Abstract
Purpose
Herniated nucleus pulposus fragments are recognized by the immune system as a foreign-body, which results in an autoimmune reaction. Human activation-inducible tumor necrosis factor receptor (AITR) and its ligand, AITRL, are important costimulatory molecules in the pathogenesis of autoimmune diseases. Despite the importance of these costimulatory molecules in autoimmune disease, their role in the autoimmune reaction to herniated disc fragments has yet to be explored. The purpose of the present study is to investigate whether the overexpression of AITR and AITRL might be associated with lumbar disc herniation.
Materials and Methods
The study population consisted of 20 symptomatic lumbar disc herniation patients. Ten macroscopically normal control discs were obtained from patients with spinal fractures managed with anterior procedures that involved a discectomy. Peripheral blood samples from both the study patients and controls were collected. The expression levels of AITR and AITRL were investigated by flow cytometric analysis, confocal laser scanning microscopy, immunohistochemistry and by reverse transcriptase-polymerase chain reaction (RT-PCR). The soluble AITR and AITRL serum levels were measured by an enzyme-linked immunosorbent assay.
Results
Flow cytometric analysis revealed significantly higher levels of both AITR and AITRL in the lumbar disc herniation patients than in the controls. The AITRL expression levels were also increased in patients with lumbar disc herniation, shown by using confocal laser scanning microscopy, immunohistochemistry and RT-PCR. Finally, soluble AITR and AITRL were elevated in the patients with lumbar disc herniations.
Conclusion
The AITR and AITRL are increased in both the herniated disc tissue and the peripheral blood of patients with lumbar disc herniation.
Keywords: Lumbar disc herniation, costimulatory molecule
INTRODUCTION
Lumbar disc herniation is one of the most common orthopaedic conditions to cause low back pain and/or sciatica,1 which may be caused by an inflammatory response to tissue injury or to a direct chemical irritation.2 However, it has recently been discovered that the nucleus pulposus can be recognized as a foreign-body by the immune system, inciting an autoimmune reaction3-6 which is mediated by pathologically autoreactive T cells. Preventing these autoreactive T-cells from running amok are the regulatory cells, called CD4+CD25+ T cells, which play an essential role in preventing and suppressing autoimmunity.7,8
AITR (Activation-Inducible Tumor necrosis factor Receptor) and AITRL (AITR Ligand) are both costimulatory molecules, which are pairs of molecules that interact with each other and are expressed at high levels on the surface of CD4+CD25+ T cells.9 When these costimulatory molecules are activated, they inhibit the ability of the CD4+CD25+ T cells to suppress the autoreactive T-cells.10 This results in an increased autoimmune response.
There is considerable evidence with animal-models to support that these costimulatory molecules are important in autoimmunity. The GITR (Glucocorticoid-Induced Tumor necrosis factor Receptor) is the mouse equivalent of the human AITR. Antagonistic and agonistic monoclonal antibodies, specific to GITR, have been therapeutically used to both block and promote autoimmune disease progression in animal models.9-12
Although AITR and AITRL are considered to play an important role in the pathogenesis of autoimmune diseases, their role in lumbar disc herniation has yet to be explored. The purpose of the present study is to determine if AITR and AITRL are overexpressed in symptomatic lumbar disc herniation patients.
MATERIALS AND METHODS
Clinical samples
The Institutional Review Board approved the present study and all of the patients provided their informed consent. The study population consisted of 20 patients who were suffering from herniated lumbar nucleus pulposus. These patients were classified as the patients group. In order to be included in the study, a patient had to have a diagnosis of sciatica with predominantly radicular symptoms, which is defined as having more lower-extremity symptoms than back or buttock symptoms, and a confirmation of disc herniation by preoperative magnetic resonance imagery. Surgery was offered to the patient if they did not recover after six weeks of nonoperative treatment, had intolerable sciatica, or had severe neurological loss, such as motor loss or symptoms or signs of cauda equina syndrome. In addition, the symptoms had to be attributable to a single intervertebral disc level, and the patient had to agree to return for follow-up examinations according to the protocol. The average age at the time of surgery in the disc herniation group was 35.0 years, ranging from 22 to 54 years. The 20 patients (12 men and 8 women) in this group had on average a symptom duration of 47.8 days (Table 1). The types of disc herniation in the patient group were either extrusion or sequestration.
Table 1.
Clinical Information about Age, Gender and Duration of Symptom in the Normal Control Groups (Control) and disc Herniation Groups (Patients)
Values are mean ± SD.
Our controls were ten macroscopically normal discs obtained from patients with spinal fractures that were managed with an anterior discectomy. All ten patients had magnetic resonance images to confirm that their discs were not herniated, degenerated, or otherwise injured by the fracture. Peripheral blood samples were collected from both the patients and the healthy controls during the same period of time. Immunohistochemistry was performed on the fresh specimens following surging. The average age at the time of surgery in the normal control group was 33.4 years, ranging from 23 to 52 years. There were 7 men and 3 women. The age and gender of the normal control group were matched to those of the disc herniation group (Table 1).
Cell isolation
Various immune cells (infiltrated T cells, CD4+CD25+ regulatory T cells, B cells, and dendritic cells such as macrophages and monocytes) and tissue cells (fibroblasts from the annulus fibrosus) were separated from the disc tissue. The chondrogenic nucleus pulposus cells could not be obtained from the disc tissue samples. Markers, such as CD4, CD8, CD19, CD14, CD25, CD55, and CD68, were identified from various immune and tissue cells as well as from the disc tissue samples during the flow cytometric analysis after tissue disaggregation. Peripheral blood mononuclear cells (PBMC) were isolated from freshly drawn heparinized peripheral venous blood using the Ficoll-Hypaque density gradient centrifugation method.
Antibodies
Monoclonal antibodies (mAbs) against AITR (anti-AITR mAb; clone 621) and AITRL (anti-AITRL mAb; clone CE2) were purchased from ImmunoMics (Ulsan, Korea). Anti-AITR and anti-AITRL mAbs were conjugated with fluorescein-isothiocyanate (FITC). Phycoerythrin (PE)-conjugated mAbs (mouse IgG1) directed against CD4, CD8, CD19, CD 14, CD55, CD68, and Cy-conjugated mAbs (mouse IgG1) against CD25 were used. The FITC, PE- and Cy-conjugated mouse IgG1 antibodies were used as controls (R&D Systems, Minneapolis, MN, USA).
Flow cytometric analysis
The disc cells and PBMC were subjected to two-color flow cytometry in order to determine the expression profiles of AITR and AITRL. The disc cells and PBMC were stained with FITC-conjugated anti-AITR or anti-AITRL mAbs and with the PE-conjugated mAbs to the T cell marker; CD4 and CD8, B cell marker; CD19, monocyte marker; CD14, dendritic cell marker; CD68, and fibroblast marker; CD55. Using two-color flow cytometry on the FACSCalibur and CELLQuest (Beckton Dickinson, Mountain View, CA), the proportion of AITR+ and AITRL+ cells and the mean fluorescence intensities (MFI) of the AITR or AITRL antigen on the specific cell subsets were analyzed. The absolute number of both specific cell subsets was calculated from their proportions. The PBMC was also stimulated with the anti-CD3 monoclonal antibody (OKT3) and flow cytometric analysis was then performed to obtain a clear distinction between the expression levels of the patients and control groups.
Confocal laser scanning microscopy
The disc cells, which are the fibroblast from the annulus fibrosus, were cultured in a monolayer for one week. The slides were briefly washed, blocked, and stained with both FITC-conjugated anti-AITR mAb and anti-AITRL mAb. These slides were analyzed using confocal laser scanning microscopy (LSM 510, Carl Zeiss, Heidelberg, Germany), which was equipped with Ar and HeNe lasers. The control mAbs were FITC-conjugated anti-mouse IgG, with the same concentrations in each assay.
Immunohistochemistry
Disc tissues were separately snap-frozen in the Tissue-Tek OCT (Miles Diagnostics, Elkhart, IN, USA) and were stored at -80℃ until assayed. Cryostat sections 6 micrometers thick were mounted onto glass slides, boxed, and stored at -20℃ until used for immunological analysis. AITR and AITRL signals were revealed as a substrate according to the instructions provided with the Histostain-Plus Kit (Zymed Laboratories, South San Francisco, CA, USA). Finally, the slides were counterstained with hematoxylin and sealed with Aqueous Mounting Media (Zymed). Control mAbs were anti-mouse IgG, with the same concentrations in each assay.
Reverse transcriptase-polymerase chain reaction
The mRNA expression of AITR and AITRL were determined by reverse transcriptase-polymerase chain reaction (RT-PCR). The total RNA was extracted from freshly isolated disc cells in a monolayer culture and from the PBMC using the RNeasy kit (Qiagen, Countaboeuf, France) following the manufacturer's instructions. The single-strand cDNA was synthesized by using 1 microgram of the total RNA for reverse-transcription using an oligo-dT primer (GenoTec, Korea).
Soluble serum AITR and AITRL
Serum levels of soluble AITR and AITRL were measured by an enzyme-linked immunosorbent assay (ELISA). Purified human recombinant AITR-GST mAb or AITRL-GST mAb (Immunomics) were used as standards.
Cytokine assay
ELISA kits for inflammatory cytokines, such as interleukin-1-b (IL-1β), interleukin-2 (IL-2), interleukin-6 (IL-6), interleukin-8 (IL-8), and tumor necrosis factor-alpha (TNF-α), were purchased from R&D systems. Serum samples of the patients and the healthy controls were analyzed with these kits. The ELISA was conducted according to the manufacturer's protocol. Cytokine levels were expressed as the number of picograms of each factor per milliliter of sample homogenate.
Statistical analysis
Statistical analysis was performed with SPSS 10 software. An independent-samples t test was used to compare variables. The data from the patients and control groups were checked first for equal variances, and the p value was confirmed according to the equality of these variances. A p value less than 0.05 was considered significant.
RESULTS
Flow cytometry
The herniation patients had significantly higher levels of AITR and AITRL than did the controls; these levels are expressed as the percentages of cells from the disc tissue (mean ± SD), AITR: 12.15 ± 5.16% versus 6.84 ± 1.76%, p < 0.05, AITRL: 39.43 ± 8.57 versus 18.71 ± 5.56%, p < 0.05) (Fig. 1). The same was true for the percentages of PBMC (AITR: resting state 10.36 ± 5.51% versus 2.18 ± 1.05%, p < 0.05, stimulated state 27.79 ± 8.54% versus 10.30 ± 3.56%, p < 0.05, AITRL: resting state 23.15 ± 3.78% versus 17.02 ± 4.57%, p < 0.05, stimulated state 45.00 ± 10.75% versus 24.96 ± 4.75%, p < 0.05) (Fig. 2).
Fig. 1.
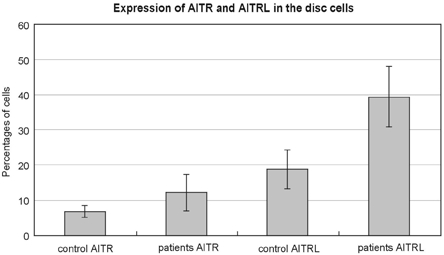
Expression of AITR and AITRL in the disc cells. In the flow cytometric analysis the patients with lumbar disc herniation (patients) had significantly higher levels than the normal controls (control), which is expressed as the percentages of cells from the disc tissue (mean ± SD), AITR: 12.15 ± 5.16% versus 6.84 ± 1.76%, p < 0.05, AITRL: 39.43 ± 8.57 versus 18.71 ± 5.56%, p < 0.05).
Fig. 2.
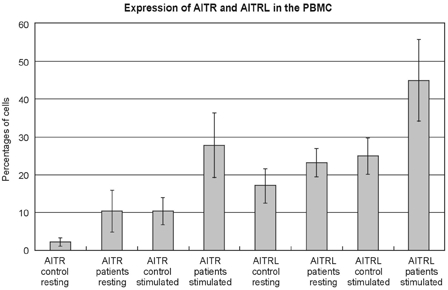
Expression of AITR and AITRL in the PBMC. In the flow cytometric analysis of AITR and AITRL, the patients with lumbar disc herniation (patients) had significantly higher levels than did normal controls (control) expressed as percentages of peripheral blood mononuclear cells (PBMC) (mean ± SD, AITR: resting state 10.36 ± 5.51% versus 2.18 ± 1.05%, p < 0.05, stimulated state 27.79 ± 8.54% versus 10.30 ± 3.56%, p < 0.05, AITRL: resting state 23.15 ± 3.78% versus 17.02 ± 4.57%, p < 0.05, stimulated state 45.00 ± 10.75% versus 24.96 ± 4.75%, p < 0.05).
The flow cytometric analysis demonstrated that the patients with lumbar disc herniations had statistically higher levels of dendritic cells, including macrophages, than did the controls, when expressed either as a percentage of cells from the disc tissue (12.11 ± 3.84% versus 6.26 ± 1.14%, p < 0.05) or by the PBMC (resting state 8.21 ± 2.48% versus 0.63 ± 0.31%, p < 0.05, stimulated state 13.56 ± 5.99% versus 0.79 ± 0.42%, p < 0.05) (Fig. 3). In addition, the disc herniation patients had statistically lower levels of CD4+CD25+ regulatory T cells when compared with the controls, whether it is expressed either as a percentage of cells from the disc tissue (0.07 ± 0.09% versus 1.72 ± 0.48%, p < 0.05) or by the PBMC (resting state 1.15 ± 1.02% versus 4.80 ± 1.82%, p < 0.05, stimulated state 7.51 ± 1.01% versus 17.03 ± 3.17%, p < 0.05) (Fig. 4).
Fig. 3.
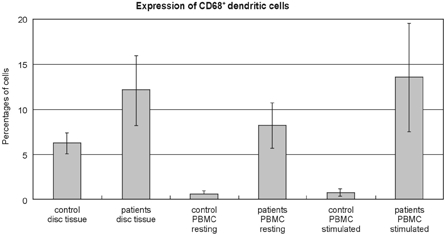
Expression of CD68+ dendritic cells, including macrophages, in the flow cytometric analysis. The patients with lumbar disc herniation (patients) had statistically higher levels of CD68+ dendritic cells, including macrophages, than did the normal controls (control), when expressed either as percentages of cells from disc tissue (mean ± SD), 12.11 ± 3.84% versus 6.26 ± 1.14%, p < 0.05) or as the peripheral blood mononuclear cells (PBMC) (resting state 8.21 ± 2.48% versus 0.63 ± 0.31%, p < 0.05, stimulated state 13.56 ± 5.99% versus 0.79 ± 0.42%, p < 0.05).
Fig. 4.
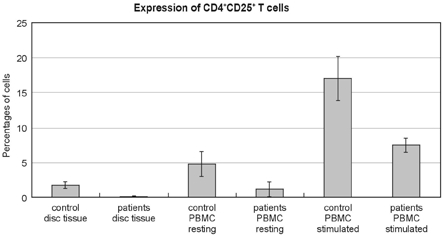
Expression of CD4+CD25+ T cells in the flow cytometric analysis. The patients with lumbar disc herniation (patients) had statistically lower levels of CD4+CD25+ T cells than did normal controls (control), when expressed either as percentages of cells from disc tissue (mean ± SD, 0.07 ± 0.09% versus 1.72 ± 0.48%, p < 0.05) or peripheral blood mononuclear cells (PBMC) (resting state 1.15 ± 1.02% versus 4.80 ± 1.82%, p < 0.05, stimulated state 7.51 ± 1.01% versus 17.03 ± 3.17%, p < 0.05).
No significant differences could be found between the patients and the controls for the other cell markers, such as CD4, CD8, CD19, and CD55, from the disc tissue cells or the PBMC.
Confocal laser scanning microscopy
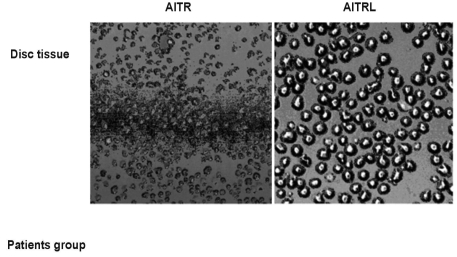
AITR and AITRL expression was apparent on the herniated disc cells (Fig. 5), but not on the control discs.
Fig. 5.
AITR and AITRL in patients with lumbar disc herniation (patients group) expressed by confocal laser microscopy. Many round-shaped cells expressing AITR and AITRL are evident. Confocal laser microscopic staining × 200.
Immunohistochemistry
There was increased expression of AITRL and slightly increased expression of AITR on the herniated disc cells, but not in the controls (Fig. 6).
Fig. 6.

AITR and AITRL, expressed by immunohistochemical staining, in the disc tissue of the patients with spinal fracture which was managed with anterior discectomy as a control group of healthy individuals (control group) and the patients with lumbar disc herniation (patients group). Many spindle-shaped cells expressing AITR and AITRL are evident inside the disc tissue. Immunohistochemical staining × 200.
The expression of AITRL mRNA was in creased in the patients when compared to the controls, but there was no difference in AITR mRNA expression between the two groups (Fig. 7).
Fig. 7.
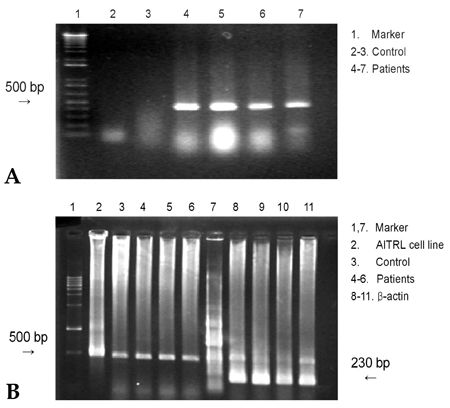
Representative RT-PCR derived from mRNA of tissue cells (A) and PBMC (B) following culture in control groups (control) and disc herniation groups (patients). RT-PCR for AITRL and β-actin was performed. The cDNA for β-actin was used as the control. The PCR products of AITRL and β-actin were 513 bp and 230 bp fragments, respectively.
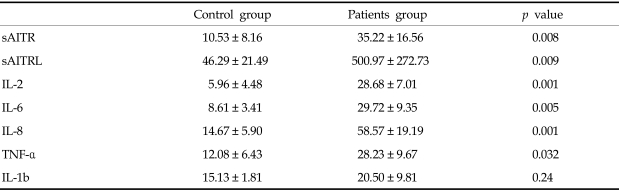
Mean serum concentrations of soluble AITR, soluble AITRL, IL-2, IL-6, IL-8, and TNF-α were significantly higher in the herniation patients, whereas no significant differences in the serum concentration of IL-1β could be found between the patients and control groups (Table 2).
Table 2.
Quantitative Analysis of Serum Concentrations of Soluble AITR (sAITR), Soluble AITRL (sAITRL), IL-2, IL-6, IL-8, TNF-α and IL-1β in Normal Control Groups (Control) and Disc Herniation Groups (Patients)
Values are mean ± SD (Picogram/milliliter).
DISCUSSION
Although AITR and AITRL play important roles in the pathogenesis of autoimmune diseases, their roles in symptomatic lumbar disc herniation have not yet been investigated. The aim of the present study is to investigate whether AITR and AITRL are overexpressed in lumbar disc herniation patients.
The discs and PBMC from patients with symptomatic herniations were found to have significantly higher levels of AITR and/or AITRL than matched controls. These findings are based on the results of flow cytometry, confocal laser scanning microscopy, immunohistochemistry, and RT-PCR. The herniated disc patients had a higher percentage of macrophages and lower levels of CD4+CD25+ regulatory T cells. Mean serum concentrations of soluble AITR, soluble AITRL, IL-2, IL-6, IL-8, and TNF-α were significantly higher in the patients with lumbar disc herniations than in the controls.
These results confirm and expand the findings of other investigators. The herniated nucleus pulposus may be recognized as a foreign-body by the immune system, resulting in an autoimmune reaction.3-6 Antigen-antibody complexes (IgG, IgM) seem to be commonly present in herniated disc tissue, but not in healthy discs.3,4 Patients with lumbar disc herniation show a significant increase in preoperative serum IgG and IgM levels, which decrease post-operatively.5,6 Macrophages have been found to be abundantly present in acute and chronic lumbar disc herniations.13 Activated T cells and B cells are found in acute lumbar disc herniations.13 Inflammatory cytokines, such as TNF-α, vascular endothelial growth factor (VEGF), basic fibroblast growth factor (BFGF), prostaglandin E2, interleukin-1-alpha (IL-1 alpha), IL-8, and matrix metalloproteinase-3 (MMP-3), are overexpressed in herniated lumbar disc tissue.14-16 The autoimmune reaction related with AITR and AITRL might briefly initiate the inflammatory reactions, resulting in the resorption of the herniated disc.
Pathologic, autoreactive T cells are responsible for autoimmune reactions. Autoimmunity is normally prevented by another type of T cells, which are a lineage of the CD4+CD25+ regulatory T-cells, that suppress pathologic T cells.7,8 Both the AITR and AITRL have been shown to interact with one another in order to inhibit these autoimmunity-suppressing CD4+CD25+ regulatory T cells.10,17 Furthermore, blocking these costimulatory molecules restores the capacity of the CD4+CD25+ regulatory T cells to suppress the pathogenic, autoreactive T cells, thereby preventing autoimmunity.17-20
The disc is immune privileged and, once the disc is herniated, the body identifies it as foreign leading to an autoimmune reaction.3-6 The cellular elements of the disc must rely on a diffusional system with the vessels that lie adjacent to the disc, because the normal adult disc is avascular. Likewise, antibodies need to be delivered to the disc tissue by diffusion in order to incite the autoimmune reactions along with the ingrowth of granulation tissue and blood vessels. In a similar fashion, AITR and AITRL might be increased in both the disc tissue and PBMC in patients with a lumbar disc herniation.
However, one limitation of this study is that all of the data were obtained from patients who required operative treatment of their lumbar disc herniations, whereas the vast majority of patients improve spontaneously. Another limitation of this study is that the data of the control group were obtained from patients with spinal fractures during the anterior discectomy and fusion as the second-stage operation for circumferential fusion. Even though the tissue was harvested from patients with spinal fractures in the subacute phase, they were not healthy controls. Also, the herniated nucleus pulposus lies somewhere in the spectrum between organ specific and non-organ specific autoimmune diseases. The exact position in this spectrum is impossible to determine based on the data in this study.
This may be the first study to demonstrate that patients with lumbar disc herniation have a significantly increased AITR and AITRL expression in herniated disc tissue and in peripheral blood. This phenomenon was not observed in healthy control groups, suggesting that the increased expression of AITR and AITRL might play a specific pathogenic role in lumbar disc herniation. These results suggest the intriguing possibility of using biological agents, such as AITR blocking antibodies to treat symptomatic disc herniations. Both the AITR and AITRL increased in the herniated disc tissue and peripheral blood of the patients with lumbar disc herniation, but decreased in the healthy controls. These costimulatory molecules could be considered to play a special role in the pathogenesis of lumbar disc herniation.
Footnotes
This work was supported by the Korea Research Foundation Grant, which was funded by the Korean Government (MOEHRD) (KRF-2005-041-E00249).
References
- 1.Deyo RA, Tsui-Wu YJ. Descriptive epidemiology of low-back pain and its related medical care in the United States. Spine. 1987;12:264–268. doi: 10.1097/00007632-198704000-00013. [DOI] [PubMed] [Google Scholar]
- 2.Kawaguchi S, Yamashita T, Yokogushi K, Murakami T, Ohwada O, Sato N. Immunophenotypic analysis of the inflammatory infiltrates in herniated intervertebral discs. Spine. 2001;26:1209–1214. doi: 10.1097/00007632-200106010-00008. [DOI] [PubMed] [Google Scholar]
- 3.Habtemariam A, Grönblad M, Virri J, Seitsalo S, Ruuskanen M, Karaharju E. Immunocytochemical localization of immunoglobulins in disc herniations. Spine. 1996;21:1864–1869. doi: 10.1097/00007632-199608150-00005. [DOI] [PubMed] [Google Scholar]
- 4.Satoh K, Konno S, Nishiyama K, Olmarker K, Kikuchi S. Presence and distribution of antigen-antibody complexes in the herniated nucleus pulposus. Spine. 1999;24:1980–1984. doi: 10.1097/00007632-199910010-00003. [DOI] [PubMed] [Google Scholar]
- 5.Spiliopoulou I, Korovessis P, Konstantinou D, Dimitracopoulos G. IgG and IgM concentration in the prolapsed human intervertebral disc and sciatica etiology. Spine. 1994;19:1320–1323. doi: 10.1097/00007632-199406000-00003. [DOI] [PubMed] [Google Scholar]
- 6.Yüceer N, Arasil E, Temiz C. Serum immunoglobulins in brain tumours and lumbar disc diseases. Neuroreport. 2000;11:279–281. doi: 10.1097/00001756-200002070-00011. [DOI] [PubMed] [Google Scholar]
- 7.Morris GP, Chen L, Kong YC. CD137 signaling interferes with activation and function of CD4+CD25+ regulatory T cells in induced tolerance to experimental autoimmune thyroiditis. Cell Immunol. 2003;226:20–29. doi: 10.1016/j.cellimm.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 8.Herman AE, Freeman GJ, Mathis D, Benoist C. CD4+CD25+ T regulatory cells dependent on ICOS promote regulation of effector cells in the prediabetic lesion. J Exp Med. 2004;199:1479–1489. doi: 10.1084/jem.20040179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Uraushihara K, Kanai T, Ko K, Totsuka T, Makita S, Iiyama R, et al. Regulation of murine inflammatory bowel disease by CD25+ and CD25- CD4+ glucocorticoid-induced TNF receptor family-related gene+ regulatory T cells. J Immunol. 2003;171:708–716. doi: 10.4049/jimmunol.171.2.708. [DOI] [PubMed] [Google Scholar]
- 10.Kohm AP, Williams JS, Miller SD. Cutting edge: ligation of the glucocorticoid-induced TNF receptor en hances autoreactive CD4+ T cell activation and experimental autoimmune encephalomyelitis. J Immunol. 2004;172:4686–4690. doi: 10.4049/jimmunol.172.8.4686. [DOI] [PubMed] [Google Scholar]
- 11.Suri A, Shimizu J, Katz JD, Sakaguchi S, Unanue ER, Kanagawa O. Regulation of autoimmune diabetes by non-islet-specific T cells-a role for the glucocorticoid-induced TNF receptor. Eur J Immunol. 2004;34:447–454. doi: 10.1002/eji.200324599. [DOI] [PubMed] [Google Scholar]
- 12.Shimizu J, Yamazaki S, Takahashi T, Ishida Y, Sakaguchi S. Stimulation of CD25(+)CD4(+) regulatory T cells through GITR breaks immunological self-tolerance. Nat Immunol. 2002;3:135–142. doi: 10.1038/ni759. [DOI] [PubMed] [Google Scholar]
- 13.Habtemariam A, Grönblad M, Virri J, Seitsalo S, Karaharju E. A comparative immunohistochemical study of inflammatory cells in acute-stage and chronic-stage disc herniations. Spine. 1998;23:2159–2166. doi: 10.1097/00007632-199810150-00003. [DOI] [PubMed] [Google Scholar]
- 14.Ahn SH, Cho YW, Ahn MW, Jang SH, Sohn YK, Kim HS. mRNA expression of cytokines and chemokines in herniated lumbar intervertebral discs. Spine. 2002;27:911–917. doi: 10.1097/00007632-200205010-00005. [DOI] [PubMed] [Google Scholar]
- 15.Haro H, Crawford HC, Fingleton B, Shinomiya K, Spengler DM, Matrisian LM. Matrix metalloproteinase-7-dependent release of tumor necrosis factor-alpha in a model of herniated disc resorption. J Clin Invest. 2000;105:143–150. doi: 10.1172/JCI7091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kato T, Haro H, Komori H, Shinomiya K. Sequential dynamics of inflammatory cytokine, angiogenesis inducing factor and matrix degrading enzymes during spontaneous resorption of the herniated disc. J Orthop Res. 2004;22:895–900. doi: 10.1016/j.orthres.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 17.Ehrenstein MR, Evans JG, Singh A, Moore S, Warnes G, Isenberg DA, et al. Compromised function of regulatory T cells in rheumatoid arthritis and reversal by anti-TNFalpha therapy. J Exp Med. 2004;200:277–285. doi: 10.1084/jem.20040165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Salazar-Fontana LI, Sanz E, Mérida I, Zea A, Sanchez-Atrio A, Villa L, et al. Cell surface CD28 levels define four CD4+ T cell subsets: abnormal expression in rheumatoid arthritis. Clin Immunol. 2001;99:253–265. doi: 10.1006/clim.2001.5003. [DOI] [PubMed] [Google Scholar]
- 19.Foell J, Strahotin S, O'Neil SP, McCausland MM, Suwyn C, Haber M, et al. CD137 costimulatory T cell receptor engagement reverses acute disease in lupus-prone NZB x NZW F1 mice. J Clin Invest. 2003;111:1505–1518. doi: 10.1172/JCI17662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Foell JL, Diez-Mendiondo BI, Diez OH, Holzer U, Ruck P, Bapat AS, et al. Engagement of the CD137 (4-1BB) costimulatory molecule inhibits and reverses the autoimmune process in collagen-induced arthritis and establishes lasting disease resistance. Immunology. 2004;113:89–98. doi: 10.1111/j.1365-2567.2004.01952.x. [DOI] [PMC free article] [PubMed] [Google Scholar]