Abstract
Objective
Numerous studies have shown higher responsiveness and/or a lack of habituation to sensory stimuli of various modalities in migraine. The present study investigated psychophysiological responses to aversive acoustic stimuli in children at risk for migraine.
Methods
We measured eyeblink responses to aversive acoustic stimuli (40ms bursts of white noise at 102dB) during anticipation of unpleasant stimuli in 74 adolescents (40 females, age 17.6±2.9). A mixed effects linear model was applied to test group differences in startle reactivity during baseline, safe and threat conditions among adolescents by maternal and personal history of migraine.
Results
The strongest association with migraine vulnerability emerged for baseline startle reactivity, which was significantly elevated in high risk youth with a history of maternal migraine. This group of offspring also had enhanced startle response during the threat condition and the threat-safe difference.
Conclusion
Our findings indicate that migraine is associated with higher acoustic startle responsiveness to aversive stimuli that is present already in children at risk for developing the disorder. Significance: The significant effect of both maternal history of anxiety disorder and migraine on baseline startle indicates that these two diagnostic entities might in part share common pathophysiological mechanisms and that the anxiety-migraine comorbidity should be considered when investigating each of these disorders.
Keywords: Migraine, anxiety, startle, children, genetic
Introduction
A substantial amount of research has investigated the pathogenesis of migraine but the specific mechanisms involved still remain elusive (Goadsby, 2007). Higher responsiveness and/or a lack of habituation to sensory stimuli of various modalities including aversive and painful stimuli (Katsarava et al., 2003; Sandrini et al., 2002; Weissman-Fogel et al., 2003; Zohsel et al., 2006) suggest that migraine may be characterized by cortical hypersensitivity and/or a lack of cortical inhibition (for review see (Gantenbein and Sandor, 2006; Schoenen et al., 2003)). Hypersensitivity to aversive or noxious stimuli could also be related to sensitization of pain pathways resulting from repeated intense nociceptive stimulation during migraine attacks (Burstein et al., 2000; Woolf and Salter, 2000). Hence, higher responsiveness to noxious stimuli, as part of the crossmodal hypersensitivity, may represent a useful index of vulnerability for migraine (Di Clemente et al., 2007).
Migraine has been shown to be strongly familial with substantial evidence of vertical transmission (Merikangas, 1996) suggesting a maternal inheritance pattern (Mortimer et al., 1992; Wang et al., 2004). The results of twin studies show that genetic factors underlie migraine, but the patterns of genetic transmission are complex, and the identification of specific genes has not yet been successful (Haan et al., 1997; Russell et al., 1995). One approach to identify sources of heterogeneity in the familial transmission of migraine has been investigation of vulnerability markers among relatives of migraine probands. Indeed, alterations in sensory and cognitive evoked potentials (Sandor et al., 1999; Siniatchkin et al., 2000; Siniatchkin et al., 2001) as well as hypersensitivity to aversive stimuli (Di Clemente et al., 2007) have been reported to aggregate in families of people who have migraine and have been suggested to represent an index of vulnerability for migraine. However, none of these studies tried to address the specificity of these findings with respect to anxiety and mood disorders that have been shown to co-occur in individuals with families with migraine.
Although comorbidity of migraine with mood and anxiety disorders has been well-established (for review see (Radat and Swendsen, 2005)), there is a lack of prospective research on the specific subtypes of these conditions and patterns of onset with migraine. We have demonstrated that in young adults, the onset of anxiety disorders tends to precede that of migraine followed by the onset of depression (Merikangas et al., 1990). In order to examine this question prospectively, we have examined the offspring of parents with these conditions in order to identify vulnerability factors and early forms of expression of the anxiety disorders and migraine. Our previous research has shown that offspring of parents with anxiety disorders have increased psychophysiological startle responsiveness to aversive and nonaversive stimuli (Grillon et al., 1997; Grillon et al., 1998). The major aims of the present study are: (1) to examine whether children of mothers with migraine exhibit increased baseline startle or startle reactivity to threat; (2) to determine whether children with migraine have differential patterns of startle; and (3) to evaluate whether differences in startle among offspring of mothers with migraine or among children with migraine are associated with parental or child anxiety disorders.
Methods
Participants
The present data have been collected as part of a family and high risk study of anxiety disorders that examined comorbidity of anxiety with a range of other physical and mental disorders. Probands were recruited from outpatient primary care of mental health clinics, or from the general population in the Greater New Haven area through a random digit dialing procedure. The probands were required to have an anxiety disorder (either social phobia or panic disorder) or have no lifetime history of major psychiatric disorder (comparison probands). Probands were also screened for migraine as part of a comprehensive evaluation of both mental and physical disorders. All probands were required to have biological offspring between the ages 7 and 17 and to provide consent for them to be interviewed. The study was approved by the Institutional Review Board of the Yale University School of Medicine.
The age and sex distribution of the offspring is presented in Table 1. There was a total of 74 offspring of mothers with and without migraine. The mean age of the sample was 17.6±2.9. No significant differences in age and gender were found between the groups stratified by offspring migraine (age: t(72) = −0.59, NS; gender: X2 = 0.04, NS), or by maternal migraine (age: t(72) = 0.14, NS; gender: X2 = 0.04, NS) (Table 1). Maternal migraine is used in these analyses because there was no association between paternal and child migraine in this sample (Merikangas et al, under review).
Table 1.
Age and gender of offspring by personal and maternal history of migraine
| Offspring Migraine | Maternal Migraine | |||
|---|---|---|---|---|
| No (N=58) | Yes (N=16) | No (N=58) | Yes (N=16) | |
| Age (Mean, SE) | 16.7 (0.4) | 17.2 (1.0) | 16.8 (0.4) | 16.7 (0.9) |
| Gender (Female/Male) | 31/27 | 9/7 | 31/27 | 9/7 |
| Proportion of Offspring with Migraine | 16% | 44% | ||
Assessment of Mental Disorders and Migraine
Criteria for mental disorders were assessed with the Schedule for Affective Disorders and Schizophrenia (SADS) (Endicott and Spitzer, 1978) modified extensively to obtain research diagnostic criteria for DSM-IV diagnoses (Diagnostic and Statistical Manual of Mental Disorders, 2000). The diagnosis of migraine was based on 1988 International Headache Society (IHS) criteria for migraine (Classification and diagnostic criteria for headache disorders, 1988). The migraine interview was conducted with all probands and relatives who indicated a history of headaches in the medical history interview.
Fear potentiated startle procedure
The startle stimuli consisted of 40-msec duration 102-dBA bursts of white noise presented binaurally through headphones. The aversive stimulus was an intense blast of air. The anticipation of airblast has been shown to yield robust and highly replicable fear-potentiated startle in children and adolescents (Grillon and Ameli, 1998; Grillon et al., 1999). The system that produced the airblast consisted of a compressed air cylinder, a regulator, a solenoid valve controlled by an AC switch, and 4-mm internal diameter polyethylene tubing. The airblast had a duration of 100 msec and an intensity of 60 psi (measured at the level of the regulator). The tubing was taped on the subjects’ neck and was directed to the throat at the level of the larynx.
The eyeblink component of the startle reflex was measured by recording the electromyographic activity below the left eye with two electrodes. The ground electrode was placed on the forehead. Impedance level was kept below 5 k. Electromyographic (EMG) activity was filtered (1–500 Hz), digitized at 1 kHz for 250 msec from the onset of the acoustic stimuli, and rectified.
Following the attachment of the electrodes and of tubing, the subjects sat in a reclining chair facing two colored lights (60-W bulbs): green and blue. One of the lights (e.g., green) signaled the possible administration of the airblast (threat signal). The other light (e.g., blue) signaled that the airblast would not be administered. The association between colored lights and safe/threat conditions was counterbalanced across subjects. Participants were told that the safe/threat lights would be turned on and off during testing. They were informed that the airblast would be administered with a 50% probability when the threat light was turned on, but that no airblast could be delivered when the safe light was turned on. The airblast was administered once as an example before the beginning of the experiment. Following the administration of this initial airblast, the startle experiment was started. The fear-potentiated startle test consisted of four blocks of two safe and two threat signals randomly presented (for a total of eight safe and eight threat signals). The duration of each threat and safe signal was 12 sec. The time interval between the onset of two successive signals varied from 17 to 42 sec, the length of the intertrial interval (ITI) was 5 to 30 sec. In each block, six startle probes were delivered: one during each threat and each safe signal, and two during the ITI. For each signal type (safe and threat), half the startle stimuli were delivered 4 sec following signal onset, and the other half were presented 7 sec following signal onset. Aversive stimuli were administered randomly on half the threat signal (2 sec before signal offset), with the exception that it was never administered in the first block.
Peak amplitude of the blink reflex was determined in the 21–120-msec time frame following stimulus onset relative to a baseline EMG value. The baseline EMG value was calculated by taking the average of the maximum and minimum values recorded during the first 20 msec. Trials with baseline EMG activity above 25 mV were rejected. The rate of rejected trials (less than 1.5%) did not differ among risk groups or gender. Eyeblink magnitudes and onset latency to startle probes presented during the safe, the threat, and the ITI conditions were separately averaged over the four blocks.
Data analysis
The Statistical Analytic Software (SAS) 9.1 was used for statistical analyses. T-test and Chi-square test were used to test differences in descriptive characteristics (age, sex) in groups formed by personal or maternal history of migraine. T-tests were used to compare startle in threat and safe condition.
A mixed effects linear model with random (family) and fixed effects (age, offspring or maternal anxiety) was applied to test group differences in startle reactivity during ITI, safe and threat conditions among adolescents by personal and maternal history of migraine. This model accounts for both the between- and within-family variance. It considers correlation among subjects in the same families by modeling the covariance structure (Littel et al. 2006).
Models testing the ITI startle and the threat-safe difference were adjusted for age and maternal anxiety. Models testing the safe startle and threat startle were adjusted for age, maternal anxiety and ITI startle amplitude. Models comparing for offspring migraine used offspring lifetime anxiety instead of maternal anxiety. In models with statistically significant main effects, up to 3 way interactions between the predictor variable (offspring migraine, maternal migraine) and adjustment variables (age, offspring or maternal anxiety) were tested.
The Likelihood Ratio Test was used for model selection by comparing -2 Log Likelihood between models. This test uses -2 x log (likelihood ratio) to construct a test statistic that has an approximate chi-square distribution with degrees of freedom given by the difference in the number of parameters between the null and alternative hypotheses (Morrell 1998).
A mixed effects linear model with random (family) and fixed effects (age, offspring or maternal anxiety) was applied to test group differences in startle reactivity during ITI, safe and threat conditions among adolescents by personal and maternal history of migraine. Models testing the ITI startle and the threat-safe difference were adjusted for age and maternal anxiety. Models testing the safe startle and threat startle were adjusted for age, maternal anxiety and ITI startle amplitude. Models comparing for offspring migraine used offspring lifetime anxiety instead of maternal anxiety. In models with statistically significant main effects, up to 3 way interactions between the predictor variable (offspring migraine, maternal migraine) and adjustment variables (age, offspring or maternal anxiety) were tested. The Likelihood Ratio Test was used for model selection by comparing -2 Log Likelihood between models.
Results
Sixteen of the 74 offspring had a lifetime history of migraine, sixteen had a positive maternal history of migraine, and 7 had both a personal and maternal history of migraine (Table 1).
The amplitude of startle across all groups was greater in the threat compared to the safe condition (fear-potentiated startle) (t(73) = 9.8, P < 0.001), documenting the potentiation of startle by fear. There was no significant difference among children with and without personal history of migraine in the magnitude of startle in the ITI (F(1,21) = 1.11; NS), safe (F(1,20) = 1.36; NS) and threat (F(1,20) < 1; NS) condition and the threat-safe difference (F(1,20) < 1; NS) (Table 2A).
Table 2.
Mean (± SE) amplitudes and statistics of startle in ITI, safe, threat conditions, and threat-safe difference by A) offspring migraine and B) maternal migraine.
| Table 2.A | Offspring Migraine | Effects of best fitting model | ||||
|---|---|---|---|---|---|---|
| No | Yes | Age | Offspring Migraine | Offspring Anxiety | ITI startle | |
| ITI | 175.6 ± 17.1 | 229.6 ± 31.9 | F(1,21)=10.6, P<0.01 | F(1,21)=1.1, NS | F(1,21)<1, NS | |
| Safe | 175.7 ± 16.8 | 238.2 ± 34.1 | F(1,20)<1, NS | F(1,20)=1.3 6, NS | F(1,20)<1, NS | F(1,20)=1273, P<0.01 |
| Threat | 276.4 ± 21.4 | 333.5 ± 39.0 | F(1,20)=1.8, NS | F(1,20)<1, NS | F(1,20)<1, NS | F(1,20)=215.7, P<0.01 |
| Threat-safe difference | 100.6 ± 11.0 | 95.4 ± 25.5 | F(1,21)<1, NS | F(1,21)<1, NS | F(1,21)<1, NS | |
| Table 2.B | Maternal Migraine | Effects of the best fitting model | |||||
|---|---|---|---|---|---|---|---|
| No | Yes | Age | Maternal Migraine | Maternal Anxiety | ITI startle | Age x anxiety interaction | |
| ITI | 170.1 ± 17.5 | 249.5 ± 25.4 | F(1,22)=12.7, P<0.01 | F(1,22)=6.5, P<0.05 | F(1,22)=4.4, P<0.05 | F(1,22)=4.6, p<0.05 | |
| Safe | 172.6 ± 17.6 | 249.6 ± 26.0 | F(1,22)<1, NS | F(1,22)<1, NS | F(1,22)=1.4, NS | F(1,22)=1215, P<0.01 | |
| Threat | 260.4 ± 20.4 | 391.5 ± 37.2 | F(1,22)=1.2, NS | F(1,22)=4.3, P<0.05 | F(1,22)<1, NS | F(1,22)=202, P<0.01 | |
| Threat-safe difference | 87.8 ± 9.2 | 142.0 ± 31.8 | F(1,23)<1, NS | F(1,23)=5.7, P<0.05 | F(1,23)=1.4, NS | ||
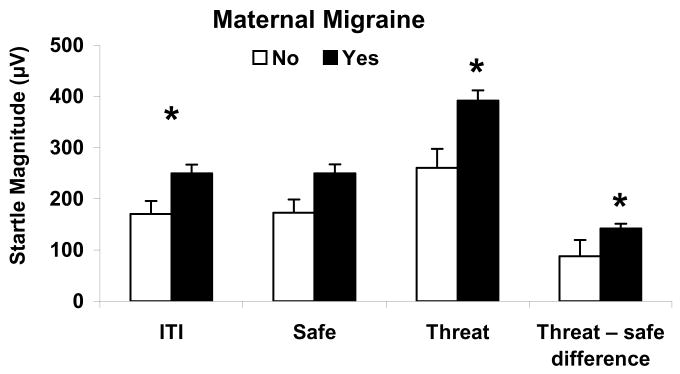
The effect of maternal migraine was significantly associated with an elevation of startle during ITI (F(1,22) = 6.5; P < 0.05), and during the safe (F(1,22) = 6.8; P < 0.05) and threat (F(1,22) = 10.7; P < 0.01) conditions, as well as during fear-potentiated startle (threat safe difference score) (F(1,22) = 5.7; P < 0.05). After adjusting for ITI startle, there was no significant effect of maternal migraine on startle during the safe condition (F(1,22) < 1; NS), but there was still significant effect of maternal migraine on startle amplitude during the threat condition (F(1,22) = 4.3; P < 0.05) and the threat-safe difference (F(1,23)=5.7; P < 0.05) (Figure 1, Table 2B). Because ITI startle did not have a significant main effect on fear potentiated startle (threat safe difference score), it was not included in the final model for this variable. The best fitting model for ITI startle also showed a significant main effect of maternal history of anxiety (F(1,22) = 4.4; P < 0.05) and an interaction between maternal history of anxiety disorder and age (F(1,22) = 4.6; P < 0.05) (Table 2B). Stratification by maternal anxiety and age revealed that the response to the startle during ITI was largest in offspring with positive maternal history of anxiety who were older than 18 years. No significant interactions were found in best fitting models for startle amplitude during the threat condition and the threat-safe difference.
Figure 1.
Mean (+ SE) amplitudes of startle in ITI, safe, threat conditions, and threat-safe difference by maternal migraine. Statistics: * - p<0.05 for the effect of maternal migraine in a Mixed effects linear model adjusted for age, ITI startle amplitude (threat only) and maternal anxiety.
Discussion
The major finding of the present study is that offspring of mothers with migraine exhibited an increase in startle reactivity during ITI, as well as during the threat condition after controlling for comorbid anxiety disorders, (see Fig. 1). To our knowledge this is the first report describing acoustic startle reflex in children selected on the basis of parental migraine at risk for migraine. This finding indicates that in addition to the possible trigeminal hyper responsiveness resulting from repeated intense nociceptive stimulation during migraine attacks (Burstein et al., 2000; Weissman-Fogel et al., 2003), migraine may also be associated with greater responsivity to aversive stimuli as indexed here by acoustic startle. The finding of increased reactivity among children who have not yet developed migraine suggests that increased physiological reactivity may be an index of vulnerability for migraine.
These findings support previous studies that documented increased responsivity to visual, acoustic, somatosensory and nociceptive stimuli in people with migraine (Afra et al., 2000; Katsarava et al., 2003; Lang et al., 2004) and supports the hypothesis of crossmodal hypersensitivity in migraine. One possible explanation for such hypersensitivity is thought to be related to low neuronal pre-excitation allowing larger range of activation [for review see (Ambrosini and Schoenen, 2003; Giffin and Kaube, 2002)]. Since the core neural pathway underlying the acoustic startle reflex is formed by cochlear nuclei (Yeomans et al., 2002), the hypersensitivity of startle is can be present at the brain stem level or it can be caused by a top-down modulation. One possible neurobiological mechanism for such hypersensitivity top down modulation could be related to altered central serotonergic transmission. Central serotonergic system is known to modulate acoustic startle (Liechti et al., 2001; Quednow et al., 2004) and altered serotonergic function is believed to be responsible for some of the electrophysiological abnormalities observed in migraine (Wang et al., 1996).
Startle is also very sensitive to fear and anxiety. As seen in this study, startle was potentiated by fear in the threat condition. Baseline startle reactivity is also increased by contextual threat (e.g., participation in an experiment where aversive stimuli are anticipated) (Grillon and Baas, 2003). It is therefore possible that the increased ITI startle was caused by anticipatory anxiety, suggesting greater sensitivity to contextual threat in children of migraineurs compared to those of non migraineurs. Similar results have been reported in children of parents with an anxiety disorder (Grillon et al., 1998). In addition to higher ITI startle, the analysis revealed that children at risk for migraine had higher responses during the threat condition and the threat-safe difference. Discrete threat cues and contextual danger elicit functionally different aversive states, a phasic fear response and a more sustained anxiety state mediated by separate neural systems (Grillon and Baas, 2003; Walker et al., 2003). The present study therefore suggests impairment in both the ‘anxiety’ system as well as the ‘fear’ system in children of migraineurs.
The present study is the first to investigate psychophysiological function as a potential vulnerability factor for the development of migraine in the context of comorbid anxiety disorders. The significant effect of both maternal anxiety and maternal migraine on ITI startle is in accordance with our previous reports (Grillon et al., 1997; Grillon et al., 1998) and indicates that these two diagnostic entities might in part share common pathophysiological mechanisms. Therefore, anxiety-migraine comorbidity needs to be taken into account when investigating either of these disorders alone. The significant interaction between a maternal history of anxiety and age of the offspring on increased ITI startle was attributable to increased amplitude of startle response in older youth, a finding that is consistent with results from a parallel study that demonstrated increased potentiated startle after puberty (Grillon et al. in preparation). This might be related to the behavioral sensitization observed in puberty (Rudolph and Flynn, 2007) and might represent a marker of vulnerability for developing anxiety disorder. There was no significant association between maternal history of anxiety and startle during the threat condition or the threat-safe difference, suggesting that children at risk for anxiety exhibit impairment in the ‘anxiety’ but not the ‘fear’ system. Although the lack of an association between lifetime migraine and baseline startle among the children was unexpected (Katsarava et al., 2003; Sandrini et al., 2002; Weissman-Fogel et al., 2003; Zohsel et al., 2006)., it is likely that this is attributable to the large proportion of youth who had not progressed through the period of risk for migraine (Rasmussen, 1993).
Although maternal inheritance of migraine has been reported in several prior studies (Baier, 1985; Dalsgaard-Nielsen, 1965; Mortimer et al., 1992; Wang et al., 2004), the evidence is inconclusive. Maternal inheritance could be attributable to mitochondrial transmission, pre- or peri-natal complications and/or endocrine factors, or a lower threshold for expression of migraine among women (Merikangas, 1990). The strong predictive value of maternal history of migraine on startle observed in the present study might indicate that this increased startle responsiveness is one of several vulnerability factors involved in the pathogenesis of migraine. This is consistent with the current understanding of migraine as a genetically complex multigenetic disorder (Wessman et al., 2007) and suggests that in combination with other tests, the startle paradigm could be used as a marker of vulnerability for developing migraine.
These findings should be considered in the context of several limitations. First, the sample was originally recruited for a study of comorbidity of anxiety disorders and substance use disorders. Therefore, the migraine probands were either recruited as controls or for comorbid anxiety disorders which might have affected the generalizability of our results. However, relatives of probands in case control studies have been shown to have similar rates to general population samples (Low et al. 2008). HoweverFurthermore, the sampling strategy was controlled in all the analyses and did not influence the associations reported herein. Second, a large proportion of the children had not passed through the age at risk for the development of migraine; therefore, we could not study the association between psychophysiological markers and migraine. Third, although the diagnosis of migraine in this study was based on the I.H.S. criteria (1988), we did not collect comprehensive data on migraine subtypes or on other types of headache.
Strengths of this study include the population based recruitment of probands and controls, inclusion of diagnostic information on both migraine and anxiety/mood disorders in probands and offspring, the prospective design of high risk study, interviewers of children were unaware of the diagnostic status of parents, and the use of a semi-structured diagnostic interview in both parents and offspring.
In conclusion, the present study reports higher responsiveness to acoustic startle in children at high risk for developing migraine and anxiety disorder. This highlights the importance of assessing migraine and anxiety comorbidity when investigating the pathophysiology of these disorders and suggests that acoustic startle may be a possible marker for estimating vulnerability for developing these disorders. Further studies need to be performed to test the role of affective disorders and to verify this hypothesis by follow-up assessments of high-risk children. It also might be worthwhile to investigate the actual neurobiological mechanisms involved through the application of paradigms tapping into systems involved in central regulatory mechanisms in migraine.
Acknowledgments
This research was supported in part by the Intramural Program of the National Institute of Mental Health, National Institutes of Health.
Footnotes
Disclaimer
The viewpoints expressed in this article do not necessarily represent those of the National Institutes of Health or the Department of Health and Human Services
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Afra J, Proietti Cecchini A, Sandor PS, Schoenen J. Comparison of visual and auditory evoked cortical potentials in migraine patients between attacks. Clin Neurophysiol. 2000;111:1124–9. doi: 10.1016/s1388-2457(00)00271-6. [DOI] [PubMed] [Google Scholar]
- Ambrosini A, Schoenen J. The electrophysiology of migraine. Curr Opin Neurol. 2003;16:327–31. doi: 10.1097/01.wco.0000073945.19076.1f. [DOI] [PubMed] [Google Scholar]
- Baier WK. Genetics of migraine and migraine accompagnee: a study of eighty-one children and their families. Neuropediatrics. 1985;16:84–91. doi: 10.1055/s-2008-1052549. [DOI] [PubMed] [Google Scholar]
- Burstein R, Yarnitsky D, Goor-Aryeh I, Ransil BJ, Bajwa ZH. An association between migraine and cutaneous allodynia. Ann Neurol. 2000;47:614–24. [PubMed] [Google Scholar]
- Headache Classification Committee of the International Headache Society. Classification and diagnostic criteria for headache disorders, cranial neuralgias and facial pain. Cephalalgia. 1988;8(Suppl 7):1–96. [PubMed] [Google Scholar]
- Dalsgaard-Nielsen T. Migraine and heredity. Acta Neurol Scand. 1965;41:287–300. doi: 10.1111/j.1600-0404.1965.tb04300.x. [DOI] [PubMed] [Google Scholar]
- Di Clemente L, Coppola G, Magis D, Fumal A, De Pasqua V, Di Piero V, et al. Interictal habituation deficit of the nociceptive blink reflex: an endophenotypic marker for presymptomatic migraine? Brain. 2007;130:765–70. doi: 10.1093/brain/awl351. [DOI] [PubMed] [Google Scholar]
- Diagnostic and Statistical Manual of Mental Disorders. 4, Text Revision. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- Endicott J, Spitzer RL. A diagnostic interview: the schedule for affective disorders and schizophrenia. Arch Gen Psychiatry. 1978;35:837–44. doi: 10.1001/archpsyc.1978.01770310043002. [DOI] [PubMed] [Google Scholar]
- Gantenbein AR, Sandor PS. Physiological parameters as biomarkers of migraine. Headache. 2006;46:1069–74. doi: 10.1111/j.1526-4610.2006.00500.x. [DOI] [PubMed] [Google Scholar]
- Giffin NJ, Kaube H. The electrophysiology of migraine. Curr Opin Neurol. 2002;15:303–9. doi: 10.1097/00019052-200206000-00013. [DOI] [PubMed] [Google Scholar]
- Goadsby PJ. Recent advances in understanding migraine mechanisms, molecules and therapeutics. Trends Mol Med. 2007;13:39–44. doi: 10.1016/j.molmed.2006.11.005. [DOI] [PubMed] [Google Scholar]
- Grillon C, Dierker L, Merikangas KR. Startle modulation in children at risk for anxiety disorders and/or alcoholism. J Am Acad Child Adolesc Psychiatry. 1997;36:925–32. doi: 10.1097/00004583-199707000-00014. [DOI] [PubMed] [Google Scholar]
- Grillon C, Ameli R. Effects of threat and safety signals on startle during anticipation of aversive shocks, sounds, and airblasts. Journal of Psychophysiology. 1998;12:329–337. [Google Scholar]
- Grillon C, Dierker L, Merikangas KR. Fear-potentiated startle in adolescent offspring of parents with anxiety disorders. Biol Psychiatry. 1998;44:990–7. doi: 10.1016/s0006-3223(98)00188-7. [DOI] [PubMed] [Google Scholar]
- Grillon C, Merikangas KR, Dierker L, Snidman N, Arriaga RI, Kagan J, et al. Startle potentiation by threat of aversive stimuli and darkness in adolescents: a multi-site study. Int J Psychophysiol. 1999;32:63–73. doi: 10.1016/s0167-8760(99)00002-1. [DOI] [PubMed] [Google Scholar]
- Grillon C, Baas J. A review of the modulation of the startle reflex by affective states and its application in psychiatry. Clin Neurophysiol. 2003;114:1557–79. doi: 10.1016/s1388-2457(03)00202-5. [DOI] [PubMed] [Google Scholar]
- Haan J, Terwindt GM, Ferrari MD. Genetics of migraine. Neurol Clin. 1997;15:43–60. doi: 10.1016/s0733-8619(05)70294-2. [DOI] [PubMed] [Google Scholar]
- Katsarava Z, Giffin N, Diener HC, Kaube H. Abnormal habituation of ‘nociceptive’ blink reflex in migraine--evidence for increased excitability of trigeminal nociception. Cephalalgia. 2003;23:814–9. doi: 10.1046/j.1468-2982.2003.00591.x. [DOI] [PubMed] [Google Scholar]
- Lang E, Kaltenhauser M, Neundorfer B, Seidler S. Hyperexcitability of the primary somatosensory cortex in migraine--a magnetoencephalographic study. Brain. 2004;127:2459–69. doi: 10.1093/brain/awh295. [DOI] [PubMed] [Google Scholar]
- Liechti ME, Geyer MA, Hell D, Vollenweider FX. Effects of MDMA (ecstasy) on prepulse inhibition and habituation of startle in humans after pretreatment with citalopram, haloperidol, or ketanserin. Neuropsychopharmacology. 2001;24:240–52. doi: 10.1016/S0893-133X(00)00199-8. [DOI] [PubMed] [Google Scholar]
- Littell RC, Milliken GA, Stroup WW, Wolfinger RD, Schabenberger O. SAS for Mixed Models. 2. SAS Publishing; 2006. [Google Scholar]
- Low NC, Cui L, Merikangas KR. Community versus clinic sampling: effect on the familial aggregation of anxiety disorders. Biol Psychiatry. 2008;63:884–90. doi: 10.1016/j.biopsych.2007.08.011. [DOI] [PubMed] [Google Scholar]
- Merikangas KR. Genetic epidemiology of migraine. Oxford: Oxford University Press; 1990. [Google Scholar]
- Merikangas KR, Angst J, Isler H. Migraine and psychopathology. Results of the Zurich cohort study of young adults. Arch Gen Psychiatry. 1990;47:849–53. doi: 10.1001/archpsyc.1990.01810210057008. [DOI] [PubMed] [Google Scholar]
- Merikangas KR. Genetics of migraine and other headache. Curr Opin Neurol. 1996;9:202–5. doi: 10.1097/00019052-199606000-00008. [DOI] [PubMed] [Google Scholar]
- Morrell CH. Likelihood Ratio Testing of Variance Components in the Linear Mixed-Effects Model Using Restricted Maximum Likelihood. Biometrics. 1998;54:1560–1568. [PubMed] [Google Scholar]
- Mortimer MJ, Kay J, Jaron A, Good PA. Does a history of maternal migraine or depression predispose children to headache and stomach-ache? Headache. 1992;32:353–5. doi: 10.1111/j.1526-4610.1992.hed3207353.x. [DOI] [PubMed] [Google Scholar]
- Quednow BB, Kuhn KU, Hoenig K, Maier W, Wagner M. Prepulse inhibition and habituation of acoustic startle response in male MDMA (‘ecstasy’) users, cannabis users, and healthy controls. Neuropsychopharmacology. 2004;29:982–90. doi: 10.1038/sj.npp.1300396. [DOI] [PubMed] [Google Scholar]
- Radat F, Swendsen J. Psychiatric comorbidity in migraine: a review. Cephalalgia. 2005;25:165–78. doi: 10.1111/j.1468-2982.2004.00839.x. [DOI] [PubMed] [Google Scholar]
- Rasmussen BK. Migraine and tension-type headache in a general population: precipitating factors, female hormones, sleep pattern and relation to lifestyle. Pain. 1993;53:65–72. doi: 10.1016/0304-3959(93)90057-V. [DOI] [PubMed] [Google Scholar]
- Rudolph KD, Flynn M. Childhood adversity and youth depression: influence of gender and pubertal status. Dev Psychopathol. 2007;19:497–521. doi: 10.1017/S0954579407070241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell MB, Iselius L, Olesen J. Inheritance of migraine investigated by complex segregation analysis. Hum Genet. 1995;96:726–30. doi: 10.1007/BF00210307. [DOI] [PubMed] [Google Scholar]
- Sandor PS, Afra J, Proietti-Cecchini A, Albert A, Schoenen J. Familial influences on cortical evoked potentials in migraine. Neuroreport. 1999;10:1235–8. doi: 10.1097/00001756-199904260-00015. [DOI] [PubMed] [Google Scholar]
- Sandrini G, Proietti Cecchini A, Milanov I, Tassorelli C, Buzzi MG, Nappi G. Electrophysiological evidence for trigeminal neuron sensitization in patients with migraine. Neurosci Lett. 2002;317:135–8. doi: 10.1016/s0304-3940(01)02447-8. [DOI] [PubMed] [Google Scholar]
- Schoenen J, Ambrosini A, Sandor PS, Maertens de Noordhout A. Evoked potentials and transcranial magnetic stimulation in migraine: published data and viewpoint on their pathophysiologic significance. Clin Neurophysiol. 2003;114:955–72. doi: 10.1016/s1388-2457(03)00024-5. [DOI] [PubMed] [Google Scholar]
- Siniatchkin M, Kirsch E, Kropp P, Stephani U, Gerber WD. Slow cortical potentials in migraine families. Cephalalgia. 2000;20:881–92. doi: 10.1046/j.1468-2982.2000.00132.x. [DOI] [PubMed] [Google Scholar]
- Siniatchkin M, Kropp P, Gerber WD. Contingent negative variation in subjects at risk for migraine without aura. Pain. 2001;94:159–67. doi: 10.1016/S0304-3959(01)00350-5. [DOI] [PubMed] [Google Scholar]
- Walker DL, Toufexis DJ, Davis M. Role of the bed nucleus of the stria terminalis versus the amygdala in fear, stress, and anxiety. Eur J Pharmacol. 2003;463:199–216. doi: 10.1016/s0014-2999(03)01282-2. [DOI] [PubMed] [Google Scholar]
- Wang Q, Ito M, Adams K, Li BU, Klopstock T, Maslim A, et al. Mitochondrial DNA control region sequence variation in migraine headache and cyclic vomiting syndrome. Am J Med Genet A. 2004;131:50–8. doi: 10.1002/ajmg.a.30323. [DOI] [PubMed] [Google Scholar]
- Wang W, Timsit-Berthier M, Schoenen J. Intensity dependence of auditory evoked potentials is pronounced in migraine: an indication of cortical potentiation and low serotonergic neurotransmission? Neurology. 1996;46:1404–9. doi: 10.1212/wnl.46.5.1404. [DOI] [PubMed] [Google Scholar]
- Weissman-Fogel I, Sprecher E, Granovsky Y, Yarnitsky D. Repeated noxious stimulation of the skin enhances cutaneous pain perception of migraine patients in-between attacks: clinical evidence for continuous sub-threshold increase in membrane excitability of central trigeminovascular neurons. Pain. 2003;104:693–700. doi: 10.1016/S0304-3959(03)00159-3. [DOI] [PubMed] [Google Scholar]
- Wessman M, Terwindt GM, Kaunisto MA, Palotie A, Ophoff RA. Migraine: a complex genetic disorder. Lancet Neurol. 2007;6:521–32. doi: 10.1016/S1474-4422(07)70126-6. [DOI] [PubMed] [Google Scholar]
- Woolf CJ, Salter MW. Neuronal plasticity: increasing the gain in pain. Science. 2000;288:1765–9. doi: 10.1126/science.288.5472.1765. [DOI] [PubMed] [Google Scholar]
- Yeomans JS, Li L, Scott BW, Frankland PW. Tactile, acoustic and vestibular systems sum to elicit the startle reflex. Neurosci Biobehav Rev. 2002;26:1–11. doi: 10.1016/s0149-7634(01)00057-4. [DOI] [PubMed] [Google Scholar]
- Zohsel K, Hohmeister J, Oelkers-Ax R, Flor H, Hermann C. Quantitative sensory testing in children with migraine: preliminary evidence for enhanced sensitivity to painful stimuli especially in girls. Pain. 2006;123:10–8. doi: 10.1016/j.pain.2005.12.015. [DOI] [PubMed] [Google Scholar]