Abstract
Concern about potential health impacts of low level exposures to organophosphorus (OP) pesticides, bisphenol A (BPA), and phthalates among the general population is increasing. We measured levels of six dialkyl phosphate (DAP) metabolites of OP pesticides, a chlorpyrifos-specific metabolite (3,5,6-trichloro-2-pyridinol, TCPy), BPA, and fourteen phthalate metabolites in urine samples of 100 pregnant women from the Generation R study, the Netherlands. The unadjusted and creatinine-adjusted concentrations were reported, and compared to National Health and Nutrition Examination Survey and other studies. In general, these metabolites were detectable in the urine of the women from the Generation R study and compared with other groups, they had relatively high level exposures to OP pesticides and several phthalates but similar exposure to BPA. The median concentrations of total dimethyl (DM) metabolites was 264.0 nmol/g creatinine (Cr) and of total DAP was 316.0 nmol/g Cr. The median concentration of mono-ethyl phthalate (MEP) was 222.0 µg/g Cr; the median concentrations of mono-isobutyl phthalate (MiBP) and mono-n-butyl phthalate (MnBP) were above 50 µg/g Cr. The median concentrations of the three secondary metabolites of di-2-ethylhexyl phthalate (DEHP) were greater than 20 µg/g Cr. The data indicate that the Generation R study population provides a wide distribution of selected environmental exposures. Reasons for the relatively high levels and possible health effects need investigation.
Keywords: Organophosphorus (OP) pesticides, Bisphenol A (BPA), Phthalates, Prenatal exposure, Biological monitoring
1. Introduction
Potential health effects of low level exposures to organophosphorus (OP) pesticides, bisphenol A (BPA), and phthalates among the general population have been investigated (Eskenazi et al., 2007; Swan et al., 2005; vom Saal., 2007). For example, associations of prenatal OP pesticide exposure with infant adverse neurodevelopment have been reported (Engel et al., 2007; Eskenazi et al., 2007). The National Toxicology Program (NTP) Review Panel (National Toxicology Program, 2007) and a Chapel Hill Expert Panel (vom Saal et al., 2007) reported on adverse health effects of low level exposure to BPA in experimental animal studies. Experimental animal studies have also found reproductive and developmental toxicity of some phthalates (Kavlock et al., 2002) and in humans an association between prenatal phthalate exposure at general population levels and reduced anogenital distance has been reported (Swan et al., 2005). Each of these potential effects, however, requires greater study in humans. For such investigations, we need to identify environmentally exposed populations where health effects can be studied.
The widespread use of OP pesticides results in human exposure through a variety of sources including residues in food (Lu et al., 2006; National Research Council, 1993). Upon intake, most OP pesticides are metabolized to one or more of six dialkyl phosphate (DAP) metabolites (Duggan et al., 2003). Thus, levels of DAP metabolites in urine reflect exposure to one or more OP pesticides (Barr et al., 2004; Barr and Needham, 2002; Bradman et al., 2005; Duggan et al., 2003; Wessels et al., 2003). Chlorpyrifos is the most widely used OP pesticide worldwide. The major chlorpyrifos-specific metabolite in humans is 3,5,6-trichloro-2-pyridinol (TCPy). Levels of TCPy in urine have frequently been used as a biomarker of exposure to chlorpyrifos, triclopyr, and chlorpyrifos-methyl (Barr and Angerer, 2006).
BPA is used to manufacture polycarbonate plastics and epoxy resins. Human exposure to BPA can arise from multiple sources, particularly from food in contact with BPA containing materials and in some dental sealants (Kang et al., 2006). BPA is rapidly glucuronidated and excreted in urine (Tsai, 2006). Other minor metabolites identified include sulfate conjugates and glucuronide/sulfate diconjugates.
Phthalates are a family of related compounds used for a variety of purposes including personal care products and as a plasticizer in polyvinyl chloride plastics. Humans are exposed to phthalates through ingestion, inhalation and dermal contact (Latini, 2005). After entering the body, phthalates are rapidly metabolized to their respective monoesters, some of which can be further metabolized to oxidative metabolites (Hauser and Calafat, 2005). All these metabolites can be glucuronidated and excreted in the urine and feces. Measurements of metabolites in body fluids (mainly urine) are usually better biomarkers of exposure than those of the parent phthalates because the latter are easily affected by laboratory contamination (Barr et al., 2003). In most cases, the metabolite is more toxic than the parent phthalate (Peck and Albro, 1982).
In this paper, we report biological monitoring data on metabolites of OP pesticides, BPA, and phthalates among 100 pregnant women, a subset of participants in the Generation R study in Rotterdam, the Netherlands. This analysis was conducted to better understand exposure status of these pollutants among the general population. To our knowledge, this is the first report on the biological monitoring of these compounds among a general population in the Netherlands.
2. Materials and Methods
2.1. Study population
The Generation R study is a population-based birth cohort study in the city of Rotterdam that has been described in detail previously (Jaddoe et al., 2006). Briefly, all mothers who resided in the study area and had a delivery date between April 2002 and January 2006 were eligible. Mothers could be enrolled during pregnancy or in the first months after the birth of their child when newborns visited the routine child health centers. Among the 9,778 mothers who participated in the study, 91% (n=8,880) were enrolled during pregnancy while the remaining 9% (n=898) were enrolled shortly after the birth of their child. Participants were asked to sign written consent forms. Their offspring are currently being followed to young adulthood. The study addresses four primary areas: (1) growth and physical development; (2) behavioral and cognitive development; (3) diseases in childhood; and (4) health and health care for pregnant women and children.
2.2. Urine collection and analysis
Details of biological specimen collection have been described elsewhere (Jaddoe et al., 2007). Women enrolled during pregnancy were asked to provide a spot urine sample at their first study visit, usually early in pregnancy (<18 weeks of gestational age). On February 24 of 2004, NIEHS supported an increase in the number of times urine specimens were collected from each woman, from 1 to 3 [i.e., added collection in mid pregnancy (gestational age 18–25 weeks) and late pregnancy (gestational age >25 weeks)]. All samples were collected between 8 a.m. and 8 p.m. in 100 mL polypropylene urine collection containers that were kept maximally 20 hours in a cold room (4 °C) before being frozen in 20 mL portions in 25 mL polypropylene vials at -20°C. In April 2006, we randomly selected 100 urine samples from women whose pregnancy resulted in a live birth, who enrolled after February of 2004, and who had only a single specimen available collected after 20 weeks of gestation. This kept the subset of subjects with multiple urine collections as large as possible for future studies.
Measurements of six nonspecific DAP metabolites of organophosphorous insecticides, TCPy, bisphenol A, and 14 phthalate metabolites (see the note to Table 2) were conducted at the Institute of Occupational, Social and Environmental Medicine of the University of Erlangen-Nürnberg, Germany, using gas chromatography coupled with tandem mass spectrometry (GCMS/ MS). The analytical methods for the determination of organophosphorus insecticide metabolites and phthalates have been described in detail previously (Bravo et al., 2004; Koch et al., 2003a; Preuss et al., 2005). For the determination of TCPy and bisphenol A, a gas chromatography and mass spectrometry (GC-MS) method originally used for the determination of TCPy, was modified and used (Koch and Angerer, 2001).
Table 2.
Unadjusted levels of OP pesticides, bisphenol A, phthalates or their metabolites in the urine of the 100 pregnant women from the Generation R study
| Metabolites | LOD | Detection | GM | GSD | Min. | Percentile |
Max. | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Frequency (%) | 5th | 25th | 50th | 75th | 95th | ||||||
| OP metabolites (nmol/L) | |||||||||||
| DMP | 0.79 | 99 | 79.9 | 2.8 | <LOD | 16.2 | 49.7 | 85.7 | 173.0 | 284.0 | 765.0 |
| DMTP | 0.70 | 100 | 60.9 | 3.2 | 0.7 | 8.4 | 28.7 | 74.6 | 137.0 | 319.0 | 774.0 |
| DMDTP | 0.63 | 96 | 2.3 | 2.8 | <LOD | 0.6 | 1.3 | 2.5 | 5.0 | 14.8 | 25.8 |
| Total DM | 157.0 | 3.0 | 16.7 | 30.5 | 85.4 | 179.0 | 310.0 | 584.0 | 1540.0 | ||
| DEP | 0.65 | 99 | 13.0 | 2.5 | <LOD | 1.9 | 6.5 | 13.0 | 22.4 | 83.9 | 253.0 |
| DETP | 0.59 | 96 | 4.7 | 3.2 | <LOD | 0.6 | 1.8 | 4.7 | 10.3 | 82.9 | 218.0 |
| DEDTP | 0.05 | 81 | 0.2 | 4.2 | <LOD | 0.1 | 0.1 | 0.2 | 0.3 | 1.4 | 26.8 |
| Total DE | 19.7 | 3.1 | 1.3 | 3.2 | 9.6 | 19.2 | 35.7 | 160.0 | 350.0 | ||
| Total DAP | 183.0 | 2.5 | 23.1 | 34.2 | 97.3 | 200.0 | 370.0 | 659.0 | 1890.0 | ||
| TCPy (nmol/L) | 6.3 | 3.0 | 0.8 | 1.2 | 2.8 | 5.9 | 11.9 | 32.0 | 797.0 | ||
| TCPy (µg/L) | 0.15 | 100 | 1.2 | 3.0 | 0.2 | 0.2 | 0.6 | 1.2 | 2.4 | 6.4 | 158.0 |
| BPA (µg/L) | 0.26 | 82 | 1.1 | 3.8 | <LOD | <LOD | 0.5 | 1.2 | 2.5 | 8.6 | 46.0 |
| Phthalates metabolites (µg/L) | |||||||||||
| MMP | 1.00 | 36 | - | - | <LOD | <LOD | <LOD | <LOD | 3.5 | 20.1 | 76.9 |
| MEP | 1.00 | 97 | 112.0 | 5.6 | <LOD | 7.2 | 37.9 | 117.0 | 425.0 | 1150.0 | 4330.0 |
| MOP | 0.50 | 2 | - | - | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | 39.1 |
| MCPP | 0.25 | 94 | 0.8 | 2.8 | <LOD | 0.1 | 0.5 | 1.0 | 1.6 | 3.5 | 8.2 |
| MnBP | 1.00 | 100 | 43.2 | 3.1 | 4.7 | 8.0 | 21.2 | 42.7 | 86.6 | 197.0 | 420.8 |
| MiBP | 1.00 | 100 | 41.3 | 3.0 | 5.3 | 7.9 | 21.7 | 42.1 | 72.8 | 249.0 | 759.0 |
| MBzP | 0.25 | 100 | 8.9 | 2.8 | 1.3 | 1.9 | 3.6 | 7.5 | 16.8 | 95.8 | 320.0 |
| MEHP | 0.25 | 96 | 6.9 | 2.7 | <LOD | 0.3 | 3.3 | 6.9 | 17.3 | 82.8 | 392.0 |
| MEHHP | 0.25 | 100 | 14.3 | 4.7 | 0.5 | 2.9 | 6.9 | 14.0 | 30.0 | 86.2 | 494.0 |
| MEOHP | 0.25 | 100 | 15.0 | 2.6 | 0.7 | 3.2 | 7.2 | 14.5 | 27.4 | 104.0 | 514.0 |
| MECPP | 0.25 | 100 | 19.4 | 2.8 | 1.2 | 4.2 | 9.6 | 18.4 | 31.5 | 141.0 | 421.0 |
| MCMHP | 0.25 | 100 | 6.2 | 3.2 | 0.5 | 1.4 | 2.9 | 6.2 | 11.1 | 33.4 | 67.3 |
| 7oxo-MMeOP | 0.25 | 96 | 2.5 | 3.7 | <LOD | 0.3 | 1.2 | 2.2 | 5.1 | 30.0 | 152.0 |
| 7oh-MMeOP | 0.25 | 98 | 3.0 | 3.6 | <LOD | 0.5 | 1.4 | 2.5 | 6.5 | 38.3 | 122.0 |
Abbreviations: GM, geometric mean; GSD, geometric standard deviation; DMP, dimethylphosphate; DMTP, dimethylthiophosphate; DMDTP, dimethyldithiophosphate ; DM, dimethyl; DEP, diethylphosphate; DETP, diethylthiophosphate; DEDTP, diethyldithiophosphate; DE, diethyl; DAP, dialkyl phosphate; TCPy, 3,5,6-trichloro-2-pyridinol; MMP, mono-methyl phthalate; MEP, mono-ethyl phthalate; MOP, mono-n-octyl phthalate; MCPP, mono-3-carboxypropyl phthalate; MnBP, mono-n-butyl phthalate; MiBP, mono-isobutyl phthalate; MBzP, mono-benzyl phthalate; MEHP, mono-2-ethylhexyl phthalate ; MEHHP, mono-2-ethyl-5-hydroxyhexyl phthalate; MEOHP, mono-2-ethyl-5-oxohexyl phthalate; MECPP, mono-2-ethyl-5-carboxypentyl phthalate; MCMHP, mono-2- carboxymethylhexyl phthalate; 7oxo-MMeOP, tono-4-methyl-7-oxooctyl phthalate; 7oh-MMeOP, mono-4-methyl-7- hydroxyoctyl phthalate.
-, Not calculated because of low detection frequencies.
Total DM=DMP+DMTP+DMDTP
Total DE=DEP+DETP+DEDTP
Total DAP= Total DM + Total DE
To measure DAPs, acidified urine samples (2 mL) were spiked with isotopically labeled internal standards and were lyophilized overnight. The lyophilate was dissolved in diethylether and acetonitrile, and metabolites were extracted and derivatized using pentafluorobenzylbromide (PFBBr) at 40°C for 15 hours. After further liquid-liquid extraction with hexane, analytes were detected and quantified by GC-MS/MS. The recovery of the method was determined at two concentrations by spiking six ‘‘blank’’ urine samples (2.0 ml) with the appropriate native standard, and ranged from 85% to 99%. Quality control samples were prepared at two concentrations (8 and 20 mg/l) and inserted blindly among the study samples. The limit of detection (LOD) was 0.01 µg/L for diethyldithiophosphate (DEDTP) and 0.1 µg/L for the other five DAP metabolites. The between-day coefficient of variation (CV) ranged from 7% to 14%. Molar concentrations were used for comparisons with other studies.
For determination of TCPy and BPA, analytes were hydrolyzed and separated from 1 mL of urine using semi-automated steam distillation and solid-phase extraction. The analytes were then converted into their tert-butyldimethylsilyl derivative with N-tert-butyldimethylsilyl-N-methyltrifluoroacetamide (MTBSTFA) and injected into a GC-MS/MS system. The mean recoveries were 94% for TCPy and 105% for BPA. For BPA, the between-day CV was determined to be 8.3 or 4.2 % at concentrations of 2.8 or 45.4 µg/L. For BPA, a mean blank value of 0.39 µg/L had been determined and was subtracted from each measured value. The LOD was 0.15 µg/L for TCPy and 0.26 µg/L for BPA. The between-day CV for the determination of TCPy was 11.1 or 10.6 % at TCPyr concentrations of 6.5 or 46.0 μg/L, respectively.
After β-glucuronidase hydrolysis at 37°C for 2h, the phthalate metabolites were extracted from 1 mL of urine by on-line extraction on a restricted access material precolumn. After this clean-up and enrichment step, analytes were transferred to a reversed-phase high performance liquid chromatography (HPLC) for separation. Eluting metabolites were detected and quantified by tandem mass spectrometry in negative ionization mode. The mean recovery of each analyte ranged from 85% to 106%. The LOD was 0.5 -2.0 µg/L. For quality assurance, one low concentration control sample (water with 10 mg of each monoester per liter) and one high concentration control sample (pooled urine from laboratory personnel with 100 mg monoester per liter after freezing and filtering the urine) was included in each analytical series. Compared to the measured values, there were relatively small reagent blank values for mono-2-ethylhexyl phthalate (MEHP), mono-isobutyl phthalate (MiBP), and mono-n-butyl phthalate (MnBP) (usually <1 µg/L), which were considered in each analytical series. The between-day CV was less than 20% with the exception of mono-2-carboxymethylhexyl phthalate (MCMHP) (23.8%) and MEHP (25.3%).
Urinary creatinine concentrations were determined using the method described by Larsen (Larsen, 1972).
2.3. Statistical analysis
We followed the procedure of Hormung and Reed (Hornung and Reed, 1990) and imputed the missing levels by the value of the LOD divided by the square root of 2 if the geometric standard deviation (GSD) was less than 3; otherwise, we imputed the LOD divided by 2. Three dimethyl (DM) metabolites (dimethylphosphate [DMP], dimethylthiophosphate [DMTP] and dimetyldithiophosphate [DMDTP]) were summed as total DM (nmol/L) and three diethyl (DE) metabolites (diethylphosphte [DEP], diethylthiophosphate [DETP] and diethyldithiophosphate [DEDTP]) were summed as total DE (nmol/L). Total DAP concentrations (nmol/L) were calculated by summing all six metabolites. Urinary values were calculated with and without adjustment for creatinine. Descriptive statistics (geometric mean, median, percentiles, and range) were calculated using SPSS 15.0 (SPSS Inc, Chicago, IL). The associations between women’s characteristics (age, education, ethnicity, house hold income, and gestational age) and metabolite levels were also examined by comparing levels by categories of characteristics (Kolmogorov-Smirnov and Kruskal-Wallis Tests). To compare the levels in the Generation R study to the levels in the United States, median levels of OP pesticide metabolites, TCPy and phthalates in the urine of pregnant women (n=118) women aged 15–44 years in the National Health and Nutrition Examination Survey (NHANES) in 2001–2002 were calculated. The median concentration of BPA was calculated for 86 pregnant women aged 15–44 years in NHANES 2003–2004 (BPA was not measured in NHANES 2001–2002). The NHANES design used a complex sampling strategy, appropriate sample weights and clustering factors were used in the calculations to make our estimates of levels nationally representative, according to NHANES analytical guidelines (http://www.cdc.gov/nchs/data/nhanes/nhanes_03_04/nhanes_analytic_guidelines_dec_2005.pdf). The sample weights for NHANES are based on population estimates that incorporate census counts. These weights were determined for each subject according to the sampling design and were incorporated into the relevant datasets for use.
3. Results
The subjects’ age ranged from 18 to 41 years, with a median of 30 years (Table 1). Nearly half (46%) of the subjects stated they were Dutch. Among 82 subjects who gave their education levels, 35% of them had at least some higher education. Sixty percent of the subjects stated that they did not smoke. Urine samples were collected during weeks 21–38 of pregnancy (median= 30 weeks). Urinary creatinine ranged from 0.14 to 2.49 g/L (median=0.68 g/L).
Table 1.
Characteristics of 99 pregnant women selected from the Generation R study a
| Median age (years) | 30 (18–41) b |
| Ethnicity (n) | |
| Dutch | 46 |
| Non-Dutch | 40 |
| Not stated | 13 |
| Median BMI before pregnancy(kg/m2)c | 23.1 (16.6–37.8) |
| Educational level (n) | |
| Primary education | 12 |
| Secondary education, first phase | 14 |
| Secondary education, second phase | 27 |
| Higher education, first phase | 17 |
| Higher education, second phase | 12 |
| Not stated | 17 |
| Smoked in the past 3 months (n) | |
| No | 60 |
| Yes | 17 |
| Smoker 0–9 cig/day | 16 |
| Smoker 10–19 cig/day | 1 |
| Not stated | 22 |
| Gravidity (n) | |
| 1 | 40 |
| 2 | 20 |
| 3 | 23 |
| ≥3 | 13 |
| Not stated | 3 |
| Parity | |
| 0 | 48 |
| 1 | 26 |
| ≥2 | 21 |
| Not stated | 4 |
| Median gestational age at urine collection (weeks) d | 30 (21–38.4) |
| Median urine creatinine (g/L) | 0.68 (0.14–2.49) |
One subject withdrew after providing urine sample and no questionnaire information was collected
range shown in parentheses
n=72
n=97
Urinary metabolite levels are summarized for all analytes, unadjusted and adjusted for creatinine, in Table 2 and Table 3 respectively. DAP metabolites were detectable in all urine specimens, with the lowest detection frequency being 81% for DEDTP. DM metabolites accounted for nearly 90% of total DAP metabolites. DMP and DMTP were the OP metabolites present at the highest levels. DMDTP was the DM metabolite with the lowest concentration at around one-thirtieth of DMP and DMTP. DEP, the major DE metabolite, comprised 73% of the total DE metabolites, with a median of 19.2 nmol/g Cr (13.0 nmol/L). Finally, the metabolite of chlorpyrifos, TCPy, had median concentration of 1.6 µg/g Cr (1.2 µg/L).
Table 3.
Creatinine-adjusted OP pesticides, bisphenol A, phthalates or their metabolites in the urine of the 100 pregnant women from the Generation R study
| Metabolite | GM | GSD | Min | Percentile |
Max | ||||
|---|---|---|---|---|---|---|---|---|---|
| 5th | 25th | 50th | 75th | 95th | |||||
| OP metabolites (nmol/g Cr) | |||||||||
| DMP | 123.0 | 2.2 | 0.9 | 42.3 | 86.9 | 140.0 | 192.0 | 359.0 | 627.0 |
| DMTP | 94.4 | 2.6 | 5 | 18.5 | 53.5 | 109.0 | 184.0 | 367.0 | 634.0 |
| DMDTP | 3.6 | 2.5 | 0.4 | 0.7 | 1.8 | 3.7 | 6.7 | 16.6 | 20.2 |
| Total DM | 240.0 | 1.9 | 51.3 | 74.3 | 163.1 | 264.3 | 379.2 | 655.9 | 1265.0 |
| DEP | 20.0 | 2.6 | 0.7 | 4.9 | 10.2 | 19.2 | 35.6 | 120.5 | 265.0 |
| DETP | 7.2 | 3.4 | 0.7 | 1.3 | 3.0 | 6.2 | 15.1 | 68.5 | 287.0 |
| DEDTP | 0.3 | 3.1 | 0.04 | 0.05 | 0.1 | 0.3 | 0.5 | 2.3 | 23.5 |
| Total DE | 30.5 | 2.5 | 5.8 | 8.2 | 16.6 | 26.9 | 51.4 | 166.0 | 457.0 |
| Total DAP | 282.0 | 1.9 | 74.5 | 83.7 | 183.0 | 316.0 | 432.0 | 810.0 | 1552.0 |
| TCPy (nmol/g Cr) | 9.3 | 2.5 | 1.4 | 2.7 | 5.4 | 8.1 | 12.9 | 49.7 | 814.0 |
| TCPy (µg/g Cr) | 1.9 | 2.5 | 0.3 | 0.5 | 1.1 | 1.6 | 2.6 | 12.20 | 162.0 |
| BPA (µg/g Cr) | 1.7 | 2.8 | 0.1 | 0.3 | 0.8 | 1.6 | 3.9 | 8.32 | 22.7 |
| Phthalate metabolites (µg/g Cr) | |||||||||
| MMP | - | - | <LOD | <LOD | <LOD | <LOD | 3.9 | 30.3 | 197.0 |
| MEP | 173.0 | 4.7 | <LOD | 13.6 | 71.9 | 222.0 | 487.0 | 1810.0 | 7160.0 |
| MOP | - | - | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD | 32.1 |
| MCPP | 1.3 | 2.3 | <LOD | 0.2 | 0.9 | 1.4 | 2.5 | 4.6 | 8.9 |
| MnBP | 67.1 | 2.2 | 15.3 | 21.7 | 41.6 | 62.2 | 106.0 | 228.0 | 351.0 |
| MiBP | 64.1 | 2.1 | 14.4 | 20.9 | 35.1 | 57.1 | 99.4 | 327.0 | 640.0 |
| MBzP | 13.9 | 2.0 | 3.0 | 3.6 | 7.6 | 11.7 | 22.6 | 84.7 | 405.0 |
| MEHP | 10.8 | 2.1 | <LOD | 1.2 | 6.9 | 9.9 | 20.9 | 88.7 | 321.0 |
| MEHHP | 22.2 | 3.4 | 3.7 | 7.5 | 13.2 | 20.3 | 32.3 | 99.5 | 371.0 |
| MEOHP | 23.3 | 2.0 | 5.3 | 8.4 | 15.6 | 20.9 | 30.7 | 126.0 | 386.0 |
| MECPP | 30.1 | 2.3 | 8.1 | 10.5 | 18.9 | 25.8 | 41.4 | 155.0 | 317.0 |
| MCMHP | 9.7 | 2.6 | 1.6 | 3.3 | 5.5 | 9.1 | 16.1 | 36.1 | 73.6 |
| 7oxo-MMeOP | 3.9 | 3.2 | <LOD | 0.8 | 1.9 | 3.4 | 5.9 | 43.9 | 119.0 |
| 7oh-MMeOP | 4.6 | 2.9 | <LOD | 1.1 | 2.3 | 4.2 | 7.0 | 53.0 | 95.7 |
Note: * See Table 1 for abbreviations.
BPA was detectable among 82% of samples. The creatinine-adjusted median concentration was 1.6 µg/g Cr, and the unadjusted median concentration was 1.2 µg/L.
Most phthalate metabolites were detectable in nearly all women (Table 2). Mono-ethyl phthalate (MEP) was the phthalate metabolite with the highest adjusted median level—222 µg/g Cr and an unadjusted median of 117 µg/L. Twelve women had MEP levels greater than 1000 µg/g Cr. The next biggest contributors were the di-n-butyl phthalate (DBP) metabolite MnBP and the di-iso-butyl phthalate (DIBP) metabolite MiBP. The concentrations of three oxidative metabolites (mono-2-ethyl-5-hydroxyhexyl phthalate [MEHHP], mono-2-ethyl-5-oxohexyl phthalate [MEOHP], and mono-2-ethyl-5-carboxypentyl [MECPP]) of di-2-ethylhexyl phthalate (DEHP) were higher than that of another oxidative metabolite, mono-2-carboxymethylhexyl phthalate (MCMHP) and the monoester MEHP. The median ratios were 1.9 (range=0.16–66.5) for MEHHP/MEHP, 2.0 (range=0.14–93.0) for MEOHP/MEHP and 1.0 (range=0.5–1.8) for MEHHP/MEOHP. The creatinine-adjusted median concentrations of total DEHP metabolites (sum of MEHP, MEHHP, MEOHP, MECPP, and MCMHP) was 88.4 µg/g Cr and the unadjusted median concentration was 62.3 µg/L. The unadjusted median concentrations of butylbenzyl phthalate (MBzP) and two di-iso-nonylphthalate metabolites (7oxo-MMeOP and 7oh-MMeOP) were less than 10 µg/g Cr. MMP (dimethyl phthalate metabolite) and MOP (di-n-octyl phthalate metabolite), however, were detectable among only 36% and 2% of samples, respectively. Mono-3-carboxypropyl phthalate (MCPP), another metabolite of di-n-octyl phthalate metabolite, was detectable among 94% of samples but the median was near 1 µg/L (1.4 µg/g Cr).
Some differences in metabolite concentrations were found among women with different characteristics (Table 4). Women aged ≤30 years had a 30% higher concentration of MnBP than women aged >30 years. Women who had higher education had the highest concentrations of total DM and total DAPs, but the lowest concentrations of the phthalate metabolites MnBP and MBzP. Compared with Dutch women, women of other ethnicity had higher levels of TCPy and several phthalate metabolites (MnBP, MiBP, MBzP, and total DEHP). Higher concentrations of MnBP, MiBP, and MBzP were also found among women whose household income was higher than 2200 Euros/month, compared with women with lower income. The levels of DM and DAP in the urine samples collected before 26 weeks of gestation were higher than those in the urine samples collected after 26 weeks.
Table 4.
Comparisons of creatinine-adjusted medians by population characteristics*
| Metabolites | Age (Year) |
Education |
Ethnicity |
Household Income (Euro/month) |
Gestational Age (Week) |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| <=30 | >30 | Primary | Secondary | Higher | Dutch | Others | <= 2200 | >2200 | <=26 | >26 | |
| N† | 50 | 49 | 12 | 36 | 26 | 41 | 37 | 29 | 25 | 17 | 83 |
| Total DM (nmol/g Cr) | 258.0 | 273.0 | 180.0 | 271.0 | 310.0 | 299.0 | 232.0 | 256.0 | 312.0 | 373.0 | 251.0 |
| 0.650 | 0.03 | 0.26 | 0.37 | 0.04 | |||||||
| Total DE (nmol/g Cr) | 26.7 | 31.5 | 19.5 | 27.4 | 38.1 | 24.7 | 34.2 | 19.4 | 35.0 | 33.2 | 24.5 |
| 0.73 | 0.14 | 0.46 | 0.045 | 0.37 | |||||||
| Total DAP (nmol/g Cr) | 322.0 | 315.0 | 204.0 | 297.0 | 354.0 | 336.0 | 265.0 | 280.0 | 359.0 | 415.0 | 285.0 |
| 0.82 | 0.03 | 0.45 | 0.18 | 0.05 | |||||||
| TCPy (µg/g cr) | 1.3 | 1.6 | 1.7 | 1.3 | 1.9 | 1.3 | 2.4 | 1.6 | 1.3 | 1.9 | 1.6 |
| 0.37 | 0.16 | 0.002 | 0.19 | 0.49 | |||||||
| BPA (µg/g cr) | 1.5 | 1.9 | 1 | 1.7 | 2.1 | 1.6 | 2 | 1.5 | 2.4 | 1.8 | 1.6 |
| 0.95 | 0.32 | 0.62 | 0.25 | 0.85 | |||||||
| MEP (µg/g cr) | 193.0 | 241.0 | 255.0 | 248.0 | 230.0 | 301.0 | 241.0 | 152.0 | 301.0 | 109.0 | 246.0 |
| 0.84 | 0.52 | 0.20 | 0.19 | 0.28 | |||||||
| MnBP (µg/g cr) | 75.6 | 56.7 | 90.5 | 62.2 | 52.5 | 46.7 | 75.6 | 72.4 | 41.7 | 75.6 | 59.7 |
| 0.04 | 0.04 | 0.006 | 0.02 | 0.72 | |||||||
| MiBP (µg/g cr) | 63.4 | 47.8 | 68.9 | 58.4 | 42.9 | 44.8 | 68.9 | 62.1 | 39.7 | 50.4 | 59.4 |
| 0.13 | 0.19 | 0.03 | 0.05 | 0.75 | |||||||
| MBzP (µg/g cr) | 14.0 | 10.5 | 14.7 | 13.6 | 8.5 | 7.8 | 13.6 | 12.2 | 8.6 | 11.5 | 11.9 |
| 0.07 | 0.005 | 0.001 | 0.002 | 0.89 | |||||||
| DEHP (µg/g cr) | 89 | 88.3 | 84 | 84.8 | 72.7 | 73.7 | 94.2 | 94.219 | 73.4 | 92.7 | 88.3 |
| 0.61 | 0.75 | 0.009 | 0.09 | 0.21 | |||||||
| Total phthalate (µg/g cr) | 573 | 537 | 678 | 561 | 413 | 601 | 527 | 482 | 601 | 555 | 562 |
| 0.31 | 0.14 | 0.98 | 0.57 | 0.87 | |||||||
Nonparametic tests (Kolmogorov-Smirnov test or Kruskal-Wallis test)
N is sample size and ≤100 due to missing value.
4. Discussion
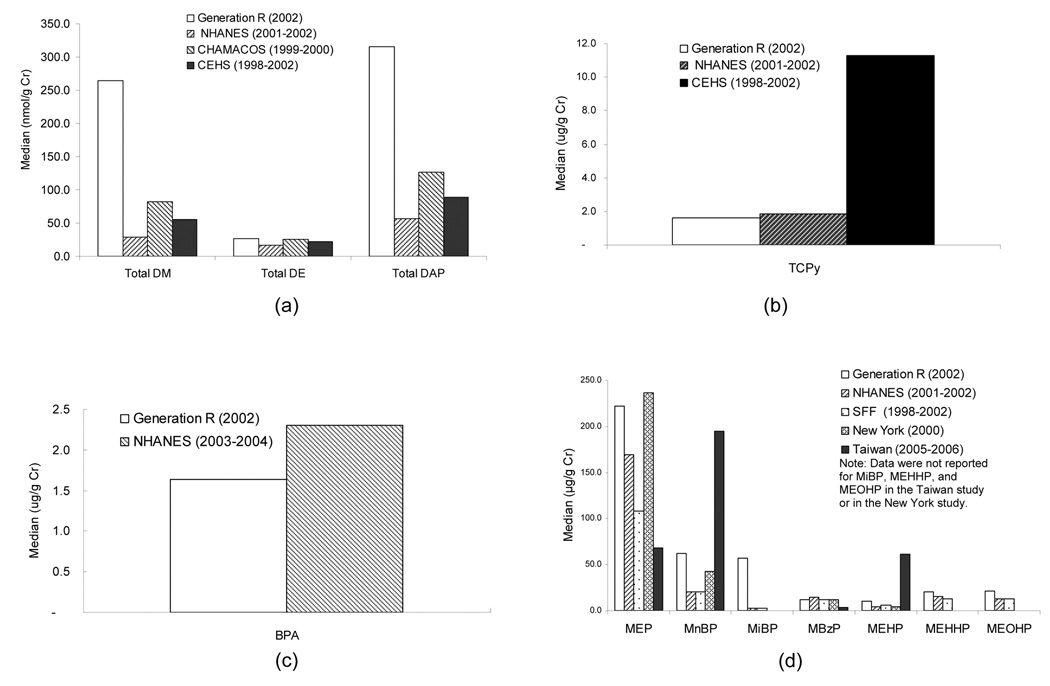
The creatinine-adjusted median concentrations (nmol/g Cr) of total DM and total DAP among 100 pregnant women in the Generation R study (Figure 1a) were higher than that of NHANES 2001–2002 pregnant women aged 15–44 years (Centers for Disease Control and Prevention, 2008), higher than that of pregnant women residing in an agricultural community in the Salinas Valley (The Center for the Health Assessment of Mothers and Children of Salinas, CHAMACOS), California (Bradman et al., 2005), and higher than that of pregnant women who were studied at Mount Sinai Hospital (The Children’s Environmental Health Study, CEHS) in New York City (Wolff et al., 2007). Urinary DE levels were similar to those reported in other populations. As observed in other populations, DE levels were lower than that of DM. This may be due to less exposure to diethyl organophosphorus pesticides or because their metabolites are less stable and more difficult to detect. The creatinine-adjusted median level of TCPy in the Generation R study, however, was similar to that of NHANES 2001–2002 pregnant women and was much lower (7 fold) than that of women from the CEHS study (Figure 1b) (Berkowitz et al., 2003). The unadjusted median of TCPy in the Generation R study was also lower than in the CHAMACOS cohort (Eskenazi et al. 2007). This suggests that this urban population studied in the Netherlands was exposed to a different mixture of OP pesticides than subjects in the U.S.
Figure 1. Comparisons of creatinine-adjusted median concentrations of metabolites in urine of pregnant women.
References: NHANES 2001–2002 and NHANES 2003–2004 (Centers for Disease Control and Prevention, 2008); CHAMCOS 1999–2000 (Bradman et al., 2005); CEHS 1998–2002 (Berkowitz et al., 2003; Wolff et al., 2007); SFF 1999–2002 (Marsee et al., 2006); Taiwan (2005–2006) (Huang et al., 2007); New York (2000) (Adibi et al., 2003).
Urinary free BPA accounts for only a small percentage of total BPA, and most human biomonitoring studies, including ours, reported urinary concentrations of total BPA (free BPA plus glucuronide/sulfate conjugated BPA). Urinary median BPA concentration in the Generation R study was slightly lower than that in the pregnant women in NHANES 2003–2004, as shown in Figure 1c. The unadjusted BPA levels were much higher in Korean women (not shown in figure because creatinine-adjusted results not available) (Yang et al., 2006).
As shown in Figure 1d, the creatinine-adjusted median MEP concentration in the present study was similar to the creatinine-adjusted median for 25 New York pregnant women (Adibi et al., 2003) and was higher than in pregnant women from NHANES 2001–2002, the pregnant women in the SFF (Study for Future Families) conducted in several cities in the United States (Marsee et al., 2006), and pregnant women from Taiwan (Huang et al., 2007). The median level of MnBP (DBP metabolite) in the present study was higher than in NHANES 2001–2002, in the SFF study, and in New York study, but lower than in Taiwan. Pregnant women in the present study had a median level of MiBP that was more than 10 times higher, compared with NHANES 2001–2002 and the SFF study. The concentrations of three DEHP metabolites (MEHP, MEHHP and MEOHP) were similar to that of women in NHANES 2001–2002 and the SFF study, but lower than in Taiwan. Ratios of the median levels of two secondary DEHP metabolites (MEHHP and MEOHP) to that of MEHP (primary metabolite) are indicators of metabolic capacity and vary across populations. The ratios of MEHHP/MEHP (1.9) and MEOHP/MEHP (2.0) were somewhat lower in the present study compared to other studies’ in which ratios were found to be greater than 3 (Barret al., 2003; Becker et al., 2004; Fromme et al., 2007; Koch et al., 2003b). This finding indicates a difference in metabolism, but still suggests that MEHHP and MEOHP are more sensitive biomarkers of exposure than is MEHP. The median concentration of MBzP, a metabolite of butylbenzyl phthalate which is used mainly as a plasticizer (PVC for vinyl floor tile, vinyl foams, and carpet backing), was slightly lower than in women in NHANES 2001–2002 and the SFF study (Marsee et al., 2006), but higher than in Taiwan (Huang et al., 2007).
The laboratory we used regularly takes part in an external quality assessment scheme for the determination of phthalates and organophosphates. In particular, the analytical laboratory at the National Center for Environmental Health, U.S. Centers for Disease Control and Prevention participated in this scheme and that laboratory did the measurements in all other studies shown in figures 1a–1d except for the Taiwan study. Thus, the values for phthalate and DAP metabolites are comparable across laboratories (e.g. similar LODs and recoveries) for the present study and for the U.S. studies. However, the interlaboratory variability may partially contribute to the differences in BPA between the Generation R study and NHANES, and the differences in phthalates between western and Taiwanese women.
The differences in OP metabolites may be due to differences in pesticide use among different countries. In 2003, 14.4% of tested foods in the Netherlands had pesticide residues above maximum residue levels (MRLs), the highest percentage of any country in the European monitoring program (European Commission, 2007). The most frequently detected pesticides were dichlorvos, pirimiphos-methyl, malathion, chlorpyriphos-ethyl, and methamidophos, which are primarily metabolized to DM, supporting our finding that urinary DM metabolite levels were relatively high in the present study. Pesticide residues in foods imported from developing countries are more often higher than MRLs and may be another factor relevant to higher DAP levels (European Commission, 2007). In the U.S., violative pesticide residues (a residue which exceeds a tolerance or a residue at a level of regulatory significance for which no tolerance has been established in the sampled food) were found in 2.4% of domestic foods and in 6.1% of imported foods in 2003 (Food and Drug Administration, 2005). The higher concentration of TCPy in the CEHS study and the CHAMACOS study might be because chlorpyrifos and chlorpyrifos-methyl were more commonly used in these areas. Chlorpyrifos-methyl was the one of five most frequently detected chemicals in food items from U.S. markets (Food and Drug Administration, 2005). The chlorpyrifos degradation product TCPy has also been frequently detected in U.S. food and environmental media (Morgan et al., 2005). In the Netherlands, however, none of three TCPy parent pesticides (chlorpyrifos, triclopyr and chlorpyrifos-methyl) has been frequently detected in food items (European Commission, 2007).
Phthalates, including di-n-butyl phthalates (DBP), diethyl phthalate (DEP) and di-2-ethylhexyl phthalate (DEHP) are used as plasticizers and ingredients in cosmetic products (bath preparations, eye shadows, perfumes and other fragrance preparations, hair sprays, nail polish, and skin care preparations), building materials, household furnishings, clothing, food packaging, cleaning materials, and insecticides (Schettler, 2006). A survey in 2002 (DiGangi and Norin, 2002) showed that more than half of cosmetic products in the European market contained more than one type of phthalate and about 40% of the products contained either DEHP or DBP, which were subsequently banned by the European Union in 2002 for use in personal care and cosmetic products. Di-iso-butyl phthalate (DiBP) has similar properties to DBP and has now replaced DBP in many applications including cosmetics. The relatively high levels of MnBP and MiBP in the Generation R women may be related to use of personal care and household products.
Few studies have examined the correlates of exposure to these contaminants and their metabolites. The higher concentrations of some phthalate metabolites among younger women (MnBP), women whose household income was less than 2200 Euros/month (MnBP, MiBP, and MBzP), women with primary education only (MnBP and MBzP), and non- Dutch women (MnBP, MiBP, MBzP, and total DEHP) may be related to more use of personal care products containing phthalates. The higher total DEHP concentrations in urine may also be related to DEHP exposure from diet and exposure to dust and air in the indoor environment. The lower level of the total DAP among women who provided a urine sample after 26 weeks of gestation was consistent with the increasing urine volume during pregnancy (Maikranz et al., 1989). The higher levels of total DAP among women who had higher education and of TCPy among non-Dutch women may be related to diet or use of pesticides in the home.
The metabolites analyzed in this study were selected in part so as to be comparable to previous studies; however, caution should be used when interpreting these levels as biomarkers of exposure. DAP metabolites are regarded as good indicators of OP exposure although several limitations exist. Measures of DAP metabolites may also reflect exposure to OP breakdown products in the environment and in the food supply and the contribution of some industrial chemicals and drugs (Barr and Needham, 2002). DAP metabolites have been used for biomonitoring in workers and other exposed populations but the interpretation of DAP metabolite levels in the general population is less clear. DAP metabolites are not toxic, so that the component that consists of "pass through" metabolites via food is, as far as we know at this time, of no toxicological significance. Because phthalates can be contaminants in the laboratory, measurement of parent phthalates is useful only for highly exposed populations (Barr et al., 2003). Phthalate metabolite concentrations in urine samples are the most frequently used biomarkers for evaluating phthalate exposure in the general population. The selection of metabolites for measurement needs careful attention because some intermediate metabolites can be further metabolized. For example, the simple monoester mono-iso-nonyl phthalate (MINP) has been used for human exposure assessment of di-iso-nonyl phthalate (DINP) (Silva et al., 2004) and was recently shown to be an unreliable biomarker because it is further metabolized to oxidative metabolites before excretion in urine (Koch et al., 2007; Silva et al., 2006). Investigations by two independent groups have shown that oxidative metabolites mono-4-methyl-7-hydroxy-octyl phthalate (7oh-MMeOP), mono-4-methyl-7-oxo-octyl phthalate (7oxo-MMeOP) and mono-4-methyl-7-carboxy-heptyl phthalate (7carboxy-MMeHP) are better biomarkers of DINP exposure (Koch et al., 2007; Silva et al., 2006). As observed before (Barr et al., 2003), concentrations of secondarily oxidized metabolites (MEHHP and MEOHP) were higher than that of MEHP in the present study.
The choice of matrix for biomonitoring depends on pharmacokinetics, access to samples, and analytical capability. For the agents considered in the present study, urine is the most frequently used matrix for human studies because of the ready availability of large amounts of sample and relatively higher concentrations than in blood (Barr and Needham, 2002). Collection of spot urine is easier and more frequently done than 24-hr or first morning urine samples. Spot urine samples were collected in all previous studies on these compounds with the exception of two studies of phthalates in Germany that used first morning void urine samples (Fromme et al., 2007; Wittassek et al., 2007).
One concern about measurements in spot urine is within-individual variability over time. Significant inter-day variation of urinary phthalate metabolite levels during 8 consecutive days has been reported (intraclass correlation coefficient ranged from 0.2 to 0.5) (Fromme et al., 2007). Bradman et al. (Bradman et al., 2005) also found temporal variation of OP metabolites between the first (about 13 weeks) and the second (about 26 weeks) prenatal urine samples, and between prenatal and postpartum samples. Due to temporal variability and short half-lives (<48 hours) (Barr and Angerer, 2006; Koch et al., 2006; Tsai, 2006), a single spot urine sample usually provides only an imprecise estimate of long-term exposure to the target compounds. Thus, measuring metabolites in multiple samples across the entire pregnancy may allow improved exposure assessment, and help to identify critical windows of exposure.
Although we compared analytes among pregnant women in different studies (figure), the difference in time of gestation when urines were collected in these studies need consideration. Creatinine excretion is affected by many factors including pregnancy (Boeniger et al., 1993). Urinary creatinine level changes with the time of gestation and is about 20% lower in the 3rd trimester than in the 1st trimester of gestation (Cherry, 1991). Urine volume increases by about 25% in pregnancy and is the greatest in the 3rd trimester (Maikranz et al., 1989). The magnitude of effect on metabolite concentrations of these and other changes in clearance is unclear. In the present study, the median levels of total DM and total DAP in women who provided a urine sample after 26 weeks of gestation were statistically lower than that in women who provided a sample earlier. In CHAMACOS study, however, the median total DAP concentrations in the first urines (about 13 weeks of gestation) and in the second urines (about 26 weeks of gestation) were similar (Bradman et al., 2005). The influence of timing may contribute to, but can not fully explain the observed large differences in concentrations of DAP metabolites, TCPy, and several phthalate metabolites (MEP, MnBP, MiBP, and MEHP) across studies. Although urine osmolality or specific gravity may be a better approach to adjust for differences in urine dilution, we chose to focus and report creatinine and non-creatinine adjusted measurements to faciliate comparison with other studies.
The subjects in the present study for whom data were complete had characteristics that were not statistically significantly different from those in the Generation R women overall (Jaddoe et al., 2006). However, the proportion with missing data in our study was higher than in the Generation R study. This reflects that the analyzed urines were selected from women who had only a third trimester specimen available. Consequently, the present subset contains a relatively large proportion of women who entered the study relatively late during pregnan0063y, for whom the responses to the questionnaires in early and mid pregnancy were not available. Thus, some differences that we could not detect may have been present. Therefore, some caution in extrapolation to all Generation R subjects is in order.
Relatively high levels of OP pesticide metabolites and some phthalate metabolites were found in the Generation R subjects and the reasons merit further study. Nonetheless, because detailed birth outcomes and follow-up information were recorded, the Generation R study provides an opportunity to efficiently address questions regarding reproductive and developmental effects of prenatal exposure to some OP pesticides, BPA, and phthalates.
Acknowledgement
The Generation R study is conducted by the Erasmus MC, University Medical Center, Rotterdam, the Netherlands in close collaboration with the School of Law and Faculty of Social Sciences of the Erasmus University Rotterdam, the Municipal Health Service Rotterdam area, the Rotterdam Homecare Foundation and the Stichting Trombosedienst & Artsenlaboratorium Rijnmond (STAR), Rotterdam. We gratefully acknowledge the contribution of general practitioners, hospitals, midwives and pharmacies in Rotterdam. The first phase of the Generation R study is made possible by financial support from the Erasmus MC, Rotterdam, the Erasmus University Rotterdam and the Netherlands Organization for Health Research and Development (ZonMw).
Funding source: This study was supported by the Intramural Research Program, NIEHS and the Harvard NIEHS Environmental Health Center Pilot Project Grant P30ES000002.
This study was approved by ethical committees in National Institutes of Health (NIH) and Erasmus MC. 4 106
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adibi JJ, et al. Prenatal exposures to phthalates among women in New York City and Krakow, Poland. Environ Health Perspect. 2003;111:1719–1722. doi: 10.1289/ehp.6235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr DB, Needham LL. Analytical methods for biological monitoring of exposure to pesticides: a review. J Chromatogr B Analyt Technol Biomed Life Sci. 2002;778:5–29. doi: 10.1016/s1570-0232(02)00035-1. [DOI] [PubMed] [Google Scholar]
- Barr DB, et al. Assessing human exposure to phthalates using monoesters and their oxidized metabolites as biomarkers. Environ Health Perspect. 2003;111:1148–1151. doi: 10.1289/ehp.6074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr DB, et al. Concentrations of dialkyl phosphate metabolites of organophosphorus pesticides in the U.S. population. Environ Health Perspect. 2004;112:186–200. doi: 10.1289/ehp.6503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr DB, Angerer J. Potential uses of biomonitoring data: a case study using the organophosphorus pesticides chlorpyrifos and malathion. Environ Health Perspect. 2006;114:1763–1769. doi: 10.1289/ehp.9062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker K, et al. DEHP metabolites in urine of children and DEHP in house dust. Int J Hyg Environ Health. 2004;207:409–417. doi: 10.1078/1438-4639-00309. [DOI] [PubMed] [Google Scholar]
- Berkowitz GS, et al. Exposure to indoor pesticides during pregnancy in a multiethnic, urban cohort. Environ Health Perspect. 2003;111:79–84. doi: 10.1289/ehp.5619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeniger MF, et al. Interpretation of urine results used to assess chemical exposure with emphasis on creatinine adjustments: a review. Am Ind Hyg Assoc J. 1993;54:615–627. doi: 10.1080/15298669391355134. [DOI] [PubMed] [Google Scholar]
- Bradman A, et al. Organophosphate urinary metabolite levels during pregnancy and after delivery in women living in an agricultural community. Environ Health Perspect. 2005;113:1802–1807. doi: 10.1289/ehp.7894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo R, et al. Measurement of dialkyl phosphate metabolites of organophosphorus pesticides in human urine using lyophilization with gas chromatography-tandem mass spectrometry and isotope dilution quantification. J Expo Anal Environ Epidemiol. 2004;14:249–259. doi: 10.1038/sj.jea.7500322. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. [Accessed in 2008];Hyattsville, MD: U.S. Department of Health and Human Services; National Health and Nutrition Examination Survey Data. Centers for Disease Control and Prevention,[ http://www.cdc.gov/nchs/about/major/nhanes/datalink.htm]
- Cherry S. Laboratory assessment and values important in pregnancy. In: Cherry S, Merkatz I, editors. Complications of pregnancy : medical, surgical, gynecologic, psychosocial, and perinatal. Baltimore: Williams & Wilkins; 1991. pp. 1249–1251. [Google Scholar]
- DiGangi J, Norin H. Pretty Nasty- Phthalates in European Cosmetic Products. Health Care Without Harm; Sweden: 2002. [Google Scholar]
- Duggan A, et al. Di-alkyl phosphate biomonitoring data: assessing cumulative exposure to organophosphate pesticides. Regul Toxicol Pharmacol. 2003;37:382–395. doi: 10.1016/s0273-2300(03)00031-x. [DOI] [PubMed] [Google Scholar]
- Engel SM, et al. Prenatal Organophosphate Metabolite and Organochlorine Levels and Performance on the Brazelton Neonatal Behavioral Assessment Scale in a Multiethnic Pregnancy Cohort. Am J Epidemiol. 2007;165:1397–1404. doi: 10.1093/aje/kwm029. [DOI] [PubMed] [Google Scholar]
- Eskenazi B, et al. Organophosphate Pesticide Exposure and Neurodevelopment in Young Mexican-American Children. Environ Health Perspect. 2007;115:792–798. doi: 10.1289/ehp.9828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Commission. Annual EU-wide Pesticide Residues Monitoring Report-2005. Brussels, Belgium: 2007. [Google Scholar]
- Food and Drug Administration. Pesticide residue monitoring program-2003. Atlanta, GA: 2005. [Google Scholar]
- Fromme H, et al. Occurrence and daily variation of phthalate metabolites in the urine of an adult population. Int J Hyg Environ Health. 2007;210:21–33. doi: 10.1016/j.ijheh.2006.09.005. [DOI] [PubMed] [Google Scholar]
- Hauser R, Calafat AM Phthalates and human health. Occupational and Environmental Medicine. Vol. 62. 2005. pp. 806–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornung RW, Reed LD. Estimation of average concentration in the presence of non-detectable values. Appl Occup Environ Hyg. 1990;5:46–51. [Google Scholar]
- Huang PC, et al. Associations between urinary phthalate monoesters and thyroid hormones in pregnant women. Hum Reprod. 2007;22:2715–2722. doi: 10.1093/humrep/dem205. [DOI] [PubMed] [Google Scholar]
- Jaddoe VW, et al. The Generation R Study: Design and cohort profile. Eur J Epidemiol. 2006;21:475–484. doi: 10.1007/s10654-006-9022-0. [DOI] [PubMed] [Google Scholar]
- Jaddoe VW, et al. The Generation R Study biobank: a resource for epidemiological studies in children and their parents. Eur J Epidemiol. 2007;22:917–923. doi: 10.1007/s10654-007-9209-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang JH, et al. Human exposure to bisphenol A. Toxicology. 2006;226:79–89. doi: 10.1016/j.tox.2006.06.009. [DOI] [PubMed] [Google Scholar]
- Kavlock R, et al. NTP Center for the Evaluation of Risks to Human Reproduction: phthalates expert panel report on the reproductive and developmental toxicity of di-n-octyl phthalate. Reprod Toxicol. 2002;16:721–734. doi: 10.1016/s0890-6238(02)00031-x. [DOI] [PubMed] [Google Scholar]
- Koch HM, Angerer J. Analysis of 3,5,6-trichloro-2-pyridinol in urine samples from the general population using gas chromatography-mass spectrometry after steam distillation and solid-phase extraction. Chromatogr B Biomed Sci Appl. 2001;759:43–49. doi: 10.1016/s0378-4347(01)00209-2. [DOI] [PubMed] [Google Scholar]
- Koch HM, et al. On-line clean-up by multidimensional liquid chromatography-electrospray ionization tandem mass spectrometry for high throughput quantification of primary and secondary phthalate metabolites in human urine. J Chromatogr B Analyt Technol Biomed Life Sci. 2003a;784:169–182. doi: 10.1016/s1570-0232(02)00785-7. [DOI] [PubMed] [Google Scholar]
- Koch HM, et al. Internal exposure of the general population to DEHP and other phthalates--determination of secondary and primary phthalate monoester metabolites in urine. Environ Res. 2003b;93:177–185. doi: 10.1016/s0013-9351(03)00083-5. [DOI] [PubMed] [Google Scholar]
- Koch HM, et al. Di(2-ethylhexyl)phthalate (DEHP): human metabolism and internal exposure-- an update and latest results. Int J Androl. 2006;29:155–165. doi: 10.1111/j.1365-2605.2005.00607.x. discussion 181-5. [DOI] [PubMed] [Google Scholar]
- Koch HM, et al. Determination of secondary, oxidised di-iso-nonylphthalate (DINP) metabolites in human urine representative for the exposure to commercial DINP plasticizers. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;847:114–125. doi: 10.1016/j.jchromb.2006.09.044. [DOI] [PubMed] [Google Scholar]
- Larsen K. Creatinine assay in the presence of protein with LKB 8600 Reaction Rate Analyser. Clin Chim Acta. 1972;38:475–476. doi: 10.1016/0009-8981(72)90146-5. [DOI] [PubMed] [Google Scholar]
- Latini G. Monitoring phthalate exposure in humans. Clin Chim Acta. 2005;361:20–29. doi: 10.1016/j.cccn.2005.05.003. [DOI] [PubMed] [Google Scholar]
- Lu C, et al. Organic diets significantly lower children's dietary exposure to organophosphorus pesticides. Environ Health Perspect. 2006;114:260–263. doi: 10.1289/ehp.8418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maikranz P, et al. Gestational hypercalciuria causes pathological urine calcium oxalate supersaturations. Kidney Int. 1989;36:108–113. doi: 10.1038/ki.1989.168. [DOI] [PubMed] [Google Scholar]
- Marsee K, et al. Estimated daily phthalate exposures in a population of mothers of male infants exhibiting reduced anogenital distance. Environ Health Perspect. 2006;114:805–809. doi: 10.1289/ehp.8663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan MK, et al. Exposures of preschool children to chlorpyrifos and its degradation product 3,5,6-trichloro-2-pyridinol in their everyday environments. J Expo Anal Environ Epidemiol. 2005;15:297–309. doi: 10.1038/sj.jea.7500406. [DOI] [PubMed] [Google Scholar]
- National Research Council. Pesticides in the diets of infants and children. Wasthing, D.C.: National Academy Press; 1993. [PubMed] [Google Scholar]
- National Toxicology Program. National Toxicology Program, U.S. Department of Health and Human Services; Research Triangle Park, NC: NTP-CERHR expert panel report on the reproductive and developmental toxicity of bisphenol A. 2007
- Peck CC, Albro PW. Toxic potential of the plasticizer Di(2-ethylhexyl) phthalate in the context of its disposition and metabolism in primates and man. Environ Health Perspect. 1982;45:11–17. doi: 10.1289/ehp.824511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preuss R, et al. Biological monitoring of the five major metabolites of di-(2-ethylhexyl)phthalate (DEHP) in human urine using column-switching liquid chromatography-tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2005;816:269–280. doi: 10.1016/j.jchromb.2004.11.048. [DOI] [PubMed] [Google Scholar]
- Schettler T. Human exposure to phthalates via consumer products. Int J Androl. 2006;29:134–139. doi: 10.1111/j.1365-2605.2005.00567.x. discussion 181-5. [DOI] [PubMed] [Google Scholar]
- Silva MJ, et al. Urinary levels of seven phthalate metabolites in the U.S. population from the National Health and Nutrition Examination Survey (NHANES) 1999–2000. Environ Health Perspect. 2004;112:331–338. doi: 10.1289/ehp.6723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva MJ, et al. Oxidative metabolites of diisononyl phthalate as biomarkers for human exposure assessment. Environ Health Perspect. 2006;114:1158–1161. doi: 10.1289/ehp.8865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swan SH, et al. Decrease in anogenital distance among male infants with prenatal phthalate exposure. Environ Health Perspect. 2005;113:1056–1061. doi: 10.1289/ehp.8100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai WT. Human health risk on environmental exposure to Bisphenol-A: a review. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2006;24:225–255. doi: 10.1080/10590500600936482. [DOI] [PubMed] [Google Scholar]
- vom Saal FS, et al. Chapel Hill bisphenol A expert panel consensus statement: integration of mechanisms, effects in animals and potential to impact human health at current levels of exposure. Reprod Toxicol. 2007;24:131–138. doi: 10.1016/j.reprotox.2007.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessels D, et al. Use of biomarkers to indicate exposure of children to organophosphate pesticides: implications for a longitudinal study of children's environmental health. Environ Health Perspect. 2003;111:1939–1946. doi: 10.1289/ehp.6179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittassek M, et al. Internal phthalate exposure over the last two decades - A retrospective human biomonitoring study. Int J Hyg Environ Health. 2007;210:319–333. doi: 10.1016/j.ijheh.2007.01.037. [DOI] [PubMed] [Google Scholar]
- Wolff MS, et al. Prenatal pesticide and PCB exposures and birth outcomes. Pediatr Res. 2007;61:243–250. doi: 10.1203/pdr.0b013e31802d77f0. [DOI] [PubMed] [Google Scholar]
- Yang M, et al. Urinary concentrations of bisphenol A in relation to biomarkers of sensitivity and effect and endocrine-related health effects. Environ Mol Mutagen. 2006;47:571–578. doi: 10.1002/em.20230. [DOI] [PubMed] [Google Scholar]