Summary
Indoleamine 2,3-dioxygenase (IDO) has been well defined as one of the important immunosuppressive properties for TH1 cell mediated immune responses, but its function in TH2 dominant system is poorly understood. Recently, an appreciable number of publications suggest that the role of IDO in TH2 cell regulation may be different from that of TH1 immune responses. Here we review the evidence on the regulatory function of IDO and tryptophan metabolites in TH1/TH2 differentiation. We propose that IDO-kynurenine pathway can serve as a negative feed-back loop for TH1 cells but it may play a distinct role in up-regulating TH2 dominant immune responses.
Keywords: T cells, immune regulation, dendritic cells, tryptophan, and immune diseases
Introduction
Indoleamine 2,3-dioxygenase (IDO) is a rate-limiting enzyme for tryptophan metabolism [1]. IDO protein is widely expressed in most of tumor cells, dendritic cells, macrophages, microglia, esinophils, fibroblasts and endotherial cells [2–6]. In immune cells, IDO expression is regulated and mainly induced by cytokines (e.g IFN-γ, IFN-α, IFN-β and IL-10), by signaling through TLRs (e.g. LPS, CpG/ODN and other bacterial antigens) and by CTLA4-B7 interaction [7–9]. Since the revolutionary findings by Munn and Mellor that IDO expression in the placenta plays an important role in preventing rejection of the fetus during pregnancy in mice, an increasing body of experimental data in the last decade has shown that IDO is an important immunosuppressive property for tumor immune resistance, pathogen-induced immune surveillance, induction of immune suppression to antigens and prevention of autoimmune responses [8,10,11]. The immunosuppressive roles of the IDO-kynurenine pathway have been well reviewed by others previously [12–15]. Two main models have been suggested to interpret mechanisms underlying the immune inhibitory function of the IDO-kynurenine pathway: one is IDO-mediated tryptophan depletion in the microenvironment that results in starvation and stress of immune cells, and subsequently reduces cell function; the other is IDO-mediated accumulation of cytotoxic catabolites from down-stream of kynurenine-metabolism pathway [1,13,16,17]. Notably, the immunosuppressive effects of IDO are mostly described in models with TH1 dominant responses. In contrast, the roles of IDO in TH2 dominant system are poorly understood. Recently, a sizable number of publications suggest that the role of IDO in TH2 cell regulation may be different from that in TH1 immune responses. In this paper, we focus on the recent data related to the function of IDO and tryptophan metabolites in TH2 cell-meditated immune responses and propose that IDO may play a role in up-regulating TH2 dominant immune responses.
Evidence for the different roles of IDO in the regulation of type 1 and type 2 T helper cells
IDO has been well confirmed as a critical suppressive property in induction of immune tolerance and in inhibition of TH1 cell mediated diseases including EAE, EAU and colitis [18–20]. Upregulation of IDO activity in DCs by IDO inducing signals or lack of negative regulator, e.g. DAP12, reduces Th1 cell responses [21,22]. In contrast, inhibition of IDO function by IDO inhibitors, e.g. 1-methyl-tryptophan (1-MT), will enhance the severity of TH1 cell-mediated diseases [23]. In non-obese diabetic (NOD) mice, impaired tryptophan catabolism in DCs was correlated with enhanced autoimmune responses [24] and expression of IDO in DCs down-regulated type 1 diabetes, in which both CD4+ and CD8+ T cells as well as B cells are involved [25,26]. It has also been documented that induction of IDO by IFN-γ or treatment with tryptophan metabolites inhibit IL-17 production from γδT cells and reduce TH17 responses in mouse chronic granulomatous disease [27]. However data on the function of IDO and tryptophan metabolism in TH2 cell mediated immune responses are controversial. The first evidence for the differential regulation of TH1/TH2 responses by IDO and kynurenine pathway-derived metabolites is from the observation that 3-hydroxyanthranilic and quinolinic acids induce apoptosis of TH1 cells but not TH2 cells in vitro [28]. Such treatment causes the rapid death of effector T cells, which subsequently lead to selective survival of TH2 cells. The impact of IDO on the TH1/ TH 2 balance was also observed by Clark et al. in humans and stress-treated or pre-immunized mice with pregnancy failure [29]. They found that the reduced expression of IDO in uteri correlated with an increased TH1/TH2 ratio although it was not necessary associated with pregnancy failure. Interestingly, the increase of the TH1/TH2 ratio in the stressed mice is due to a significant increase in the percentage of TH1 cells; when mice were subjected to both stress and immunization, IDO levels dropped further and led to a striking drop in the levels of TH2 cytokine-positive cells, which resulted in a further increase in the ratio of TH1/TH2. These data imply that high levels of IDO may be needed for limiting TH1 cell proliferation but a relatively low concentration of IDO may be required for the maintenance of the TH2 population. In a study on the role of human eosinophils in the maintenance of asthmatic TH2 cell responses, Odemuyiwa et al. showed that eosinophils constitutively expressed functional IDO. The IDO expression by eosinophils was further enhanced by ligation of CD28 or treatment with IFN-γ. When kynurenine-producing eosinophils were co-cultured with either a TH1 cell line or TH2 clone, they selectively inhibited anti-CD3 induced proliferation of TH1 but not TH2 cells, suggesting that IDO-expressing eosinophils may create a tryptophan-depleted microenvironment in vivo and produce cytotoxic metabolites that inhibit the function of bystander TH1 cells and maintain the imbalance between TH1 and TH2 populations [4]. A similar phenomenon was also observed in mouse spleen cell culture, that IDO expression or addition of tryptophan metabolites in culture down-regulates TH1 but up-regulates TH2 cytokine production [30]. The potential roles of IDO in promoting TH2 type cellular immune responses is also supported by Platten et al’s data from EAE model, in which MBP Ac1-11[4Y] (a peptide promoting TH2 response and displaying therapeutic effects in EAE mice)-activated T cells express 70 fold higher IDO mRNA in comparison with MBP Ac1-11(an encephalitogenic peptide)-activated T cells. When they used natural tryptophan metabolites and a synthetic derivative N-(3, 4-dimeththoxycinnnamoyl) anthranilic acid (3,4-DAA) to treat EAE mice, the treatment reduced TH1 cytokines and increased TH2 cytokine production [20]. The data described above strongly suggest that IDO-expressing cells play a different role in the regulation of TH1/TH2 immune responses: suppressing TH1 cells but promoting TH2 immune responses. Indeed, our data from an OVA-induced chronic asthma model also showed that IDO-deficient mice displayed a weaker TH2 response and lower levels of serum antigen-specific IgE in comparison with WT control mice when they were immunized with OVA and challenged by respiratory inhalation of the antigen [31]. The reduced TH2 responses in IDO-deficient mice are possibly related to the lower expression of MHC class II and co-stimulatory molecules on the lung resident dendritic cells, which subsequently lead to a reduced co-stimulation between DCs and T cells (data not shown). Interestingly, Chen et al. showed that in the mouse anterior chamber associated immune deviation (ACAID) model, a TH2 cell-mediated ocular immune disease, the inhibition of IDO by 1-MT treatment reduced IL-4 levels, partially revived IFN-γ production and prevented the development of ACAID [32].
In contrast, Hayashi et al. found that ISS-ODN, a TLR 9 ligand, induced IDO expression in lungs and inhibited both TH2- and TH1-mediated lung inflammation in experimental asthma; administration of 1-MT reversed the inhibition of TH2 cell responses by ISS-ODN treatment [33]. Since ISS-ODN itself is a strong inducer of IFN-γ production and IFN-γ may also directly affect the development of TH2-mediated pulmonary inflammation by impacting TH1/TH2 balance in the IDO-independent pathway [34,35], the contribution of IDO and tryptophan catabolism pathway in this system may thus need to be evaluated carefully. Further, the authors find that ISS-ODN administration enhanced cell death of the TH2 cell line in the lungs and this enhancement was reversed by 1-MT treatment. TH2 cells presumably underwent apoptosis in this study and 1-MT treatment implies the role of IDO in the programmed cell death. This seems at variance with the observation by others that TH1 cells but not TH2 cells are sensitive to tryptophan catabolism pathway-induced apoptosis [28]. Further experiments are therefore necessary to determine the reasons for the different results from TH2 models. Another observation by Gordon et al. showed that spleen CD8+ dendritic cells, which are documented to be IDO+ and tolerogenic, decreased TH2 cytokine responses of asthmatic T cells in vitro and reduced airway hyperresponsiveness in vivo [36]. As the authors pointed out, these CD8+ dendritic cells secrete high levels of IL-10 and TGF-β, which are considered to be major factors leading to immune suppression. Addition of IDO inhibitor 1-MT to the co-culture of CD8+ DCs and asthmatic T cells only somewhat less efficiently reduced dendritic cell-driven tolerance. Indeed, 1-MT has been shown to induce the production of IFN-γ through TLR, which is independent of its inhibitory effect on the enzymatic activity of IDO [7] and may directly lead to a TH1 shift. IDO independent effects of 1-MT must therefore be taken into account when evaluating the contribution of IDO in CD8+ DC driven-tolerance.
Table 1 shows the experimental data supporting the possibility that IDO may exert different effects on TH2 vs. TH1 cells. Although an appreciable amount of evidence show that IDO-expressing cells are in favor of TH2 type immune responses, further investigation is required to elucidate the mechanism underlying the enhancement of TH2 responses and to explain why contradictory results were observed in different in vitro and in vivo TH2 models.
Table 1.
Influence of tryptophan metabolism on T cell responses
| Model | Alternation of tryptophan metabolism | Effects on immune cells
|
Comments | References | |||
|---|---|---|---|---|---|---|---|
| TH1 | TH2 | CD8+ | NK | ||||
| EAE | IDO expression↑ or administration of tryptophan catabolites | ↓ | ↑ | 1-MT treatment exacerbated EAE; absence of DAP12 caused enhanced IDO function and reduced EAE | [20,23,59] [22] | ||
| MLR and islet transplantation | IDO ↑in DCs
IDO ↑in islets |
↓? | ↓? | IDO expression in DCs reduced MLR in vitro and the IDO expression in islets prolong graft survival | [60–62] | ||
| EAU | IDO↑in DCs | ↓ | Anti-4-1 BB induced IDO expression and reduced EAU | [18] | |||
| OVA induced ACAID model | IDO↓ by 1-MT | ↑ | ↓ | IDO expression is associated with ACAID and 1-MT mediated inhibition of IDO prevents ACAID | [32] | ||
| Mouse colitis model (TH1 dominant) | IDO↓ by 1-MT | ↑ | IDO↓ increased inflammation and mortality | [19] | |||
| Tumor and co- culture of T cells with IDO- transfected tumor cells | IDO↑in Tumor | ↓ | IDO expressing tumor cells block CD8+ T cell expansion | [6,63,64] | |||
| T cell activation in vitro | Addition of IDO or tryptophan catabolites | ↓? | ↓ | ↓ | Priming T cells with purified IDO or tryptophan catabolites in vitro | [65] | |
| Nasal tolerance model | IDO↓ by 1-MT | ↑ | Blockade of IDO inhibits nasal tolerance induction | [66] | |||
| GVHD model | IDO−/− | ↑ | ↑ | IDO−/− mice display increased GVHD; histone deacetylase (HDAC) inhibitor reduced GVHD through IDO | [67,68] | ||
| Stress-induced pregnancy failure | IDO↓ | ↑ | ↓ | Reduced IDO in uterus is associated with an increased ratio of TH1/TH2 | [29] | ||
| Asthma model | IDO↓ by 1-MT or intratracheal treatment with 3-HAA after OVA challenge through airways | ↑ | 1-MT treatment caused severer asthma in adoptive transfer model; 3- HAA inhibited polarized TH2 cells | [33,58] | |||
| DC and T cell co-culture | IDO↓ in CD8+ DCs by 1-MT | ↑ | These DCs secret high levels of IL-10 and TGF-β beside expression of IDO | [36] | |||
| Aspergillus- induced allergic airway inflammation | IDO↑in DCs | ↓ | IDO↑ is associated with Treg induction and inhibits TH2 through IL-10 and TGF-β production | [69] | |||
| Eosinophils and T cell coculture | IDO↑in eosinophils | ↓ | ↑ | IDO in eosinophils selectively inhibited TH1 proliferation but promotes TH2 responses | [4] | ||
| Allergic airway inflammation | IDO↓ by 1-MT in vivo | ? | No change on TH2 response when mice treated by 1- MT alone | [70] | |||
| Mouse spleen cell culture | IDO↓ | ↑ | ↓ | IDO and tryptophan catabolites down- regulated TH1 but up-regulated TH2 | [30] | ||
| Addition of tryptophan catabolites | ↓ | ↑ | |||||
IDO-mediated modulation: a negative feedback regulatory loop for peripheral TH1 responses?
It has been suggested by MacKenzie et al that IDO from antigen presenting cells functions as a critical negative feedback regulator in T cell responses [17]. To date, two types of DCs have been found to be closely associated with IDO-mediated immune suppression: plasmacytoid DCs (pDCs) expressing TLR 7 and TLR9, and myeloid DCs (mDCs) expressing TLR 2 and TLR 4 [37]. In response to stimulation of bacterial or viral DNA, or synthetic CpG-ODN through TLR9, pDCs produce type I IFN in an autocrine manner, that will positively feedback to activate IDO expression in DCs [38]. On the other hand, most antigens and pathogens are presented by mDCs for T cell activation. Upon stimulation through TLR 2 or TLR 4, mDCs secret IL-12, which not only skews TH0 cells into TH1 pathway but also strongly enhances IFN-γ production from T cells and NK cells. IFN-γ is a key inducer for IDO expression and activation since its efficiency in up-regulation of IDO activity is far higher than that of type I IFN [39,40]. In the periphery, DCs does not express functional IDO until its expression is triggered by stimulation of IFN-γ, IFN-α, IFN-β or through TLR-ligand signaling [2,14]. It has been documented that murine DCs are capable of releasing IFN-γ triggered by infection with intracellular pathogens, e.g. Listeria monocytogenes and Toxoplasma gondii, or a stimulating signal through CTLA 4-B7-1 ligation [41,42]. Human dendritic cells also produce IFN-γ in response to bacterial stimuli through TLR 2 [43]. IFN-γ production from DCs can be further enhanced by autocrine or exogenous IL-12 [42,44,45]. In addition to DC-released IFN-γ, DC-produced IL-12 can stimulate bystander NK cells to produce IFN-γ [46]. NK cells are thus also an important source of IFN-γ at the antigen priming stage, which contribute to creating TH1 skewing conditions. As naïve T cells differentiate into TH1 cells, they will produce IFN-γ [2,47]. The increased levels of IFN-γ can have two consequences: establishing a TH1 dominant microenvironment, which subsequently inhibits TH2 development, and stimulating DCs to express functional IDO, which will deplete tryptophan in the microenvironment and limit T cell activation (most likely TH1 cells at this stage). Since tryptophan catabolites selectively induce apoptosis in TH1 but not TH2 cells, IDO-mediated inhibition of TH1 cells will limit TH1 cell responses and lead to a selective survival of TH2 cells. Therefore, the TH1↑→IFN-γ→IDO→TH1↓ axis consists of a negative feedback regulatory loop to self-limit TH1 responses.
Another mechanism for the inhibition of T cell activation through regulatory T cells has been suggested by Mellor and Munn [2]. Findings from Fallarino’s study showed that Treg cells are capable of inducing IDO expression in DCs by CTLA4-B7 interaction [41]. The possibility that IDO expression in DCs induces the generation of regulatory T cells was initially suggested in a previous study, in which CD4+ naïve T cells converted to FoxP3+ functional regulatory T cells upon exposure to LT/kynurenine or IDO+ DCs [16]. In humans, naïve T cells will convert to FoxP3+ Treg cells when they are co-cultured with IDO+ AML (acute myeloid leukemia) cells and this conversion is completely abrogated by IDO inhibitor 1-MT treatment [48]. IDO may upregulate the function of Treg cells directly or indirectly. In humans, it has been found that IDO expression in DC contributes to DC maturation, which subsequently leads to expansion of Treg cells [49]. The direct role of IDO in activation of Treg cells has been confirmed by a recent study from Munn’s laboratory, that IDO activates Treg cells via GCN2 pathway and the IDO-activated Treg cells exert suppression on target cells through PD-/PD-L interaction [50,51]. Thus, IDO-activated Treg cells will subsequently suppress antigen-induced TH cell responses in a “time delayed manner”. IDO-induced Treg cells may therefore contribute to the negative-feedback suppression of T cell responses. But the difference from IDO-directly suppressive role on T cells is that Treg cells inhibit both TH1 and TH2 type immune responses. In addition to inhibition of specific TH1 and TH2 responses, IDO-induced Treg may also mediate bystander suppression of cellular immune responses to “the third party antigens” [52].
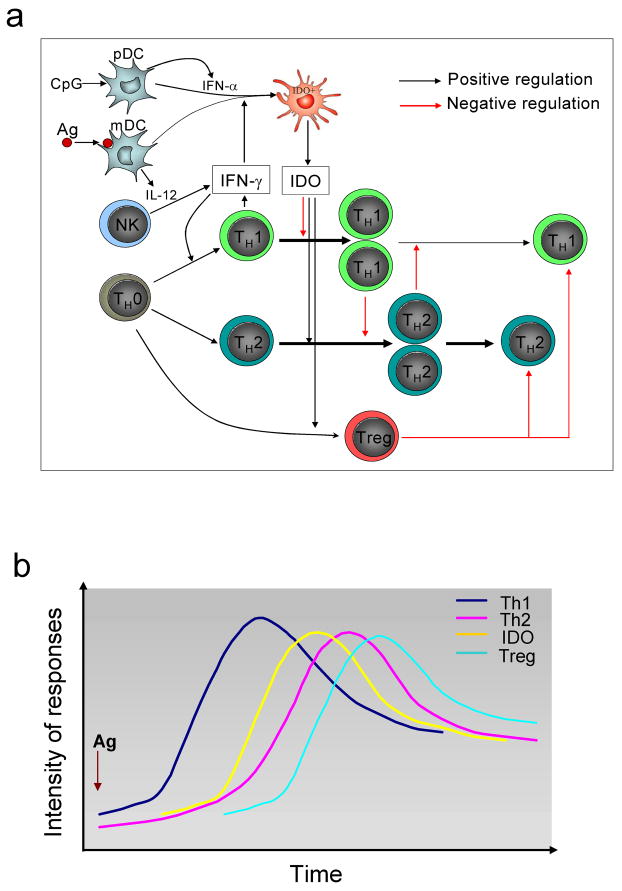
In Fig.1, we propose a model which interprets the regulatory roles of IDO in TH1/ TH2 differentiation. Fig.1a summarizes the cross-regulatory network mainly consist of the dual effects of IDO from DCs and interaction between TH1 and TH2 responses as well as Treg cells. Fig.1b displays kinetics of IDO activity and T cell responses, which may explain the relationship between temporally controlled IDO levels and dynamics of T cell responses: antigen-induced DC activation and TH1 differentiation trigger increasing IDO expression; the increased IDO activity will reduce TH1 responses but lead to upregulation of TH2 responses and induction of Treg cells. Emergence of Treg cells will counteract hyperactive immune responses and bring the immune system close to physiological balance.
Figure 1.
Dynamics of antigen-induced cell immune responses: a. IDO expression and its influence on T helper cell differentiation. DCs uptake antigen and activate naïve T cells to initiate T helper cell differentiation. IFN-γ production from DCs and bystander NK cells will skew TH0 cells into the TH1 pathway. Polarized TH1 cells produce IFN-γ, which subsequently suppresses TH2 differentiation and induces DCs to express functional IDO. The increase in IDO expression will inhibit proliferation of TH1 cells and release TH2 cells from suppression by TH1. In the meantime, IDO will directly induce the development of TH2 cells, which leads to an enhanced TH2 response. In addition, IDO also induces the generation of regulatory T cells, which further limit both TH1 and TH2 responses. b. Kinetics of T cell responses and IDO expression. Antigen stimulation initiates TH1 response followed by an increasing IDO expression. As IDO climbs to high levels, TH1 response will decline, which is accompanied by enhanced TH2 response. As IDO-induced Treg cell responses occur, Treg cells will inhibit both TH1 and TH2 cells and bring the immune system close to physiological balance.
IDO favors TH2 type immune responses: beyond a negative feedback effect
DCs are critical to antigen presentation and T cell activation. Immature DCs express TLRs and other surface molecules that facilitate recognizing and uptaking antigens. Once DCs are stimulated by antigens they undergo maturation and up-regulate expression of co-stimulatory molecules, which interact with T cells and cause T cell activation [53]. Although it is unclear whether IDO expression in DCs may directly be involved in up-regulation and maintenance of TH2 type immune responses, it has been shown that appropriate tryptophan metabolism through the kynurenine pathway is important for phenotypic maturation of DCs; inhibition of IDO function by 1-MT treatment can reduce the expression of co-stimulatory molecules in DCs and cause an inefficient co-stimulation for T cells [54]. Our recent data from a murine asthma model show that although IDO is not constitutively expressed in spleen DCs, which is consistent with other observations [55,56], it does constitutively express in lung resident DCs [31]. Moreover, IDO deficient mice are fully susceptible to the induction of immune tolerance by OVA inhalation, suggesting that IDO is not absolutely required for induction of immune tolerance. Whether the deficiency of IDO in the knockout mice is compensated by other alternatives, e.g. kynurenine pathway enzymes downstream of IDO[57], remains to be determined. Phenotype analysis of in vitro-stimulated lung DCs showed that the OVA-induced expression of co-stimulatory molecules (CD86, OX40L and MHC class II) in lung DCs from IDO deficient mice was reduced in comparison with that from WT control mice. When CD4+ T cells from DO11.10 TCR transgenic mice were co-cultured with lung DCs from either IDO deficient or WT control mice, OVA-induced TH2 cytokine production from IDO−/− lung DC-stimulated T cells was significantly lower than that from WT lung DC-stimulated T cells (data not shown). Similarly when DO11.10 CD4+ T cells were adoptively transferred into IDO−/− mice, expansion of the transferred T cells declined in comparison with that of WT control mice [31]. These data suggest that deficiency of IDO function in lung DCs impairs the expression of co-stimulatory molecules in these cells and result in reduced TH2 cell expansion and decreased TH2 cytokine production in response to antigen stimulation. One of the potential explanations for the reduced TH2 responses observed in our study is that deficiency of IDO function in IDO−/− mice may genetically create “TH1 dominant status”, which may overwhelm TH2 cell responses. However, our data from an OVA-induced asthma model showed that IFN-γ production in IDO deficient mice was only slightly increased with very low concentration [31]. It seems that such a low amount of IFN-γ may not completely account for the reduction of TH2 cell-mediated airway inflammation in our study. Although more extensive studies from multiple angles may be needed to further confirm the direct role of IDO in DCs in TH2 cell activation, available information suggests that IDO function in DCs, at least in lung tissue, may play a role in mounting TH2 cell responses independent of negative-feedback mechanism from TH1 cells. Our data raise the possibility that IDO in DCs may have dual roles: physiological levels of IDO may be needed for maintenance of DC function, which is necessary for antigen presentation, but increased levels of IDO and accumulation of IDO-mediated cytotoxic metabolites from the kynurenine pathway will hamper cell function by cell starvation or apoptosis, including activated T cells and DCs themselves.
Our data from the asthma model with IDO deficient mice are not consistent with the observations by Hayashi et al, in which 1-MT treatment reduced TH2 cell apoptosis and enhanced TH2 type inflammation in an experimental asthma model adoptively transferred with an in vitro differentiated OVA-specific TH2 cell line [33]. Their further study showed that treatment of asthmatic mice with HAA, a kynurenine metabolite, after intratracheal challenge by OVA, suppressed TH2 type lung inflammation [58]. Similar treatment also induced TH2 cell apoptosis in this model although induction of TH2 cell apoptosis by this compound was modest [58]. Notably, in Hayashi’s experiments IDO inhibition and HAA treatment are performed either with polarized TH2 cells or in a “relatively pure TH2 cell environment” while in our studies IDO deficiency impacts antigen priming and early stage of T cell differentiation in lung lymph node or spleen, where both TH2 and TH1 cells are likely involved. Given that TH1 cells are more sensitive than TH2 cells to induction of apoptosis by IDO and tryptophan metabolites [28], these differences suggest that tryptophan depletion and cellular toxic catabolites from downstream of the kynurenine metabolism pathway may similarly influence function of all cells in general. However, such a mechanism will impact the ratio of TH1/TH2 when both TH1 and TH2 responses are involved (this is likely the case in most natural immune responses regardless of whether it is TH1 or TH2 dominant), which will skew T cells into the TH2 pathway. In Table 2, we summarize the effects of IDO on polarized TH1 and TH2 cells and show potential outcomes under three different situations, which may explain the various observations by different investigators.
Table 2.
Effects of IDO on polarized TH1 and TH2 cells
| Type of immune response | Effect of IDO- kynurenine pathway |
|---|---|
| TH1 | TH1 ↓ |
| TH1/TH2 | TH1 ↓ TH2 ↑ |
| TH2 | TH2 ↓ |
Concluding remarks
IDO in DCs likely plays different roles in the regulation of TH1 and TH2 immune responses especially under natural conditions. Expression of functional IDO is suppressive for the development of TH1 type responses, which constitute a key step in “self-limiting loops” to avoid “over-reaction” of cellular responses to antigen exposure. In contrast, IDO in DCs may be immune-stimulatory for TH2 type responses, at least under some circumstances, e.g., IDO-expressing immature DCs in tissue, which may represent a new regulatory mechanism of IDO for TH2 cell differentiation. Obviously, extensive investigations in different settings are required for further confirmation of this concept. If proven true, it will impact the design of therapeutic strategies targeting kynurenine pathway of tryptophan metabolism, e.g., 1-MT treatment. Treatment with IDO inhibitors can enhance anti-tumor immunity, which is currently undergoing clinical trails or is in active preclinical development. On the other hand, such a therapeutic strategy may also be of benefit in the treatment of TH2 type cell-mediated inflammation. Thus, targeting the role of IDO in TH2 cell-mediated immune responses may open a new avenue for the potential development of drugs for the inhibition of TH2 type cell-mediated inflammation.
Acknowledgments
We are grateful to Katherine Regan for critical review of this manuscript.
Abbreviations used
- IDO
Indoleamine 2,3-dioxygenase
- DC
dendritic cell
- TH
T helper cell
- Treg
regulatory T cell
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Stone TW, Darlington LG. Endogenous kynurenines as targets for drug discovery and development. Nat Rev Drug Discov. 2002;1:609–620. doi: 10.1038/nrd870. [DOI] [PubMed] [Google Scholar]
- 2.Mellor AL, Munn DH. IDO expression by dendritic cells: tolerance and tryptophan catabolism. Nat Rev Immunol. 2004;4:762–774. doi: 10.1038/nri1457. [DOI] [PubMed] [Google Scholar]
- 3.Beutelspacher SC, Tan PH, McClure MO, Larkin DF, Lechler RI, George AJ. Expression of indoleamine 2,3-dioxygenase (IDO) by endothelial cells: implications for the control of alloresponses. Am J Transplant. 2006;6:1320–1330. doi: 10.1111/j.1600-6143.2006.01324.x. [DOI] [PubMed] [Google Scholar]
- 4.Odemuyiwa SO, Ghahary A, Li Y, Puttagunta L, Lee JE, Musat-Marcu S, et al. Cutting edge: human eosinophils regulate T cell subset selection through indoleamine 2,3-dioxygenase. J Immunol. 2004;173:5909–5913. doi: 10.4049/jimmunol.173.10.5909. [DOI] [PubMed] [Google Scholar]
- 5.Munn DH, Mellor AL. IDO and tolerance to tumors. Trends Mol Med. 2004;10:15–18. doi: 10.1016/j.molmed.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 6.Uyttenhove C, Pilotte L, Theate I, Stroobant V, Colau D, Parmentier N, et al. Evidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2,3-dioxygenase. Nat Med. 2003;9:1269–1274. doi: 10.1038/nm934. [DOI] [PubMed] [Google Scholar]
- 7.Agaugue S, Perrin-Cocon L, Coutant F, Andre P, Lotteau V. 1-Methyl-tryptophan can interfere with TLR signaling in dendritic cells independently of IDO activity. J Immunol. 2006;177:2061–2071. doi: 10.4049/jimmunol.177.4.2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Munn DH. Indoleamine 2,3-dioxygenase, tumor-induced tolerance and counter-regulation. Curr Opin Immunol. 2006;18:220–225. doi: 10.1016/j.coi.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 9.Puccetti P, Fallarino F. Generation of T cell regulatory activity by plasmacytoid dendritic cells and tryptophan catabolism. Blood Cells Mol Dis. 2008;40:101–105. doi: 10.1016/j.bcmd.2007.06.026. [DOI] [PubMed] [Google Scholar]
- 10.Mellor AL, Munn DH. Creating immune privilege: active local suppression that benefits friends, but protects foes. Nat Rev Immunol. 2008;8:74–80. doi: 10.1038/nri2233. [DOI] [PubMed] [Google Scholar]
- 11.Terness P, Kallikourdis M, Betz AG, Rabinovich GA, Saito S, Clark DA. Tolerance signaling molecules and pregnancy: IDO, galectins, and the renaissance of regulatory T cells. Am J Reprod Immunol. 2007;58:238–254. doi: 10.1111/j.1600-0897.2007.00510.x. [DOI] [PubMed] [Google Scholar]
- 12.Katz JB, Muller AJ, Prendergast GC. Indoleamine 2,3-dioxygenase in T-cell tolerance and tumoral immune escape. Immunol Rev. 2008;222:206–221. doi: 10.1111/j.1600-065X.2008.00610.x. [DOI] [PubMed] [Google Scholar]
- 13.Munn DH, Mellor AL. Indoleamine 2,3-dioxygenase and tumor-induced tolerance. J Clin Invest. 2007;117:1147–1154. doi: 10.1172/JCI31178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Puccetti P, Grohmann U. IDO and regulatory T cells: a role for reverse signalling and non-canonical NF-kappaB activation. Nat Rev Immunol. 2007;7:817–823. doi: 10.1038/nri2163. [DOI] [PubMed] [Google Scholar]
- 15.Terness P, Chuang JJ, Opelz G. The immunoregulatory role of IDO-producing human dendritic cells revisited. Trends Immunol. 2006;27:68–73. doi: 10.1016/j.it.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 16.Fallarino F, Grohmann U, You S, McGrath BC, Cavener DR, Vacca C, et al. The combined effects of tryptophan starvation and tryptophan catabolites down-regulate T cell receptor ζ-chain and induce a regulatory phenotype in naive T cells. J Immunol. 2006;176:6752–6761. doi: 10.4049/jimmunol.176.11.6752. [DOI] [PubMed] [Google Scholar]
- 17.MacKenzie CR, Heseler K, Muller A, Daubener W. Role of indoleamine 2,3-dioxygenase in antimicrobial defence and immuno-regulation: tryptophan depletion versus production of toxic kynurenines. Curr Drug Metab. 2007;8:237–244. doi: 10.2174/138920007780362518. [DOI] [PubMed] [Google Scholar]
- 18.Choi BK, Asai T, Vinay DS, Kim YH, Kwon BS. 4-1BB-mediated amelioration of experimental autoimmune uveoretinitis is caused by indoleamine 2,3-dioxygenase-dependent mechanisms. Cytokine. 2006;34:233–242. doi: 10.1016/j.cyto.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 19.Gurtner GJ, Newberry RD, Schloemann SR, McDonald KG, Stenson WF. Inhibition of indoleamine 2,3-dioxygenase augments trinitrobenzene sulfonic acid colitis in mice. Gastroenterology. 2003;125:1762–1773. doi: 10.1053/j.gastro.2003.08.031. [DOI] [PubMed] [Google Scholar]
- 20.Platten M, Ho PP, Youssef S, Fontoura P, Garren H, Hur EM, et al. Treatment of autoimmune neuroinflammation with a synthetic tryptophan metabolite. Science. 2005;310:850–855. doi: 10.1126/science.1117634. [DOI] [PubMed] [Google Scholar]
- 21.Orabona C, Puccetti P, Vacca C, Bicciato S, Luchini A, Fallarino F, et al. Toward the identification of a tolerogenic signature in IDO-competent dendritic cells. Blood. 2006;107:2846–2854. doi: 10.1182/blood-2005-10-4077. [DOI] [PubMed] [Google Scholar]
- 22.Orabona C, Tomasello E, Fallarino F, Bianchi R, Volpi C, Bellocchio S, et al. Enhanced tryptophan catabolism in the absence of the molecular adapter DAP12. Eur J Immunol. 2005;35:3111–3118. doi: 10.1002/eji.200535289. [DOI] [PubMed] [Google Scholar]
- 23.Kwidzinski E, Bunse J, Aktas O, Richter D, Mutlu L, Zipp F, et al. Indolamine 2,3-dioxygenase is expressed in the CNS and down-regulates autoimmune inflammation. Faseb J. 2005;19:1347–1349. doi: 10.1096/fj.04-3228fje. [DOI] [PubMed] [Google Scholar]
- 24.Grohmann U, Fallarino F, Bianchi R, Orabona C, Vacca C, Fioretti MC, et al. A defect in tryptophan catabolism impairs tolerance in nonobese diabetic mice. J Exp Med. 2003;198:153–160. doi: 10.1084/jem.20030633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saxena V, Ondr JK, Magnusen AF, Munn DH, Katz JD. The countervailing actions of myeloid and plasmacytoid dendritic cells control autoimmune diabetes in the nonobese diabetic mouse. J Immunol. 2007;179:5041–5053. doi: 10.4049/jimmunol.179.8.5041. [DOI] [PubMed] [Google Scholar]
- 26.Ueno A, Cho S, Cheng L, Wang J, Hou S, Nakano H, et al. Transient upregulation of indoleamine 2,3-dioxygenase in dendritic cells by human chorionic gonadotropin downregulates autoimmune diabetes. Diabetes. 2007;56:1686–1693. doi: 10.2337/db06-1727. [DOI] [PubMed] [Google Scholar]
- 27.Romani L, Fallarino F, De Luca A, Montagnoli C, D'Angelo C, Zelante T, et al. Defective tryptophan catabolism underlies inflammation in mouse chronic granulomatous disease. Nature. 2008;451:211–215. doi: 10.1038/nature06471. [DOI] [PubMed] [Google Scholar]
- 28.Fallarino F, Grohmann U, Vacca C, Bianchi R, Orabona C, Spreca A, et al. T cell apoptosis by tryptophan catabolism. Cell Death Differ. 2002;9:1069–1077. doi: 10.1038/sj.cdd.4401073. [DOI] [PubMed] [Google Scholar]
- 29.Clark DA, Blois S, Kandil J, Handjiski B, Manuel J, Arck PC. Reduced uterine indoleamine 2,3-dioxygenase versus increased Th1/Th2 cytokine ratios as a basis for occult and clinical pregnancy failure in mice and humans. Am J Reprod Immunol. 2005;54:203–216. doi: 10.1111/j.1600-0897.2005.00299.x. [DOI] [PubMed] [Google Scholar]
- 30.Molano A, Illarionov PA, Besra GS, Putterman C, Porcelli SA. Modulation of invariant natural killer T cell cytokine responses by indoleamine 2,3-dioxygenase. Immunol Lett. 2008;117:81–90. doi: 10.1016/j.imlet.2007.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu H, Oriss TB, Fei M, Henry AC, Melgert BN, Chen L, et al. Indoleamine 2,3-dioxygenase in lung dendritic cells promotes Th2 responses and allergic inflammation. Proc Natl Acad Sci U S A. 2008;105:6690–6695. doi: 10.1073/pnas.0708809105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen X, Liu L, Yang P, Wu C, Jin H, Xing L, et al. Indoleamine 2,3-dioxygenase (IDO) is involved in promoting the development of anterior chamber-associated immune deviation. Immunol Lett. 2006;107:140–147. doi: 10.1016/j.imlet.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 33.Hayashi T, Beck L, Rossetto C, Gong X, Takikawa O, Takabayashi K, et al. Inhibition of experimental asthma by indoleamine 2,3-dioxygenase. J Clin Invest. 2004;114:270–279. doi: 10.1172/JCI21275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ishii KJ, Akira S. Innate immune recognition of, and regulation by, DNA. Trends Immunol. 2006;27:525–532. doi: 10.1016/j.it.2006.09.002. [DOI] [PubMed] [Google Scholar]
- 35.Kline JN. Effects of CpG DNA on Th1/Th2 balance in asthma. Curr Top Microbiol Immunol. 2000;247:211–225. doi: 10.1007/978-3-642-59672-8_15. [DOI] [PubMed] [Google Scholar]
- 36.Gordon JR, Li F, Nayyar A, Xiang J, Zhang X. CD8α+, but not CD8α−, dendritic cells tolerize Th2 responses via contact-dependent and -independent mechanisms, and reverse airway hyperresponsiveness, Th2, and eosinophil responses in a mouse model of asthma. J Immunol. 2005;175:1516–1522. doi: 10.4049/jimmunol.175.3.1516. [DOI] [PubMed] [Google Scholar]
- 37.Colonna M, Trinchieri G, Liu YJ. Plasmacytoid dendritic cells in immunity. Nat Immunol. 2004;5:1219–1226. doi: 10.1038/ni1141. [DOI] [PubMed] [Google Scholar]
- 38.Puccetti P. On watching the watchers: IDO and type I/II IFN. Eur J Immunol. 2007;37:876–879. doi: 10.1002/eji.200737184. [DOI] [PubMed] [Google Scholar]
- 39.Adams O, Besken K, Oberdorfer C, MacKenzie CR, Takikawa O, Daubener W. Role of indoleamine-2,3-dioxygenase in alpha/beta and gamma interferon-mediated antiviral effects against herpes simplex virus infections. J Virol. 2004;78:2632–2636. doi: 10.1128/JVI.78.5.2632-2636.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Taylor MW, Feng GS. Relationship between interferon-γ, indoleamine 2,3-dioxygenase, and tryptophan catabolism. Faseb J. 1991;5:2516–2522. [PubMed] [Google Scholar]
- 41.Fallarino F, Grohmann U, Hwang KW, Orabona C, Vacca C, Bianchi R, et al. Modulation of tryptophan catabolism by regulatory T cells. Nat Immunol. 2003;4:1206–1212. doi: 10.1038/ni1003. [DOI] [PubMed] [Google Scholar]
- 42.Suzue K, Asai T, Takeuchi T, Koyasu S. In vivo role of IFN-γ produced by antigen-presenting cells in early host defense against intracellular pathogens. Eur J Immunol. 2003;33:2666–2675. doi: 10.1002/eji.200323292. [DOI] [PubMed] [Google Scholar]
- 43.Fricke I, Mitchell D, Mittelstadt J, Lehan N, Heine H, Goldmann T, et al. Mycobacteria induce IFN-γ production in human dendritic cells via triggering of TLR2. J Immunol. 2006;176:5173–5182. doi: 10.4049/jimmunol.176.9.5173. [DOI] [PubMed] [Google Scholar]
- 44.Fujii S, Liu K, Smith C, Bonito AJ, Steinman RM. The linkage of innate to adaptive immunity via maturing dendritic cells in vivo requires CD40 ligation in addition to antigen presentation and CD80/86 costimulation. J Exp Med. 2004;199:1607–1618. doi: 10.1084/jem.20040317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stober D, Schirmbeck R, Reimann J. IL-12/IL-18-dependent IFN-γ release by murine dendritic cells. J Immunol. 2001;167:957–965. doi: 10.4049/jimmunol.167.2.957. [DOI] [PubMed] [Google Scholar]
- 46.Trinchieri G. Immunobiology of interleukin-12. Immunol Res. 1998;17:269–278. doi: 10.1007/BF02786451. [DOI] [PubMed] [Google Scholar]
- 47.Berenson LS, Ota N, Murphy KM. Issues in T-helper 1 development--resolved and unresolved. Immunol Rev. 2004;202:157–174. doi: 10.1111/j.0105-2896.2004.00208.x. [DOI] [PubMed] [Google Scholar]
- 48.Curti A, Pandolfi S, Valzasina B, Aluigi M, Isidori A, Ferri E, et al. Modulation of tryptophan catabolism by human leukemic cells results in the conversion of CD25− into CD25+ T regulatory cells. Blood. 2007;109:2871–2877. doi: 10.1182/blood-2006-07-036863. [DOI] [PubMed] [Google Scholar]
- 49.Hill M, Tanguy-Royer S, Royer P, Chauveau C, Asghar K, Tesson L, et al. IDO expands human CD4+CD25high regulatory T cells by promoting maturation of LPS-treated dendritic cells. Eur J Immunol. 2007;37:3054–3062. doi: 10.1002/eji.200636704. [DOI] [PubMed] [Google Scholar]
- 50.Sharma MD, Baban B, Chandler P, Hou DY, Singh N, Yagita H, et al. Plasmacytoid dendritic cells from mouse tumor-draining lymph nodes directly activate mature Tregs via indoleamine 2,3-dioxygenase. J Clin Invest. 2007;117:2570–2582. doi: 10.1172/JCI31911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Munn DH, Sharma MD, Baban B, Harding HP, Zhang Y, Ron D, et al. GCN2 kinase in T cells mediates proliferative arrest and anergy induction in response to indoleamine 2,3-dioxygenase. Immunity. 2005;22:633–642. doi: 10.1016/j.immuni.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 52.Derks RA, Jankowska-Gan E, Xu Q, Burlingham WJ. Dendritic cell type determines the mechanism of bystander suppression by adaptive T regulatory cells specific for the minor antigen HA-1. J Immunol. 2007;179:3443–3451. doi: 10.4049/jimmunol.179.6.3443. [DOI] [PubMed] [Google Scholar]
- 53.Quah BJ, O'Neill HC. Maturation of function in dendritic cells for tolerance and immunity. J Cell Mol Med. 2005;9:643–654. doi: 10.1111/j.1582-4934.2005.tb00494.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hwang SL, Chung NP, Chan JK, Lin CL. Indoleamine 2, 3-dioxygenase (IDO) is essential for dendritic cell activation and chemotactic responsiveness to chemokines. Cell Res. 2005;15:167–175. doi: 10.1038/sj.cr.7290282. [DOI] [PubMed] [Google Scholar]
- 55.Mellor AL, Baban B, Chandler P, Marshall B, Jhaver K, Hansen A, et al. Cutting edge: induced indoleamine 2,3 dioxygenase expression in dendritic cell subsets suppresses T cell clonal expansion. J Immunol. 2003;171:1652–1655. doi: 10.4049/jimmunol.171.4.1652. [DOI] [PubMed] [Google Scholar]
- 56.Grohmann U, Bianchi R, Orabona C, Fallarino F, Vacca C, Micheletti A, et al. Functional plasticity of dendritic cell subsets as mediated by CD40 versus B7 activation. J Immunol. 2003;171:2581–2587. doi: 10.4049/jimmunol.171.5.2581. [DOI] [PubMed] [Google Scholar]
- 57.Belladonna ML, Grohmann U, Guidetti P, Volpi C, Bianchi R, Fioretti MC, et al. Kynurenine Pathway Enzymes in Dendritic Cells Initiate Tolerogenesis in the Absence of Functional IDO. J Immunol. 2006;177:130–137. doi: 10.4049/jimmunol.177.1.130. [DOI] [PubMed] [Google Scholar]
- 58.Hayashi T, Mo JH, Gong X, Rossetto C, Jang A, Beck L, et al. 3-Hydroxyanthranilic acid inhibits PDK1 activation and suppresses experimental asthma by inducing T cell apoptosis. Proc Natl Acad Sci U S A. 2007;104:18619–18624. doi: 10.1073/pnas.0709261104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sakurai K, Zou JP, Tschetter JR, Ward JM, Shearer GM. Effect of indoleamine 2,3-dioxygenase on induction of experimental autoimmune encephalomyelitis. J Neuroimmunol. 2002;129:186–196. doi: 10.1016/s0165-5728(02)00176-5. [DOI] [PubMed] [Google Scholar]
- 60.Alexander AM, Crawford M, Bertera S, Rudert WA, Takikawa O, Robbins PD, et al. Indoleamine 2,3-dioxygenase expression in transplanted NOD Islets prolongs graft survival after adoptive transfer of diabetogenic splenocytes. Diabetes. 2002;51:356–365. doi: 10.2337/diabetes.51.2.356. [DOI] [PubMed] [Google Scholar]
- 61.Funeshima N, Fujino M, Kitazawa Y, Hara Y, Hara Y, Hayakawa K, et al. Inhibition of allogeneic T-cell responses by dendritic cells expressing transduced indoleamine 2,3-dioxygenase. J Gene Med. 2005;7:565–575. doi: 10.1002/jgm.698. [DOI] [PubMed] [Google Scholar]
- 62.Swanson KA, Zheng Y, Heidler KM, Mizobuchi T, Wilkes DS. CDllc+ cells modulate pulmonary immune responses by production of indoleamine 2,3-dioxygenase. Am J Respir Cell Mol Biol. 2004;30:311–318. doi: 10.1165/rcmb.2003-0268OC. [DOI] [PubMed] [Google Scholar]
- 63.Mellor AL, Keskin DB, Johnson T, Chandler P, Munn DH. Cells expressing indoleamine 2,3-dioxygenase inhibit T cell responses. J Immunol. 2002;168:3771–3776. doi: 10.4049/jimmunol.168.8.3771. [DOI] [PubMed] [Google Scholar]
- 64.von Bergwelt-Baildon MS, Popov A, Saric T, Chemnitz J, Classen S, Stoffel MS, et al. CD25 and indoleamine 2,3-dioxygenase are up-regulated by prostaglandin E2 and expressed by tumor-associated dendritic cells in vivo: additional mechanisms of T-cell inhibition. Blood. 2006;108:228–237. doi: 10.1182/blood-2005-08-3507. [DOI] [PubMed] [Google Scholar]
- 65.Frumento G, Rotondo R, Tonetti M, Damonte G, Benatti U, Ferrara GB. Tryptophan-derived catabolites are responsible for inhibition of T and natural killer cell proliferation induced by indoleamine 2,3-dioxygenase. J Exp Med. 2002;196:459–468. doi: 10.1084/jem.20020121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.van der Marel AP, Samsom JN, Greuter M, van Berkel LA, O'Toole T, Kraal G, et al. Blockade of IDO inhibits nasal tolerance induction. J Immunol. 2007;179:894–900. doi: 10.4049/jimmunol.179.2.894. [DOI] [PubMed] [Google Scholar]
- 67.Jasperson LK, Bucher C, Panoskaltsis-Mortari A, Taylor PA, Mellor AL, Munn DH, et al. Indoleamine 2,3-dioxygenase is a critical regulator of acute graft-versus-host disease lethality. Blood. 2008;111:3257–3265. doi: 10.1182/blood-2007-06-096081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Reddy P, Sun Y, Toubai T, Duran-Struuck R, Clouthier SG, Weisiger E, et al. Histone deacetylase inhibition modulates indoleamine 2,3-dioxygenase-dependent DC functions and regulates experimental graft-versus-host disease in mice. J Clin Invest. 2008;118:2562–2573. doi: 10.1172/JCI34712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Montagnoli C, Fallarino F, Gaziano R, Bozza S, Bellocchio S, Zelante T, et al. Immunity and tolerance to Aspergillus involve functionally distinct regulatory T cells and tryptophan catabolism. J Immunol. 2006;176:1712–1723. doi: 10.4049/jimmunol.176.3.1712. [DOI] [PubMed] [Google Scholar]
- 70.Grohmann U, Volpi C, Fallarino F, Bozza S, Bianchi R, Vacca C, et al. Reverse signaling through GITR ligand enables dexamethasone to activate IDO in allergy. Nat Med. 2007;13:579–586. doi: 10.1038/nm1563. [DOI] [PubMed] [Google Scholar]