Abstract
Liposomes containing bisphosphonates have been shown to deplete circulating monocytes and reduce experimental restenosis. However, acceptable shelf life was not achieved, and the disruption extent and rate of the vesicles in the circulation has not been examined. Designing an optimal liposomal formulation in general, and for an anti-inflammatory effect in particular, requires careful consideration of the factors that contribute to their in vitro stability and integrity in the blood after injection. An improved liposomal alendronate formulation was prepared by a modified thin lipid film hydration technique followed by extrusion, resulting in relatively smaller size vesicles, narrow size distribution, and low drug to lipid ratio in comparison to the reverse phase evaporation method. In order to rule out premature leakage of the drug, the integrity of the vesicles was examined by means of size-exclusion chromatography in vitro and in vivo, with subsequent analysis of size, drug (fractions of encapsulated and free) and lipid concentrations. Vesicles were found to be stable in serum, with 15 ± 3% leakage of the drug after 10 min in rabbit’s circulation, and intact liposomes were detected in the circulation 24 h following administration. It is concluded that the new formulation results in increased stability (2.5 years) as determined by the insignificant changes in vesicle size, drug leakage, lipid and drug stability, in vitro bioactivity (macrophages inhibition), as well as in vivo in depleting circulating monocytes and inhibition of restenosis in rabbits. Our in vitro stability results regarding dilution in serum paralleled in vivo data. Thus, in vitro assessment may provide a valuable tool in assessing in vivo integrity of liposomal formulations.
Key words: alendronate, formulation, leakage, liposomes, macrophages, monocytes, restenosis, stability, vesicles
INTRODUCTION
It has been demonstrated that partial systemic inactivation and transient depletion of monocytes and macrophages by liposomal bisphosphonates (BP) reduces neointimal hyperplasia and restenosis in animal models (1–4). Restenosis, reobstruction of the coronary arteries following percutaneuos coronary interventions such as stent angioplasty, can be prevented by diminishing the inflammatory trigger of SMC proliferation and migration (1–3,5–7). One single IV injection of liposomes incorporating a BP such as clodronate or alendronate, at the time of injury, can treat several vessels with a long-term effect and minimal side effects (1–3). The intracellular effects of BP on osteoclasts, utilized clinically in bone-related disorders, are enabled only after ingestion of bone-adsorbed drug (8) since BP do not cross the cell membrane (9). Thus, only when BP in a particulate dosage form, such as liposomes, are endocytosed by monocytes/macrophages, would intracellular BP release and action result (5,10).
Designing an optimal liposomal formulation in general, and for an anti-inflammatory effect in particular, requires careful consideration of the factors that contribute to their in vitro stability and in vivo integrity upon dilution in the blood after injection. The physicochemical properties of liposomes including size, charge, lipid composition, and cholesterol content are governing factors of stability, blood integrity, and clearance rate from the circulation (11–13). Premature discharge of the encapsulated drug would reduce bioactivity since less drug will be delivered to the phagocytic cell and may increase the possibility of untoward effects. The formulation of alendronate liposomes examined so far by our group (2,3) was of a wide range of size distribution (100 to 500 nm, with fractions >1 μm), the process was not always reproducible, shelf life stability was less than 1 month, and could not be easily up-scaled for manufacturing (14). All liposomal bisphosphonate formulations reported heretofore were prepared by the reverse-phase evaporation (REV) method (1,10,15–17) or the evaporation technique (18,19); encapsulation yield was low (3% and 5–7%, for alendronate (20) and clodronate liposomes (19), respectively), stability in vitro was less than 1 month (19,20), in vivo stability had not been determined, liposome size was characterized with a wide-size distribution (7–20), and filter-sterilization was impossible.
We report here on an improved liposomal formulation of alendronate manufactured by the thin layer film hydration method. The facile and reproducible method resulted in a narrower size distribution of the vesicles, and the preparation has a projected stability of over 2.5 years verified by unchanged physicochemical and bioactivity properties. Moreover, in order to rule out premature leakage of the drug upon interaction with blood, the integrity of the vesicles was examined by means of size-exclusion chromatography following in vitro incubation studies and IV administration in rabbits, with subsequent analysis of size, drug and lipid concentrations in the fractions collected over time. An in vitro/in vivo correlation was obtained providing a valuable tool for determining in vivo integrity of liposomal formulations.
MATERIALS AND METHODS
Preparation of Liposomes
Small unilamellar vesicles were prepared by a modified thin lipid film hydration method (21). 1,2-Distearoyl-sn-glycero-3-phosphocholine (DSPC, Lipoid, Ludwigshafen, Germany), negatively charged distearoyl-phosphatidylglycerol (DSPG, Lipoid), and cholesterol (Sigma-Aldrich, Israel), at a molar ratio of 3:1:2 were dissolved in t-butanol and lyophilized overnight. The lyophilized cake was hydrated with an aqueous solution containing sodium alendronate (Unipharm, Tel-Aviv, Israel) at 55–60°C, and left to stand for 1 h. The suspension was then extruded in a thermobarrel extruder (Northern Lipids Inc, Vancouver, Canada), at 70°C, through double polycarbonate membranes of 0.8 and 0.4 μm (×3 times each), and followed through 0.2 μm pore size membrane (×7 times, Nucleopore, CA, USA). The obtained liposomes were purified on a Sephadex G-50 column and eluted in 2-(N-morpholino) ethanesulfonic acid (MES)/4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) isotonic buffer pH 7.2 50 mM MES, 50 mM 4-(2-hydroxyethyl) piperazine-1-ethanesulfonic acid (HEPES), 75 mM NaCl, pH 7.2) to remove free alendronate. The liposomes were suspended at a concentration of 24 mM total lipids in isotonic MES/HEPES buffer, and sterilized by microfiltration (Minisart 0.22 μm, Sartorius). Drug-free liposomes were prepared by the same procedure, omitting the drug.
Liposome Characterization
To evaluate alendronate and lipid concentration inside and outside the liposomes, a centricon separation filter (Millipore, 30,000 MW) was used. Liposome size and morphology was determined by photon correlation spectroscopy (ALV-GmBH, Langen Germany) and cryogenic transmission electron microscopy (cryo-TEM, Phillips CM 120). The multilamellarity was calculated by counting the number of multilamellar liposomes in 50 different sections of the Cryo-TEM comparing three batches prepared by the reverse-phase evaporation method to the thin-film hydration method. The zeta potential was measured by means of a NanoZ (Malvern instruments, Malvern, UK).
The concentration of DSPC and DSPG was determined colorimetrically by the Bartlett assay (22) which assessed the amount of phosphorus following hydrolysis of the phospholipids, with 1 mol of phosphorus equivalent to 1 mol of phospholipids. A TLC method was used for the determination of polar and nonpolar impurities and concomitant components of the phospholipids. The sample was applied to a TLC–aluminum foil with different amounts of the corresponding reference substances, stearic acid, L-α-monostearoyl-phosphatidylcholine (LPC), 1-oleoyl-2-hydroxy-sn-glycero-3- [phosphor-rac-(1-glycerol)] (sodium salt) (LPG), and distearoyl L-α-phosphatidic acid (C18:0, DSPA), and then developed with CuSO4 for visualization. Analysis was done in comparison to corresponding reference standards. An isocratic high-performance liquid chromatography (HPLC) method using a photodiode array detector was used for the determination of cholesterol and cholesterol impurities (23). The main expected degradation products, 5α-cholestan-3β-ol-7-one (7-ketocholesterol) and 5α-cholestene-3β,7β-diol (7β-hydroxycholesterol) were analyzed as reference standards.
Alendronate concentration was determined by a gradient reversed-phase HPLC method based on the United Stated Pharmacopeia (24). Stability of 4-amino-1-hydroxyl-butanylidene-1,1-bisphosphate (alendronate) was monitored by chromatography for the degradation product, 4-aminobutyric acid. For the analysis of alendronate in serum, 1 ml of serum was diluted with water (3 ml) and 0.4 mg/ml pamidronate was added as an internal standard. Protein was precipitated by the addition of 250 μl 10% trichloroacetic acid, vortexed and centrifuged for 10 min at 4,000 × g at 4°C. This precipitation procedure was repeated six times for optimal recovery. The supernatant and precipitate were analyzed separately. Alendronate was recovered from 1 ml supernatant by direct precipitation of its calcium salt by adding 4 0 μl 0.5 M NaH2PO4, 25 μl of 2.5 M CaCl2, and adjustment of the pH to 12 with 6.25 M NaOH, followed by vigorous mixing and centrifugation as described above. The supernatant was discarded, and the pellet was washed with 3 ml distilled water and centrifuged as described. The resulting precipitate was dissolved in 200 μl, 1 M HCl, followed by three cycles of precipitation, with the final precipitate dissolved in 400 μl 0.13 M Na2EDTA and the pH adjusted to 10 by 6.25 M NaOH to a final volume of 1 ml. The serum precipitate obtained during deproteinization was dissolved with octyl b-d-glucopyranosile (OGP, Sigma-Aldrich, Israel) and subsequently underwent the same process as the supernatant.
Following derivatization of alendronate and pamidronate (a BP, serving as an internal standard) with fluorescamine, their concentration was determined on a Nucleosil C18 column (10 nm particle size, Macherey-Nagel, Germany) by fluorescence, excitation, and emission at 395 and 480 nm, respectively. The standard peaks of alendronate and pamidronate were eluted at 6.5 min and 4.6 min, respectively. The limit of detection, at a signal-to-noise ratio of 3:1, was 5 ng/ml, and therefore, the detection threshold was set at 10 ng/ml. This method had been developed and fully validated for the range between 10 to 100 mg/ml (R2 = 0.9899).
In Vitro Stability
The stability of liposomal alendronate was determined in different buffers [MES/HEPES, phosphate-buffered saline (PBS), 5% dextrose, 2.2% glycerol] and at three different incubation temperatures, namely, 4°C, 25°C, and 37°C. Stability was determined by examining changes in vesicle size, zeta potential, phospholipid integrity, drug and lipid leakage (disruption of the membrane), and bioactivity changes over time. At specific time points, 400 μl of the liposomal formulation was centrifuged with a centricon separation filter (Millipore, 30,000 MW) at 3,500 × g, for 60 min, at 4°C. The liposomes were retained in the upper chamber, 100–150 μl of the filtrate was recovered from the lower chamber, and the drug and lipid concentrations were determined. Leakage, phospholipid integrity, size distribution, and zeta potential were evaluated each month for 2.5 years.
Liposomes Integrity in Serum In Vitro
In order to rule out premature leakage of the drug upon interaction with serum and blood, the integrity of the vesicles in serum was examined by means of size-exclusion chromatography of samples from in vitro incubation studies and following intravenous (IV) administration to rabbits. The in vitro integrity of alendronate liposomes in serum was examined following dilution with rabbit serum vs. buffer control at 37°C. Five milliliters of alendronate liposomes, empty liposomes, spiked liposomes, or free drug (611 μg/ml alendronate) were incubated with 40 ml of freshly collected rabbit serum at 37°C, with stirring. The dilution factor was chosen based on the estimated concentration of alendronate in vivo following an IV injection of a dose of 30 mg/kg to rabbits of 3.5–4 kg, assuming a blood volume of 57–70 ml/kg, and a hematocrit of 30–40% (25). Aliquots of 2.5 ml, after 10 min, 60 min, 5 h, and 24 h incubation period, were chromatographed on Sephadex G-50 size exclusion column and were eluted with MES/HEPES buffer. The eluted fractions were collected for each time point, 20 fractions of 2 ml each, and stored at −70°C until analysis. Liposome integrity was evaluated as a function of drug concentration (encapsulated and free alendronate), lipids content, drug to lipids ratio, and size distribution in each fraction. Protein concentration was determined by the bicinchoninic acid protein assay (BCA, Pierce USA) and a modified BCA protein assay (26). Since liposomal phospholipids and blood phospholipids may interfere with this protein assay, protein concentration was also determined, in each fraction, by a modified BCA assay that reduces lipid interference (with sodium dodecylsulfate). The findings of both assays were similar, with somewhat lower absolute amounts, but not relative amounts, of proteins in the modified assay, which was used in the data analysis. The results were compared to free drug, empty liposomes, and empty liposomes spiked with free alendronate that underwent the same procedure.
Liposomes Integrity In Vivo
Alendronate liposomes (30 mg/kg) were injected via the ear vein of three rabbits (3.5–4 kg). This dose allowed a suitable detection limit in all assays. For comparison, animals (N = 3 in each group) were similarly treated with empty liposomes (dose normalized to lipids content, 112 mg/kg), empty liposomes spiked with alendronate (30 mg/kg), and saline (same volume). Blood samples were collected after 10 min, 60 min, 5 h, and 24 h, allowed to clot for 60 min on ice, and centrifuged (4,000 × g, 10 min, 4°C). Aliquots (2.5 ml) of the serum were applied to the columns as above (two columns per each specimen at each time point). Eluted fractions were collected for each time point, 20 fractions of 2 ml each, and stored at −70°C until analysis. Vesicle integrity was evaluated as a function of drug concentration (encapsulated and free alendronate), lipid content, drug to lipid ratio, and size distribution in each fraction in comparison to other groups.
Bioactivity
Effect on Cell Growth
Inhibition of RAW 264 proliferation by the various formulations was determined for evaluating bioactivity over time. A murine macrophage cell line (RAW 264, ATCC, Rockville, MD, USA) was utilized. Cells were grown in Dulbecco’s modified Eagle’s medium supplemented with 10% fetal calf serum (FCS), 2 mM l-glutamine, 100 U/ml penicillin and 100 μg/ml streptomycin in 5% CO2 atmosphere at 37°C. Cells were plated at 2 × 104 cells per well in 24-well plates and were allowed to grow overnight. The cells were incubated with the formulations tested for stability (6 month, 1, 2, and 2.5 years) in comparison to freshly prepared liposomes. After 48 h, the number of viable cells was counted by means of a coulter counter (Coulter Corporation, Miami, FL, USA) and the 3-(4, 5-dimethylthiazolyl-2)-2, 5-diphenyltetrazolium bromide (MTT ) assay (Sigma, USA). Unless otherwise noted, all materials for cell cultures were purchased from Biologic Industries (Beit Haemek, Israel).
Depletion of Blood Monocytes
Another measure for preserved bioactivity of the liposomes over time was the depletion of blood monocytes. The effect of the freshly prepared liposomes in comparison to formulation tested for stability (6 month, 1, 2, and 2.5 years) on circulating monocytes in rabbits was assayed by fluorescence-activated cell sorting (FACS) analysis as described previously (1). In brief, whole blood (200 μl) was labeled with mouse antihuman R-phycoerythin-conjugated anti-CD14 (Dako, Denmark), red blood cells were lysed (FACS lysing solution, Becton & Dickinson, USA), the cell suspension was washed twice with PBS containing 1% FCS. The monocytes percentage of total white blood cells was recorded on the basis of relative size, light side-scattering, and fluorescence (Becton & Dickinson).
Inhibition of Restenosis
Inhibition of vascular stenosis in a rabbit model of restenosis was examined for evaluating bioactivity of the liposomes over time. New Zealand White rabbits (Harlan Laboratories, Jerusalem, Israel) weighing 2.5–3.5 kg were used in accordance with the guidelines for animal care of the Hebrew University of Jerusalem conforming to the National Institutes of Health (USA). Animals were fed an atherogenic diet of 2% cholesterol and 6% peanut oil, starting 28 days before angioplasty. Hypercholesterolemia was ascertained (plasma cholesterol >1,200 mg/dl). Animals were anesthetized by xylazine (7 mg/kg) and ketamine (40 mg/kg). Heparin (200 U/kg), atropine (0.05 mg), and norfloxacin nicotinate (70 mg) were given. Balloon injury was performed on the left common carotid artery with a 3-mm angioplasty balloon catheter (Cordis, 2X1-minute inflation at 8 atm). An investigator blinded to the type of experimental group performed the procedure as well as the analyses below. Animals were randomly assigned to treatment and control groups. On the day of injury, animals were injected with alendronate liposomes (3 mg/kg) or empty liposomes intravenously. Animals were sacrificed 28 days after injury by pentothal, and the arteries were perfusion-fixed in situ with 150 ml of 4% formaldehyde solution (pH 7.4) for morphometric analysis. The arterial segments were then embedded in paraffin and cut at eight to ten sites (600 μm apart), and sections of 6 μm were mounted and stained with Verhoeff elastin staining, Mayer hematoxylin and eosin, and modified Movat pentachrome (1).
Morphometric Analysis
Eight to ten sections in each slide were analyzed by means of computerized morphometric analysis (NIH Image) by an investigator blinded to the type of the experimental group. The section with the greatest luminal narrowing by neointima was analyzed as previously described (1). The residual lumen, the area bounded by the internal elastic lamina (original lumen), and the area circumscribed by the external elastic lamina (total arterial area) were measured directly. The degree of neointimal thickening was expressed as the ratio between the area of the neointima and the original lumen (% stenosis) and as the ratio between the neointimal area to the area of the media (N/M).
Statistics
Data are expressed as mean ± SD. Statistical differences between groups in the in vitro and in vivo experiments were assessed by two-way ANOVA, using InStat software.
RESULTS
Characterization of the Liposomal Formulations
The spherical and mainly unilamellar liposomes of the present formulation are demonstrated in Fig. 1. Drug to lipids ratio was 1:3.7, and the encapsulation yield was 30 ± 5%. The concentration of encapsulated alendronate and lipids in the liposomes was 5.5 ± 0.2 mg/ml and 20.4 ± 1.6 mg/ml, respectively, and the free drug concentration was less than 0.05 mg/ml. The liposomes obtained had a mean diameter of 160 ± 24, 161 ± 21, and 161 ± 21 nm, for alendronate loaded, empty, and spiked liposomes, respectively, with a polydispersity index below 0.1. Negative zeta potentials were obtained, –26.4 ± 2.1, –29.2 ± 1.9, and –29.1 ± 1.9 mV, alendronate loaded, empty, and spiked liposomes, respectively.
Fig. 1.
Transmission electron microscopy photomicrographs of liposomal alendronate and empty liposomes
In vitro Shelf-Life Stability
Stability determined in different buffers (MES/HEPES, PBS, 5% dextrose, and 2.2% glycerol) revealed that alendronate liposomes were stable for >2.5 years at 4°C and 25°C. A lower stability was detected at 37°C (3 month). Drug leakage was less than 10%, and no significant changes in liposome size, zeta potential, drug to lipid ratio, and morphology were detected over that period of storage. In addition, lipid and cholesterol degradation products were below 0.1%, and no degradation of alendronate was noted. In vitro assay for bioactivity on RAW 264 macrophages showed no change in the concentration of maximal bioactivity, at 50 μM alendronate concentration 90 ± 10% inhibition of proliferation was observed, similar to freshly prepared liposomes (similar bioactivity was also obtained at lower drug concentrations of 1, 5, 10, 15, and 25 μM). In vivo bioactivity studies supported the unchanged bioactivity over time (see below). The advantageous physicochemical properties of the developed formulation in comparison to the previous formulation are delineated in Table I.
Table I.
Summary of the Improved Physicochemical Properties of the Liposomal Alendronate Formulation Prepared in this Study
| Formulation | |||
|---|---|---|---|
| Pre sent study | Previous study | ||
| Size and morphology | nm ± SD | 160 ± 24 | 254 ± 322,a/161 ± 603,a |
| Polydispersity index | <0.1 | >0.3 | |
| Multilamellarityb (%) | 10 | 40 | |
| Stability | Projected Shelf-life | >2.5 year | 1 month |
| Drug leakage (%) | <5 | >20 | |
| Yield | Drug encapsulation (%) | 30 ± 5 | 10 ± 2 |
aCharacterized by >15% of the vesicles at 400 nm, and 1 to 5% in the micron size range
bEstimated % of multilamellar vesicles from the cryo-TEM images
Liposome Integrity in Serum In Vitro
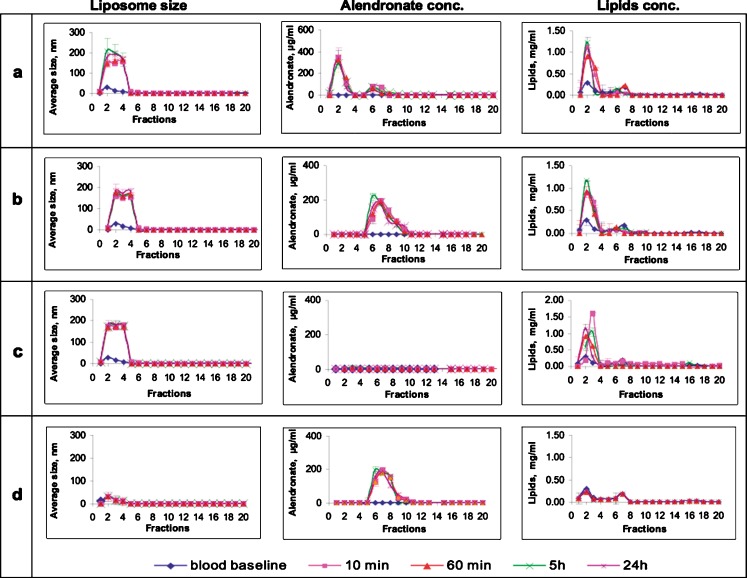
In preliminary experiments, liposomes were diluted with buffer, and the vesicles and free drug elution profiles were determined in terms of changes in size, and drug and lipids concentration in 20 fractions (2 ml each). Intact liposomes were detected in fractions 1 to 3. Fraction 4 was considered as a transition phase, which contained neither liposomes nor free drug, and in fractions 5 to 10, free drug was eluted (to be on the safe side, all 20 fractions were analyzed for size, drug, lipid, and protein concentrations). Since the column separates particles according to their size, it is unlikely to detect liposomes of larger size in any other fractions than 1 to 3. In size determinations, but not by drug and lipids concentration determinations, exceedingly low amounts of liposomes were detected in fraction 4. Background noise, obtained from the elution profile of serum incubated with MES/HEPES buffer at the same volumes ratio, was subtracted.
A similar elution profile to that found upon dilution in buffer was observed in liposomes diluted with rabbit’s serum (Fig. 2). Size determinations of liposomes in serum revealed that intact liposomes were eluted in fractions 1 to 3, fraction 4 contained exceedingly low amounts of liposomes detected by size but not by drug and lipid concentration determinations, free drug was eluted in fractions 5–10, and no liposomes could be detected in fractions 5 to 20. Liposome integrity, in terms of average size distribution, was unaffected, and their diameter, 148 to 180 nm, remained the same for over 24 h in serum (Fig. 2, left column).
Fig. 2.
Elution profile (size-exclusion chromatography columns) of alendronate liposomes as function of size (diameter, left column), alendronate concentration (middle column), and lipid concentration (right column) in specimens recovered over time following incubation in rabbit’s serum in vitro, a alendronate liposomes (611 μg/ml), b empty liposomes spiked with alendronate (611 μg/ml), c empty liposomes, and d free alendronate (611 μg/ml). In this system of size-exclusion chromatography, intact liposomes are detected in fractions 1 to 3, fraction 4 is a transitional phase, and free drug is detected in fractions 5–10 (total of 20 fractions, 2 ml each). Background noise, obtained from the elution’s profile of serum incubated with buffer at the same volumes ratio, was subtracted
The concentration of the drug was determined in all fractions (1–20) as an additional measure of vesicle integrity (Fig. 2, middle column). As expected, no drug was found in the empty formulation (Fig. 2c, middle column). In the spiked formulation, alendronate was detected in fractions 5 to 10 (Fig. 2b, middle column) corresponding to free alendronate, and exceedingly low concentrations of the drug near the detection limit were found in other fractions (11–20).
A “burst effect” of drug leakage (initial high release of drug content, wt.%) was noted after 10 min (Fig. 2a, middle column). Of the drug, 25 ± 3% was detected in fractions 5–10 (free drug), 75 ± 11% of the drug was detected in fractions 1 to 3 (encapsulated), and nothing was detected in fraction 4 (transitional phase). At all other time points (60 min, 5 and 24 h), no additional release was observed. The assay of lipids corroborated the previous observations (Fig. 2, right column). As expected, lipids in empty liposome elutions were detected mainly in fractions 1 to 3, and nothing was detected in fraction 4 (Fig. 2c, right column). Certain concentration of lipids was detected in fractions 5 to 20, indicating partial liposome disruption or residual lipids from serum. Similar patterns were observed for alendronate-liposomes (Fig. 2a, right column) and for spiked liposomes (Fig. 2b, right column).
Negatively charged liposomes are likely to adsorb proteins (opsonized) following incubation in serum by electrostatic force of positively charged proteins. In order to determine protein adsorption, protein concentration was determined in all fractions (data not shown). Proteins were detected mainly in fractions 1–3 (fraction 4 was empty), as would be expected from protein adsorption on intact liposomes. Increase in protein adsorption over time was observed in empty liposomes incubated in serum. The protein content was 8.9, 13.5, and 17.1 mg/ml at 10 min, 60 min, and 24 h, respectively. This pattern of protein adsorption was not observed in the spiked, free alendronate and alendronate-loaded liposomes.
Combining the elution profiles of all four variables: size and concentrations of alendronate, lipid and proteins, allowed us to consider fractions 1–3 of all formulations as those containing intact liposomes, fraction 4 as a transition phase, while disrupted liposomes, free drug and free lipids are eluted in fractions 5 to 10. It can be concluded that 25 ± 3% of encapsulated alendronate is released after 10 min of incubation in serum without further release, after a “burst effect” (Fig. 2a, middle column). Additionally, another indication of liposome integrity was the drug to lipids ratio, 1:3.5 in fractions 1–3, similar to that found in intact formulation (1:3.7). Furthermore, a mass balance was obtained since 94 ± 5.4% of the initial drug content was recovered when calculating total drug in fractions 1 to 20.
Liposome Integrity In Vivo
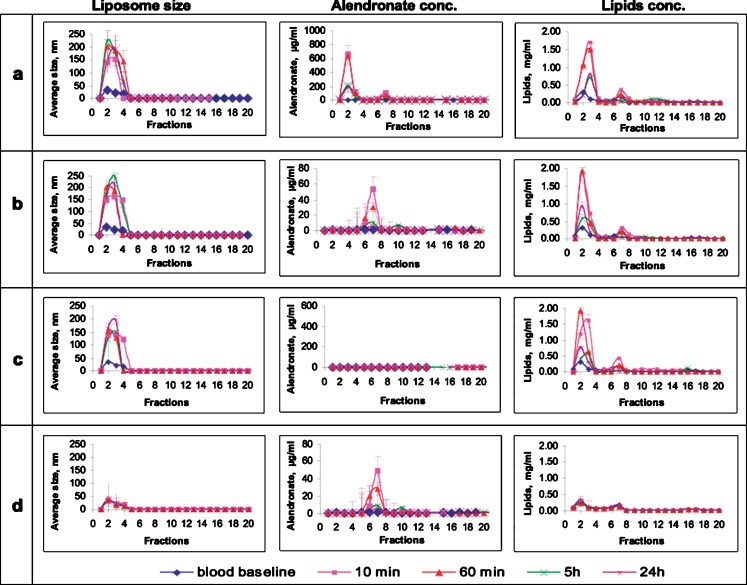
A similar elution profile to that found in vitro was observed in vivo, with an increase in liposome average size, from 160 to 220 nm, which was most pronounced after 5 and 24 h (Fig. 3a–d, left column). As expected from the preliminary studies and from the in vitro experiments, intact liposomes were eluted in fractions 1 to 3, fraction 4 contained exceedingly low amounts of liposomes detected by size but not by drug and lipids concentration determinations, free drug was eluted in fractions 5–10, and no liposomes could be detected in fractions 5 to 20.
Fig. 3.
In vivo elution profile (size-exclusion chromatography columns) of alendronate liposomes as function of size (diameter, left column), alendronate concentration (middle column), and lipid concentrations (right column) in specimens recovered over time from rabbits’ blood following IV injection (30 mg/kg) of a alendronate liposomes, b empty liposomes spiked with alendronate (30 mg/kg), c empty liposomes, and d free alendronate (30 mg/kg). See legend of Fig. 2 for additional information regarding fractions and detection
Drug concentration was determined in all fractions (Fig. 3a–d, middle column). As expected, no drug was found in the empty formulation (Fig. 3c, middle column). In the spiked formulation, alendronate was detected in fractions 5 to 8 (Fig. 3b, middle column), corresponding to free alendronate (Fig. 3d). A “burst effect” of drug leakage was noted 10 min postinjection (Fig. 3a, middle column). Approximately 15 ± 3% of the drug was detected in fractions 5–10 (free drug), 85 ± 5% of the drug was detected in fractions 1 to 3 (encapsulated), and nothing was detected in fraction 4 (transition phase). At 1 and 24 h postinjection, 91 ± 3%, and 98 ± 3% of the recovered drug was found in encapsulated form, respectively. It can be concluded that, at all time points, most of the drug in circulation is encapsulated in liposomes (Fig. 3a, middle column). Moreover, the drug to lipid ratio of liposomes recovered in vivo was similar to that determined prior to injection, 1:3.54 and 1:3.70, respectively, indicating vesicle integrity of over 85 ± 5%.
Lipids concentration was determined in the same fractions (Fig. 3a–d, right column). As expected, lipids of alendronate liposomes were detected mainly in fractions 1 to 3 (Fig. 3a, right column), corresponding to intact liposomes (fraction 4 was empty). A certain concentration of lipids was detected in fractions 5 to 20 (less than 15% after 10 min, and lower concentrations at later time points). Lipids in fractions 5–20 indicated partial disruption of liposomes or residual lipids from serum. Similar patterns were observed for empty (Fig. 3c, right column) and spiked liposomes (Fig. 3b, right column). Of the lipids, 81 ± 2% were detected in fractions 1 to 3 after 10 min, corresponding to the 85 ± 5% of drug that was detected in same fractions, indicating over 80% of intact liposomes.
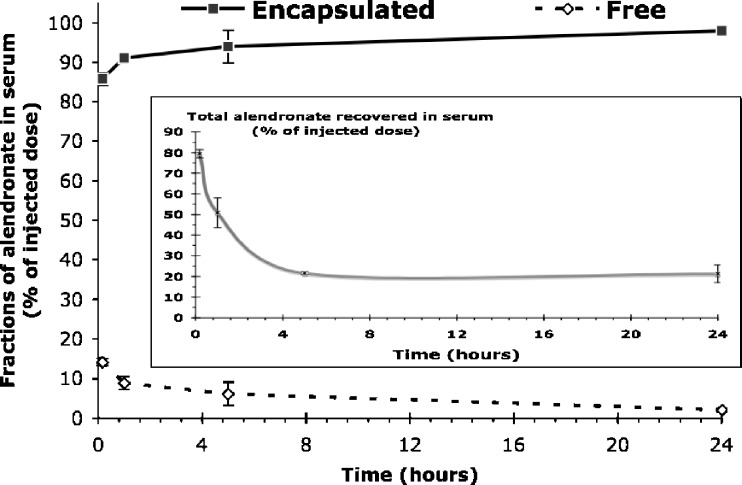
As can be seen from Fig. 4, which summarizes the findings of alendronate clearance from circulation as encapsulated and in free form, at any given time point more than 85% of the total recovered drug was in encapsulated form. In contrast, free alendronate was hardly detected in the blood, probably due to the rapid elimination of the drug.
Fig. 4.
The concentration of encapsulated and free alendronate fractions in serum of rabbits over time after IV administration of 30 mg/kg liposomal alendronate (large graph). The decline of total alendronate concentration in serum is shown in the inset
Depletion of Monocytes and Inhibition of Restenosis
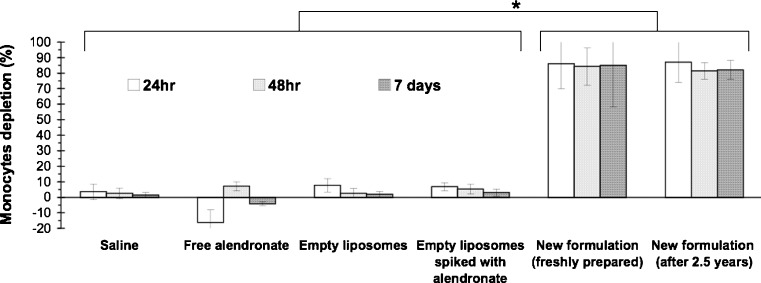
In vivo bioactivity studies supported the unchanged bioactivity over time. Over 80 ± 5% depletion of circulating monocytes in rabbits was obtained following a 3-mg/kg dose of freshly prepared liposomes in comparison to the new formulation aged for 2.5 years (Fig. 5). No effect was observed following treatment with saline, empty liposomes, and empty liposomes spiked with alendronate.
Fig. 5.
Depletion of monocytes in peripheral blood of rabbits analyzed by FACS following treatment with the new formulation after 2.5 years storage at 4°C in comparison to freshly prepared old formulation and to treatment with the control groups (free alendronate, empty liposomes spiked with alendronate and saline). N = 6 at each group, *p < 0.01, no significant difference between freshly prepared old formulation to the new formulation aged for 2.5 years
In order to evaluate the bioactivity of the developed formulation in a pathological disorder, the hypercholesterolemic rabbit model of restenosis was utilized. IV administration of alendronate liposomes (3 mg/kg, day 0, n = 12) resulted in a significantly reduced neointima formation triggered by balloon injury in comparison to empty liposomes (n = 10). Decrease of both, stenosis and neointima to media ratio was obtained (Fig. 6). Stenosis was 40.1 ± 10.2 and 73.5 ± 17.0%, treatment and control groups, respectively (p < 0.01). Neointima to media ratio was 0.7 ± 0.2 and 1.9 ± 0.7, alendronate liposomes and empty liposomes treatment groups, respectively (p < 0.02, Fig. 5).
Fig. 6.
Inhibition of restenosis in the hypercholesterolemic rabbit carotid artery injury model 28 days after injury (n = 12, each group). Photomicrographs of Verhoeff tissue elastin staining of sections from untreated (empty liposomes) and from rabbits treated with alendronate liposomes (3 mg/kg, IV on the day of injury). Bar graphs show the extent of neointima to media ratio, and the extent of stenosis (%)
DISCUSSION
An improved formulation of liposomal alendronate was achieved characterized by a facile and reproducible method of preparation, a projected stability of over 2.5 years, in the nano-size range (~160 nm), negatively charged, and possessing potent immunomodulating effects in vivo. As opposed to our former formulation of liposomal bisphosphonates (1–3), the improved formulation described here was prepared by lyophilization, a modified thin film hydration technique, rather than by the reverse-phase evaporation (REV) method and, more importantly, followed by extrusion instead of sonication. This is the first report on a liposomal bisphosphonate formulation not prepared by the REV method (1,10,16–19). The developed formulation resulted in a narrow size distribution having a polydispersity index below 0.1 and encapsulation yield of 30 ± 5%. In contrast, the former formulation (2) is characterized by both relatively large SD and PDI, with more of 15% of the vesicles at 400 nm size, and 1 to 5% in the micron size range (Table I). The drug to lipid ratio was reduced to 1:3.7 instead of 1:5–6 minimizing the burden of lipids in vivo, resulting in a better encapsulation yield, and making the formulation suitable for filter-sterilization as well as scale-up production [without the utilization of non-biocompatible solvents such as chloroform (19)]. Moreover, the stability of the former formulation was limited to 1 month because aggregation and phase separation as well as drug leakage (>20%) were observed at longer storage time. Vesicle morphology in the improved formulation is characterized by more than 90% unilamellar liposomes in comparison to the relatively large population of multilamellar liposomes in the previous formulation (Fig. 1). By controlling vesicle size by extrusion, aggregation cores might be eliminated, resulting in both narrower size distribution and increased stability.
The advantages of a long shelf life and reproducible formulation are self-evident. It seems that an optimal average size of the liposomes was achieved, for maximizing both efficacy and safety. Large vesicles (>1 μm) are known to cause adverse effects after injection accumulating in the lungs and causing thrombosis (27), whereas vesicles smaller than 100 nm are prone to nonphagocytic cells escaping the mononuclear phagocyte system (MPS) (28). Since our target cells are circulating monocytes, liposome size of 150–250 nm is preferable. This is because, while vesicles larger than 100 nm are eliminated from the blood stream exclusively by monocyte/macrophage uptake (29,30), vesicles under ~250 nm can be readily filter-sterilized.
A charged membrane enhances the internalization of liposomes into phagocytic cells through adsorptive endocytosis (30,31), and opsonization mediates efficient internalization (32,33). It should be noted that the Food and Drug Administration in the United States has not yet approved positively charged lipids for clinical use. Negatively charged liposomes prevent the adsorption of negatively charged proteins that could induce leakage of the encapsulated content in biological fluids (10,13,34). Therefore, the surface charge density of the liposomes has to be optimized, minimizing leakage on one hand and maximizing internalization, on the other. The zeta potential obtained (−26 to −29 mV) meets these requirements, since a zeta potential of |30| is considered optimal for conferring stability (5), and the developed formulation exhibited circulation integrity as well as efficient internalization accompanied by potent bioactivity.
It is also important to choose lipids with specific transition phases that are intrinsically related to the effect of charge and stability (35). Premature leakage can be avoided by the inclusion of high-phase transition lipids (36,37), such as DSPC and DSPG, and of cholesterol (11,38), utilized in our formulations. Thus, the formulation developed meets all of the above requirements; a negatively charged membrane, composed of DSPC/DSPG/cholesterol (molar ratio of 3:2:1), having an average size of 160 ± 24 nm (Fig. 1), characterized with a small PDI and low multilamellarity (Table I). The utilization of the modified thin lipid film hydration method followed by extrusion resulted in improved characteristics including the long-time stability in different buffers (PBS, MES/HEPES, 2.2% glycerol and 5% dextrose) exceeding 2.5 years.
The contact of liposome vesicles with rabbit serum caused a “burst effect” of 25% and 15% drug leakage after 10 min, in vitro and in vivo, respectively, with a corresponding decrease of lipid concentration and drug to lipids ratio (Figs. 2 and 3). This is probably due to protein interactions and fluid-induced leakage (32,33). The in vivo integrity assay proved that intact liposomes could be detected in the blood during 24 h post-administration (Fig. 3).
Up to 24 h post-administration, at any given time point, more than 85% of the total recovered drug was in encapsulated form. In contrast, free alendronate was hardly detected in the blood, probably due to the rapid elimination of the drug. More than 85% of the total recovered drug in serum during 24 h post-administration was in encapsulated form, whereas only 2 ± 3% of free drug was detected after 24 h (Fig. 4). In addition, the total concentration of alendronate in the blood after 24 h was 21 ± 4% of the administered dose (Fig. 4, inset). Taken together, the exact serum fractions of alendronate at later time points, whether encapsulated or free, are irrelevant to therapeutic activity and to toxicity.
The various methods of chromatographic size-exclusion separation and the parallel determination of size, drug and lipid concentration, and protein adsorption corroborate this technique for assessing liposome vesicles integrity. As can be evidenced, a good parallelism between the in vitro (rabbit serum) and in vivo (rabbit blood) elution profile patterns of the various formulations was obtained (compare Figs. 2–3). Following incubation of liposomes in serum in vitro or IV administration, the size and the drug to lipid ratio was maintained, and most of the drug remains encapsulated; 25 ± 3% and 15 ± 3% leakage of the drug was observed after 10 min, in vitro and in the rabbit’s circulation, respectively, with no additional release. An apparent limitation of the in vivo method to determine liposome integrity is that certain fractions were not accounted for, including those internalized by monocytes, liposomes, and free drug that are eliminated, adsorbed to the bone, and/or excreted. Thus, the in vitro method for studying liposomal formulation integrity upon dilution in blood is suggested for other liposomal formulations, since it can provide useful insights into liposome integrity in vivo without the need of cumbersome mass-balance studies.
Our earlier works have clearly demonstrated that the monocytes, in particular, play a crucial role in vascular injury and repair. Although the permeability of the injured artery is increased, the accumulation of liposomes as well as monocytes (the carrier) in the injured arterial wall is significantly reduced following treatment with alendronate or clodronate liposomes due to the systemic depletion of monocytes (1–3). The suppression of circulating monocytes and their stimulatory factors inhibit intimal hyperplasia following stenting and balloon injury. The therapeutic effect of the present formulation was examined in the hypercholesterolemic rabbit model of restenosis. A single IV injection of 3 mg/kg alendronate liposomes, at the time of injury, resulted in both significant depletion of circulating monocytes (Fig. 5) and inhibition of neointimal proliferation (p < 0.01, Fig. 6). This bioactivity is similar to previous results with the former formulation of liposomal alendronate (2,3), inhibition of restenosis with liposomal alendronate was 43.8 ± 12% and 36 ± 11%, in comparison to 45 ± 17% in this work. Thus, the results confirm that the changed physicochemical properties of the new formulation were not associated with impaired bioactivity.
In conclusion, we successfully formulated an improved liposomal alendronate formulation that possesses in vitro stability (>2.5 years) and bioactivity. The parallel results found between the in vitro and in vivo data on liposome integrity upon dilution in serum, provides a valuable new tool in assessing in vivo integrity of new liposomal formulations.
Acknowledgements
This work was supported in part by grants from Biorest Ltd., Israel (GG). GG and HE are affiliated with the David R. Bloom Center for Pharmacy at The Hebrew University of Jerusalem. This work is part of HE Ph.D. dissertation.
References
- 1.Danenberg H. D., Fishbein I., Gao J., et al. Macrophage depletion by clodronate-containing liposomes reduces neointimal formation after balloon injury in rats and rabbits. Circulation. 2002;106(5):599–605. doi: 10.1161/01.CIR.0000023532.98469.48. [DOI] [PubMed] [Google Scholar]
- 2.Danenberg H. D., Fishbein I., Epstein H., et al. Systemic depletion of macrophages by liposomal bisphosphonates reduces neointimal formation following balloon-injury in the rat carotid artery. J. Cardiovasc. Pharmacol. 2003;42(5):671–679. doi: 10.1097/00005344-200311000-00014. [DOI] [PubMed] [Google Scholar]
- 3.Danenberg H. D., Golomb G., Groothuis A., et al. Liposomal alendronate inhibits systemic innate immunity and reduces in-stent neointimal hyperplasia in rabbits. Circulation. 2003;108(22):2798–2804. doi: 10.1161/01.CIR.0000097002.69209.CD. [DOI] [PubMed] [Google Scholar]
- 4.Epstein H., Berger V., Levi I., et al. Nanosuspensions of alendronate with gallium or gadolinium attenuate neointimal hyperplasia in rats. J. Controlled Rel. 2007;117(3):322–332. doi: 10.1016/j.jconrel.2006.10.030. [DOI] [PubMed] [Google Scholar]
- 5.Cohen-Sela E., Rosenzweig O., Gao J., et al. Alendronate-loaded nanoparticles deplete monocytes and attenuate restenosis. J. Control. Release. 2006;113(1):23–30. doi: 10.1016/j.jconrel.2006.03.010. [DOI] [PubMed] [Google Scholar]
- 6.Colombo A., Sangiorgi G. The monocyte: the key in the lock to reduce stent hyperplasia? J. Am. Coll. Cardiol. 2004;43(1):24–26. doi: 10.1016/j.jacc.2003.10.015. [DOI] [PubMed] [Google Scholar]
- 7.Libby P., Shcwartz D., Brogi E., Tanaka H., Clinton S. A cascade model for restenosis. A special case of atherosclerosis progression. Circulation. 1992;86(6S):III47–III52. [PubMed] [Google Scholar]
- 8.Rodan G. A. Mechanisms of action of bisphosphonates. Ann. Rev. Pharmacol. Toxicol. 1998;38:375–388. doi: 10.1146/annurev.pharmtox.38.1.375. [DOI] [PubMed] [Google Scholar]
- 9.Lin J. H., Russell G., Gertz B. Pharmacokinetics of alendronate: an overview. Int. J. Clin. Pract. 1999;101:18–26. [PubMed] [Google Scholar]
- 10.Monkkonen J., Liukkonen J., Taskinen M., Heath T. D., Urtti A. Studies on liposome formulations for intraarticular delivery of clodronate. J. Control Release. 1995;35(2–3):145–154. doi: 10.1016/0168-3659(95)00031-3. [DOI] [Google Scholar]
- 11.Davis C., Gregoriadis G. The effect of lipid composition on the stability of liposomes in vivo [proceedings] Biochem. Soc. Trans. 1979;7(4):680–682. doi: 10.1042/bst0070680. [DOI] [PubMed] [Google Scholar]
- 12.Harashima H., Hiraiwa T., Ochi Y., Kiwada H. Size dependent liposome degradation in blood: in vivo/in vitro correlation by kinetic modeling. J. Drug. Target. 1995;3(4):253–261. doi: 10.3109/10611869509015954. [DOI] [PubMed] [Google Scholar]
- 13.Juliano R. L., Stamp D. The effect of particle size and charge on the clearance rates of liposomes and liposome encapsulated drugs. Biochem. Biophys. Res. Commun. 1975;63(3):651–658. doi: 10.1016/S0006-291X(75)80433-5. [DOI] [PubMed] [Google Scholar]
- 14.H. Epstein-Barash. Immunomodulation by liposomal delivery systems for vascular healing. Jerusalem: Dept. of Pharmaceutics, School of Pharmacy, Faculty of Medicine, The Hebrew University of Jerusalem, 2007.
- 15.Mönkkönen J., Valjakka R., Hakasalo M., Urtti A. The effects of liposome surface charge and size on the intracellular delivery of clodronate and gallium in vitro. Int. J. Pharm. 1994;107:187–197. doi: 10.1016/0378-5173(94)90433-2. [DOI] [Google Scholar]
- 16.Cohen H., Alferiev I. S., Monkkonen J., et al. Synthesis and preclinical pharmacology of 2-(2-aminopyrimidinio) ethylidene-1,1-bisphosphonic acid betaine (ISA-13–1)-a novel bisphosphonate. Pharm. Res. 1999;16(9):1399–1406. doi: 10.1023/A:1018951025493. [DOI] [PubMed] [Google Scholar]
- 17.Mönkkönen J., Taskinen M., Auriola S. O. K., Urtti A. Growth inhibition of macrophage-like and other cell types by liposome-encapsulated, calcium-bound, and free bisphosphonates in vitro. J Drug Targeting. 2003;11:279–286. doi: 10.1080/10611860310001636539. [DOI] [PubMed] [Google Scholar]
- 18.Van Rooijen N., Sanders A. Liposome-mediated depletion of macrophages - mechanism of action, preparation of liposomes and applications. J. Immunol. Meth. 1994;174(1–2):83–93. doi: 10.1016/0022-1759(94)90012-4. [DOI] [PubMed] [Google Scholar]
- 19.van Rooijen N., van Kesteren-Hendrikx E. “In vivo” depletion of macrophages by liposome-mediated “suicide”. Methods Enzymol. 2003;373:3–16. doi: 10.1016/S0076-6879(03)73001-8. [DOI] [PubMed] [Google Scholar]
- 20.Makkonen N., Salminen A., Rogers M. J., et al. Contrasting effects of alendronate and clodronate on RAW 264 macrophages: The role of a bisphosphonate metabolite. Eur. J. Pharm. Sci. 1999;8(2):109–118. doi: 10.1016/S0928-0987(98)00065-7. [DOI] [PubMed] [Google Scholar]
- 21.Szoka F., Jr., Papahadjopoulos D. Comparative properties and methods of preparation of lipid vesicles (liposomes) Ann. Rev. Biophys. Bioeng. 1980;9:467–508. doi: 10.1146/annurev.bb.09.060180.002343. [DOI] [PubMed] [Google Scholar]
- 22.Bartlett G. R. Phosphorus assay in column chromatography. J. Biol. Chem. 1959;234(3):466–468. [PubMed] [Google Scholar]
- 23.Lang J. Quantitative determination of cholesterol in liposome drug products and raw materials by high-performance liquid chromatography. J. Chromat. 1990;507:157–163. doi: 10.1016/S0021-9673(01)84191-1. [DOI] [PubMed] [Google Scholar]
- 24.Convention TUSP. USP 29 The United States Pharmacopeia. Vol 29. Rockville: The United States Pharmacopeial Convention, 2005.
- 25.J. Nichols. Rabbit Formulary Updated. Vol 2004: University Veterinarian, Middlebury College, http://www.middlebury.edu/administration/stss/animal_facility/veterinary/handouts/rabbit.htm, 2001.
- 26.Morton R. E., Evans T. A. Modification of the bicinchoninic acid protein assay to eliminate lipid interference in determining lipoprotein protein content. Anal. Biochem. 1992;204(2):332–334. doi: 10.1016/0003-2697(92)90248-6. [DOI] [PubMed] [Google Scholar]
- 27.Rodrigueza W. V., Pritchard P. H., Hope M. J. The influence of size and composition on the cholesterol mobilizing properties of liposomes in vivo. Biochim. Biophys. Acta. 1993;1153(1):9–19. doi: 10.1016/0005-2736(93)90270-A. [DOI] [PubMed] [Google Scholar]
- 28.Sato Y., Kiwada H., Kato Y. Effects of dose and vesicle size on the pharmacokinetics of liposomes. Chem. Pharm. Bull. (Tokyo) 1986;34(10):4244–4252. doi: 10.1248/cpb.34.4244. [DOI] [PubMed] [Google Scholar]
- 29.Kao Y. J., Juliano R. L. Interactions of liposomes with the reticuloendothelial system. Effects of reticuloendothelial blockade on the clearance of large unilamellar vesicles. Biochim. Biophys. Acta. 1981;677(3–4):453–461. doi: 10.1016/0304-4165(81)90259-2. [DOI] [PubMed] [Google Scholar]
- 30.Schroit A. J., Madsen J., Nayar R. Liposome–cell interactions: in vitro discrimination of uptake mechanism and in vivo targeting strategies to mononuclear phagocytes. Chem. Phys. Lipids. 1986;40(2–4):373–393. doi: 10.1016/0009-3084(86)90080-0. [DOI] [PubMed] [Google Scholar]
- 31.Lee K. D., Hong K., Papahadjopoulos D. Recognition of liposomes by cells—In vitro binding and endocytosis mediated by specific lipid headgroups and surface-charge density. Biochimica. Et. Biophysica. Acta. 1992;1103(2):185–197. doi: 10.1016/0005-2736(92)90086-2. [DOI] [PubMed] [Google Scholar]
- 32.Patel H. M. Serum opsonins and liposomes: Their interaction and opsonophagocytosis. Crit. Rev. Ther Drug Carrier Syst. 1992;9(1):39–90. [PubMed] [Google Scholar]
- 33.Patel H. M., Moghimi S. M. Serum-mediated recognition of liposomes by phagocytic cells of the reticuloendothelial system—The concept of tissue specificity. Adv. Drug Deliv. Rev. 1998;32(1–2):45–60. doi: 10.1016/s0169-409x(97)00131-2. [DOI] [PubMed] [Google Scholar]
- 34.Senior J., Gregoriadis G. Is half-life of circulating liposomes determined by changes in their permeability? FEBS Lett. 1982;145(1):109–114. doi: 10.1016/0014-5793(82)81216-7. [DOI] [PubMed] [Google Scholar]
- 35.Senior J. H. Fate and behavior of liposomes in vivo: A review of controlling factors. Crit. Rev. Ther. Drug Carrier Syst. 1987;3(2):123–193. [PubMed] [Google Scholar]
- 36.Barza M., Stuart M., Szoka Jr F. Effect of size and lipid composition on the pharmacokinetics of intravitreal liposomes. Invest. Ophthalmol. Vis. Sci. 1987;28(5):893–900. [PubMed] [Google Scholar]
- 37.Kimelberg H. K. Influence of lipid phase transitions and cholesterol on protein–lipid interaction. Cryobiology. 1978;15(2):222–226. doi: 10.1016/0011-2240(78)90028-7. [DOI] [PubMed] [Google Scholar]
- 38.Kirby A., Clarke J., Gregoriadis G. Effect of the cholesterol content of small unilamellar liposomes on their stability in vivo and in vitro. Biochem. J. 1980;186(2):591–598. doi: 10.1042/bj1860591. [DOI] [PMC free article] [PubMed] [Google Scholar]