Abstract
The objective was to directly compare the four different “calculation” methods of assessing P-gp inhibition potential using experimental data obtained from ~60 structurally diverse internal research and marketed compounds. Bidirectional studies for digoxin (probe for P-gp substrate) were performed with and without test compounds (at 10 μM). Four different calculation methods were applied to the same dataset (raw bidirectional permeability values) to obtain the “percent inhibition of P-gp” for these compounds using the different methods. Significantly different inhibition potential was obtained with the “exact” same experimental dataset depending on the calculation method used. Subsequently, entirely different conclusions regarding the “inhibition potential” of test compound was reached due to the different calculation methods. Based on the direct comparison of these methods, method no. 3 (i.e., inhibition of B to A permeability of digoxin) is recommended as the calculation method ideal during screening stages due to its high throughput amenability. The methodology is capable of rapidly screening compounds with adequate reliability for early stage drug discovery. Method no. 3 provides an abridged version of a bidirectional study that is fully capable of identifying all non-inhibitors (0–20%), moderate inhibitors (20–60%), and potent inhibitors (>60%) and demonstrates high correlation with method no. 1 (inhibition based on both A to B and B to A permeability of digoxin). Nevertheless, method no. 1 might be appropriate for more detailed mechanistic studies required in late stage discovery and development.
Key words: drug–drug interactions, efflux ratio, in vitro models, P-gp inhibition, permeability
INTRODUCTION
P-glycoprotein (P-gp) is a member of the ATP binding cassette (ABC) transporter family that is the most widely studied and best understood amongst all drug transporter proteins. Common features of a pharmaceutically relevant drug transporter that can significantly affect drug disposition are: strategic and adequate expression in several target organs, ability to identify a wide spectrum of structurally diverse compounds as substrates/inhibitors, and adequate capacity and ability to be inhibited by xenobiotics. P-gp has all of the above characteristics and thus has a unique ability to modulate pharmacokinetics of drugs. P-gp is a major determinant of absorption, distribution, and elimination of a wide variety of drugs (1–8). It is well known to limit the oral absorption of drugs such as cyclosporin and taxol; it can limit entry of drugs such as HIV protease inhibitors into brain and CNS; and it can actively facilitate excretion of drugs via biliary and urinary routes. Since P-gp can play such a pivotal role in the pharmacokinetics and eventually distribution of drugs into target organs, increasing efforts are being made in early discovery and development to identify compounds that can potentially interact with P-gp. Regulatory agencies have also identified P-gp as a key drug transporter that can impact ADME of new chemical entities and have published a draft guidance (9) on in vitro drug–drug interaction studies with P-gp.
There are literature reports of various in vitro and in vivo models used for assessing P-gp interactions (10–19). In vitro assays such as ATPase activity, rhoadmine-123 uptake, calcein AM uptake, cell-based bidirectional permeability, radioligand binding along with in vivo models such as transgenic (knockout mice) animal models are most commonly used in discovery settings. Cell-based bidirectional permeability assay using digoxin as a probe is currently accepted as the “method of choice” for determining the P-gp inhibition potential of test compounds in drug discovery laboratories (9,10,14,16,17). The FDA guidance document also recommends conducting bidirectional permeability studies using Caco-2 cells or other cell lines (e.g., MDCK, LLC-PK1 etc.), either wild-type or transfected (with P-gp), to determine the P-gp inhibition potential of test compounds.
The cell-based bidirectional permeability assay is the standard methodology for P-gp inhibition studies across the industry. The experimental protocol for this assay is well established and quite uniform across different laboratories. Typically, bidirectional permeability of digoxin (a well-accepted P-gp substrate) is assessed alone and in the presence of a single concentration (10 μM) of test compound to estimate the inhibition potential. However, currently, there is no universally accepted “calculation” procedure provided as guidance from any regulatory agencies to standardize the results, and therefore, different laboratories utilize their own unique calculation methods to estimate the extent of P-gp inhibition potential (9,14,17,20–23). This lack of a uniform “calculation” method leaves a lot of room for error and misinterpretation of data. Several laboratories (including FDA) suggest using the efflux ratio [ratio of permeabilities: basolateral to apical (B to A)/apical to basolateral (A to B)] to calculate the percent inhibition of P-gp. Some investigators suggest incorporating both the A to B and B to A permeability values of probe (in the presence and absence of test compounds) to calculate the percent inhibition of P-gp. Others suggest using only one of the permeability values (A to B or B to A) to calculate the percent inhibition of P-gp.
To date, no definitive report compares the different calculation methods used by various investigators to assess the percent inhibition of P-gp and, subsequently, the IC50 values. The current study describes a head-to-head comparison of these diverse “calculation” methods. Using data from ~60 structurally diverse BMS internal research and marketed compounds (some well known to be potent P-gp inhibitors), an empirical analysis was performed to compare these “calculation” methods and to help establish the most appropriate method during drug discovery stage. It is intriguing that vastly different percent inhibition values were obtained for the “exact” same experimental dataset based on the calculation methods used. It is clear that entirely different conclusions would be drawn from the same experimental dataset if different “calculation” methods are used. Therefore, currently, there is an urgent need to standardize the “calculation” method for the quantitative assessment of P-gp inhibition potential. A standardized ‘calculation” method would ensure consistent interpretation of data across published literature and across different laboratories.
EXPERIMENTAL
Materials and Methods
Caco-2 cells (passage no. 17) were obtained from the American Type Culture Collection (Rockville, MD, USA). Dulbecco’s modified Eagle medium, nonessential amino acids, and antibiotic were purchased from JHR Biosciences (Lenexa, KS, USA). Fetal bovine serum was obtained from Hyclone Lab. Inc. (Logan, UT, USA). HTS-24-Transwell® inserts (surface area, 0.33 cm2) with a polycarbonate membrane (0.4-μm pore size) were purchased from Costar (Cambridge, MA, USA). Hank’s balanced salt solution (HBSS) and N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (HEPES) were purchased from Sigma Chemical Co. (St. Louis, MO, USA). All solvents were analytical grade. 3H-Digoxin and 14C Mannitol were obtained from Perkin Elmer Life Sciences (Boston, MA, USA). All other test compounds were obtained from Sigma Chemical Co. and Bristol-Myers Squibb compound distribution.
Caco-2 Cell Culture Procedure
Caco-2 cells were seeded onto filter membrane at a density of ~100,000 cells/cm2 in 24-well transwell plates. The cells were grown in culture medium consisting of Dulbecco’s modified Eagle’s medium supplemented with 10% fetal bovine serum, 1% nonessential amino acids, 1% l-glutamine, 100 U/mL penicillin-G, and 100 μg/mL streptomycin. The culture medium was replaced every 2 days and the cells were maintained at 37°C, 95% relative humidity, and 5% CO2. Permeability studies were conducted with the monolayers cultured for approximately 21 days with the cell passage numbers between 40 and 60. Physiologically and morphologically well-developed Caco-2 cell monolayers with transepithelial electrical resistance (TEER) values greater than 500 Ω cm2 were used for the studies reported in this manuscript.
P-gp Inhibition Assay
The transport medium used for the P-gp inhibition studies was modified HBSS buffer containing 10 mM HEPES. The pH of both the apical and basolateral compartments was 7.4. Prior to all experiments, each monolayer was washed twice with buffer and TEER was measured to ensure the integrity of the monolayers. Both the apical to basolateral (A to B) transport as well as the basolateral to apical (B to A) transport of [3H]-digoxin was measured in the absence and presence of the test compound. The concentration of digoxin used was 5 μM, which was much below its Km value of ~60 μM (24). The concentration of test compounds was chosen to be 10 μM in this assay. The studies were initiated by adding an appropriate volume of buffer containing digoxin to either the apical (A to B transport) or basolateral (B to A transport) side of the monolayer. The volumes of the apical and basolateral compartments were 200 and 600 μL, respectively, and the test compound (as an inhibitor) was added to both sides of the monolayer at a concentration of 10 μM. The monolayers were then incubated for 2 h at 37°C. Samples are taken from either the apical (B to A transport) or basolateral (A to B transport) compartment at the end of the 2-h incubation period and analyzed for [3H]-digoxin using LSC. The A to B as well as the B to A permeability coefficient (Pc) of digoxin was calculated in the presence and absence of the test compound. Efflux ratio (ratio of permeability: B to A/A to B) was also assessed with and without test compound. For assessment of IC50 value, a range of inhibitor concentration (0.01 to 50 μM) was used. On all study occasions, permeability of 14C Mannitol was performed (in the same plate) to confirm the formation of the tight junctions and viability of the cells for transport studies.
Methods for Calculation of Percent Inhibition of P-gp
Different calculation methods were applied to the same dataset to obtain the “percent inhibition of P-gp” for all compounds tested.
Method No. 1 (Cumulative AB and BA)
The A to B and B to A Pc of digoxin was calculated in the presence and absence of the test compound. Calculation of percent inhibition of P-gp was performed using the equation below (21):
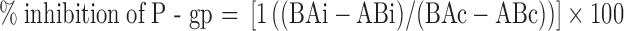 |
where BAc and ABc are the B to A and A to B permeability of digoxin alone. BAi and ABi are the B to A and A to B permeability of digoxin in the presence of the test compound. Therefore, the calculation depended on the individual A to B and B to A Pc values obtained for control (digoxin only) as well as in the presence of inhibitor.
Method No. 2 (Efflux Ratio −1)
The A to B and B to A Pc of digoxin was calculated in the presence and absence of the test compound. Efflux ratio (ratio of B to A/A to B permeability) was calculated for digoxin in the absence and presence of test compound. Calculation of percent inhibition of P-gp was performed using the equation below (modified from FDA method) (9):
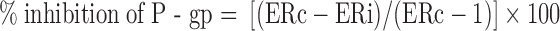 |
where ERc was the efflux ratio of digoxin alone and ERi was the efflux ratio of digoxin in the presence of test compound. Therefore, the calculation depended on the overall efflux ratio for control (digoxin only) as well as in the presence of inhibitor rather than the individual A to B and B to A permeability values. In this method, the denominator was corrected by subtracting 1 which is the residual efflux ratio when complete inhibition of P-gp has been achieved (i.e., A to B and B to A digoxin permeability is identical).
Method No. 3 (Inhibition of Corrected BA)
For this methodology, only the B to A Pc of digoxin (in the presence and absence of test compound) was utilized. Calculation of percent inhibition of P-gp was performed using the equation below [modified from (17)]:
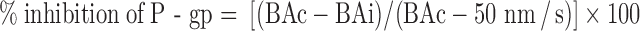 |
where BAc and BAi are the B to A permeability value in nanometers per second of digoxin in the absence and presence of test compound, respectively. Therefore, the calculation depended only on the B to A permeability value for digoxin (alone as well as in the presence of inhibitor) rather than both the individual A to B and B to A permeability values. In this method, the denominator used was BAc–50 rather than BAc–ABc (which is the maximum mathematically possible range of inhibition of B to A permeability value). The correction factor of 50 used in the denominator is based on an experimental value obtained from several potent inhibitors studied. In Table I, the last five compounds (which include ketoconazole and GF120918) are potent P-gp inhibitors that completely knock out the efflux ratio of digoxin and practically lead to similar permeability value in both directions. Upon complete inhibition of P-gp/efflux, the B to A permeability of digoxin is reduced to a minimum value of ~50 nm/s. Therefore, the lowest B to A permeability achievable (for digoxin in presence of an inhibitor) was ~50 nm/s and not the control A to B value. Thus, an accurate empirical “maximum” dynamic range of inhibition of B to A permeability value for digxoin is BAc − 50 that forms the denominator in this method. Further details on the rational for the selection of 50 as the normalization factor is presented in “DISCUSSION”.
Table I.
Comparison of Percent P-gp Inhibition Values Via Different Methods for Test Compounds
| Test | A to B | B to A | Efflux ratio | Method no. 1 | Method no. 2 | Method no. 3 | Method no. 4 |
|---|---|---|---|---|---|---|---|
| Digoxin (control) | 13 | 169 | 13.0 | ||||
| Compound-1 | 22 | 177 | 8.0 | 1 | 41 | −7 | 24 |
| Compound-2 | 17 | 168 | 9.9 | 3 | 26 | 1 | 11 |
| Compound-3 | 15 | 170 | 11.3 | 1 | 14 | −1 | 5 |
| Compound-4 | 13 | 166 | 12.8 | 2 | 2 | 3 | 0 |
| Compound-5 | 13 | 165 | 12.7 | 3 | 3 | 3 | 0 |
| Compound-6 | 18 | 168 | 9.3 | 4 | 31 | 1 | 14 |
| Compound-7 | 21 | 171 | 8.1 | 4 | 40 | −2 | 22 |
| Compound-8 | 10 | 159 | 15.9 | 4 | −24 | 8 | −8 |
| Compound-9 | 20 | 168 | 8.4 | 5 | 38 | 1 | 19 |
| Compound-10 | 23 | 169 | 7.3 | 6 | 47 | 0 | 27 |
| Compound-11 | 35 | 179 | 5.1 | 8 | 66 | −8 | 59 |
| Compound-12 | 24 | 168 | 7.0 | 8 | 50 | 1 | 30 |
| Compound-13 | 13 | 156 | 12.0 | 8 | 8 | 11 | 0 |
| Compound-14 | 30 | 171 | 5.7 | 10 | 61 | −2 | 46 |
| FTC | 18 | 158 | 8.8 | 10 | 35 | 9 | 14 |
| Compound-15 | 34 | 172 | 5.1 | 12 | 66 | −3 | 57 |
| Compound-16 | 23 | 161 | 7.0 | 12 | 50 | 7 | 27 |
| Compound-17 | 23 | 160 | 7.0 | 12 | 50 | 8 | 27 |
| Compound-18 | 17 | 153 | 9.0 | 13 | 33 | 13 | 11 |
| Compound-19 | 29 | 165 | 5.7 | 13 | 61 | 3 | 43 |
| Compound-20 | 21 | 157 | 7.5 | 13 | 46 | 10 | 22 |
| Compound-21 | 24 | 159 | 6.6 | 13 | 53 | 8 | 30 |
| Compound-22 | 13 | 146 | 11.2 | 15 | 15 | 19 | 0 |
| Compound-23 | 34 | 163 | 4.8 | 17 | 68 | 5 | 57 |
| Compound-24 | 18 | 146 | 8.1 | 18 | 41 | 19 | 14 |
| Compound-25 | 37 | 162 | 4.4 | 20 | 72 | 6 | 65 |
| Compound-26 | 39 | 163 | 4.2 | 21 | 74 | 5 | 70 |
| Compound-27 | 40 | 161 | 4.0 | 22 | 75 | 7 | 73 |
| Compound-28 | 13 | 133 | 10.2 | 23 | 23 | 30 | 0 |
| Compound-29 | 25 | 145 | 5.8 | 23 | 60 | 20 | 32 |
| Compound-30 | 19 | 137 | 7.2 | 24 | 48 | 27 | 16 |
| Compound-31 | 38 | 156 | 4.1 | 24 | 74 | 11 | 68 |
| Compound-32 | 39 | 156 | 4.0 | 25 | 75 | 11 | 70 |
| Indinavir | 13 | 125 | 9.6 | 28 | 28 | 37 | 0 |
| MK571 | 32 | 144 | 4.5 | 28 | 71 | 21 | 51 |
| Compound-33 | 15 | 126 | 8.4 | 29 | 38 | 36 | 5 |
| Compound-34 | 36 | 146 | 4.1 | 29 | 75 | 19 | 62 |
| Compound-35 | 45 | 151 | 3.4 | 32 | 80 | 15 | 86 |
| Compound-36 | 41 | 146 | 3.6 | 33 | 79 | 19 | 76 |
| Compound-37 | 56 | 157 | 2.8 | 35 | 85 | 10 | 116 |
| Compound-38 | 30 | 129 | 4.3 | 37 | 73 | 34 | 46 |
| Compound-39 | 40 | 134 | 3.4 | 40 | 80 | 29 | 73 |
| Compound-40 | 28 | 116 | 4.1 | 44 | 74 | 45 | 41 |
| Compound-41 | 37 | 118 | 3.2 | 48 | 82 | 43 | 65 |
| Compound-42 | 12 | 92 | 7.7 | 49 | 44 | 65 | −3 |
| Compound-43 | 34 | 108 | 3.2 | 53 | 82 | 51 | 57 |
| Saquinavir | 28 | 96 | 3.4 | 56 | 80 | 61 | 41 |
| Compound-44 | 16 | 80 | 5.0 | 59 | 67 | 75 | 8 |
| Verapamil | 33 | 90 | 2.7 | 63 | 86 | 66 | 54 |
| Compound-45 | 42 | 95 | 2.3 | 66 | 89 | 62 | 78 |
| Compound-46 | 36 | 87 | 2.4 | 67 | 88 | 69 | 62 |
| Compound-47 | 26 | 66 | 2.5 | 74 | 87 | 87 | 35 |
| Compound-48 | 54 | 94 | 1.7 | 74 | 94 | 63 | 111 |
| Compound-49 | 67 | 102 | 1.5 | 78 | 96 | 56 | 146 |
| Ketoconazole | 43 | 58 | 1.3 | 90 | 97 | 93 | 81 |
| Compound-50 | 32 | 45 | 1.4 | 92 | 97 | 104 | 51 |
| Compound-51 | 54 | 66 | 1.2 | 92 | 98 | 87 | 111 |
| GF120918 (2 μM) | 61 | 65 | 1.1 | 97 | 99 | 87 | 130 |
| Compound-52 | 38 | 41 | 1.1 | 98 | 99 | 108 | 68 |
Method No. 4 (Enhancement of Corrected AB)
For this methodology, only the A to B Pc of digoxin (in the presence and absence of test compound) was utilized. Calculation of percent inhibition of P-gp was performed using the equation below [modified from (17)]:
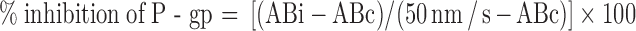 |
where ABc and ABi are the A to B permeability value in nanometers per second of digoxin alone and of digoxin in the presence of test compound. Therefore, the calculation depended only on the A to B permeability value for digoxin (alone as well as in the presence of inhibitor) rather than both the individual A to B and B to A permeability values. In this method, the denominator was 50–ABc rather than BAc–ABc (maximum possible range of increase of A to B permeability value). The correction factor of 50 used in the denominator is again an empirical value obtained from several potent inhibitor studied. In Table I, the last five compounds (which include ketoconazole and GF120918) are potent P-gp inhibitors that completely knock out the efflux ratio of digoxin and practically lead to similar permeability value in both directions. In all those incidents of complete inhibition of P-gp/efflux, the A to B permeability of digoxin increased to a maximum value of ~50 nm/s. Therefore, in complete absence of any efflux, the highest A to B permeability achieved (for digoxin in presence of an inhibitor) was ~50 nm/s and not the control B to A value. Thus, an accurate empirical “maximum” dynamic range of increase of A to B permeability value for digoxin is 50–ABc which forms the denominator in this method. Further details on the rational for the selection of 50 as the normalization factor is presented in “DISCUSSION”.
Assessment of IC50 Value
A handful of compounds were also studied at various concentrations to obtain the IC50 of inhibition of P-gp. For this exercise, the percent inhibition at each concentration was calculated using the different “calculation” methods described above followed by estimation of IC50 using XLfit, version 2. The data were fitted using the Hill equation given below:
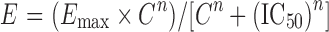 |
where E = percent inhibition of P-gp at a particular concentration of inhibitor, C = concentration of inhibitor, Emax = maximum inhibition of P-gp, and n = Hill coefficient.
RESULTS
The bidirectional permeability values of digoxin in the presence of ~60 BMS (internal research) and marketed compounds are listed in Table I. In the absence of any inhibitor, the control digoxin permeability values for A to B and B to A were 13 ± 4 and 169 ± 17 nm/s, respectively (N > 10 repeats). Based on the bidirectional permeability values of digoxin in the presence of test compounds, the percent inhibition of P-gp was calculated using four different “calculation” methods listed earlier. The test compounds are categorized into three different classification bins based on the extent of P-gp inhibition:
 |
Comparison of Percent Inhibition of P-gp
Table I illustrates that the percent inhibition of tested compounds was substantially different depending on the calculation method used. In other words, in spite of using identical datasets, the four calculation methods generated significantly different percent inhibition values due to the different mathematical treatment afforded by each method. It is important to note that not only were the absolute percent inhibition values different but, more importantly, the designated classification were different as well via the four different methods. For example, compound-11 is identified as a non-inhibitor of P-gp based on methods no. 1 and no. 3 (8% and −8%, respectively). However, it is identified as a potent/moderate inhibitor based on methods no. 2 and no. 4 (66% and 59%, respectively). Several other compounds such as compound-15, 16, 19, and 23 also demonstrate a similar trend (non-inhibitor via method nos. 1 and 3, but moderate to potent inhibitor via method nos. 2 and 4). On the other hand, another example (compound-42) showed moderate/potent inhibition (44–65%) based on method nos. 1, 2, and 3, but it showed no inhibition (−3%) based on method no. 4.
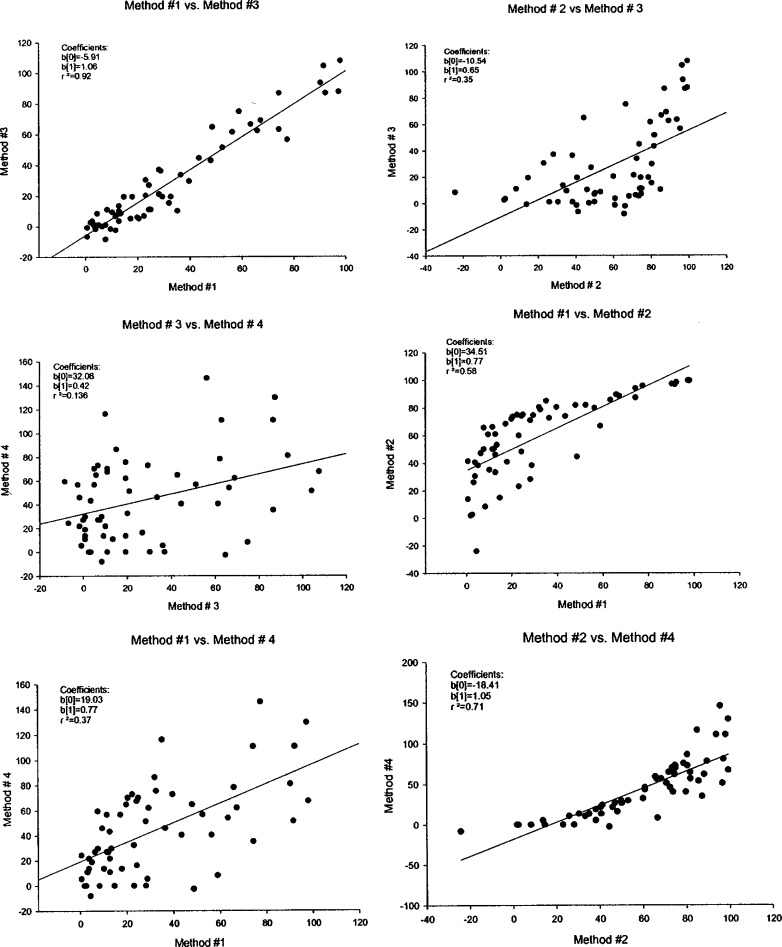
The percent P-gp inhibition values obtained for all the test compounds via the four methods were plotted against each other to assess the relative correlation between the different methods. As shown in Table II, there was an excellent correlation found between methods no. 1 and no. 3 with a correlation coefficient of 0.92. Figure 1 shows six plots correlating data from four methods. Methods no. 1 and no. 3 not only had the best correlation but also showed a slope of ~1, demonstrating a 1:1 correlation. Method no. 1 requires bidirectional permeability of digoxin in the presence and absence of test compounds, whereas method no. 3 only requires the B to A permeability of digoxin in the presence and absence of test compounds (i.e., method no. 3 requires approximately half the number of samples and the functional cell monolayers compared to method no. 1). Amongst the four different methods, method no. 4 consistently had the lowest correlation with all other methods. The poorest correlation was noted between methods no. 3 and no. 4. The smaller dynamic range (i.e., range of A to B permeability in the absence or presence of inhibitor) coupled with a very low initial A to B permeability value (13 nm/s for digoxin) may explain the highly variable nature of percent inhibition values from method no. 4 compared to other methods. Since the percent inhibition values depended solely on changes in A to B permeability, a small change in A to B permeability with no change in B to A would suggest significant inhibition. This highly variable nature of data using method no. 4 for calculation of percent inhibition of P-gp might make it prone to errors in data interpretations.
Table II.
Correlation Coefficient Observed for the Different Methods for their Percent P-gp Inhibition Values
| Method no. 1 | Method no. 2 | Method no. 3 | Method no. 4 | |
|---|---|---|---|---|
| Method no. 1 | 1.00 | 0.58 | 0.92 | 0.37 |
| Method no. 2 | 0.58 | 1.00 | 0.35 | 0.71 |
| Method no. 3 | 0.92 | 0.35 | 1.00 | 0.14 |
| Method no. 4 | 0.37 | 0.71 | 0.14 | 1.00 |
Fig. 1.
Correlation of “percent P-gp inhibition” values obtained via the four different calculation methods for internal research and marketed compounds. Percent P-gp inhibition values were calculated using the bidirectional permeability values for digoxin (alone as well as in presence of 10 μM test compound). Parameters obtained were: b(0) = intercept, b(1) = slope, r 2 = regression
Comparison of IC50 Values
IC50 values of P-gp inhibition were determined with a few test compounds at a range of concentrations (0.01 to 50 μM). The percent inhibition was calculated using the four methods listed earlier, which was followed by IC50 assessment using the Hill equation. Four different IC50 values were calculated using the percent P-gp inhibition values from different methods. MK571 was selected as a test probe for which IC50 was assessed via the four methods. In the earlier study (at 10 μM), MK571 was identified as a non/mild inhibitor (21–28% inhibition) via method nos. 1 and 3, but as moderate/potent inhibitor (51–71% inhibition) via method nos. 2 and 4 (Table I). As expected, a significantly lower IC50 value was derived via method nos. 2 and 4 (5.6 and 9.8 μM, respectively) compared to method nos. 1 and 3 (17.3 and 21.3 μM, respectively). Therefore, in spite of using identical experimental data, there was a fourfold variation in the IC50 values based on the calculation method used (with significantly higher potency of inhibition observed with calculation method no. 2). Similarly, several other compounds such as compound-15, 16, 19, and 23 that demonstrate a similar trend (significantly higher percent P-gp inhibition values via method nos. 2 and 4, around 60%, compared to method nos. 1 and 3, less than 20%) are also expected to reveal significantly lower IC50 value via method nos. 2 and 4. Thus, these compounds belonging to the class of “low/moderate inhibitors” can potentially demonstrate significantly different IC50 values based on the method used for data analysis. On the other hand, a similar exercise performed with several compounds belonging to the class of “potent inhibitors” such as compound-42, 52, and GF120918 demonstrated very similar IC50 values irrespective of the method used for data analysis. The IC50 values via the four methods varied minimally within a very tight and acceptable range for these potent inhibitors (between 0.04 and 0.1 μM for GF120918; between 3.2 and 4.4 μM for compound-45; and between 2.3 and 3.2 μM for compound-52). Thus, the impact of different calculation methods on IC50 value depended on the intrinsic property of the compound with greatest impact for “low/moderate inhibitors” and no tangible impact for “potent inhibitors”.
DISCUSSION
There has been a steady increase in the level of interest in P-gp both by pharmaceutical industry and regulatory agencies due to potential drug–drug interactions that can be mediated by this important efflux transporter. Early identification of compounds that can potentially interact with P-gp either as a substrate or an inhibitor has become a routine task during the optimization and selection of drug candidate. In vitro P-gp assays play an important role in triaging compounds as well as providing critical data for decision making regarding progression of lead compounds. Better predictability of in vitro assays is important, since costly human clinical studies might be initiated based solely on in vitro observations. With regards to the P-gp inhibition assay, the cell-based bidirectional permeability assay is the most widely adopted methodology (9,10,14,16,21). Caco-2 cells or transfected cell lines (MDCK or LLC-PK1 cells overexpressing P-gp) are most commonly used for assessing the substrate and inhibition potential of test compounds. By virtue of their overexpression of P-gp, the P-gp transfected cells might be preferred for these interaction studies and provide some selectivity from other efflux transporters. However, Caco-2 cells are acceptable for these interaction studies as well due to their extensive historical use and widespread popularity across the industry. Moreover, Caco-2 cells are known to express significant amounts of P-gp (in addition to several efflux transporters) that are functionally relevant leading to efflux ratio’s higher than 1 for classical P-gp substrates (digoxin, vinblastine, dexamethasone, etc.). Digoxin is used as a P-gp probe substrate in the cell-based P-gp inhibition assay. It is a sensitive P-gp substrate that is chemically and metabolically stable in cell model and demonstrates a high efflux ratio (~10), making it a popular substrate probe for P-gp inhibition studies. FDA guidance document (9) provides a decision tree to determine whether an investigational drug is an inhibitor of P-gp and whether follow-up in vivo drug interaction studies are warranted with digoxin in the clinic. This decision tree provides a tiered approach with initial assessment of inhibition potential at a single (or a few) concentrations of test compound (inhibitor) followed by a thorough IC50 assessment. There is universal acceptance with standardized “experimental” protocol across laboratories for this cell-based inhibition assay. However, currently, there is no universally accepted “calculation” procedure provided as guidance to standardize the results. Therefore, different laboratories utilize their own unique calculation methods to determine the extent of P-gp inhibition. This lack of uniformity leaves a lot of flexibility in the hands of end-users that can lead to potentially erroneous calculations ultimately leading to erroneous interpretation of results.
Several laboratories (including FDA) suggest using the efflux ratio to calculate the percent inhibition of P-gp. FDA draft guidance document states a detailed in vitro IC50 or Ki determination is warranted for a test compound if “net flux ratio of a probe substrate (like digoxin) decreases with increasing concentration of investigational drug”. Change in efflux ratio as an independent parameter to assess the percent inhibition of P-gp is associated with several shortcomings.
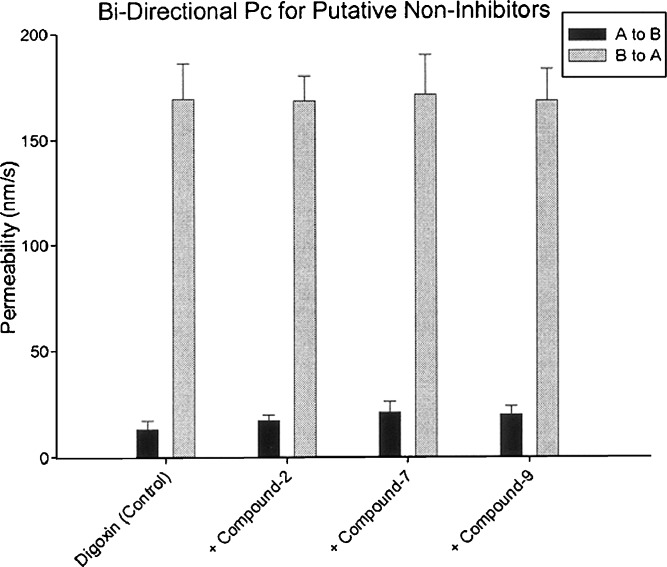
First, the use of efflux ratio (method no. 2) makes the model unreliable when there are small changes in A to B and B to A permeability of digoxin (with or without test compounds). Table III lists the bidirectional digoxin permeability in the absence and presence of three test compounds. The data highlight the overly “sensitive” nature of the “calculation” method no. 2. For three test compounds included in this exercise, the A to B permeability of digoxin in the presence of test compounds is not different statistically from the A to B permeability of control (i.e., digoxin alone). The statistical p values were ~0.23, 0.10, and 0.10. Similarly, the B to A permeability of digoxin in the presence of test compounds is also not different statistically from the B to A permeability of control (i.e., digoxin alone). The p values were ~0.94, 0.90, and 0.94. Thus, it is clear that these compounds did not produce any significant inhibitory effect on the bidirectional permeability of digoxin, and they should be considered non-inhibitors of P-gp. Figure 2 provides a pictorial of the bidirectional permeability of digoxin (with and without test compounds) and provides additional visual evidence confirming the minimal effect of these compounds on digoxin’s permeability. However, based on the efflux ratio obtained in the presence of these compounds and subsequent percent inhibition of P-gp using method no. 2 (which uses the efflux ratio as the independent parameter), these compounds would be identified as moderate P-gp inhibitors. The percent inhibition ranged between 26% and 40% (Table III) for these compounds, even though the A to B and B to A permeability values are statistically unchanged from the control (digoxin only). This clearly demonstrates that “calculation” method no. 2 that factors “efflux ratio” into obtaining percent inhibition of P-gp can be overly sensitive to A to B permeability values and potentially lead to increase in “false positives” (i.e., non-inhibitors being identified as inhibitors), triggering unnecessary assessment of IC50 and leading to wasted resources and time. Similarly, method no. 4 (increase of corrected AB method), like the efflux-ratio-based methods, also appeared to be overly sensitive and showed 11–22% inhibition. Contrary to the efflux-ratio-based methods, method no. 1 (cumulative AB and BA method) and method no. 3 (inhibition of corrected BA method) correlated well and predicted <5% inhibition for these test compounds that seems consistent with the fact that these test compounds did not change the bidirectional permeability values of digoxin.
Table III.
Percent P-gp Inhibition Values Obtained by Using Different Methods for Putative Non-inhibitors
| Test | A to B | B to A | Efflux ratio | Method no. 1 | Method no. 2 | Method no. 3 | Method no. 4 |
|---|---|---|---|---|---|---|---|
| Digoxin (control) | 13 ± 4 | 169 ± 17 | 13.0 | ||||
| Compound-2 | 17 ± 3 | 168 ± 12 | 9.9 | 3 | 26 | 1 | 11 |
| Compound-7 | 21 ± 5 | 171 ± 19 | 8.1 | 4 | 40 | −2 | 22 |
| Compound-9 | 20 ± 4 | 168 ± 15 | 8.4 | 5 | 38 | 1 | 19 |
Fig. 2.
Bidirectional permeability values for digoxin in presence and absence of putative non-inhibitors of P-gp. Each column represents the mean ± SD of at least three data points
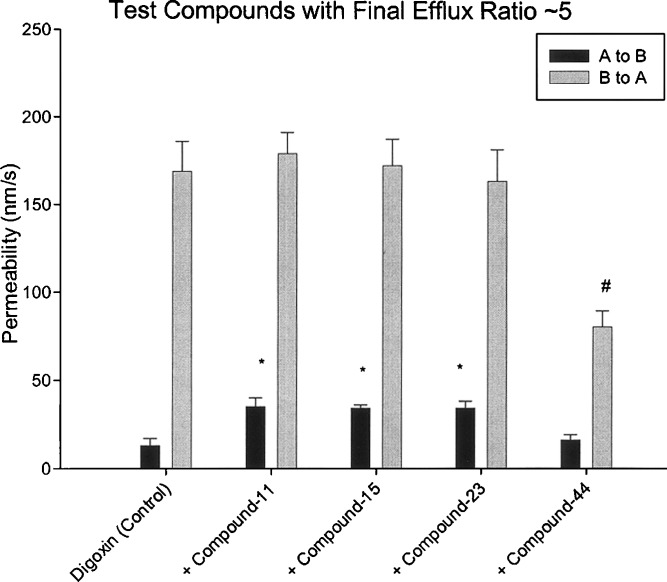
Second, the use of efflux ratio (i.e., method no. 2) makes the model inaccurate again when different test compounds (used as inhibitors) lead to similar efflux ratio despite having different A to B (and B to A) values. Table IV showed the bidirectional digoxin permeability in the absence and presence of several test compounds (compound-11, 15, 23, and 44). All four test compounds produced a similar final efflux ratio of ~5 for digoxin despite the fact that the final A to B and B to A permeability values are quite different. Compared to the digoxin control, the first three compounds (compound-11, 15, and 23) consistently demonstrated significantly higher A to B permeability values (p values 0.001 to 0.004) with no significant effect on B to A permeabilities (p values 0.45 to 0.83). The fourth compound (compound-44) on the other hand demonstrated significantly lower B to A permeability (p = 0.01) with no significant change in A to B permeability (p = 0.36). However, based on the method no. 2, all four compounds produced similar percent inhibition (~67%). Figure 3 provides a pictorial of the bidirectional permeability of digoxin (with and without these four test compounds). It is clear from the figure that in spite of their similar final efflux ratios, they demonstrate vastly different effects on A to B and B to A digoxin permeability. The percent inhibition observed for compound-44 should clearly be significantly greater than the first three compounds. Incidentally, method no. 1 (cumulative AB and BA method) again seems to predict the trends for these four test compounds with low percent inhibition for the compound-11, 15, and 23 (8–17%) but significantly higher inhibition (~60%) for compound-44. Like method no. 1, method no. 3 (inhibition of corrected BA method) also demonstrates negligible inhibition (<10%) for compound-11, 15, and 23; however, it also identifies the potent inhibition (~75%) by compound-44. Method no. 4 (enhancement of corrected AB) provided the most disparate results with significant inhibition (~57–59%) for compound-11, 15, and 23 with negligible inhibition (<10%) for compound-44. It is worthwhile to point out that it is quite possible that the mechanism of inhibition of first three compounds could be entirely different from the fourth compound, leading to the fact that some compounds impact the A to B permeability (without any effect on B to A permeability), whereas others impact the B to A permeability (without any effect on A to B permeability). In the absence of a clear mechanistic understanding of the inhibition process, it might be prudent to use a method that relies on using both the A to B and B to A permeability values to avoid any interpretation errors. Hence, both method no. 1 (relies on both permeability values) and method no. 3 (relies only on B to A, however, is correlated very well with method no. 1) should be acceptable during P-gp screening stages in early discovery.
Table IV.
Percent P-gp Inhibition Values Obtained by Using Different Methods for Compounds with Final Efflux Ratio ~5
| Test | A to B | B to A | Efflux ratio | Method no. 1 | Method no. 2 | Method no. 3 | Method no. 4 |
|---|---|---|---|---|---|---|---|
| Digoxin (control) | 13 ± 4 | 169 ± 17 | 13.0 | ||||
| Compound-11 | 35 ± 5 | 179 ± 12 | 5.1 | 8 | 66 | −8 | 59 |
| Compound-15 | 34 ± 2 | 172 ± 15 | 5.1 | 12 | 66 | −3 | 57 |
| Compound-23 | 34 ± 4 | 163 ± 18 | 4.8 | 17 | 68 | 5 | 57 |
| Compound-44 | 16 ± 3 | 80 ± 9 | 5.0 | 59 | 67 | 75 | 8 |
Fig. 3.
Bidirectional permeability values for digoxin in presence and absence of test compounds that result in a final efflux ratio ~5. Each column represents the mean ± SD of at least three data points (* = A to B permeability in presence of test compound is significantly higher than A to B for digoxin control, # = B to A permeability in presence of test compound is significantly lower than B to A for digoxin control; p < 0.05)
With the advent of high-throughput paradigms in drug discovery organizations, assessment of P-gp inhibition is often mandated at early stages with a large number of compounds. Thus, the P-gp inhibition assay should provide meaningful, reliable, and interpretable data at the lowest cost with minimal usage of resources and time. Based on our empirical analysis of experimental data, it is clear that a remarkably good correlation is obtained when percent inhibition values are compared using calculation method no. 1 (cumulative AB and BA method) vs. method no. 3 (inhibition of corrected BA method). Method no. 1 requires that bidirectional permeability studies be performed for digoxin with and without test compound. However, method no. 3 requires only B to A permeability data (not bidirectional data) for digoxin with and without test compound. Therefore, it is evident that performance of an “abridged” version of the study (only B to A) provides a similar high-quality data as is obtained from a full-fledged bidirectional study (i.e., permeability study in B to A direction followed by “calculation” method no. 3 should suffice to get preliminary P-gp inhibition data at single concentration for test compounds). Moreover, for method no. 3, the denominator value (i.e., the possible range of inhibition of B to A permeability) was chosen to be BAc-50 and not the theoretically possible BAc-ABc. The value of 50 is experimentally obtained value from several potent P-gp inhibitors listed at the bottom (last five compounds) of Table I. On closer analysis of A to B and B to A values obtained for digoxin in the presence of these potent inhibitors, it is clear that all of these afford near-complete percent inhibition of P-gp (>90%). The final average A to B permeability values for these inhibitors is ~46 nm/s and the average B to A permeability values is ~55 nm/s. Therefore, it is clear that for every occasion where near-complete inhibition of P-gp is observed, the permeability value in both directions collapsed to ~50 nm/s (average of final A to B and B to A values). This value of 50 nm/s is referred to as the “mean collapse value” by the authors. Caution should be exercised while using this value of 50 nm/s in calculation method no. 3 by other labs. Keeping in mind the wide diversity of permeability values commonly observed for the same compound across labs (due to differences in cell origin, cell culture techniques, study protocols, etc.), it is advisable that each lab determines its own empirical “mean collapse value” based on the permeability values obtained in the presence of potent inhibitors of P-gp. Positive controls (for potent P-gp inhibitors) such as GF120918, cyclosporin A, ketoconazole, tariquidar, LY335979, quinidine, etc. at 10 μM or higher concentrations can be used to ensure near-complete inhibition of P-gp and obtain the “mean collapse value” for the lab. It is also essential to be aware of the fact that “mean collapse value” of 50 nm/s is a “unique” property of the substrate probe that one uses in the cell-based inhibition assay. Our analysis was performed using digoxin as the P-gp probe. FDA document and several leading labs (9,10,16,17,21,25,26) have suggested the use of digoxin as an ideal probe for such inhibition studies. However, several other probes such as taxol, vinblastine, quinidine, and vincristine can also be used in this cell-based model for P-gp inhibition. Preliminary studies in our lab (data not reported) suggest that the “mean collapse value” for each of these compounds is quite unique and could be different from 50 nm/s observed for digoxin. Therefore, it is critical to perform key control studies (bidirectional permeability for selected P-gp substrate probe alone as well as in presence of classical potent inhibitors) for accurate assessment of maximum possible range of inhibition of B to A permeability prior to using method no. 3 for calculation of percent inhibition of P-gp. Moreover, with regards to the applicability of method no. 3 for assessing the P-gp inhibition potential of test compounds, it is important to realize that the method is independent of cell line (i.e., applies to Caco-2 cells a well as other P-gp cell lines) as well as the probe substrate (digoxin or other classical P-gp substrates such as vinblastine, taxol, etc.) once the appropriate control studies are performed. Additionally, it is also imperative to understand that though method no. 3 has broad application and is suitable in high-throughput screening stages; further detailed methodologies (like method no. 1 where both A to B and B to A studies are performed) are often required to investigate mechanistic questions regarding advanced discovery and development compounds. This could be due to the different inhibitory characteristics of test compounds—some compounds effect B to A permeability without impacting A to B permeability, while others can effect A to B permeability without impacting the B to A permeability. The exact scientific rationale for such a differential effect or its functional (clinical) impact is not fully comprehensible to this time, but it might point towards the possibility of a more pronounced effect on P-gp at the intestinal level (impacting absorption) vs. tissue level (impacting disposition).
CONCLUSIONS
To date, there is no definitive report that compares the different “calculation” methods routinely used by various investigators to assess the percent inhibition of P-gp and, subsequently, the IC50 values. This study describes the very first head-to-head comparison of these diverse “calculation” methods using real experimental data from ~60 structurally diverse internal research and marketed compounds. These empirical analyses were performed to compare these different “calculation” methods and help establish the most appropriate method for drug discovery efforts. It is intriguing that vastly different percent inhibition values were obtained for the “exact” same experimental dataset based on the calculation method one used. It is clear that entirely different conclusions regarding the inhibition potential of test compounds would be reached from the same experimental dataset if different “calculation” methods were used. The commonly used calculation method dependent only on final efflux ratio (method no. 2) requires studies in bidirectional mode and can lead to results that are oversensitive in the “low’ inhibition range which can potentially result in too many false positives (i.e., compounds that are actually non-inhibitors but get identified as inhibitors in this method). Generation of too many false positives is undesirable at early stages, as it can trigger more comprehensive IC50 assessments and clinical studies for many unwarranted compounds, thus negatively impacting the effectiveness of discovery organizations.
Based on the direct comparison between the various methods, we recommend the use of “calculation” method no. 3 for initial assessment of percent inhibition of P-gp. This assay methodology will involve the conduct of only B to A permeability study with digoxin (or any other acceptable P-gp substrate probe) in the presence and absence of discovery compound as a test inhibitor. This abridged version of a full bidirectional study is amenable to high throughput required for rapid screening of compounds but still fully capable of identifying all non-inhibitors (0–20%), moderate inhibitors (20–60%), and potent inhibitors (>60%).
Abbreviations
- A to B
Apical to basolateral
- ADME
Absorption, distribution, metabolism, elimination
- B to A
Basolateral to apical
- BCRP
Breast cancer resistance protein
- CNS
Central nervous system
- FDA
Food and drug administration
- HBSS
Hank’s balanced salt solution
- HEPES
N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid
- LSC
Liquid scintillation counting
- MDCK
Mardin Darby canine kidney
- MRP
Multi-drug resistance protein
- PAMPA
Parallel artificial membrane permeability assay
- P-gp
P-glycoprotein
- Pc
Permeability coefficient
- TEER
Transepithelial electrical resistance
References
- 1.Leahey E., et al. Interaction between quinidine and digoxin. JAMA. 1978;240:533–534. doi: 10.1001/jama.240.6.533. [DOI] [PubMed] [Google Scholar]
- 2.Lin J. Drug–drug interaction mediated by inhibition and induction of P-glycoprotein. Adv. Drug Deliv. Rev. 2003;55:53–81. doi: 10.1016/S0169-409X(02)00171-0. [DOI] [PubMed] [Google Scholar]
- 3.Lin J., Yamazaki M. Role of P-glycoprotein in pharmacokinetics. Clin Pharmacokinet. 2003;42:59–98. doi: 10.2165/00003088-200342010-00003. [DOI] [PubMed] [Google Scholar]
- 4.Matheny C., Lamb M., Brouwer K., Pollak G. Pharmacokinetic and pharmacodynamic implications of P-gp modulation. Pharmacotherapy. 2001;21:778–796. doi: 10.1592/phco.21.9.778.34558. [DOI] [PubMed] [Google Scholar]
- 5.Polli J., et al. Role of P-gp on CNS disposition of amprenavir, an HIV protease inhibitor. Pharm. Res. 1999;16:1206–1212. doi: 10.1023/A:1018941328702. [DOI] [PubMed] [Google Scholar]
- 6.Polli J. W., et al. P-glycoprotein influences the brain concentrations of cetirizine (Zyrtec), a second-generation non-sedating antihistamine. J. Pharm. Sci. 2003;92:2082–2089. doi: 10.1002/jps.10453. [DOI] [PubMed] [Google Scholar]
- 7.Sparreboom A., et al. Limited oral bioavailability and active epithelial excretion of paclitaxel (Taxol) caused by P-gp in the intestine. Proc. Natl. Acad. Sci. 1997;94:2031–2035. doi: 10.1073/pnas.94.5.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Watanabe T., et al. Kinetic analysis of hepatobiliary transport of vincristine in perfused rat liver: Possible roles of P-gp in biliary excretion of vincristine. J. Hepatol. 1992;16:77–88. doi: 10.1016/S0168-8278(05)80098-4. [DOI] [PubMed] [Google Scholar]
- 9.FDA Draft Guidance. Drug interaction studies—Study design, data analysis, and implications for dosing and labeling. 2006. [DOI] [PubMed]
- 10.Balimane P. V., Han Y. H., Chong S. Current industrial practices of assessing permeability and P-glycoprotein interaction. AAPS J. 2006;8:E1–13. doi: 10.1208/aapsj080101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cutler L., Howes C., Deeks N. J., Buck T. L., Jeffrey P. Development of a P-glycoprotein knockout model in rodents to define species differences in its functional effect at the blood-brain barrier. J. Pharm. Sci. 2006;95:1944–1953. doi: 10.1002/jps.20658. [DOI] [PubMed] [Google Scholar]
- 12.Feng B., et al. In vitro p-glycoprotein assays to predict the in vivo interactions of p-glycoprotein with drugs in the central nervous system. Drug. Metab. Dispos. 2008;36:268–275. doi: 10.1124/dmd.107.017434. [DOI] [PubMed] [Google Scholar]
- 13.Hidalgo I. Assessing the absorption of new pharmaceuticals. Current Topics in Medicinal Chemistry. 2001;1:385–401. doi: 10.2174/1568026013395010. [DOI] [PubMed] [Google Scholar]
- 14.Keogh J. P., Kunta J. R. Development, validation and utility of an in vitro technique for assessment of potential clinical drug–drug interactions involving P-glycoprotein. Eur. J. Pharm. Sci. 2006;27:543–554. doi: 10.1016/j.ejps.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 15.Kerns E., et al. Combined application of parallel artificial membrane permeability assay and Caco-2 permeability assays in drug discovery. J. Pharm. Sci. 2004;93:1440–1453. doi: 10.1002/jps.20075. [DOI] [PubMed] [Google Scholar]
- 16.Polli J., et al. Rational use of in vitro P-gp assays in drug discovery. J. Pharmacol. Exp. Ther. 2001;299:620–628. [PubMed] [Google Scholar]
- 17.Rautio J., et al. In vitro p-glycoprotein inhibition assays for assessment of clinical drug interaction potential of new drug candidates: a recommendation for probe substrates. Drug Metab. Dispos. 2006;34:786–792. doi: 10.1124/dmd.105.008615. [DOI] [PubMed] [Google Scholar]
- 18.Tang F., Horie K., Borchardt R. Are MDCK cells transfected with the human MDR1 gene a good model of the human intestinal mucosa. Pharm. Res. 2002;19:765–772. doi: 10.1023/A:1016140429238. [DOI] [PubMed] [Google Scholar]
- 19.Ungell A.-L. Caco-2 replace or refine. Drug Discov. Today. 2004;1:423–430. doi: 10.1016/j.ddtec.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 20.Hsiao P., Bui T., Ho R. J., Unadkat J. D. In vitro-to-in vivo prediction of P-glycoprotein-based drug interactions at the human and rodent blood–brain barrier. Drug. Metab. Dispos. 2008;36:481–484. doi: 10.1124/dmd.107.018176. [DOI] [PubMed] [Google Scholar]
- 21.Kim R., et al. Interrelationship between substrates and inhibitors of human CYP3A and P-gp. Pharm. Res. 1999;16:408–414. doi: 10.1023/A:1018877803319. [DOI] [PubMed] [Google Scholar]
- 22.Perloff M., Stromer E., von Moltke L., Greenblatt D. Rapid assessment of P-gp inhibition and induction in vitro. Pharm. Res. 2003;20:1177–1183. doi: 10.1023/A:1025092829696. [DOI] [PubMed] [Google Scholar]
- 23.Troutman M. D., Thakker D. R. Efflux ratio cannot assess P-glycoprotein-mediated attenuation of absorptive transport: Asymmetric effect of P-glycoprotein on absorptive and secretory transport across Caco-2 cell monolayers. Pharm. Res. 2003;20:1200–1209. doi: 10.1023/A:1025049014674. [DOI] [PubMed] [Google Scholar]
- 24.Stephens R., et al. Kinetic profiling of P-gp mediated drug efflux in rat and human intestinal epithelia. J. Pharmacol. Exp. Ther. 2001;296:584–591. [PubMed] [Google Scholar]
- 25.Balimane P. V., Chong S. Cell culture-based models for intestinal permeability: A critique. Drug Discov. Today. 2005;10:335–343. doi: 10.1016/S1359-6446(04)03354-9. [DOI] [PubMed] [Google Scholar]
- 26.Balimane P. V., Patel K., Marino A., Chong S. Utility of 96 well Caco-2 cell system for increased throughput of P-gp screening in drug discovery. Eur. J. Pharm. Biopharm. 2004;58:99–105. doi: 10.1016/j.ejpb.2004.02.014. [DOI] [PubMed] [Google Scholar]