Abstract
The genetic mechanism of aspirin intolerant acute urticaria (AIAU) is unknown. To demonstrate an association between the β2 adrenergic receptor (ADRB2) polymorphism and the phenotype of AIAU, one hundred fourteen patients with AIAU, 110 patients with aspirin intolerant chronic urticaria (AICU), and 498 normal healthy controls (NC) based on a Korean population were enrolled. The genotype of ADRB2 at 46 A > G was analyzed using a direct sequencing method. The ADRB2 polymorphism at 46 A > G showed a significant difference between AIAU and NC; the frequency of the major genotype was significantly higher in the AIAU group (p = 0.017 in recessive model), while no differences were noted in allele and genotype frequencies between AICU and NC. In conclusion, the ADRB2 (46 A > G) gene polymorphism may contribute to the development of the phenotype of AIAU.
Keywords: ADRB2 polymorphism, aspirin sensitivity, urticaria
Aspirin intolerant acute urticaria (AIAU) presents with the immediate onset of urticaria after aspirin ingestion, which often resolves within 1 - 2 weeks.1 Aspirin ingestion could aggravate chronic urticaria, and this condition is defined as aspirin intolerant chronic urticaria (AICU).2 The prevalence of aspirin intolerant urticaria is approximately 0.3% of the normal population.3 It is generally agreed that aspirin triggers or aggravates chronic urticaria in 20% to 40% of patients.4
The beta 2-adrenergic receptor having a potential anti-inflammatory action of stabilizing mast cell degranulation and releasing inflammatory mediators5 has been postulated to be involved in the pathogenic mechanism of urticaria. In this study, we analyzed ADRB2 polymorphisms in two phenotypes of aspirin-induced urticaria, AIAU and AICU, which were also compared with those of normal healthy controls (NC) in the Korean population.
One hundred fourteen patients with AIAU, 110 patients with AICU, and 498 normal healthy controls (NC) were enrolled from the Department of Allergy and Rheumatology, in Ajou University Hospital, Suwon, Korea. AIAU was defined as a condition in which patients suffer acute urticaria when they are exposed to aspirin as confirmed by an aspirin (Rhonal®, KunWha Pharmaceutical Co., Seoul, Korea) oral provocation test as described in a previous study.6 The AICU phenotype was defined presenting with daily or near-daily spontaneous urticaria for a duration longer than 6 weeks and showing positive results (appearance of urticaria within 4 hr without any changes of FEV1) in an aspirin oral provocation test. Because there has been abundant evidence to suggest an association between the ADRB2 polymorphism at 46A > G and 79C > G with asthma related phenotypes,7,8 we excluded the patients with both AIU and AIA to better analyze any associations between the ADRB2 polymorphism and aspirin intolerant urticaria in this study. All subjects provided informed consent, and the protocol was approved by the ethics committee of Ajou University Hospital, Suwon, Korea. Atopy was determined by a positive skin test to at least one common aeroallergen including Dermatophagoides pteronyssinus, Dermatophagoides farinae, cats, dogs, cockroaches, tree pollen mixture, grass pollen mixture, mugwort, ragweed, Hop Japanese, Aspergillus, Alternaria (Bencard, Bradford, U.K.).
Genomic DNA for the all subjects in the study was extracted using the PUREGENE® DNA purification kit (Gentra systems Inc., MN, USA) according to the manufacturer's instructions. Since the minor allele frequency at 79 C > G polymorphism was very low in this cohort, we analyzed only 46A > G exon polymorphisms by a single base extension method, which was performed with the SNaPshot ddNTP primer extension kit (Applied Biosystems, CA, USA). The sequences of the forward, reverse, and extension primers used for 46A > G were 5'-caggaaacagctatgaccgcacataacgggcagaac-3', 5'-tgtaaaacgacggccagtctgacacaatccacaccatc-3', and 5'-acgtcgtggtccggcgcatggcttc-3'.
The differences in clinical characteristics among the three groups were determined by ANOVA and t-test analysis for continuous variables, and by a chi-square test with multiple comparisons for categorical variables. Differences in genotypic frequency between the groups were tested by χ2 test using SPSS ver. 12.0 (SPSS, Chicago, IL), and three logistic regression models (codominant, dominant, and recessive) were used after controlling for age, sex, and atopy as covariates. The level of statistical significance was set at p ≤ 0.05.
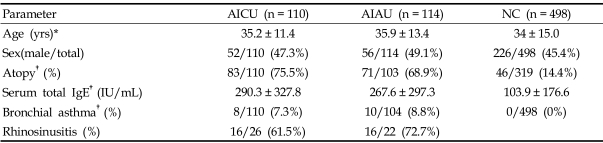
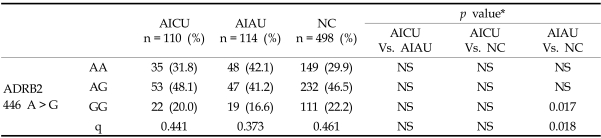
The prevalence of atopy and total IgE are significantly higher in patients with AIAU and AICU than in NC (p < 0.05 for AIAU vs. NC and AICU vs. NC, respectively), while between AICU and AIAU, no significant differences were noted in age, sex, atopy, serum total IgE level or prevalence of allergic rhinitis or asthma (p > 0.05, respectively) (Table 1). The genotype data demonstrated that the ADRB2 polymorphism at 46 A > G showed a significant difference between AIAU and NC; frequencies of the major allele and genotype were significantly higher in the AIAU group (p = 0.017 in recessive model), while no differences were noted in allele and genotype frequencies between AICU and AIAU (Table 2). Within the AIAU group, there were no significant differences in other clinical parameters between the two genotype groups (p > 0.05, respectively).
Table 1.
Clinical Characteristics of the Three Study Groups
AICU, aspirin intolerant chronic urticaria; AIAU, aspirin intolerant acute urticaria; NC, normal controls.
*Values presented as means ± SD.
†p value < 0.01 (AICU vs. NC and AIAU vs. NC, ANOVA and t-test for continuous variables and chi-square test with multiple comparison for categorical variables).
Table 2.
The Genotype and Allelic Frequencies of ADRB2 46A > G Polymorphism
AICU, aspirin intolerant chronic urticaria; AIAU, aspirin intolerant acute urticaria; NC, normal controls, q, minor allele frequency.
*Each p value was calculated with co-dominant, dominant and recessive models. Differences in genotypic frequency between the groups were tested by χ2 test.
In this study, we demonstrated that the ADRB2 46 A > G polymorphism was significantly associated with the phenotype of AIAU.
β2 adrenergic receptors on mast cells could be involved in IgE mediated mediator release from skin mast cells.9,10 Desensitization in the inhibition of histamine release has been observed with the use of β2 agonists.11 The ADRB2 polymorphism in the β2-adrenergic receptor influences the degree of desensitization : mutants (Gly16) are more resistant to desensitization than the wild type (Arg16).12 Under the same receptor desensitization condition, attenuation of histamine release inhibition was more potent than that of PGD2 and leukotriene. In this study, the frequencies of the major alleles and genotypes at ADRB2 46 A > G were significantly higher in AIAU than in NC, suggesting that patients with the major homozygous genotype may be more resistant to the inhibitory effects of β2-agonists on mast cell degranulation. Therefore, we concluded that the β2-adrenergic receptors on mast cells of subjects with the major genotype at ADRB2 46 A > G are more susceptible to histamine release from skin mast cells when they are exposed to aspirin. Further studies would be helpful to investigate how this association is present in the AIAU phenotype, but absent in the AICU phenotype.
This study suggests that the genetic polymorphism of the beta 2-adrenergic receptor at 46 A > G may affect the ability of mast cells to release histamine, which could contribute to the development of the AIAU phenotype in the Korean population.
Footnotes
This study was supported by a grant of the Korea Health 21 R&D project, Ministry of Health & Welfare, R.O.K. (A050571).
References
- 1.Grattan CE. Aspirin sensitivity and urticaria. Clin Exp Dermatol. 2003;28:123–127. doi: 10.1046/j.1365-2230.2003.01228.x. [DOI] [PubMed] [Google Scholar]
- 2.Kim SH, Park HS. Genetic markers for differentiating aspirin-hypersensitivity. Yonsei Med J. 2006;47:15–21. doi: 10.3349/ymj.2006.47.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Settipane RA, Constantine HP, Settipane GA. Aspirin intolerance and recurrent urticaria in normal adults and children. Epidemiology and review. Allergy. 1980;35:149–154. doi: 10.1111/j.1398-9995.1980.tb01730.x. [DOI] [PubMed] [Google Scholar]
- 4.Mastalerz L, Setkowicz M, Sanak M, Szczeklik A. Hypersensitivity to aspirin: common eicosanoid alterations in urticaria and asthma. J Allergy Clin Immunol. 2004;113:771–775. doi: 10.1016/j.jaci.2003.12.323. [DOI] [PubMed] [Google Scholar]
- 5.Kay LJ, Peachell PT. Mast cell beta2-adrenoceptors. Chem Immunol Allergy. 2005;87:145–153. doi: 10.1159/000087641. [DOI] [PubMed] [Google Scholar]
- 6.Kim SH, Choi JH, Holloway JW, Suh CH, Nahm DH, Ha EH, et al. Leukotriene-related gene polymorphisms in patients with aspirin-intolerant urticaria and aspirinintolerant asthma: differing contributions of ALOX5 polymorphism in Korean population. J Korean Med Sci. 2005;20:926–931. doi: 10.3346/jkms.2005.20.6.926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Contopoulos-Ioannidis DG, Manoli EN, Ioannidis JP. Meta-analysis of the association of beta2-adrenergic receptor polymorphisms with asthma phenotypes. J Allergy Clin Immunol. 2005;115:963–972. doi: 10.1016/j.jaci.2004.12.1119. [DOI] [PubMed] [Google Scholar]
- 8.Thakkinstian A, McEvoy M, Minelli C, Gibson P, Hancox B, Duffy D, et al. Systematic review and meta-analysis of the association between {beta}2-adrenoceptor polymorphisms and asthma: a HuGE review. Am J Epidemiol. 2005;162:201–211. doi: 10.1093/aje/kwi184. [DOI] [PubMed] [Google Scholar]
- 9.Hughes JM, Seale JP, Temple DM. Effect of fenoterol on immunological release of leukotrienes and histamine from human lung in vitro: selective antagonism by beta-adrenoceptor antagonists. Eur J Pharmacol. 1983;95:239–245. doi: 10.1016/0014-2999(83)90640-4. [DOI] [PubMed] [Google Scholar]
- 10.Undem BJ, Peachell PT, Lichtenstein LM. Isoproterenolinduced inhibition of immunoglobulin E-mediated release of histamine and arachidonic acid metabolites from the human lung mast cell. J Pharmacol Exp Ther. 1988;247:209–217. [PubMed] [Google Scholar]
- 11.Chong LK, Suvarna K, Chess-Williams R, Peachell PT. Desensitization of beta2-adrenoceptor-mediated responses by short-acting beta2-adrenoceptor agonists in human lung mast cells. Br J Pharmacol. 2003;138:512–520. doi: 10.1038/sj.bjp.0705050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chong LK, Chowdry J, Ghahramani P, Peachell PT. Influence of genetic polymorphisms in the beta2-adrenoceptor on desensitization in human lung mast cells. Pharmacogenetics. 2000;10:153–162. doi: 10.1097/00008571-200003000-00007. [DOI] [PubMed] [Google Scholar]