
- 14.Lee KY, Sohn YH, Baik JS, Kim GW, Kim JS. Arterial pulsatility as an index of cerebral microangiopathy in diabetes. Stroke. 2000;31:1111–1115. doi: 10.1161/01.str.31.5.1111. [DOI] [PubMed] [Google Scholar]
- 15.Kidwell CS, el-Saden S, Livshits Z, Martin NA, Glenn TC, Saver JL. Transcranial Doppler pulsatility indices as a measure of diffuse small-vessel disease. J Neuroimaging. 2001;11:229–235. doi: 10.1111/j.1552-6569.2001.tb00039.x. [DOI] [PubMed] [Google Scholar]
- 16.Lippera S, Gregorio F, Ceravolo MG, Lagalla G, Provinciali L. Diabetic retinopathy and cerebral hemodynamic impairment in type II diabetes. Eur J Ophthalmol. 1997;7:156–162. doi: 10.1177/112067219700700207. [DOI] [PubMed] [Google Scholar]
- 17.Lee KO, Park JH, Choi YC, Han SW, Nam HS, Heo JH, et al. Increased pulsatility index in acute lacunar infarction with type II diabetes. J Korean Neurol Assoc. 2005;23:457–462. [Google Scholar]
- 18.Tkác I, Troscák M, Javorský M, Petrík R, Tomcová M. Increased intracranial arterial resistance in patients with type 2 diabetes mellitus. Wien Klin Wochenschr. 2001;113:870–873. [PubMed] [Google Scholar]
- 19.Felberg RA, Christou I, Demchuk AM, Malkoff M, Alexandrov AV. Screening for intracranial stenosis with transcranial Doppler: the accuracy of mean flow velocity thresholds. J Neuroimaging. 2002;12:9–14. doi: 10.1111/j.1552-6569.2002.tb00083.x. [DOI] [PubMed] [Google Scholar]
- 20.Rorick MB, Nichols FT, Adams RJ. Transcranial Doppler correlation with angiography in detection of intracranial stenosis. Stroke. 1994;25:1931–1934. doi: 10.1161/01.str.25.10.1931. [DOI] [PubMed] [Google Scholar]
- 21.Sierra C, de la Sierra A, Chamorro A, Larrousse M, Domenech M, Coca A. Cerebral hemodynamics and silent cerebral white matter lesions in middle-aged essential hypertensive patients. Blood Press. 2004;13:304–309. doi: 10.1080/08037050410024448. [DOI] [PubMed] [Google Scholar]
- 22.Kwon JH, Kim JS, Kang DW, Bae KS, Kwon SU. The thickness and texture of temporal bone in brain CT predict acoustic window failure of transcranial Doppler. J Neuroimaging. 2006;16:347–352. doi: 10.1111/j.1552-6569.2006.00064.x. [DOI] [PubMed] [Google Scholar]
- 23.Itoh T, Matsumoto M, Handa N, Maeda H, Hougaku H, Hashimoto H, et al. Rate of successful recording of blood flow signals in the middle cerebral artery using transcranial Doppler sonography. Stroke. 1993;24:1192–1195. doi: 10.1161/01.str.24.8.1192. [DOI] [PubMed] [Google Scholar]
- 24.Halsey JH. Effect of emitted power on waveform intensity in transcranial Doppler. Stroke. 1990;21:1573–1578. doi: 10.1161/01.str.21.11.1573. [DOI] [PubMed] [Google Scholar]
- 25.Dawson SL, Panerai RB, Potter JF. Serial changes in static and dynamic cerebral autoregulation after acute ischaemic stroke. Cerebrovasc Dis. 2003;16:69–75. doi: 10.1159/000070118. [DOI] [PubMed] [Google Scholar]
- 26.Toyoda K, Okada Y, Fujimoto S, Hagiwara N, Nakachi K, Kitazono T, et al. Blood pressure changes during the initial week after different subtypes of ischemic stroke. Stroke. 2006;37:2637–2639. doi: 10.1161/01.STR.0000242781.80832.cc. [DOI] [PubMed] [Google Scholar]