Abstract
Antiphospholipid syndrome (APS) is primarily considered to be an autoimmune pathological condition that is also referred to as "Hughes syndrome". It is characterized by arterial and/or venous thrombosis and pregnancy pathologies in the presence of anticardiolipin antibodies and/or lupus anticoagulant. APS can occur either as a primary disease or secondary to a connective tissue disorder, most frequently systemic lupus erythematosus (SLE). Damage to the nervous system is one of the most prominent clinical constellations of sequelae in APS and includes (i) arterial/venous thrombotic events, (ii) psychiatric features and (iii) other non-thrombotic neurological syndromes. In this overview we compare the most important vascular ischemic (occlusive) disturbances (VIOD) with neuro-psychiatric symptomatics, together with complete, updated classifications and hypotheses for the etio-pathogenesis of APS with underlying clinical and laboratory criteria for optimal diagnosis and disease management.
Keywords: Antiphospholipid syndrome, antiphospholipid antibodies, ischemic, occlusive, neurological, classifications, etio-pathogenesis, criteria
INTRODUCTION
Antiphospholipid (antibody) syndrome (APS) is a pathological condition that is also referred to as "Hughes syndrome." It originates from excess accumulation of blood clots by antiphospholipid antibodies (aPLs). The syndrome may occur as a primary condition (primary APS) or along with the autoimmune disease, systemic lupus erythematosus (SLE or lupus). SLE is a chronic disease that affects certain organs, blood vessels, or the skin. The main signs of APS include blotchy skin, migraine, memory loss, fatigue, deep vein thrombosis, pulmonary embolism, and stroke. Primary APS may affect heart valves and present with such damage in 30% of patients. In pregnant women with APS, miscarriages may occur.
In this overview, we present an up-to-date description and synthesis of the main vascular ischemic (occlusive) diseases (VIOD) with neuropsychiatric symptomatics in APS. The recognition that a number of SLE manifestations have a thrombotic rather than an inflammatory basis can be considered one of the most important recent contributions to rheumatology and immunology. The "anticardiolipin syndrome" described by Graham Hughes in the 1980s,1 which was subsequently renamed antiphospholipid (Hughes) syndrome, appeared as a frequent condition in patients with SLE2 but also was present in others without SLE or other autoimmune diseases. In such cases it was termed a "primary" APS (PAPS)3.
Almost 20 years after its definition, APS has crossed over into many fields of medicine. However, the full spectrum of the syndrome has yet to be defined, and significant advances in the diagnosis and management of patients with APS have been made. In addition, a consensus about very important questions, such as the use of alternative tests for anticardiolipin (aCL) antibodies and lupus anticoagulant (LA) assays, the treatment of pregnancy failure, or the intensity of anticoagulant therapy, has not yet been achieved.
CLASSIFICATIONS, ETIO-PATHOGENESIS AND CRITERIA FOR APS
An expert's council at the Eighth International Symposium on antiphospholipid antibodies at Sapporo (Japan)4 has recently established the preliminary criteria for the classification of definite APS (Table 1). Clinical criteria include arterial, venous, and/or small vessel thrombosis as well as recurrent (three or more) miscarriage, one fetal death or prematurity due to severe preeclampsia or placental insufficiency. Laboratory criteria only consider aCL levels at medium to high titers or LA if any of them are positive on two separate occasions at least six weeks apart. These criteria were established for research but also provide a working basis for clinical diagnosis. The criteria have been validated in a cross-sectional trial of 243 patients with primary and secondary APS, SLE, or lupus-like disease. They have shown a 71% sensitivity and a 98% specificity with positive and negative predictive values of 95% and 88%, respectively, to correctly classify patients with APS.5 Recently, a revised version of the APS classification criteria has been presented (Table 1).
Table 1.
Classification of APS
*Definite antiphospholipid syndrome is considered to be present if at least one clinical and one laboratory criteria are met.
†Classification of APS should be avoided if less than 12 weeks or more than 5 years separate the positive aPL test and the clinical manifestation.
‡Two subgroups of APS patients should be recognized, according to: (a) the presence, and (b) the absence of additional risk factors for thrombosis. Indicative (but not exhaustive) such cases include: age (> 55 in men, and > 65 in women), and the presence of any of the established risk factors for cardiovascular disease (hypertension, diabetes mellitus, elevated LDL or low HDL cholesterol, cigarette smoking, family history of premature cardiovascular disease, body mass index > 30, microalbuminuria, estimated GFR < 60 mL min-1), inherited thrombophilias, oral contraceptives, nephrotic syndrome, malignancy, immobilization, and surgery. Thus, patients who fulfil criteria should be stratified according to contributing causes of thrombosis. A thrombotic episode in the past could be considered as a clinical criterion, provided that thrombosis is proved by appropriatediagnostic means and that no alternative diagnosis or cause of thrombosis is found. Superficial venous thrombosis is not included in the clinical criteria. Generally accepted features of placental insufficiency include: (i) abnormal or non-reassuring foetal surveillance test(s), e.g. a non-reactive non-stress test, suggestive of foetalhypoxemia, (ii) abnormal Doppler flow velocimetry waveform analysis suggestive of foetal hypoxemia, e.g. absent end-diastolic flow in the umbilical artery, (iii) oligohydramnios, e.g. an amniotic fluid index of 5 cm or less, or (iv) a postnatal birth weight less than the 10th percentile for the gestational age.
§ In studies of populations of patients who have more than one type of pregnancy morbidity, investigators are strongly encouraged to stratify groups of subjects according to a, b, or c below.
¶Investigators are strongly advised to classify APS patients in studies into one of the following categories: I, more than one laboratory criteria present (any combination);IIa, LA present alone; IIb, aCL antibody present alone; IIc, anti-b2 glycoprotein-I antibody present alone.
Antiphospholipid antibody testing
Laboratory work is crucial in the identification of patients with APS. Clinical suspicion based on recurrent thrombosis, miscarriage or other features of APS must be always confirmed by positive tests that detect the presence of aPLs. Ideally, these tests must have a high sensitivity and specificity. In addition, standardization and a high level of intra and inter-laboratory reproducibility are desirable, so patients tested at different times and places do not exhibit different results.
The two most standardized techniques for the detection of aPLs are the enzyme-linked immunosorbent assay (ELISA) for aCL and the coagulation-based assays for LA. These two methods do not necessarily identify the same antibodies and are not completely coincident (up to 30% of patients exhibit positivity to only one of them), making it necessary to routinely determine both. It is significant to note that aCL assays, which use β2-glycoprotein I (β2GPI) as a cofactor, detect antibodies of the IgG, IgM and IgA isotypes,6 but only the former two have been adopted into clinical practice as laboratory criteria by the original Sapporo panel.4 Moreover, persistent positivity (of aCL, LA or both) and medium-to-high levels of aCL on a semi-quantitative scale are required to consider them clinically significant.
The LA test consists of a combination of coagulation assays which must demonstrate that the prolongation of phospholipid-dependent tests corrects by adding reagents with a high concentration of phospholipids but not by adding normal plasma7. The most widely used tests include a modified activated partial thromboplastin time (APTT), the dilute Russell's Viper Venom Time (dRVVT) and the Kaolin Clotting Time (KCT). Recent guidelines for the detection of LA have been published.8
Various assays for the detection of antibodies to other phospholipids or phospholipid-binding proteins (phosphatidylserine, β2GPI, prothrombin, protein C, etc.) are available, but they are less standardized and are not recommended for sustaining clinical decisions in daily practice.9
According to these results, the role of these more specific but less sensitive alternative tests should be to confirm a diagnosis of APS in the context of atypical manifestations or borderline aCL and LA results, rather than to test patients with APS features who are aCL-negative. In this sense it was found that testing for anti-β2GPI does not identify additional patients with APS among those with recurrent miscarriage or fetal death who are negative for aCL.10
Etiology and pathogenetic mechanisms
APS is mainly considered an autoimmune disorder where a vascular thrombosis and/or recurrent pregnancy pathologies are observed in patients with laboratory evidence for antibodies against phospholipids or phospholipid-binding protein cofactors. As mentioned above, the clinical manifestations of the syndrome include venous and arterial thromboses and embolisms, disseminated large and small vessel thromboses with accompanying multi-organ ischaemia and infarction, stroke, premature coronary artery disease, and spontaneous pregnancy losses.11
The aetiology of APS is multifactorial,11,12 and an exact, single cause cannot always be determined. A number of hypotheses have been proposed to explain the pathophysiology of cellular, molecular, and genetic involvement in APS, as well as the pathogenesis by which the presence of antibodies reinforces its clinical appearance. The most important conjectures are summarized here.
(i) The first pathogenetic pathway implicates an activation of the endothelium. The antiphospholipid antibodies bind and activate the endothelial cells, whereas the expression of adhesion molecules is increased together with a higher secretion of cytokines and activated prostacyclin metabolism. aPLs recognize β2-glycoprotein I as being bound to resting endothelial cells, although the basis for the interaction of β2GPI with viable endothelial cells remains unclear.
(ii) The second hypothesis indicates an oxidant-mediated injury of the vascular endothelium. Oxidized low-density lipoprotein (LDL) is absorbed by macrophages thus leading to macrophage activation and subsequent damage to endothelial cells. Autoantibodies to oxidized LDL appear in association with aCL, and it is possible that a cross-reaction of aCL with oxidized LDL could take place. It is important that aCLs bind to oxidized cardiolipin-recognizing oxidized phospholipids, phospholipid-binding proteins, or both.
(iii) The third mechanism involves the interference of aPLs with or the modulation of the function of phospholipid-binding proteins involved in the coagulation regulatory system (it has been suggested that β2GPI may represent a natural anticoagulant). The high affinity of the aPL/β2GPI complex for phospholipid membranes is considered a critical step in the mechanism of APS.11 For example, molecular "mimicry" between β2GPI related synthetic peptides and structures within bacteria, viruses (e.g., cytomegalovirus) and the tetanus toxoid could explain the appearance of APS in such conditions (see below).13 Additional pathways where aPLs interfere with the regulation of protein C, annexin V, prothrombin, and tissue factor have also been suggested.12,13
(iv) The fourth approach to thrombosis in APS is related to heparin-induced thrombocytopenia (a thrombosis in multiple arterial and venous beds is observed in both pathologies). In heparin-induced thrombocytopenia, a prior cardiovascular disease determines the site of thrombosis while a high recurrence rate of similar thrombotic events is observed in APS. Notably, a "second hit" (e.g., vascular damage) may be needed for thrombosis to appear. However, it is not very clear which cellular phospholipids and phospholipid-binding proteins are bound by aPLs in vivo. The absence of anionic phospholipids on the cell surface and the lack of reactivity of aPLs with intact cells suggest that cell membrane injury may be necessary for aPLs to bind. Indeed, some aPLs bind to activated platelets and apoptotic cells after the normal asymmetric distribution of membrane phospholipids have been lost and the anionic phospholipids have been exposed on the cell surface. Both the binding to and induction of aPLs by apoptotic cells are dependent on β2GPI;
(v) The fifth "triggering" hypothesis, which has been emphasized recently although it is not always apparent in APS,13,14 is based on the abovementioned molecular mimicry with infections and/or other pathologic events. Many infections may be accompanied by increases in aPLs and, frequently, by clinical APS manifestations (e.g., 18% skin infections, 17% human immunodeficiency virus infection, 14% pneumonia, 13% hepatitis C virus infections and 10% urinary tract infections). Other, less common infections in APS include mycoplasma, pulmonary tuberculosis, malaria, Pneumocystis carinii, and leptospirosis. Although IgM isotypes of aCLs seem to be the primary products, increases in IgG have also been detected. Moreover, even though the initial reports indicated that infection-associated aPLs were β2GPI-independent and nonpathogenic, several later studies clearly documented their reactivity with β2GPI.14 It appears that APS in infection is also developed by a two-hit mechanism, which is similar to the vascular damage described previously. It is also possible that the infections occurred long before the autoimmune manifestation emerged. In particular, it was observed that "triggering" factors become increasingly apparent in catastrophic APS, and these were present in 51% of cases in a recent analysis by Cervera et al. in 2003 [as cited in 14]. Such factors included trauma (surgical, both major and minor), anticoagulation withdrawal, a variety of carcinomas, and, most importantly and commonly, infections, which were described in 24% of these patients.
MAIN CLINICAL FEATURES OF APS
The "classical" clinical features associated with APS, as originally introduced, include recurrent thrombosis and pregnancy losses. The main pregnancy-related complications consist of maternal complications: women with aPLs are more likely to develop a postpartum cardiopulmonary syndrome,15,16 chorea gravidarum17, postpartum cerebral infarct following aspirin withdrawal18, and maternal death19. It is suggested that clinical thrombosis is associated with pregnancy and the postpartum period pathologies. The other manifestation is the so-called "HELLP Syndrome." It was found that preeclamptic women may present with hemolysis (H), elevated liver enzymes (EL) and a low platelet count (P). Notably, HELLP syndrome may also occur in the absence of severe preeclampsia. Although typically encountered during pregnancy, HELLP syndrome may be atypical and persist into the postpartum period. There are doubts as to whether this syndrome represents a variant of preeclampsia or whether it represents a hypercoagulable state with thrombotic microangiopathy, and this is the reason we described these obstetric symptomatics separately from the rest of the clear vaso-ischemic pathologies. Earlier evidence for an association between this syndrome and aPLs was published in 199420 with demonstrated aCL antibodies and a refractory appearance despite delivery, corticosteroids and anticoagulation therapy. The clinical course also included a macular rash that extended to the palms. The placental pathology and skin biopsies revealed diffuse deposition of fibrin with small vessel thrombi; these were resolved by plasmapheresis, and it was postulated that aPLs may have contributed to the refractoriness. Notably, early (35.4%) or late fetal loss (16.9%), premature births (10.6%) and preeclampsia (9.5%) are the most frequent fetal and obstetric manifestations of APS.21
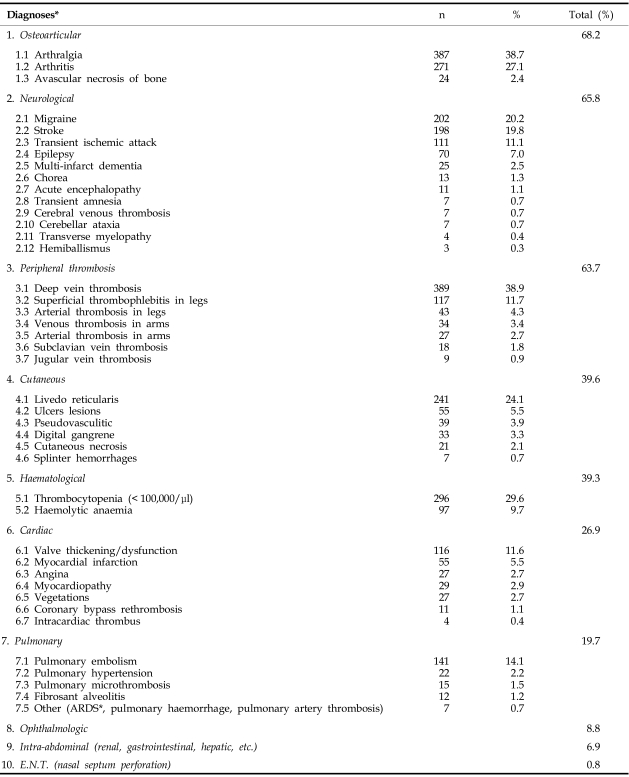
As previously mentioned, the classical clinical picture of APS is characterized by venous and arterial thromboses, fetal losses and thrombocytopenia in the presence of aPLs, LA, aCL or antibodies to the protein "co-factor" beta 2 glycoprotein 1. APS can be found in patients having neither clinical nor laboratory evidence of another definable condition (primary APS), or it may be associated with other diseases (secondary APS, e.g., after SLE). Single vessel involvement or multiple vascular occlusions may give rise to a wide variety of presentations as summarized below, beyond the obstetric symptomatics alone. Any combination of vascular occlusive events may occur in the same individual (Table 2). The time interval between these events can also vary considerably, from weeks to months or even years. Rapid chronological occlusive events, occurring over days to weeks, have been referred to as "catastrophic" APS (CAPS).22
Table 2.
APS and Cumulative Incidence of Main Clinical Diagnoses (× 1000 Patients)
*Obstetric diagnoses are not included. ARDS, adult respiratory distress syndrome. With modifications from Asherson RA, Cervera R. Unusual manifestations of the antiphospholipid syndrome (http://www.rheuma21st.com/downloads/cutting_edge_unsual_aps_asherson.pdf) Cutting Edge Reports, Rheuma21st, 2002.
The percent (relative) cumulative distribution of APS-related pathologies is presented in Table 2 as an example based on a population cohort of 1000 APS patients. According to the largest survey of APS patients to date,21 deep vein thrombosis, sometimes accompanied by pulmonary embolism, is the most frequently reported manifestation of this syndrome (38.9%). Conversely, cerebrovascular accidents, either stroke (19.8%) or transient ischemic attacks (11.1%), are the most common arterial thrombotic manifestations. In addition, several other clinical features are relatively common in these patients, i.e. thrombocytopenia (29.6%), livedo reticularis (24.1%), heart valve lesions (11.6%), hemolytic anemia (9.7%), epilepsy (7%), myocardial infarction (5.5%), leg ulcers (5.5%), and amaurosis fugax (5.4%). Below we summarize and describe the main patterns of the most important vaso-ischemic (occlusive) diseases (VIOD) in APS.
APS AND VASO-ISCHEMIC (OCCLUSIVE) DISEASES WITH NEUROPSYCHIATRIC SYMPTOMATICS
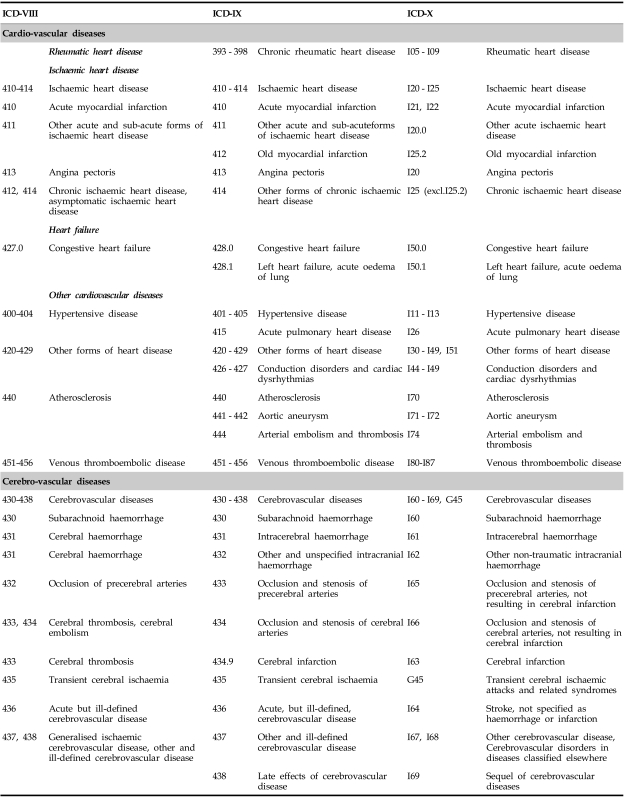
For the purpose of this overview, we describe here all VIODs in APS, and later we emphasize those with prevalently-expressed neuropsychiatric symptomatics. Since the cardiovascular and cerebrovascular pathologies in APS are potentially the most deadly and life-threatening conditions (especially in catastrophic APS), although not the most frequent (Table 2), we present here in a summarized form their evolving classifications in order to compare them (Table 3).
Table 3.
Classifications of Cardio- and Cerebrovascular Diseases (ICD-VIII, IX and X Revisions)*
*With modifications from EUROCISS Project Final Report 2006 (http://ec.europa.eu/health/ph_projects/2003/action1/docs/2003_1_10_frep_en.pdf).
Vascular-ischemic/occlusive diseases (VIOD) in APS
Cardiac complications
One of the most important groups of VIODs in APS includes those with cardiac manifestations. For instance, intracardiac thrombi in the ventricular cavities are reported in patients with aPLs.23,24 Such patients may present with systemic or pulmonary embolic symptoms (e.g. transient ischemic attacks (TIAs), stroke, pulmonary infarction) depending on the location of the thrombus (right or left ventricle; the thrombus forms on the akinetic segments of the ventricle). Occasionally, a clot may form even on a normal mitral valve.25 In other APS patients, multiple small vascular occlusions ("thrombotic microvasculopathy") develop and are responsible for acute or chronic cardiomyopathy. Acute cardiac collapse (with eventual respiratory decompensation) is frequent in catastrophic APS and is one of the most common causes of death in such patients. Isolated circulatory failure has also been reported,26 as has renal thrombotic microangiopathy. However, chronic cardiomyopathy may be global or localized, whereas a segmental ventricular dysfunction can supervene.27 Impaired left ventricular diastolic filling was also observed28 and associated with cardiomyopathy or myocardial ischaemia (the latter provoked by coronary arteriolar occlusions). Both may lead to myocardial fibrosis and a decrease in left ventricular compliance.
Cyanotic congenital heart disease with elevated aCL has also been reported (with thrombotic episodes and/or thrombocytopenia).29 At the same time, post-surgery complications in APS have also been reported: a hypercoagulable condition has been detected in 10% of patients undergoing cardiovascular surgical procedures leading to a higher incidence of early graft thrombosis (27% vs. 1.6%).30 A constellation of risk factors identified the highest-risk patient: female, young, non-smoker, and more likely to have upper extremity involvement than patients who were aPL-negative.31 Other authors32 have found that 26% of patients were aPL-positive and were 1.8 times more likely to have undergone previous lower-extremity vascular surgical procedures and 5.6 times more likely to have suffered occlusion during previous reconstructions.
Osteoarticular symptomatics
The osteoarticular syndromes have shown the highest relative cumulative frequency among all APS complications (Table 2). It has been suggested that the avascular necrosis (AVN) of bone in SLE patients is probably multifactorial.33 A possible link between AVN and aPLs has been postulated,34 but the relationship is still unproven; the lack of other risk factors points to a suspected etiological role of antiphospholipid antibodies in AVN syndrome.35
Dermatological appearances
The dermatological syndromes are the most obvious signs of APS, for instance, superficial skin necrosis,36,37 including necrosing livedo reticularis.38 To note, widespread cutaneous necrosis is associated with massive thrombosis of the small and medium-sized dermal vessels in primary APS,39 SLE,40 rheumatoid arthritis,41 and mycosis fungoides.42 Erythematous macules and painful nodules have been reported in aPL-positive patients as being due to thrombotic skin disease,43,44 and they can possibly improve with salicylates.45 Nail symptoms such as multiple subungual haemorrhages,36,46 in particular with amaurosis fugax47 or splinter haemorrhage in general48 or ischaemia-provoked aPL-associated gangrene, e.g., in SLE (different from vasculitis, cryoglobulinemia or disseminated intravascular coagulation), have been reported. The latter is a hallmark of the cutaneous complications of catastrophic APS. Additionally, other dermatological syndromes such as anetoderma,49,50 discoid LE49 or intravascular coagulation necrosis have also been observed in aPL-positive patients. The large variety of clinical events in aPL-positive patients differing from underlying thrombotic lesions (e.g. chorea) supports the multifactorial action of these antibodies (i.e., there is possibly systemic cellular involvement for extrapyramidal damage in an APS patient, as recently discussed by Atanassova and Dimitrov51), thus suggesting that long-term anticoagulation therapy may not be needed, at least in these particular cases.
Pulmonary complications
A major pulmonary arterial occlusion by thrombosis is rare;52 pulmonary microthromboses are also rare in aPL- positive patients.53 This rarity might be explained by eventual mechanical disruption of clots in the lungs (i.e., similar to cancer cells destroyed in lung microvasculature54). Adult respiratory distress syndrome (ARDS)55 has been reported in APS and CAPS, together with adrenal hypofunction. This suggests a "cause and effect" to increased cytokines following tissue damage, possibly as a part of the so-called "systemic inflammatory response syndrome" (SIRS). Intra-alveolar pulmonary haemorrhage56,57 is another sign of APS, together with pulmonary capillaritis, microvascular thrombi and bronchiolitis obliterates. Fibrosing alveolitis was also reported with APS.58 Spiking fevers, pleuritic chest pain associated with pleural effusion and patchy infiltration (postpartum syndrome) were described15,16 as forming part of the clinical picture of CAPS.
Renal diseases
The renal pathologies in ASP, which are due to VIODs, are widespread with various presentations, although they are not very common. Glomerular capillary thrombosis in lupus nephritis59-62 may affect up to 48% of APS/aPL-positive patients and is highly predictive of sclerosis progression, possibly due to inflammatory processes. Thrombotic microangiopathy,63 similar in pathological appearance to scleroderma, eclampsia, thrombotic thrombocytopenic purpura (TTP)-hemolytic uremic syndrome (HUS) conditions and transplant rejection64 may occur together with microangiopathic hemolytic anemia, schistocytes and moderate to severe thrombocytopenia.65 Such occurrences may be observed in CAPS,66 but in more benign cases the differential diagnosis from TTP may be difficult.
Adrenal pathologies
Adrenal damage has been reported with APS67,68 even in patients only 10 years old. Recently, acute adrenal insufficiency after such haemorrhage has also been reported.69 Adrenal vein thrombosis and hemorrhagic infarction are the putative causes, since increased adrenal venous pressure may provoke haemorrhage into the gland.67 In addition to such typical vascular arrangements as the "vascular dam",67 stress and several other risk factors for adrenal haemorrhage have also been reported (e.g., severe systemic illnesses, previous thromboembolic disease or post-surgery states). Additionally, this complication has also been seen in many patients who had not been on anticoagulation therapy at all.
Hepatic syndromes
Budd-Chiari syndrome was first observed in APS in 198470; since then, more cases with obstruction of the large hepatic veins resulting in hepatic congestion and liver cell necrosis in APS have been reported.71 An association of portal hypertension (possibly due to thromboembolism/cirrhosis) with aPLs has been found,72,73 as well as a correlation with pulmonary hypertension.72 Thus, it has been suggested74 that a thromboembolism associated with aCL antibodies might be the underlying pathological mechanism in both conditions. Hepatic venoocclusive disease is caused by nonthrombotic concentric narrowing of small centrilobular veins by loose connective tissue, congestion, and cell necrosis in the centrilobular areas.70 This syndrome is possibly associated with nodular liver hyperplasia as well as with bone marrow transplantation.75,76 The presence of aPLs in liver nodular regenerative hyperplasia has been suggested77 to result from a venoocclusive disease or hepatic infarction and is associated with a range of systemic autoimmune disorders.78 In particular, overt hepatic infarction is rare. It has occasionally been described in APS78 as well as during pregnancy and the postpartum period or been associated with HELLP syndrome.
Chronic hepatitis in APS has also been reported.79-81 Various incidences (from about 2% to 17%) of hepatitis C virus (HCV) infection have been observed in aCL positive thrombotic disorders.81,82 In contrast, 33% of patients with chronic HCV infection were found to be positive for aCL. In a recent study, the development of APS in the course of an HCV infection was described.83 Additionally, about half of patients with alcoholic liver disease/a history of alcohol abuse had aCL elevations.82 Positive results for aPLs may range up to as high as 80%84 in alcoholic hepatitis or cirrhosis patients. Notably, cirrhosis is inversely associated with hypercoagulation by aCL; however, one half of the patients with splanchnic thromboses (12%) in biopsy-diagnosed cirrhosis were positive for LA or aCL. At the same time it was found that 75% of LA patients were positive for HCV, which further supports the possible association between chronic HCV infection and aPLs85 Recently, a case of Wilson's disease (hepatolenticular degeneration) was reported along with primary APS in a patient presenting mainly with extrapyramidal damage.86 The latter was supposed to have had an autoimmune aPL component provoking mainly recurrent arterial/venous thrombosis and thrombocytopenia, but a possible systemic cellular involvement by aPLs in the central nervous system (besides thrombosis) may have interacted with a specific genetic background.51
Digestive manifestations
Esophageal necrosis87 and bleeding esophageal varices from portal vein thromboses have been reported in APS. Gastric ulceration with necrosis due to widespread occlusive vascular disease88 as well as small and large bowel vascular occlusions in aPL-positive patients were also reported.89,90 Gastrointestinal haemorrhage as a result of bowel ischaemia or an atypical duodenal ulcer91 occurs most frequently in CAPS patients (abdominal pain is one of the most common manifestations in such cases). Mesenteric inflammatory vasoocclusive disease leading to ischemic injury was associated with APS.92 Half of such cases are "primary" or idiopathic; often, a family history of thromboembolism suggests an inherited hypercoagulable disorder (in fact, a link between idiopathic mesenteric thrombosis and peripheral thrombosis has been shown93). At the same time, thromboembolic complications of inflammatory bowel disease were associated with aPLs (e.g., in ulcerative colitis and Crohn's disease94).
As mentioned above, the abdominal pain in APS is an important initial presentation; it may also be due to a pancreatic involvement by microangiopathy.95 Recently such involvement has been reported in CAPS as well. Acute cholecystitis has also been observed in CAPS patients.96 Last but not least, an occlusion of the splenic vessels was parallel to other vascular occlusions.97 However, splenic infarction/splenic atrophy are rare events, although at least one SLE patient with these complaints has been reported.98
Malignant diseases
Although the role of malignancies in and their associations with APS are not clear, these are the primary causes that precipitate APS development and progression. A recent report99 has shown that more than 25% of the neoplasms in patients having aPLs are haematological malignancies (e.g., B-cell lymphoma, spleen lymphoma, chronic myeloid leukaemia, and non-Hodgkin's lymphoma) and another 27% consist of solid tumours (renal cell carcinoma, lung adenocarcinoma, breast carcinoma, melanoma, and primary tumours of unknown origin). Notably, in 17 of the above 23 cases, CAPS had been possibly triggered by the cancer; it is very important to realize that thrombotic events associated with aPLs, especially in elder patients, may be the initial and/or single manifestation of the malignant disease.
Neuropsychiatric syndromes in APS (predominant vascular-ischemic / occlusive origin)
Since the description and designation of the antiphospholipid (Hughes) syndrome, the links between aPLs and diseases of the nervous system have been considered of major importance.100
The first evidence of large peripheral arterial occlusions in SLE patients appeared in the 1960s.101 Such general vascular-ischemic (occlusion) symptoms as recurring thrombophlebitis, skin infarcts and chronic leg ulcers as well as other arterial occlusions and TIAs were described in these patients. During the following years, more studies on large arterial occlusions and gangrene in SLE patients with aPLs were published.102,103 In addition, an aortic arch syndrome and SLE were also observed,104-106 whereas a positive testing for aPLs was found,105 including occlusions of the abdominal aorta in aPL-positive patients.107,108 Since the first report by Hughes in 1983,109 the cerebral symptomatics in APS patients have become more and more important. Table 4 summarizes CNS manifestations: much of these symptomatics could not be explained solely by hypercoagulability and could have had a more complex origin.51,86 In many patients with chorea, for example, focal lesions on computed tomography (CT) scan, possibly due to thrombosis, have not been found.110,111 To note, aPLs may have more direct effects; etiologically, they may bind neurons or glial cells and disrupt their function.112,113 There is evidence that aPLs may interfere with endothelial cell function and promote the procoagulant activity of endothelial cells.114,115 It has been shown that IgG fractions increase mononuclear cell adhesion to human umbilical vein endothelial cells (HUVEC) in aPL- positive patients. Recently, it was also reported that anti-β2GPI antibodies bound to and activated endothelial cells through the adherent cofactor β2GPI.116 These studies indicate that aPLs focus on the endothelium and damage the vasculature, making it more prone to leukocyte adhesion and thrombosis. It is not known why the brain and CNS are particularly vulnerable in APS patients. The aPLs involvement in thrombosis is well evidenced, e.g., APS was induced after immunization with aCL or β2GPI.117 Furthermore, immunization of BALB/c mice with monoclonal aCL resulted in APS with neurological dysfunction and impaired motor coordination.118,119
Table 4.
APS/Neuro-Psychiatric Presentations
Cerebrovascular disease/cerebral ischaemia
Strokes and transient ischaemic attack (TIA)
These are considered the second most common clinical manifestations of APS after venous thrombosis.120 Cerebrovascular disease (CVD) is the most frequent neurological manifestation in aPL-positive patients.121 The association between CVD and aPLs has been described in earlier studies122 and later confirmed by many other authors.123,124 It was also suggested that aCL and LA represent a kind of aPLs leading to cerebral vascular injury and thrombosis resulting in cerebral infarction. The cerebral ischaemia, which is mainly focal, can be transient or permanent. Recurrent disease often leads to multifocal deficits. For instance, amaurosis fugax,123 transient paresthesias, motor weakness, vertigo, and transient global ischaemia125 can all be expressions of TIAs, either initial or recurrent ones, whereas the latter may occur without a cerebral infarction. The risk for recurrent stroke appears to be increased in APS patients. Generally, the territory of the middle cerebral artery is more commonly affected.126,127 A chronic multifocal disease can produce multi-infarct dementia.123 This dementia, generally associated with a loss of cognitive functions and an impairment of skills, concentration, memory dysfunction, language impairment and judgemental defects, as described below, might be considered secondary if it occurred separately, and it does not present with peculiar characteristics.
Although not very frequent, cardiac emboli may be another cause of cerebral ischaemia in aPL-positive patients (cerebral emboli from Libman-Sacks endocarditis in SLE patients128 or from the heart chambers or the internal carotid artery). Cerebral ischemic events are more frequent in patients with valvular heart disease (e.g., left-sided valve lesions or mitral valve vegetations and mitral regurgitation in SLE patients with aPLs129). Brain magnetic resonance imaging (MRI) in ischemic stroke shows cortical abnormalities consistent with large vessel occlusion in aPL-positive patients. Often, small foci of high signals in the white matter of the brain are seen on MRI. Larger sizes and atypical topographic distributions of these lesions may also be consistent with demyelination and are sometimes difficult to differentiate from MRI pictures seen in multiple sclerosis130 (see below).
There have been reports on recurrent episodes of cerebral ischaemia in patients with livedo reticularis (Sneddon's syndrome) with aPLs suggesting the presence of APS.131,132 A more rapid progression and more severe clinical manifestations are seen in patients with increased aCL levels. At the same time, single photon emission computed tomography (SPECT) in livedo reticularis without focal neurological deficits indicated deficits of cerebral perfusion suggestive of increased risk of Sneddon's syndrome.133 This association between livedo and ischemic stroke, occasionally with hypertension, has been known since 1965.134 The syndrome is more frequent in women, and it is usually diagnosed between 40-50 years of age. A familial clustering is suggestive for a primary APS.135 Early inflammatory reactions (endothelitis) of the small arteries occur as followed by subendothelial cell proliferation leading to partial or complete occlusion.136 Therefore, there is accumulating evidence in support of the association (Sneddon's syndrome) not only in SLE and/or APS but in the general population as well. However, these results are contradictory; most studies revealed an association of aPLs with an increased risk of cerebral ischaemia,137,138 but others did not.139 Some of the results showed that the presence of IgG β2GPI-dependent aCL was characterized by a two-fold increase in the odds of stroke within 15 years versus aCL-negative patients. Antiphosphatidylserine antibodies were also associated with an increased risk for ischemic stroke in the general population.140 As previously mentioned, other studies have not confirmed the presence of aCL and LA with repeated testing, although it is well known that aCL and LA can vary over time (especially during the acute phase of thrombotic events).141 Moreover, neither aCL ELISA nor any cut-off values have all been standardized. However, it is recommended that aPLs should be confirmed again at least one to three months after the thrombotic event.
Recurrent ischemic events in aPL-positive patients are related to aCL at the time of the initial stroke. In consecutive patients with aPLs and focal cerebral ischaemia,142 cerebral infarctions within the first follow-up year supported the data that IgG aCL represented a risk factor for recurrent stroke. In a later study126 this was confirmed, and the subsequent thrombo-occlusive events were associated with IgG aCL (most frequently in patients with aCL ≥ 40 GPL), thus confirming the role of aCL as a risk factor for recurrent stroke. Other studies143 included patients with lower levels of aCL (>10 GPL) who were at lower risk of thrombotic events, but their population was older, with other cardiovascular risk factors that minimized the relative impact of aCL on recurrent stroke. To note, the above positive relationships are probably most relevant to younger populations with evidence of prothrombotic tendencies and little other risk of stroke.
Additionally, acute ischemic encephalopathy has also been observed but is a rare feature in SLE patients with aPLs. Patients are acutely ill, confused and have asymmetrical quadriparesis, hyper-reflexia, and bilateral extensor plantar responses.144 Seizures may also occur; small cortical hypodensities are evident on MRI scanning in such patients. The differential diagnosis lies between acute lupus cerebritis and even steroid psychosis in those with predominantly frontal lobe symptomatology. It is likely that cerebral thrombotic microangiopathy may lead to such acute ischemic encephalopathy as can occur within a catastrophic APS. Additionally, cerebral venous thrombosis is another uncommon appearance in APS but it may be involved in a range of hypercoagulable states,145 especially in young women.146
Transient global amnesia
Sudden, unexplained short-term memory loss was associated with aPLs.147 This was due to migrainous etiology, however, and epileptic seizures were considered pathogenic.148 Often associated with stereotypical behaviour, the transient global amnesia was related to aPL-linked ischaemia.147
Headache
One of the most common features in patients with APS is headache, which can vary from classic intermittent migraines to almost continuous incapacitating states. However, the results are controversial; demonstrating a true association between aCL positivity and migraine is difficult since (i) there is a high frequency of migraine in normal individuals and (ii) a relatively low frequency of aCL in healthy populations. aPLs were also observed in transient neurological symptoms, including migraine with aura. The available data suggest an association between the migraine-like phenomena and aPLs, but not between migraine headache and aPLs. A prospective study149 failed to find an association between the presence of aCL and migraine. Notably, aPLs, especially IgG aCL, is associated with chronic headache in SLE patients, but not with a particular subtype of headache or with migraines.150 For instance, both the aforementioned cerebral venous sinus thromboses and the dural ones have a diverse spectrum of clinical manifestations, the most common being headache accompanied by papilledema, nausea, vomiting, and visual field loss. The cerebral thrombosis leads to a syndrome called "pseudotumour cerebri" (benign intracranial hypertension), which is mainly idiopathic and associated with impaired cerebrospinal fluid dynamics.146,151,152
Epilepsy
Increased aPLs in SLE patients with seizures were found as being higher than the accepted prevalence in SLE.153 It was suggested.154 that seizures in aPLs were due to ischemic events occurring in hypercoagulability (seizures are a well-known symptom of cerebral ischaemia155). Earlier studies156 found a high prevalence of aPLs in SLE patients with seizures compared with controls, providing evidence that the IgG isotype of aCL may have a pathogenic role in SLE-associated epilepsy. This was confirmed later157 with an odds ratio of 3.7 versus no aCL. Another trial158 described in detail the brain injury seen in SLE patients with APS, finding frequent epilepsy (and stroke) with an increase of thrombotic and non-thrombotic brain injures. Angelini et al.159 suggested that aPLs play a role in the immune-mediated pathogenic mechanism for partial epilepsy. Verrot et al.160 reported that aCL was present in 20% of patients and was independent of the type of epilepsy, the antiepileptic treatment, or the age or sex of the patients. These findings were later confirmed161 by showing that the prevalence of aPLs was greater in patients with epilepsy, including those with newly-diagnosed seizure disorders. The prevalence of IgG aCL in localization-related epilepsy was almost twice that in generalized epilepsy, and IgM aCL was higher as well, indicating an immune dysregulation in epilepsy. The aCL-brain phospholipid interaction in SLE may be expressed through a direct reversible mechanism by which aPLs lower the seizure threshold.162 It was also reported that aPLs might bind directly to the ependyma and myelin in the brains of animals.163
Ocular syndromes
Ocular vascular-occlusive diseases
Ocular syndromes are frequent in APS patients.164 Amaurosis fugax is one of the most common appearances. Optic neuropathy (acute retrobulbar optic neuritis, ischemic optic atrophy and progressive optic atrophy) is a well-known ocular impairment occurring in SLE patients, and it remains one of the major causes of blindness. Bilateral optic neuropathy in SLE occurs more frequently than the monolateral form. It is associated with transverse myelitis,165 particularly in SLE patients with Devic's syndrome (probably due to such immunological mechanisms as vasculitis). Optic neuropathy was also linked with the presence of aPLs.166 Several reports have estimated that retinal vascular occlusions occur in up to 12% of aPL- positive patients.167 In addition, optic neuropathy was less frequent in APS patients without SLE and tended to be monolateral in these cases. Monolateral occurrences of optic neuropathy are considered a focal neurological disease168 due to a thrombotic event involving the ciliary vasculature. Small vessel occlusions affecting the choroid, retina, and optic nerve lead to ischaemia and even infarctions. Neovascularization may provoke secondary vitreous haemorrhage, traction retinal detachments or glaucoma.169
Cognitive dysfunctions
As shown above, seizures and transverse myelopathy, as well as affective disorders and cognitive impairments (see further), are also linked with aPLs.170 APS patients with severe impairments and rapidly progressive changes in mental status, memory disorders, confusion, and emotional lability were reported.171 Cognitive impairments range from multi-infarct dementia to subtle deficits in asymptomatic patients with aPLs. Poor memory, difficulty in concentrating or difficulty keeping attention focused for a long time are most frequent and indicate a probable preclinical phase of neurological involvement. The recognition of subtle cognitive dysfunctions was made possible by formal neuropsychological assessments in a number of trials, mainly in patients with SLE, and it has helped identify the primary etiological role of aPLs.
Dementia
Recurrent or progressive neurological deteriorations due to cerebrovascular disease can produce multi-infarct dementia (i.e., chronic multifocal disease). This was first reported in 1987, and 35 aPL-positive patients with cerebrovascular disease were described in 1989.123 Dementia, which is generally associated with a loss of cognitive functions and an impairment of skills, poor concentration, memory dysfunction, language impairment, and judgemental defects, was not peculiar and is difficult to distinguish from Alzheimer's disease, senile dementia, or other metabolic or toxic brain conditions. Later, however, brain biopsy findings from a patient with multi-infarct dementia and APS were published.172 Luminal occlusion by thrombi and marked endothelial hyperplasia of the small meningeal and cortical arterioles were found, suggesting a noninflammatory etiology associated with reactive endothelial hyperplasia and thrombosis of the small arterioles. In another study,121 cerebral atrophy and white matter hyperintensities were detected on MRI, whereas positron emission tomography (PET) scans showed a considerable, diffuse impairment of cortical glucose metabolism combined with a reduced cerebral perfusion in arterial border zones. These findings indicate that primary APS-associated vascular dementia may be due to cortical neuronal losses as caused by small vessel disease with immune-mediated intravascular thrombosis. The investigation of the link between aPLs and dementia173 indicated that 6% of patients had significantly elevated aCL IgG levels (> 20 GPL). All these patients had dementia similar to that seen in Alzheimer's except for one who had mixed dementia, but none of them had an immune-mediated disorder. Therefore, a significant number of patients with dementia were shown to have had high levels of aPLs.
Movement disorders
Chorea
A strong relationship between aPLs and chorea has been reported in retrospective studies, especially in SLE, but the occurrence of chorea in SLE is usually rare (1 - 3%). Chorea has also been documented in cases of pregnancy and as a complication of oral contraceptives.174 Chorea is more frequent in primary APS than in SLE, with a vascular pathogenesis being most probable. It has been proposed that aPLs can provoke chorea by an antigen/antibody mechanism by binding with phospholipids in the basal ganglia.175 In a review study of 50 patients with chorea and APS,17 SLE was found in 58% of the patients, primary APS-in 30%, and the rest 12% of the studied patients suffered from a 'lupus-like' syndrome while, in the same time, the chorea was bilateral in 55% of the total patient population. Cerebral infarcts on CT/MRI scans were found in 35% of the patients. It is difficult to distinguish the chorea in APS from the chorea encountered with rheumatic fever (Sydenhan's) or the inherited form (Huntington's). Chorea may appear without any obvious precipitating factors or be induced by oral contraceptive use (Cervera et al. reported in the above review17 that 96% of patients were females with a mean age of 23 years). A single episode of chorea was observed in 66% of patients, while in 34% it was recurrent (the chorea was seen bilaterally or monolaterally as starting occasionally on one side and reappearing on the other one). CT patterns are usually normal, but infarcts outside the basal ganglia themselves were seen. MRI findings were reported in 13 out of 50 cases, and three infarcts were seen in the caudate nuclei. Reversible immune-mediated response was the most likely pathogenesis of chorea,176 but a vascular hypothesis with thrombosis and infarction could not be excluded. The binding of autoantibodies to striatal interneurons may cause hypermetabolic dysfunction of these cells, notably, a striatal hypermetabolism has been described.176 Additionally, beyond chorea, hemiballismus in an aCL-positive patient was described,177 along with a cerebellar ataxia with aPL positivity.178
Dystonia-Parkinsonism
Basal ganglial involvement is often confirmed on MRI; notably, Milanov and Bogdanova179 reported an occurrence of primary APS with marked dystonic posturing, rigidity, bradykinesia, several hyperintense lesions in the basal ganglia and in the periventricular white matter, and diffuse hyperintensity of the subcortical white matter, bilaterally in the parietal regions.
Psychoses
Several cases were recorded where psychosis preceded the thrombotic symptoms in APS by many years.180 Indeed, increased aPL levels have been reported in schizophrenic patients181 as well as in those with a major depressive illness.182 However, aPL involvement was not well defined, although aPLs may be primarily associated with psychosis (i.e., the patients were not known to have autoimmune disorders but only acute psychosis).
Other neurological disorders in APS
The previous neurological complications in APS were shown to have mainly an ischemic-thrombotic origin, but a possible cellular involvement by aPLs and/or a specific interplay between immunologic and genetic factors in the central nervous system (CNS) could not be excluded. As emphasized elsewhere,51 a specific genetic background with autoimmune mechanisms may have been involved not only in the recently reported case of Wilson's disease in APS with predominant extrapyramidal symptomatics,86 but also in the aforementioned dystonia, Parkinsonism,179 or other neurological disorders. Such diseases in APS, with ischaemia and/or direct neural/neuronal tissue damage by antibody-mediated interactions, are separately described in the following sections.
Multiple sclerosis (MS)
Clinical manifestations and CNS imaging characteristics for MS with APS patterns and responses to anticoagulant therapy have been described.130,183 It was recommended that anticoagulation therapy should be considered in MS with persistent aCL at medium-high levels and/or LA, especially those with atypical MS forms, previous thromboses, obstetric complications, thrombocytopenia, and/or lupus.184 A rare syndrome of "lupoid sclerosis" was described with symptoms resembling MS and laboratory findings suggestive of SLE (e.g., spastic paraplegia185). Another finding is the so called "pseudo-multiple sclerosis" in aPL-positive patients in which the distinction between the conditions may be difficult (i.e., young patients with fluctuating/recurrent neurological events and focal/visual neurological symptoms). In such patients high signal lesions in the periventricular white matter on T2-weighted images resembled those seen in MS.186
In 1994, four patients with multiple neurological manifestations of MS (including white matter lesions, vertigo, aphasia, unilateral visual loss, diplopia or hemiparesis) were reported over several years with variable degrees of recovery after the episodes.187 IgG aCL were positive in all, and LA was also found in three of the four patients. More recent studies showed a controversial relationship between MS and aPLs. Patients with MS-like illnesses and aPLs130 were so similar to other MS patients that they should not be excluded from clinical trials.188 A study in 1998183 found a large, significant proportion of aCL-positive patients in non-classic MS (with similar patterns including progressive myelopathy, spinocerebellar syndrome, or neuromyelitis optica). Subsequently, aCL testing with an eventual anticoagulant approach was recommended in MS patients showing slower progressions and some atypical features (persistent headaches, absence of oligoclonal bands). Patients with probable MS presented with underlying connective tissue disease, uncommon findings for MS on MRI, an atypical evolution of MS, or aPL positivity.130 However, neither special examinations nor other laboratory findings or MRI were useful to distinguish APS from MS (primary APS responded to oral anticoagulants, while the outcome in secondary APS was not as favorable).
Transverse myelitis
The prevalence of aPLs has been shown to be higher in SLE patients with transverse myelitis compared with SLE patients in general.2 A study189 reported 10 of 12 patients with transverse myelitis and SLE as having aCL, and the other two exhibited evidence of venereal disease research laboratory (VDRL) positivity and prolonged activated partial thromboplastin time (aPTT). Both IgG and IgM isotype aCL were detected in 8 of the 10 patients. The authors concluded that there is a strong association between transverse myelitis in SLE and the presence of aPLs. Another report190 described a patient with refractory hiccups as the heralding symptom of transverse myelitis in association with aCL. Additionally, 14 patients with SLE and transverse myelitis were evaluated,191 and 91 additional cases were published in the literature. Forty-three percent of their patients and 64% of the patients reported in the literature were aPL-positive, confirming the strong association of transverse myelitis with aPLs. The presentation of myelitis is usually acute, with paraesthesia in the legs that ascends to the thorax within 24 to 48 hours. Paraplegia, back pain, and loss of sphincter control may follow.192 Several other papers have also shown its occurrence in the presence of aPLs.189,193 Optic neuritis may occur simultaneously with transverse myelitis, presenting with rapid visual loss accompanied by orbital pain.194 At the same time, the pathophysiology of spinal cord damage in aPL-associated myelopathy is uncertain: both ischaemia and an antibody-mediated interaction have been postulated (e.g. one patient with anterior spinal artery syndrome presented with a flaccid paraplegia, sphincter disturbances, and dissociated sensory impairment as being positive for aCL195).
Idiopathic intracranial hypertension
Idiopathic intracranial hypertension, also known as pseudotumour cerebri, is the term used to describe the occurrence of raised intracranial pressure that is not due to mass lesions, obstruction of cerebrospinal fluid flow, or focal structural abnormalities in alert and oriented patients. The term idiopathic excludes the possibility of intracranial venous sinus thrombosis. Idiopathic intracranial hypertension is frequently associated with aCL and can be the only presenting symptom of APS. The association of idiopathic intracranial hypertension with aPLs has been acknowledged only recently.196 However, its actual incidence is still unknown. A previous report197 described 11 out of 38 patients (29%) with both aPLs and idiopathic intracranial hypertension. However, only four had aCL without other prothrombotic risk factors or evidence of sinus thrombosis. Another study198 found aCL in six out of 14 patients (43%) with idiopathic intracranial hypertension. No differences were found in clinical, laboratory, or radiological findings that distinguished between patients with idiopathic intracranial hypertension in those with and without aCL. A retrospective study199 confirmed the association between idiopathic intracranial hypertension and aCL, although they also found a lower frequency of aCL in their patients. Three out of 37 patients (8.1%) were shown to be aCL positive, with a prevalence lower than that reported in the two previously published studies.
Sensori-neural hearing loss
A link between sensori-neural hearing loss and autoimmune disease has been postulated by many authors who have described the association between sensori-neural hearing loss and aPLs in several reports.200,201 An instance of sensori-neural hearing loss in a young woman diagnosed with SLE has also been presented.200 Serological tests for syphilis were false-positive and were positive for IgG aCL. The authors postulated the association of sudden profound sensori-neural hearing loss with aCL in patients with autoimmune diseases. Another woman, age 55, with a six-year history of Sjögren's syndrome who presented with IgG and IgM aCL elevations and developed a sudden onset of sensori-neural hearing loss associated with vertigo was also described.201 Another report202 described 30 patients, 11 of whom suffered from sudden deafness and 19 of whom presented with progressive sensori-neural hearing loss and had been matched to 20 healthy controls. It was found that 27% of the patients had low to moderate titers of aCL, while none of the control group presented with aCL. The authors concluded that aPLs may play an important role in the pathogenesis of this disability and speculated about the possibility of anticoagulant therapy for these patients. Six patients with SLE or a lupus-like syndrome have also been described,203 who developed sudden sensori-neural hearing loss with elevated serum levels of aCL or LA. An acute onset of sensori-neural hearing loss in the presence of aPLs may be a manifestation of APS, therefore, anticoagulation treatment was recommended for these patients.
Guillain-Barré syndrome
Guillain-Barré syndrome is a transient neurological disorder characterized by inflammatory demyelination of peripheral nerves. Although the pathogenesis of this disorder is not clear, there is increasing evidence for an autoimmune etiology. This demyelinating neuropathy, also uncommon in SLE, was associated with aPLs in the original description of Hughes syndrome. A later study204 reported on the reactivity of Guillain-Barré syndrome sera with various phospholipids, which are important constituents of myelin and serve as autoantigens in other autoimmune conditions, thus demonstrating that some Guillain-Barré syndrome patients produce autoantibodies to various phospholipids and nuclear antigens. However, these autoantibodies are probably produced as a result of the myelin damage and are not the cause of demyelination.
THERAPIES IN APS
Discussion of therapeutic approaches in APS is beyond the scope of this overview, but most recent advances, especially in CAPS which is the most life-threatening variant of APS, are listed briefly as follows:
The main therapy of APS is anticoagulant/antiaggregant medication.205 Corticosteroids may be useful in haematological manifestations (thrombocytopenia, hemolytic anemia, myelopathy and CAPS);206
Prevention of recurrent thrombosis is achieved by prolonged oral anticoagulation.206 Intensive therapy to a target internal normalized ratio (INR) higher than 3.0 is most effective. However, even in INRs < 3.0, no short-term recurrences in patients with venous thromboembolism and low-titer aCL were reported;207
The optimal approach to recurrent pregnancy loss in APS may consist of a combination of aspirin and heparin,208 but the results are controversial.205 The best mode of application would be to give low-dose aspirin to women with recurrent early miscarriage and to add low-molecular weight heparin to those with fetal losses or a history of previous thrombosis.
High-intensity anticoagulation is recommended as a standard for preventing thrombotic recurrences in APS with a lower intensity in patients at high risk of bleeding or at low risk of severe thrombosis.205,209-211
CONCLUSION
The nature of APS, as well as the evolving symptomatology of SLE, contributed to the recognition that anticoagulating approaches, rather than steroids or immunosuppressive drugs, significantly improved the outcome in a substantial number of patients with APS. We may also conclude that CNS disease in SLE is significantly associated with aPLs. Cerebral ischaemia due to vessel occlusion is considered the most important cause of CNS diseases in SLE, and aPLs play an important role in their pathogenesis. There is a strong association between aPLs and CVD, headache, cognitive dysfunction, and seizures, thus supporting the importance of occlusive vasculopathies in neuro-psychiatric lupus. Hence, testing for aPLs should be recommended not only for patients with autoimmune diseases and neuropsychiatric syndromes but also for younger patients (age < 40 years) without an underlying autoimmune disease who develop ischemic cerebral events and may have 'non-classic' MS, transverse myelitis, or atypical seizures. During brain MRI, such young individuals with multiple hyperintensity lesions without other known causes should also undergo testing for aPLs.
All patients with a target INR > 3.0 and cerebral ischaemia should undergo anticoagulation therapy to prevent recurrences. All patients with thrombosis associated with aPLs should undergo long-term (life-long) warfarin therapy. Unfortunately, low-dose aspirin alone does not prevent recurrent thrombosis. Additionally, oral anticoagulation carries an increased risk of serious haemorrhage; however, this risk, if well controlled, might be lowered to acceptable levels. Steroids and immunosuppressive drugs in aPL-positive patients and thrombosis may be justified only in life-threatening situations when episodes of thrombosis occur despite adequate anticoagulation treatment. In aPL-positive patients without previous thrombosis, low-dose aspirin (75 mg/day) indefinitely as a thromboprophylactic measure is recommended. In neuropsychiatric appearances different from CVD (e.g., headache, seizures), anticoagulation therapy should be considered for patients with more severe disease and unsatisfactory responses to traditional treatments for headache or conventional anti-epileptic drugs.
The most important points of our study are:
Newly suggested improvements in the Sapporo criteria for APS (Hughes syndrome) should be widely adopted.
-
APS appears as a recurrent thrombosis, obstetric complications and elevated serum aPL levels:
The similar prevalence of arterial and venous thrombosis in APS is a unique pattern among thrombophilic disorders.
Pregnancy/fetal complications are hallmarks of APS.
Neurological diseases (e.g., stroke, TIA), possibly due to vascular-ischemic (occlusive) disturbances, are the most life-threatening conditions, especially in CAPS and/or when recurrences appear. Other neuropsychiatric disorders (e.g., cognitive impairment, certain forms of migraine/headache, MS-like diseases) are also associated with APS.
Diagnosis and routine testing of APS should be done by β2GPI-dependent ELISA for aCL and LA.
Definite APS is diagnosed according to the persistency of aCL (high to medium concentrations) and/or LA.
The role of other antiphospholipid antibodies (e.g., phosphatidylserine, β2GPI, prothrombin, etc.) in APS, although not well defined, is likely to have a certain clinical significance;
Prolonged, even life-long oral anticoagulation at a target INR of 3.5 is the treatment of choice in thrombotic APS (e.g., warfarin);
Aspirin and heparin are recommended as treatments for pregnancy complications; corticosteroids, although considered life-saving in thrombocytopenia, hemolytic anemia and/or CAPS, do not have a decisive role in the treatment of other APS complications.
New approaches have been applied in APS such as fresh frozen plasma use during plasma exchange, rituximab therapy, anticytokine treatment, recombinant human-activated protein C, among others, but more first-level evidence is needed to make definitive recommendations.
Fig. 1.
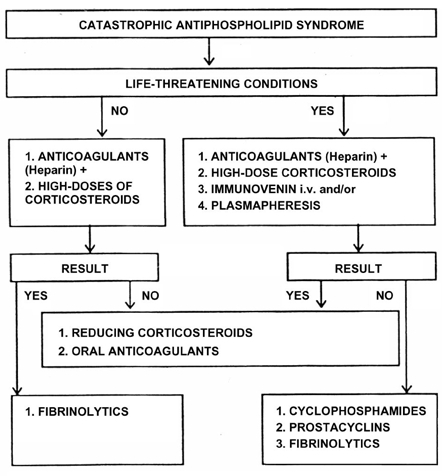
Therapeutic approach to catastrophic antiphospholipid syndrome (CAPS). With modifications from Panchovska M., Atanassova P., Despotova L. Therapeutic strategy in antiphospholipid syndrome. Rheumatology (Sofia) 2005;13;7-13 (in Bulgarian) as based on the paper by Asherson RA. The catastrophic antiphospholipid (Asherson's) syndrome in 2004 - a review. Autoimmunity Reviews 2005;4:48-54.
ACKNOWLEDGMENT
The author thanks Dr. Borislav D. Dimitrov (Italy) for his help during the preparation of this overview.
References
- 1.Hughes GR. The anticardiolipin syndrome. Clin Exp Rheumatol. 1985;3:285–286. [PubMed] [Google Scholar]
- 2.Alarcón-Segovia D, Delezé M, Oria CV, Sánchez-Guerrero J, Gómez-Pacheco L, Cabiedes J, et al. Anti-phospholipid antibodies and the antiphospholipid syndrome in systemic lupus erythematosus. A prospective analysis of 500 consecutive patients. Medicine (Baltimore) 1989;68:353–365. doi: 10.1097/00005792-198911000-00003. [DOI] [PubMed] [Google Scholar]
- 3.Asherson RA, Khamashta MA, Ordi-Ros J, Derksen RH, Machin SJ, Barquinero J, et al. The "primary" antiphospholipid syndrome: major clinical and serological features. Medicine (Baltimore) 1989;68:366–374. [PubMed] [Google Scholar]
- 4.Wilson WA, Gharavi AE, Koike T, Lockshin MD, Branch DW, Piette JC, et al. International consensus statement on preliminary classification criteria for definite antiphospholipid syndrome: report of an international workshop. Arthritis Rheum. 1999;42:1309–1311. doi: 10.1002/1529-0131(199907)42:7<1309::AID-ANR1>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 5.Lockshin MD, Sammaritano LR, Schwartzman S. Validation of the Sapporo criteria for antiphospholipid syndrome. Arthritis Rheum. 2000;43:440–443. doi: 10.1002/1529-0131(200002)43:2<440::AID-ANR26>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 6.Pierangeli SS, Gharavi AE, Harris EN. Anticardiolipin testing. In: Khamashta MA, editor. Hughes' syndrome. London: Springer; 2000. pp. 205–213. [Google Scholar]
- 7.Mackie IJ, Donohoe S, Machin SJ. Lupus anticoagulant measurement. In: Khamashta MA, editor. Hughes' syndrome. London: Springer; 2000. pp. 214–224. [Google Scholar]
- 8.Brandt JT, Triplett DA, Alving B, Scharrer I. Criteria for the diagnosis of lupus anticoagulants: an update. On behalf of the Subcommittee on Lupus Anticoagulant/Antiphospholipid Antibody of the Scientific and Standardisation Committee of the ISTH. Thromb Haemost. 1995;74:1185–1190. [PubMed] [Google Scholar]
- 9.Roubey RAS. Antiphospholipid antibody-negative syndrome-other phospholipids. In: Khamashta MA, editor. Hughes' syndrome. London: Springer; 2000. pp. 253–260. [Google Scholar]
- 10.Lee RM, Emlen W, Scott JR, Branch DW, Silver RM. Anti-beta2-glycoprotein I antibodies in women with recurrent spontaneous abortion, unexplained fetal death, and antiphospholipid syndrome. Am J Obstet Gynecol. 1999;181:642–648. doi: 10.1016/s0002-9378(99)70507-7. [DOI] [PubMed] [Google Scholar]
- 11.Rand JH. Molecular pathogenesis of the antiphospholipid syndrome. Circ Res. 2002;90:29–37. doi: 10.1161/hh0102.102795. [DOI] [PubMed] [Google Scholar]
- 12.Giannakopoulos B, Passam F, Rahgozar S, Krilis SA. Current concepts on the pathogenesis of the antiphospholipid syndrome. Blood. 2007;109:422–430. doi: 10.1182/blood-2006-04-001206. [DOI] [PubMed] [Google Scholar]
- 13.Sherer Y, Berkun Y, Blank M, Shoenfeld Y. Pathogenesis of the antiphospholipid syndrome. Pediatr Rheumatol Online J. 2004;2:492–496. [Google Scholar]
- 14.Shoenfeld Y, Blank M, Cervera R, Font J, Raschi E, Meroni PL. Infectious origin of the antiphospholipid syndrome. Ann Rheum Dis. 2006;65:2–6. doi: 10.1136/ard.2005.045443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kochenour NK, Branch DW, Rote NS, Scott JR. A New postpartum syndrome associated with antiphospholipid antibodies. Obstet Gynecol. 1987;69:460–468. [PubMed] [Google Scholar]
- 16.Kupferminc MJ, Lee MJ, Green D, Peaceman AM. Severe postpartum pulmonary, cardiac and renal syndrome associated with antiphospholipid antibodies. Obstet Gynecol. 1994;83:806–807. [PubMed] [Google Scholar]
- 17.Cervera R, Asherson RA, Font J, Tikly M, Pallarés L, Chamorro A, et al. Chorea in the antiphospholipid syndrome. Clinical, radiologic, and immunologic characteristics of 50 patients from our clinics and the recent literature. Medicine (Baltimore) 1997;76:203–212. doi: 10.1097/00005792-199705000-00006. [DOI] [PubMed] [Google Scholar]
- 18.Lê Thi Huong D, Wechsler B, Edelman P, Fournié A, Le Tallec Y, Piette JC, et al. Postpartum cerebral infarction associated with aspirin withdrawal in the antiphospholipid antibody syndrome. J Rheumatol. 1993;20:1229–1232. [PubMed] [Google Scholar]
- 19.Hochfeld M, Druzin ML, Maia D, Wright J, Lambert RE, McGuire J. Pregnancy complicated by primary antiphospholipid antibody syndrome. Obstet Gynecol. 1994;83:804–805. [PubMed] [Google Scholar]
- 20.Ornstein MH, Rand JH. An association between refractory HELLP syndrome and antiphospholipid antibodies during pregnancy: a report of 2 cases. J Rheumatol. 1994;21:1360–1364. [PubMed] [Google Scholar]
- 21.Cervera R, Piette JC, Font J, Khamashta MA, Shoenfeld Y, Camps MT, et al. Antiphospholipid syndrome: clinical and immunologic manifestations and patterns of disease expression in a cohort of 1,000 patients. Arthritis Rheum. 2002;46:1019–1027. doi: 10.1002/art.10187. [DOI] [PubMed] [Google Scholar]
- 22.Asherson RA, Cervera R, Piette JC, Shoenfeld Y. Milestones in the antiphospholipid syndrome. In: Asherson RA, Cervera R, Piette JC, Shoenfeld Y, editors. The antiphospholipid syndrome. II. Autoimmune thrombosis. Amsterdam: Elsevier; 2002. pp. 3–9. [Google Scholar]
- 23.Bruce D, Bateman D, Thomas R. Left ventricular thrombi in a patient with the antiphospholipid syndrome. Br Heart J. 1995;74:202–203. doi: 10.1136/hrt.74.2.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O'Neill D, Magaldi J, Dobkins D, Greco T. Dissolution of intracardiac mass lesions in the primary antiphospholipid antibody syndrome. Arch Intern Med. 1995;155:325–327. [PubMed] [Google Scholar]
- 25.Nickele GA, Foster PA, Kenny D. Primary antiphospholipid syndrome and mitral valve thrombosis. Am Heart J. 1994;128:1245–1247. doi: 10.1016/0002-8703(94)90760-9. [DOI] [PubMed] [Google Scholar]
- 26.Murphy JJ, Leach IH. Findings of necropsy in the heart of a patient with anticardiolipin syndrome. Br Heart J. 1989;62:61–64. doi: 10.1136/hrt.62.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leung WH, Wong KL, Lau CP, Wong CK, Liu HW. Association between antiphospholipid antibodies and cardiac abnormalities in patients with systemic lupus erythematosus. Am J Med. 1990;89:411–419. doi: 10.1007/BF01453668. [DOI] [PubMed] [Google Scholar]
- 28.Hasnie AM, Stoddard MF, Gleason CB, Wagner SG, Longaker RA, Pierangeli S, et al. Diastolic dysfunction is a feature of the antiphospholipid syndrome. Am Heart J. 1995;129:1009–1013. doi: 10.1016/0002-8703(95)90124-8. [DOI] [PubMed] [Google Scholar]
- 29.Martinez-Levin M, Fonseca C, Amigo MC, Nava A, Reyes PA, Ruiz-Arguelles A. Antiphospholipid syndrome in patients with cyanotic congenital heart disease. Clin Exp Rheumatol. 1995;13:489–491. [PubMed] [Google Scholar]
- 30.Donaldson MC, Weinberg DS, Belkin M, Whittemore AD, Mannick JA. Screening for hypercoagulable states in vascular surgical practice: A preliminary study. J Vasc Surg. 1990;11:825–831. doi: 10.1067/mva.1990.20120. [DOI] [PubMed] [Google Scholar]
- 31.Shortell CK, Ouriel K, Green RM, Condemi JJ, DeWeese JA. Vascular disease in the antiphospholipid syndrome: a comparison with the patient population with atherosclerosis. J Vasc Surg. 1992;15:158–166. doi: 10.1067/mva.1992.33160. [DOI] [PubMed] [Google Scholar]
- 32.Taylor LM, Chitwood RW, Dalman RL, Sexton G, Goodnight SH, Porter JM. Antiphospholipid antibodies in vascular surgery patients. A cross-sectional study. Ann Surg. 1994;220:545–551. doi: 10.1097/00000658-199410000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liote F, Meyer O. Osteoarticular manifestations in the antiphospholipid syndrome. In: Asherson RA, Cervera R, Piette J-C, Shoenfeld Y, editors. The Antiphospholipid Syndrome. Boca Raton: CRC Press; 1996. pp. 195–200. [Google Scholar]
- 34.Asherson RA, Liote F, Page B, Meyer O, Buchanan N, Khamashta MA, et al. Avascular necrosis of bone and antiphospholipid antibodies in systemic lupus erythematosus. J Rheumatol. 1993;20:284–288. [PubMed] [Google Scholar]
- 35.Nagasawa K, Ishii Y, Mayumi T, Tada Y, Ueda A, Yamauchi Y, et al. Avascular necrosis of bone in systemic lupus erythematosus: possible role of haemostatic abnormalities. Ann Rheum Dis. 1989;48:672–676. doi: 10.1136/ard.48.8.672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kleiner RC, Najarian LV, Schatten S, Jabs DA, Patz A, Kaplan HJ. Vaso-occlusive retinopathy associated with antiphospholipid antibodies (lupus anticoagulant retinopathy) Ophthalmology. 1989;96:896–904. doi: 10.1016/s0161-6420(89)32825-9. [DOI] [PubMed] [Google Scholar]
- 37.Dessein PH, Lamparelli RD, Phillips SA, Rubenchik IA, Zwi S. Severe immune thrombocytopenia and the development of skin infarctions in a patient with an overlap syndrome. J Rheumatol. 1989;16:1494–1496. [PubMed] [Google Scholar]
- 38.Aronoff DM, Callen JP. Necrosing livedo reticularis in a patient with recurrent pulmonary hemorrhage. J Am Acad Dermatol. 1997;37:300–302. [PubMed] [Google Scholar]
- 39.Del Castillo LF, Soria C, Schoendorff C, Garcia Garcia C, Diaz-Caballero N, Rodriguez Alen A, et al. Widespread cutaneous necrosis and antiphospholipid antibodies: Two episodes related to surgical manipulation and urinary tract infection. J Am Acad Dermatol. 1997;36:872–875. doi: 10.1016/s0190-9622(97)70045-8. [DOI] [PubMed] [Google Scholar]
- 40.Amster MS, Conway J, Zeid M, Pincus S. Cutaneous necrosis resulting from protein S deficiency and increased antiphospholipid antibody in a patient with systemic lupus erythematosus. J Am Acad Dermatol. 1993;29:853–857. doi: 10.1016/0190-9622(93)70254-q. [DOI] [PubMed] [Google Scholar]
- 41.Wolf P, Soyer HP, Auer-Grumbach P, Kerl H. Widespread cutaneous necrosis in a patient with rheumatoid arthritis associated with anticardiolipin antibodies. Arch Dermatol. 1991;127:1739–1740. [PubMed] [Google Scholar]
- 42.Hill VA, Whittaker SJ, Hunt BJ, Liddell K, Spittle MF, Smith NP. Cutaneous necrosis associated with the antiphospholipid syndrome and mycosis fungoides. Br J Dermatol. 1994;130:92–96. doi: 10.1111/j.1365-2133.1994.tb06890.x. [DOI] [PubMed] [Google Scholar]
- 43.Liano F, Mampaso F, Garcia Martin F, Pardo A, Orte L, Teruel JL. Allograft membranous glomerulonephritis and renal-vein thrombosis in a patient with a lupus anticoagulation factor. Nephrol Dial Transplant. 1988;3:684–689. doi: 10.1093/oxfordjournals.ndt.a091729. [DOI] [PubMed] [Google Scholar]
- 44.Grob JJ, Bonerandi JJ. Cutaneous manifestations associated with the presence of the lupus anticoagulant. A report of two cases and a review of the literature. J Am Acad Dermatol. 1986;15:211–219. doi: 10.1016/s0190-9622(86)70159-x. [DOI] [PubMed] [Google Scholar]
- 45.Asherson RA, Jacobelli S, Rosenberg H, Mckee p, Hughes GR. Skin nodules and macules resembling vasculitis in the antiphospholipid syndrome -- a report of two cases. Clin Exp Dermatol. 1992;17:266–269. doi: 10.1111/j.1365-2230.1992.tb02164.x. [DOI] [PubMed] [Google Scholar]
- 46.Asherson RA. Subungual splinter haemorrhages: a new sign of the antiphospholipid coagulopathy? Ann Rheum Dis. 1990;49:268. doi: 10.1136/ard.49.4.268-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Digre KB, Durcan FJ, Branch DW, Jacobson DM, Varner MW, Baringer JR. Amaurosis fugax associated with antiphospholipid antibodies. Ann Neurol. 1989;25:228–232. doi: 10.1002/ana.410250304. [DOI] [PubMed] [Google Scholar]
- 48.Fawcett RS, Linford S, Stulberg DL. Nail abnormalities: clues to systemic disease. Am Fam Physician. 2004;69:1417–1424. [PubMed] [Google Scholar]
- 49.Ruffatti A, Veller-Fornasa C, Patrassi GM, Sartori E, Tonello M, Tonetto S, et al. Anticardiolipin antibodies and antiphospholipid syndrome in chronic discoid lupus erythematosus. Clin Rheumatol. 1995;14:402–404. doi: 10.1007/BF02207672. [DOI] [PubMed] [Google Scholar]
- 50.Alarcón-Segovia D, Pérez-Vázquez ME, Villa AR, Drenkard C, Cabiedes J. Preliminary classification criteria for the antiphospholipid syndrome with systemic lupus erythematosus. Semin Arthritis Rheum. 1992;21:275–286. doi: 10.1016/0049-0172(92)90021-5. [DOI] [PubMed] [Google Scholar]
- 51.Atanassova PA, Dimitrov BD. Neurological and systemic disorders in antiphospholipid syndrome: novel constellations on a genetic basis. Clin Neurol Neurosurg. 2006;108:814. doi: 10.1016/j.clineuro.2006.06.009. [DOI] [PubMed] [Google Scholar]
- 52.Luchi ME, Asherson RA, Lahita RG. Primary idiopathic pulmonary hypertension complicated by pulmonary arterial thrombosis. Association with antiphospholipid antibodies. Arthritis Rheum. 1992;35:700–705. doi: 10.1002/art.1780350616. [DOI] [PubMed] [Google Scholar]
- 53.Brucato A, Baudo F, Barberis M, Redaelli R, Casadeo G, Allegri F, et al. Pulmonary hypertension secondary to thrombosis of the pulmonary vessels in a patient with the primary antiphospholipid syndrome. J Rheumatol. 1994;21:942–944. [PubMed] [Google Scholar]
- 54.Weiss L, Dimitrov DS, Angelova M. The hemodynamic destruction of intravascular cancer cells in relation to myocardial metastasis. Proc Natl Acad Sci U S A. 1985;82:5737–5741. doi: 10.1073/pnas.82.17.5737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kerr JE, Poe R, Kramer Z. Antiphospholipid antibody syndrome presenting as a refractory non-inflammatory pulmonary vasculopathy. Chest. 1997;112:1707–1710. doi: 10.1378/chest.112.6.1707. [DOI] [PubMed] [Google Scholar]
- 56.Crausman RS, Achenbach GA, Pluss WT, O'Brien RF, Jennings CA. Pulmonary capillaritis and alveolar hemorrhage associated with the antiphospholipid antibody syndrome. J Rheumatol. 1995;22:554–556. [PubMed] [Google Scholar]
- 57.Asherson RA, Greenblatt MA. Recurrent alveolar haemorrhage and pulmonary capillaritis in the "primary" antiphospholipid syndrome. J Clin Rheumatol. 2001;7:30–33. doi: 10.1097/00124743-200102000-00007. [DOI] [PubMed] [Google Scholar]
- 58.Savin H, Huberman M, Kott E, Lishner M, Kitai Y, Kidron D, et al. Fibrosing alveolitis associated with primary antiphospholipid syndrome. Br J Rheumatol. 1994;33:977–980. doi: 10.1093/rheumatology/33.10.977. [DOI] [PubMed] [Google Scholar]
- 59.Asherson RA, Kant KS. Antiphospholipid antibodies and the kidney. J Rheumatol. 1993;20:1268–1272. [PubMed] [Google Scholar]
- 60.Piette J-C, Kleinknecht D, Bach J-F. Renal manifestations in the antiphospholipid syndrome. In: Asherson RA, Cervera R, Piette J-C, Shoenfeld Y, editors. The Antiphospholipid Syndrome. New York, Boca Raton: CRC Press; 1996. pp. 169–181. [Google Scholar]
- 61.Frampton G, Hicks J, Cameron JS. Significance of antiphospholipid antibodies in patients with lupus nephritis. Kidney Int. 1991;39:1225–1231. doi: 10.1038/ki.1991.155. [DOI] [PubMed] [Google Scholar]
- 62.Glueck HI, Kant KS, Weiss MA, Pollak VE, Miller MA, Coots M. Thrombosis in systemic lupus erythematosus. Relation to the presence of circulating anticoagulants. Arch Intern Med. 1985;145:1389–1395. [PubMed] [Google Scholar]
- 63.Bhathena DB, Sobel BJ, Migdal SD. Noninflammatory renal microangiopathy of systemic lupus erythematosus ("lupus vasculitis") Am J Nephrol. 1981;1:144–159. doi: 10.1159/000166532. [DOI] [PubMed] [Google Scholar]
- 64.Churg J, Goldstein MH, Bernstein J. Thrombotic angiopathy including haemolytic-uremic syndrome, thrombotic thrombocytopenic purpura and postpartum renal failure. In: Tister CC, Brenner BM, editors. Renal Pathology with Clinical and Functional Correlates. Vol 2. Philadelphia: J B Lippincott; 1989. pp. 1081–1113. [Google Scholar]
- 65.Hughson MD, Nadasdy T, McCarty GA, Sholer C, Min KW, Silva T. Renal thrombotic microangiopathy in patients with systemic lupus erythematosus and the antiphospholipid syndrome. Am J Kidney Dis. 1992;20:150–158. doi: 10.1016/s0272-6386(12)80543-9. [DOI] [PubMed] [Google Scholar]
- 66.Asherson RA. The catastrophic antiphospholipid syndrome. J Rheumatol. 1992;19:508–512. [PubMed] [Google Scholar]
- 67.Rao RH, Vagnucci AH, Amico JA. Bilateral massive adrenal hemorrhage: early recognition and treatment. Ann Intern Med. 1989;110:227–235. doi: 10.7326/0003-4819-110-3-227. [DOI] [PubMed] [Google Scholar]
- 68.Oelkers W. Adrenal insufficiency. N Engl J Med. 1996;335:1206–1212. doi: 10.1056/NEJM199610173351607. [DOI] [PubMed] [Google Scholar]
- 69.Blanc PL, Forel C, Jay S, Susset V. Acute adrenal insufficiency during unilateral adrenal hemorrhage secondary to the antiphospholipid syndrome. Rev Med Interne. 2006;27:970–972. doi: 10.1016/j.revmed.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 70.Valla D, Benhamou JP. Disorders of the hepatic veins and venules. In: McIntyre N, Benhamou JP, Bircher J, Rizetto M, Rodes J, editors. Oxford Textbook of Clinical Hepatology. Oxford University Press; 1991. pp. 1004–1011. [Google Scholar]
- 71.Ouwendijk RJ, Koster JC, Wilson JH, Stibbe J, Lameris JS, Visser W, et al. Budd-Chiari syndrome in a young patient with anticardiolipin antibodies: need for prolonged anticoagulant treatment. Gut. 1994;35:1004–1006. doi: 10.1136/gut.35.7.1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.De Clerck LS, Michielsen PP, Ramael MR, Janssens E, Van Maercke YM, Van Marck EA, et al. Portal and pulmonary vessel thrombosis associated with systemic lupus erythematosus and anticardiolipin antibodies. J Rheumatol. 1991;18:1919–1921. [PubMed] [Google Scholar]
- 73.Takahaski C, Kumagai S, Tsubata R, Sorachi K, Ozaki S, Imura H, et al. Portal hypertension associated with anticardiolipin antibodies in a case of systemic lupus erythematosus. Lupus. 1995;4:232–235. doi: 10.1177/096120339500400314. [DOI] [PubMed] [Google Scholar]
- 74.Mantz FA, Craige E. Portal axis thrombosis with spontaneous portacaval shunt and resultant cor pulmonale. AMA Arch Pathol. 1951;52:91–97. [PubMed] [Google Scholar]
- 75.Nakamura H, Uehara H, Okada T, Kambe H, Kimura Y, Ito H, et al. Occlusion of small hepatic veins associated with systemic lupus erythematosus with the lupus anticoagulant and anti-cardiolipin antibody. Hepatogastroenterology. 1989;36:393–397. [PubMed] [Google Scholar]
- 76.Morio S, Oh H, Hirasawa A, Aotsuka N, Nakamura H, Asai T, et al. Hepatic veno-occlusive disease in a patient with lupus anticoagulant after allogeneic bone marrow transplantation. Bone Marrow Transplant. 1991;8:147–149. [PubMed] [Google Scholar]
- 77.Perez Ruiz F, Orte Martinez FJ, Zea Mendoza AC, Ruiz del Arbol L, Moreno-Caparros A. Nodular regenerative hyperplasia of the liver in rheumatic diseases: report of seven cases and review of the literature. Semin Arthritis Rheum. 1991;21:47–54. doi: 10.1016/0049-0172(91)90056-6. [DOI] [PubMed] [Google Scholar]
- 78.Morlà RM, Ramos-Casals M, García-Carrasco M, Cervera R, Font J, Bruguera M, et al. Nodular regenerative hyperplasia of the liver and antiphospholipid antibodies: report of two cases and review of the literature. Lupus. 1999;8:160–163. doi: 10.1191/096120399678847515. [DOI] [PubMed] [Google Scholar]
- 79.Kesler A, Pomeranz IS, Huberman M, Novis B, Kott E. Cerebral venous thrombosis and chronic active hepatitis as part of the antiphospholipid antibody syndrome. Postgrad Med J. 1996;72:690–692. doi: 10.1136/pgmj.72.853.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dourakis SP, Michael AE, Papanikolaou IS, Nomikou E, Thalassinou P, Hadziyannis SJ. Autoimmune hepatitis associated with the antiphospholipid syndrome. Eur J Gastroenterol Hepatol. 2001;13:591–593. doi: 10.1097/00042737-200105000-00021. [DOI] [PubMed] [Google Scholar]
- 81.Prieto J, Yuste JR, Beloqui O, Civeira MP, Riezu JI, Aguirre B, et al. Anticardiolipin antibodies in chronic hepatitis C: implication of hepatitis C virus as the cause of the antiphospholipid syndrome. Hepatology. 1996;23:199–204. doi: 10.1002/hep.510230201. [DOI] [PubMed] [Google Scholar]
- 82.Muñoz-Rodríguez FJ, Tàssies D, Font J, Reverter JC, Cervera R, Sánchez-Tapias JM, et al. Prevalence of hepatitis C virus infection in patients with antiphospholipid syndrome. J Hepatol. 1999;30:770–773. doi: 10.1016/s0168-8278(99)80127-5. [DOI] [PubMed] [Google Scholar]
- 83.Alric L, Oskman F, Sanmarco M, Izopet J, Bonnet E, Garcia-Ricart F, et al. Association of antiphospholipid syndrome and chronic hepatitis C. Br J Rheumatol. 1998;37:589–590. doi: 10.1093/rheumatology/37.5.589. [DOI] [PubMed] [Google Scholar]
- 84.Chedid A, Chadalawada KR, Morgan TR, Moritz TE, Mendenhall CL, Hammond JB, et al. Phospholipid antibodies in alcoholic liver disease. Hepatology. 1994;20:1465–1471. doi: 10.1002/hep.1840200614. [DOI] [PubMed] [Google Scholar]
- 85.Violi F, Ferro D, Basili S, D'Angelo A, Mazzola G, Quintarelli C, et al. Relation between lupus anticoagulant and splanchnic venous thrombosis in cirrhosis of the liver. BMJ. 1994;309:239–240. doi: 10.1136/bmj.309.6949.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Atanassova PA, Panchovska MS, Tzvetanov P, Chalakova NT, Masaldzhieva RI, Dimitrov BD. Hepatolenticular degeneration combined with primary antiphospholipid syndrome: a case report. Eur Neurol. 2006;55:42–43. doi: 10.1159/000091426. [DOI] [PubMed] [Google Scholar]
- 87.Cappell MS. Eesophageal necrosis and perforation associated with the anticardiolipin antibody syndrome. Am J Gastroenterol. 1994;89:1241–1245. [PubMed] [Google Scholar]
- 88.Kalman DR, Khan A, Romain PL, Nompleggi DJ. Giant gastric ulceration associated with antiphospholipid antibody syndrome. Am J Gastroenterol. 1996;91:1244–1247. [PubMed] [Google Scholar]
- 89.Sánchez-Guerrero J, Reyes E, Alarcón-Segovia D. Primary antiphospholipid syndrome as a cause of intestinal infarction. J Rheumatol. 1992;19:623–625. [PubMed] [Google Scholar]
- 90.Vahl AC, Gans RO, Mackaay AJ, van der Waal C, Rauwerda JA. Superior mesenteric artery occlusion andperipheral emboli caused by an aortic ulcer in a young patient with antiphospholipid syndrome. Surgery. 1997;121:588–590. doi: 10.1016/s0039-6060(97)90116-1. [DOI] [PubMed] [Google Scholar]
- 91.Cappell MS, Mikhail N, Gujral N. Gastrointestinal hemorrhage and intestinal ischemia associated with anticardiolipin antibodies. Dig Dis Sci. 1994;39:1359–1364. doi: 10.1007/BF02093805. [DOI] [PubMed] [Google Scholar]
- 92.Gül A, Inanç M, Ocal L, Koniçe M, Aral O, Lie JT. Primary antiphospholipid syndrome associated with mesenteric inflammatory veno-occlusive disease. Clin Rheumatol. 1996;15:207–210. doi: 10.1007/BF02230344. [DOI] [PubMed] [Google Scholar]
- 93.North JP, Wollenman OJ. Venous mesenteric occlusion in the course of migratory thrombophlebitis. Surg Gynecol Obstet. 1952;95:665–667. [PubMed] [Google Scholar]
- 94.Souto JC, Borrell M, Fontcuberta J, Roca M. Antiphospholipid antibodies in inflammatory bowel disease. Dig Dis Sci. 1995;40:1524–1525. doi: 10.1007/BF02285202. [DOI] [PubMed] [Google Scholar]
- 95.Chang KY, Kuo YC, Chiu CT, Wu SS, Huang CY, Wu CS, et al. Anti-cardiolipin antibody associated with acute hemorrhagic pancreatitis. Pancreas. 1993;8:654–657. doi: 10.1097/00006676-199309000-00022. [DOI] [PubMed] [Google Scholar]
- 96.Date K, Shirai Y, Hatakeyama K. Antiphospholipid antibody syndrome presenting as acute acalculous cholecystitis. Am J Gastroenterol. 1997;92:2127–2128. [PubMed] [Google Scholar]
- 97.Arnold MH, Schrieber L. Splenic and renal infarction in systemic lupus erythematosus: association with anti-cardiolipin antibodies. Clin Rheumatol. 1988;7:406–410. doi: 10.1007/BF02239202. [DOI] [PubMed] [Google Scholar]
- 98.Pettersson T, Julkunen H. Asplenia in a patient with systemic lupus erythematosus and antiphospholipid antibodies. J Rheumatol. 1992;19:1159. [PubMed] [Google Scholar]
- 99.Goómez-Puerta JA, Cervera R, Espinosa G, Aguiló S, Bucciarelli S, Ramos-Casals M, et al. Antiphospholipid antibodies associated with malignancies: clinical and pathological characteristics of 120 patients. Semin Arthritis Rheum. 2006;35:322–332. doi: 10.1016/j.semarthrit.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 100.Ruiz-Irastorza G, Khamashta MA, Hughes GR. Hughes syndrome crosses boundaries. Autoimmun Rev. 2002;1:43–48. doi: 10.1016/s1568-9972(01)00005-2. [DOI] [PubMed] [Google Scholar]
- 101.Alarcon Segovia D, Osmundson PJ. Peripheral vascular syndromes associated with systemic lupus erythematosus. Ann Intern Med. 1965;62:907–919. doi: 10.7326/0003-4819-62-5-907. [DOI] [PubMed] [Google Scholar]
- 102.Bird AG, Lendrum R, Asherson RA, Hughes GR. Disseminated intravascular coagulation, antiphospholipid antibodies, and ischaemic necrosis of extremities. Ann Rheum Dis. 1987;46:251–255. doi: 10.1136/ard.46.3.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Asherson RA, Derksen RH, Harris EN, Bingley PJ, Hoffbrand BI, Gharavi AE, et al. Large vessel occlusion and gangrene in systemic lupus erythematosus and "lupus-like" disease. A report of six cases. J Rheumatol. 1986;13:740–747. [PubMed] [Google Scholar]
- 104.Ferrante FM, Myerson GE, Goldman JA. Subclavian artery thrombosis mimicking the aortic arch syndrome in systemic lupus erythematosus. Arthritis Rheum. 1982;25:1501–1504. doi: 10.1002/art.1780251220. [DOI] [PubMed] [Google Scholar]
- 105.Asherson RA, Harris EN, Gharavi AE, Englert HE, Hughes GR. Aortic arch syndrome associated with anticardiolipin antibodies and the lupus anticoagulant: comment on Ferrante paper. Arthritis Rheum. 1985;28:594–595. doi: 10.1002/art.1780280525. [DOI] [PubMed] [Google Scholar]
- 106.Asherson RA, Ridley MG, Khamashta MA, Hughes GR. Gangrena en el lupus eritematoso sistemico. Piel. 1988;3:409–412. [Google Scholar]
- 107.Drew P, Asherson RA, Zuk RJ, Goodwin FJ, Hughes GR. Aortic occlusion in systemic lupus erythematosus associated with antiphospholipid antibodies. Ann Rheum Dis. 1987;46:612–616. doi: 10.1136/ard.46.8.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ter Borg EJ, Van der Meer J, De Wolf JT, Van Leeuwen MH, Kallenberg CG. Arterial thrombotic manifestations in young women associated with the lupus anticoagulant. Clin Rheumatol. 1988;7:74–79. doi: 10.1007/BF02284060. [DOI] [PubMed] [Google Scholar]
- 109.Hughes GR. Thrombosis, abortion, cerebral disease and the lupus anticoagulant. Br Med J (Clin Res Ed) 1983;287:1088–1089. doi: 10.1136/bmj.287.6399.1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Khamashta MA, Gil A, Anciones B, Lavilla P, Valencia ME, Pintado V, et al. Chorea in systemic lupus erythematosus: association with antiphospholipid antibodies. Ann Rheum Dis. 1988;47:681–683. doi: 10.1136/ard.47.8.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Asherson RA, Derksen RH, Harris EN, Bouma BN, Gharavi AE, Kater L, et al. Chorea in systemic lupus erythematosus and "lupus-like" disease: association with antiphospholipid antibodies. Semin Arthritis Rheum. 1987;16:253–259. doi: 10.1016/0049-0172(87)90003-5. [DOI] [PubMed] [Google Scholar]
- 112.Sun KH, Liu WT, Tsai CY, Liao TS, Lin WM, Yu CL. Inhibition of astrocyte proliferation and binding to brain tissue of anticardiolipin antibodies purified from lupus serum. Ann Rheum Dis. 1992;51:707–712. doi: 10.1136/ard.51.6.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Khalili A, Cooper RC. A study of immune responses to myelin and cardiolipin in patients with systemic lupus erythematosus (SLE) Clin Exp Immunol. 1991;85:365–372. doi: 10.1111/j.1365-2249.1991.tb05733.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Oosting JD, Derksen RH, Blokzijl L, Sixma JJ, de Groot PG. Antiphospholipid antibody positive sera enhance endothelial cell procoagulant activity - studies in a thrombosis model. Thromb Haemost. 1992;68:278–284. [PubMed] [Google Scholar]
- 115.Simantov R, LaSala JM, Lo SK, Gharavi AE, Sammaritano LR, Salmon JE, et al. Activation of cultured vascular endothelial cells by antiphospholipid antibodies. J Clin Invest. 1995;96:2211–2219. doi: 10.1172/JCI118276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Del Papa N, Guidali L, Sala A, Buccellati C, Khamashta MA, Ichikawa K, et al. Endothelial cells as target for antiphospholipid antibodies. Human polyclonal and monoclonal anti-beta 2-glycoprotein I antibodies react in vitro with endothelial cells through adherent beta 2-glycoprotein I and induce endothelial activation. Arthritis Rheum. 1997;40:551–561. doi: 10.1002/art.1780400322. [DOI] [PubMed] [Google Scholar]
- 117.Pierangeli SS, Harris EN. In vivo models of thrombosis for the antiphospholipid syndrome. Lupus. 1996;5:451–455. doi: 10.1177/096120339600500524. [DOI] [PubMed] [Google Scholar]
- 118.Ziporen L, Shoenfeld Y, Levy Y, Korczyn AD. Neurological dysfunction and hyperactive behavior associated with antiphospholipid antibodies. A mouse model. J Clin Invest. 1997;100:613–619. doi: 10.1172/JCI119572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Blank M, Krause I, Fridkin M, Keller N, Kopolovic J, Goldberg I, et al. Bacterial induction of autoantibodies to beta2-glycoprotein-I accounts for the infectious etiology of antiphospholipid syndrome. J Clin Invest. 2002;109:797–804. doi: 10.1172/JCI12337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Shah NM, Khamashta MA, Atsumi T, Hughes GR. Outcome of patients with anticardiolipin antibodies: a 10 year follow-up of 52 patients. Lupus. 1998;7:3–6. doi: 10.1191/096120398678919624. [DOI] [PubMed] [Google Scholar]
- 121.Hilker R, Thiel A, Geisen C, Rudolf J. Cerebral blood flow and glucose metabolism in multi-infarct-dementia related to primary antiphospholipid antibody syndrome. Lupus. 2000;9:311–316. doi: 10.1191/096120300680199015. [DOI] [PubMed] [Google Scholar]
- 122.Harris EN, Gharavi AE, Asherson RA, Boey ML, Hughes GR. Cerebral infarction in systemic lupus: association with anticardiolipin antibodies. Clin Exp Rheumatol. 1984;2:47–51. [PubMed] [Google Scholar]
- 123.Asherson RA, Khamashta MA, Gil A, Vazquez JJ, Chan O, Baguley E, et al. Cerebrovascular disease and antiphospholipid antibodies in systemic lupus erythematosus, lupus-like disease, and the primary antiphospholipid syndrome. Am J Med. 1989;86:391–399. doi: 10.1016/0002-9343(89)90335-5. [DOI] [PubMed] [Google Scholar]
- 124.Harris EN, Gharavi AE, Boey ML, Patel BM, Mackworth-Young CG, Loizou S, et al. Anticardiolipin antibodies: detection by radioimmunoassay and association with thrombosis in systemic lupus erythematosus. Lancet. 1983;2:1211–1214. doi: 10.1016/s0140-6736(83)91267-9. [DOI] [PubMed] [Google Scholar]
- 125.Kushner M, Simonian N. Lupus anticoagulants, anticardiolipin antibodies, and cerebral ischemia. Stroke. 1989;20:225–229. doi: 10.1161/01.str.20.2.225. [DOI] [PubMed] [Google Scholar]
- 126.Levine SR, Salowich-Palm L, Sawaya KL, Perry M, Spencer HJ, Winkler HJ, et al. IgG anticardiolipin antibody titer > 40 GPL and the risk of subsequent thrombo-occlusive events and death. A prospective cohort study. Stroke. 1997;28:1660–1665. doi: 10.1161/01.str.28.9.1660. [DOI] [PubMed] [Google Scholar]
- 127.Provenzale JM, Barboriak DP, Allen NB, Ortel TL. Patients with antiphospholipid antibodies: CT and MR findings of the brain. AJR Am J Roentgenol. 1996;167:1573–1578. doi: 10.2214/ajr.167.6.8956600. [DOI] [PubMed] [Google Scholar]
- 128.Devinsky O, Petito CK, Alonso DR. Clinical and neuropathological findings in systemic lupus erythematosus: the role of vasculitis, heart emboli, and thrombotic thrombocytopenic purpura. Ann Neurol. 1988;23:380–384. doi: 10.1002/ana.410230411. [DOI] [PubMed] [Google Scholar]
- 129.Khamashta MA, Cervera R, Asherson RA, Font J, Gil A, Coltart DJ, et al. Association of antibodies against phospholipids with heart valve disease in systemic lupus erythematosus. Lancet. 1990;335:1541–1544. doi: 10.1016/0140-6736(90)91373-i. [DOI] [PubMed] [Google Scholar]
- 130.Cuadrado MJ, Khamashta MA, Ballesteros A, Godfrey T, Simon MJ, Hughes GR. Can neurologic manifestations of Hughes (antiphospholipid) syndrome be distinguished from multiple sclerosis? Analysis of 27 patients and review of the literature. Medicine (Baltimore) 2000;79:57–68. doi: 10.1097/00005792-200001000-00006. [DOI] [PubMed] [Google Scholar]
- 131.Levine SR, Langer SL, Albers JW, Welch KM. Sneddon's syndrome: an antiphospholipid antibody syndrome? Neurology. 1988;38:798–800. doi: 10.1212/wnl.38.5.798. [DOI] [PubMed] [Google Scholar]
- 132.Kalashnikova LA, Nasonov EL, Kushekbaeva AE, Gracheva LA. Anticardiolipin antibodies in Sneddon's syndrome. Neurology. 1990;40:464–467. doi: 10.1212/wnl.40.3_part_1.464. [DOI] [PubMed] [Google Scholar]
- 133.Menzel C, Reinhold U, Grünwald F, von Smekal A, Uerlich M, Rieker O, et al. Cerebral blood flow in Sneddon syndrome. J Nucl Med. 1994;35:461–464. [PubMed] [Google Scholar]
- 134.Sneddon IB. Cerebro-vascular lesions and livedo reticularis. Br J Dermatol. 1965;77:180–185. doi: 10.1111/j.1365-2133.1965.tb14628.x. [DOI] [PubMed] [Google Scholar]
- 135.Brey RL, Escalante A, Futrell N, Asherson RA. Cerebral thrombosis and other neurologic manifestations in the Antiphospholipid Syndrome. In: Asherson RA, Cervera R, Piette JC, Shoenfeld Y, editors. The Antiphospholipid Syndrome. 1th ed. Boca Raton, FL: CRC-Press; 1996. pp. 133–150. [Google Scholar]
- 136.Stockhammer G, Felber SR, Zelger B, Sepp N, Birbamer GG, Fritsch PO, et al. Sneddon's Syndrome: diagnosis by skin biopsy and MRI in 17 patients. Stroke. 1993;24:685–690. doi: 10.1161/01.str.24.5.685. [DOI] [PubMed] [Google Scholar]
- 137.Tuhrim S, Rand JH, Wu XX, Weinberger J, Horowitz DR, Goldman ME, et al. Elevated anticardiolipin antibody titer is a stroke risk factor in a multiethnic population independent of isotype or degree of positivity. Stroke. 1999;30:1561–1565. doi: 10.1161/01.str.30.8.1561. [DOI] [PubMed] [Google Scholar]
- 138.Kenet G, Sadetzki S, Murad H, Martinowitz U, Rosenberg N, Gitel S, et al. Factor V Leiden and antiphospholipid antibodies are significant risk factors for ischemic stroke in children. Stroke. 2000;31:1283–1288. doi: 10.1161/01.str.31.6.1283. [DOI] [PubMed] [Google Scholar]
- 139.Metz LM, Edworthy S, Mydlarski R, Fritzler MJ. The frequency of phospholipid antibodies in an unselected stroke population. Can J Neurol Sci. 1998;25:64–69. doi: 10.1017/s0317167100033515. [DOI] [PubMed] [Google Scholar]
- 140.Tuhrim S, Rand JH, Wu X, Horowitz DR, Weinberger J, Goldman ME, et al. Antiphosphatidyl serine antibodies are independently associated with ischemic stroke. Neurology. 1999;53:1523–1527. doi: 10.1212/wnl.53.7.1523. [DOI] [PubMed] [Google Scholar]
- 141.Gómez-Pacheco L, Villa AR, Drenkard C, Cabiedes J, Cabral AR, Alarcón-Segovia D. Serum anti-beta2-glycoprotein-I and anticardiolipin antibodies during thrombosis in systemic lupus erythematosus patients. Am J Med. 1999;106:417–423. doi: 10.1016/s0002-9343(99)00053-4. [DOI] [PubMed] [Google Scholar]
- 142.Levine SR, Brey RL, Sawaya KL, Salowich-Palm L, Kokkinos J, Kostrzema B, et al. Recurrent stroke and thrombo-occlusive events in the antiphospholipid syndrome. Ann Neurol. 1995;38:119–124. doi: 10.1002/ana.410380119. [DOI] [PubMed] [Google Scholar]
- 143.Heinzlef O, Abuaf N, Cohen A, Amarenco P French Study of Aortic Plagues in Stroke Group. Recurrent stroke and vascular events in elderly patients with anticardiolipin antibodies: a prospective study. J Neurol. 2001;248:373–379. doi: 10.1007/s004150170176. [DOI] [PubMed] [Google Scholar]
- 144.Briley DP, Coull BM, Goodnight SH. Neurological disease associated with antiphospholipid antibodies. Ann Neurol. 1989;25:221–227. doi: 10.1002/ana.410250303. [DOI] [PubMed] [Google Scholar]
- 145.Bousser MG, Chiras J, Bories J, Castaigne P. Cerebral venous thrombosis? a review of 38 cases. Stroke. 1985;16:199–213. doi: 10.1161/01.str.16.2.199. [DOI] [PubMed] [Google Scholar]
- 146.Montón F, Rebollo M, Quintana F, Berciano J. Cerebral arterial occlusion and intracranial venous thrombosis in a woman taking oral contraceptives. Postgrad Med J. 1984;60:426–428. doi: 10.1136/pgmj.60.704.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Montalbán J, Arboix A, Staub H, Barquinero J, Martí-Vilalta J, Codina A, et al. Transient global amnesia and antiphospholipid antibodies. Clin Exp Rheumatol. 1989;7:85–87. [PubMed] [Google Scholar]
- 148.Brey RL, Gharavi AE, Lockshin MD. Neurologic complications of antiphospholipid antibodies. Rheum Dis Clin North Am. 1993;19:833–850. [PubMed] [Google Scholar]
- 149.Montalbán J, Cervera R, Font J, Ordi J, Vianna J, Haga HJ, et al. Lack of association between anticardiolipin antibodies and migraine in systemic lupus erythematosus. Neurology. 1992;42:681–682. doi: 10.1212/wnl.42.3.681. [DOI] [PubMed] [Google Scholar]
- 150.Sanna G, Cuadrado MJ, Nurchis P, Khamashta MA, Mathieu A, Hughes GRV. Prevalence of headache and relationship with the presence of antiphospholipid antibodies in patients with systemic lupus erythematosus. J Autoimmun. 2000;15:P177. (A75) [Google Scholar]
- 151.Lau SO, Bock GH, Edson JR, Michael AF. Sagittal sinus thrombosis in the nephrotic syndrome. J Pediatr. 1980;97:948–950. doi: 10.1016/s0022-3476(80)80430-6. [DOI] [PubMed] [Google Scholar]
- 152.Khoo KB, Long FL, Tuck RR, Allen RJ, Tymms KE. Cerebral venous sinus thrombosis associated with the primary antiphospholipid syndrome. Resolution with local thrombolytic therapy. Med J Aust. 1995;162:30–32. doi: 10.5694/j.1326-5377.1995.tb138408.x. [DOI] [PubMed] [Google Scholar]
- 153.Mackworth-Young CG, Hughes GR. Epilepsy: an early symptom of systemic lupus erythematosus. J Neurol Neurosurg Psychiatry. 1985;48:185. doi: 10.1136/jnnp.48.2.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Inzelberg R, Korczyn AD. Lupus anticoagulant and late onset seizures. Acta Neurol Scand. 1989;79:114–118. doi: 10.1111/j.1600-0404.1989.tb03721.x. [DOI] [PubMed] [Google Scholar]
- 155.Cocito L, Favale E, Reni L. Epileptic seizures in cerebral arterial occlusive disease. Stroke. 1982;13:189–195. doi: 10.1161/01.str.13.2.189. [DOI] [PubMed] [Google Scholar]
- 156.Herranz MT, Rivier G, Khamashta MA, Blaser KU, Hughes GR. Association between antiphospholipid antibodies and epilepsy in patients with systemic lupus erythematosus. Arthritis Rheum. 1994;37:568–571. doi: 10.1002/art.1780370418. [DOI] [PubMed] [Google Scholar]
- 157.Liou HH, Wang CR, Chen CJ, Chen RC, Chuang CY, Chiang IP, et al. Elevated levels of anticardiolipin antibodies and epilepsy in lupus patients. Lupus. 1996;5:307–312. doi: 10.1177/096120339600500412. [DOI] [PubMed] [Google Scholar]
- 158.Sabet A, Sibbitt WL, Stidley CA, Danska J, Brooks WM. Neurometabolite markers of cerebral injury in the antiphospholipid antibody syndrome of systemic lupus erythematosus. Stroke. 1998;29:2254–2260. doi: 10.1161/01.str.29.11.2254. [DOI] [PubMed] [Google Scholar]
- 159.Angelini L, Granata T, Zibordi F, Binelli S, Zorzi G, Besana C. Partial seizures associated with antiphospholipid antibodies in childhood. Neuropediatrics. 1998;29:249–253. doi: 10.1055/s-2007-973570. [DOI] [PubMed] [Google Scholar]
- 160.Verrot D, San-Marco M, Dravet C, Genton P, Disdier P, Bolla G, et al. Prevalence and signification of antinuclear and anticardiolipin antibodies in patients with epilepsy. Am J Med. 1997;103:33–37. doi: 10.1016/s0002-9343(97)90046-2. [DOI] [PubMed] [Google Scholar]
- 161.Peltola JT, Haapala A, Isojärvi JI, Auvinen A, Palmio J, Latvala K, et al. Antiphospholipid and antinuclear antibodies in patients with epilepsy or new-onset seizure disorders. Am J Med. 2000;109:712–717. doi: 10.1016/s0002-9343(00)00617-3. [DOI] [PubMed] [Google Scholar]
- 162.Liou HH, Wang CR, Chou HC, Arvanov VL, Chen RC, Chang YC, et al. Anticardiolipin antisera from lupus patients with seizures reduce a GABA receptor-mediated chloride current in snail neurons. Life Sci. 1994;54:1119–1125. doi: 10.1016/0024-3205(94)00422-6. [DOI] [PubMed] [Google Scholar]
- 163.Kent MN, Alvarez FJ, Ng AK, Rote NS. Ultrastructural localization of monoclonal antiphospholipid antibody binding to rat brain. Exp Neurol. 2000;163:173–179. doi: 10.1006/exnr.2000.7358. [DOI] [PubMed] [Google Scholar]
- 164.Wiechens B, Schröder JO, Pötzsch B, Rochels R. Primary antiphospholipid antibody syndrome and retinal occlusive vasculopathy. Am J Ophthalmol. 1997;123:848–850. doi: 10.1016/s0002-9394(14)71142-0. [DOI] [PubMed] [Google Scholar]
- 165.Gibbs AN, Moroney J, Foley-Nolan D, O'Connell PG. Neuromyelitis optica (Devic's syndrome) in systemic lupus erythematosus: a case report. Rheumatology (Oxford) 2002;41:470–471. doi: 10.1093/rheumatology/41.4.470. [DOI] [PubMed] [Google Scholar]
- 166.Reino S, Muñoz-Rodriguez FJ, Cervera R, Espinosa G, Font J, Ingelmo M. Optic neuropathy in the primary antiphospholipid syndrome: report of a case and review of the literature. Clin Rheumatol. 1997;16:629–631. doi: 10.1007/BF02247807. [DOI] [PubMed] [Google Scholar]
- 167.Montehermoso A, Cervera R, Font J, Ramos-Casals M, García-carrasco M, Formiga F, et al. Association of antiphospholipid antibodies with retinal vascular disease in systemic lupus erythematosus. Semin Arthritis Rheum. 1999;28:326–332. doi: 10.1016/s0049-0172(99)80017-1. [DOI] [PubMed] [Google Scholar]
- 168.Giorgi D, Gabrieli CB, Bonomo L. The clinico-ophthalmological spectrum of antiphospholipid syndrome. Ocul Immunol Inflamm. 1998;6:269–273. doi: 10.1076/ocii.6.4.269.4025. [DOI] [PubMed] [Google Scholar]
- 169.Labutta RJ. Ophthalmic manifestations in the antiphospholipid syndrome. In: Asherson RA, Cervera R, Piette J-C, Shoenfeld Y, editors. The Antiphospholipid Syndrome. Boca Raton, New York, London, Tokyo: CRC Press; 1996. pp. 213–218. [Google Scholar]
- 170.Navarrete MG, Brey RL, Levine SR. Cerebral disease in the antiphospholipid syndrome. In: Khamashta MA, editor. Hughes' syndrome. London: Springer; 2000. pp. 43–58. [Google Scholar]
- 171.Mikdashi J A, Chase C, Kay GG. Neurocognitive deficits in antiphospholipid syndrome. Neurology. 1996;46:A359. [Google Scholar]
- 172.Westerman EM, Miles JM, Backonja M, Sundstrom WR. Neuropathologic findings in multi-infarct dementia associated with anticardiolipin antibody. Evidence for endothelial injury as the primary event. Arthritis Rheum. 1992;35:1038–1041. doi: 10.1002/art.1780350908. [DOI] [PubMed] [Google Scholar]
- 173.Mosek A, Yust I, Treves TA, Vardinon N, Korczyn AD, Chapman J. Dementia and antiphospholipid antibodies. Dement Geriatr Cogn Disord. 2000;11:36–38. doi: 10.1159/000017211. [DOI] [PubMed] [Google Scholar]
- 174.Fernando SJ. An attack of chorea complicating oral contraceptive therapy. Practitioner. 1966;197:210–211. [PubMed] [Google Scholar]
- 175.Asherson RA, Hughes GR. Antiphospholipid antibodies and chorea. J Rheumatol. 1988;15:377–379. [PubMed] [Google Scholar]
- 176.Sundén-Cullberg J, Tedroff J, Aquilonius SM. Reversible chorea in primary antiphospholipid syndrome. Mov Disord. 1998;13:147–149. doi: 10.1002/mds.870130127. [DOI] [PubMed] [Google Scholar]
- 177.Tam LS, Cohen MG, Li EK. Hemiballismus in systemic lupus erythematosus: possible association with antiphospholipid antibodies. Lupus. 1995;4:67–69. doi: 10.1177/096120339500400114. [DOI] [PubMed] [Google Scholar]
- 178.Singh RR, Prasad K, Kumar A, Misra A, Padmakumar K, Malaviya AN. Cerebellar ataxia in systemic lupus erythematosus: Three case reports. Ann Rheum Dis. 1988;97:954–956. doi: 10.1136/ard.47.11.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Milanov I, Bogdanova D. Antiphospholipid syndrome and dystonia-parkinsonism. A case report. Parkinsonism Relat Disord. 2001;7:139–141. doi: 10.1016/s1353-8020(00)00025-0. [DOI] [PubMed] [Google Scholar]
- 180.Kurtz G, Müller N. The antiphospholipid syndrome and psychosis. Am J Psychiatry. 1994;151:1841–1842. doi: 10.1176/ajp.151.12.1841. [DOI] [PubMed] [Google Scholar]
- 181.Sirota P, Schild K, Firer M, et al. The diversity of autoantibodies in schizophrenic patients and their first degree relatives: analysis of multiple case families. Abstracts; First International Congress of the International Society of Neuro-Immune Modulation; Florence: ISNIM; 1991. 389 pp. [Google Scholar]
- 182.Maes M, Meltzer H, Jacobs J, Suy E, Calabrese J, Minner B, et al. Autoimmunity in depression: increased antiphospholipid autoantibodies. Acta Psychiatr Scand. 1993;87:160–166. doi: 10.1111/j.1600-0447.1993.tb03349.x. [DOI] [PubMed] [Google Scholar]
- 183.Karussis D, Leker RR, Ashkenazi A, Abramsky O. A subgroup of multiple sclerosis patients with anticardiolipin antibodies and unusual clinical manifestations: do they represent a new nosological entity? Ann Neurol. 1998;44:629–634. doi: 10.1002/ana.410440408. [DOI] [PubMed] [Google Scholar]
- 184.Ruiz-Irastorza G, Khamashta MA. Warfarin for multiple sclerosis? QJM. 2000;93:497–499. doi: 10.1093/qjmed/93.8.497. [DOI] [PubMed] [Google Scholar]
- 185.Marullo S, Clauvel JP, Intrator L, Danon F, Brouet JC, Oksenhendler E. Lupoid sclerosis with antiphospholipid and antimyelin antibodies. J Rheumatol. 1993;20:747–749. [PubMed] [Google Scholar]
- 186.Hughes GR. The antiphospholipid syndrome and 'multiple sclerosis'. Lupus. 1999;8:89. doi: 10.1191/096120399678847579. [DOI] [PubMed] [Google Scholar]
- 187.Scott TF, Hess D, Brillman J. Antiphospholipid antibody syndrome mimicking multiple sclerosis clinically and by CNS involvement in the antiphospholipid syndrome magnetic resonance imaging. Arch Intern Med. 1994;154:917–920. [PubMed] [Google Scholar]
- 188.Tourbah A, Clapin A, Gout O, Fontaine B, Liblau R, Batteux F, et al. Systemic autoimmune features and multiple sclerosis: a 5-year follow-up study. Arch Neurol. 1998;55:517–521. doi: 10.1001/archneur.55.4.517. [DOI] [PubMed] [Google Scholar]
- 189.Lavalle C, Pizarro S, Drenkard C, Sánchez-Guerrero J, Alarcón-Segovia D. Transverse myelitis: a manifestation of systemic lupus erythematosus strongly associated with antiphospholipid antibodies. J Rheumatol. 1990;17:34–37. [PubMed] [Google Scholar]
- 190.Ruiz-Argüelles GJ, Guzmán-Ramos J, Flores-Flores J, Garay-Martínez J. Refractory hiccough heralding transverse myelitis in the primary antiphospholipid syndrome. Lupus. 1998;7:49–50. doi: 10.1191/096120398678919633. [DOI] [PubMed] [Google Scholar]
- 191.Kovacs B, Lafferty TL, Brent LH, DeHoratius RJ. Transverse myelopathy in systemic lupus erythematosus: an analysis of 14 cases and review of the literature. Ann Rheum Dis. 2000;59:120–124. doi: 10.1136/ard.59.2.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Harisdangkul V, Doorenbos D, Subramony SH. Lupus transverse myelopathy: better outcome with early recognition and aggressive high-dose intravenous corticosteroid pulse treatment. J Neurol. 1995;242:326–331. doi: 10.1007/BF00878876. [DOI] [PubMed] [Google Scholar]
- 193.Smyth AE, Bruce IN, McMillan SA, Bell AL. Transverse myelitis: a complication of systemic lupus erythematosus that is associated with the antiphospholipid syndrome. Ulster Med J. 1996;645:91–94. [PMC free article] [PubMed] [Google Scholar]
- 194.Oppenheimer S, Hoffbrand BI. Optic neuritis and myelopathy in systemic lupus erythematosus. Can J Neurol Sci. 1986;13:129–132. doi: 10.1017/s0317167100036064. [DOI] [PubMed] [Google Scholar]
- 195.Markusse HN, Haan J, Tan WD, Breedveld FC. Anterior spinal artery syndrome in systemic lupus erythematosus. Br J Rheumatol. 1989;28:344–346. doi: 10.1093/rheumatology/28.4.344. [DOI] [PubMed] [Google Scholar]
- 196.Orefice G, De Joanna G, Coppola M, Brancaccio V, Ames PR. Benign intracranial hypertension: a non-thrombotic complication of the primary antiphospholipid syndrome? Lupus. 1995;4:324–326. doi: 10.1177/096120339500400417. [DOI] [PubMed] [Google Scholar]
- 197.Sussman J, Leach M, Greaves M, Malia R, Davies-Jones GA. Potentially prothrombotic abnormalities of coagulation in benign intracranial hypertension. J Neurol Neurosurg Psychiatry. 1997;62:229–233. doi: 10.1136/jnnp.62.3.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Leker RR, Steiner I. Anticardiolipin antibodies are frequently present in patients with idiopathic intracranial hypertension. Arch Neurol. 1998;55:817–820. doi: 10.1001/archneur.55.6.817. [DOI] [PubMed] [Google Scholar]
- 199.Kesler A, Ellis MH, Reshef T, Kott E, Gadoth N. Idiopathic intracranial hypertension and anticardiolipin antibodies. J Neurol Neurosurg Psychiatry. 2000;68:379–380. doi: 10.1136/jnnp.68.3.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Hisashi K, Komune S, Taira T, Uemura T, Sadoshima S, Tsuda H. Anticardiolipin antibody-induced sudden profound sensorineural hearing loss. Am J Otolaryngol. 1993;14:275–277. doi: 10.1016/0196-0709(93)90075-i. [DOI] [PubMed] [Google Scholar]
- 201.Casoli P, Tumiati B. Cogan's syndrome: a new possible complication of antiphospholipid antibodies? Clin Rheumatol. 1995;14:197–198. doi: 10.1007/BF02214943. [DOI] [PubMed] [Google Scholar]
- 202.Toubi E, Ben-David J, Kessel A, Podoshin L, Golan TD. Autoimmune aberration in sudden sensorineural hearing loss: association with anti-cardiolipin antibodies. Lupus. 1997;6:540–542. doi: 10.1177/096120339700600611. [DOI] [PubMed] [Google Scholar]
- 203.Naarendorp M, Spiera H. Sudden sensorineural hearing loss in patients with systemic lupus erythematosus or lupus-like syndromes and antiphospholipid antibodies. J Rheumatol. 1998;25:589–592. [PubMed] [Google Scholar]
- 204.Gilburd B, Stein M, Tomer Y, Tanne D, Abramski O, Chapman Y, et al. Autoantibodies to phospholipids and brain extract in patients with the Guillain-Barré syndrome: cross-reactive or pathogenic? Autoimmunity. 1993;16:23–27. doi: 10.3109/08916939309010644. [DOI] [PubMed] [Google Scholar]
- 205.Ruiz-Irastorza G, Khamashta MA, Hughes GR. Antiaggregant and anticoagulant therapy in systemic lupus erythematosus and Hughes' syndrome. Lupus. 2001;10:241–245. doi: 10.1191/096120301667789546. [DOI] [PubMed] [Google Scholar]
- 206.Khamashta MA. Management of thrombosis in the antiphospholipid syndrome. In: Khamashta MA, editor. Hughes' syndrome. London: Springer; 2000. pp. 391–396. [Google Scholar]
- 207.Schulman S, Svenungsson E, Granqvist S. Anticardiolipin antibodies predict early recurrence in thromboembolism and death among patients with venous thromboembolism following anticoagulant therapy. Duration of Anticoagulation Study Group. Am J Med. 1998;104:332–338. doi: 10.1016/s0002-9343(98)00060-6. [DOI] [PubMed] [Google Scholar]
- 208.Rai R, Cohen H, Dave M, Regan L. Randomised controlled trial of aspirin and aspirin plus heparin in pregnant women with recurrent miscarriage associated with phospholipid antibodies (or antiphospholipid antibodies) BMJ. 1997;314:253–257. doi: 10.1136/bmj.314.7076.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Castellino G, Cuadrado MJ, Godfrey T, Khamashta MA, Hughes GR. Characteristics of patients with antiphospholipid syndrome with major bleeding after oral anticoagulant treatment. Ann Rheum Dis. 2001;60:527–530. doi: 10.1136/ard.60.5.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Erkan D. Therapeutic and prognostics consideraitons in catastrophic antiphospholipid syndrome. Autoimmun Rev. 2006;6:98–103. doi: 10.1016/j.autrev.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 211.Ordonez L, Skromne E, Ontaneda D, Rivera VM. Multiphasic disseminated encephalomyelitis, systemic lupus erythematosus and antiphospholipid syndrome. Clin Neurol Neurosurg. 2007;109:102–105. doi: 10.1016/j.clineuro.2006.03.009. [DOI] [PubMed] [Google Scholar]