Abstract
Using the approach of peptide transduction domain (PTD)-mediated loading of interleukin-2(IL-2)-activated natural killer (A-NK) cells, tumor-seeking lymphocytes, with prodrug-activating enzymes, we primarily aim to generate a cytotoxic drug selectively within tumors and minimize damage to normal tissues. A-NK cells are able to accumulate selectively at tumor sites. While these cells by themselves possess significant antitumor effect in vivo, we suggest that they can also serve as Trojan horses, by bringing anticancer agents, such as prodrug-activating enzymes, selectively to tumors. We have successfully demonstrated in a mouse model that A-NK cells can be rapidly loaded with prodrug-activating enzymes, such as alkaline phosphatase (AP) and beta-galactosidase (beta-gal), in vitro using enzyme-conjugated peptide PTD5. Upon adoptive transfer into lung-tumor-bearing animals, the loaded A-NK cells are able to bring their cargo of the prodrug-activating enzymes selectively to pulmonary metastases. The targeting of the AP to the tumor tissues is highly specific, since more than a fivefold higher concentration of AP was found in the tumor tissues compared to the surrounding normal lung tissue at 24 h after injection. The approach of transporting prodrug-activating enzymes selectively into tumors clearly shows potential for future targeted chemotherapy. Ongoing studies in our laboratory are evaluating the antitumor efficacy of cellular-dependent enzyme prodrug therapy.
Key words: activated natural killer cells, delivery, metastases in vivo, prodrug enzymes, protein transduction domain
INTRODUCTION
The efficacy of systemically administered antineoplastic drugs for the treatment of disseminated cancer is limited by damage induced in normal tissues, in particular gut and bone marrow. To reduce this systemic toxicity, different strategies have been developed to limit the drug effects in normal tissues by targeting the antineoplastic drugs selectively to the tumor tissue. One such approach is antibody-delivered enzyme prodrug therapy (ADEPT), where a prodrug-activating enzyme is conjugated to an antibody capable of recognizing antigens expressed mainly by the tumor (1,2). Once the enzyme is targeted to the tumor, a relatively non-toxic prodrug is administered systemically. Due to the selective localization of the antibody–enzyme conjugate, the prodrug is cleaved by the enzyme into active drug mainly in the tumor tissue. Although successful in several models (3,4) and clinical trials (5,6), the overall success of this approach is limited by several factors. First, the tumor must express a suitable tumor-associated antigen (TAA), and this is not always the case. Second, a relatively large amount of an appropriate enzyme-conjugated antibody recognizing the TAA has to be available. Third, elevation of the intratumoral osmotic and hydrostatic pressures limits the delivery, by simple diffusion, of macromolecules such as enzyme–antibody conjugates to the tumor tissue (7,8).
We have previously shown that adoptively transferred interleukin-2(IL-2)-activated natural killer (A-NK) cells (9–12) are able to localize selectively into tumor tissues and that they inflict substantial damage to the tumors they infiltrate, leading to a significant reduction in the total tumor mass (13). To further boost the antitumor effect of the adoptively transferred A-NK cells and to overcome some of the above-mentioned problems associated with ADEPT, it may be possible to use the A-NK cells as carriers for the delivery of antineoplastic agents, e.g., prodrug-activating enzymes, selectively to tumor sites. Since the ratio of A-NK cells in tumor versus normal tissue at 24 h after injection is better than 5:1 (9,13), one would expect that the A-NK cells would be able to deliver fivefold more enzyme to the malignant than to the normal tissues.
Gene transfer is a commonly used strategy to ensure overexpression of a certain protein product by mammalian cells and it has been used to induce expression of potentially prodrug-activating enzymes such as alkaline phosphatase (AP) (14), beta-galactosidase (beta-gal) (15–17), and thymidine kinase (TK) (18–26) in many cell lines. However, primary lymphocytes are notoriously difficult to genetically transduce in high numbers. We have therefore developed an alternative non-genetic strategy for loading enzymes into the tumor-seeking A-NK carrier cells based on peptide transduction domains (PTDs), which have previously been shown to be able to translocate various macromolecules inside a variety of cells (27–35). Here, we demonstrate that prodrug-activating enzymes, such as AP and beta-gal, can be rapidly loaded into A-NK cells using enzyme-conjugated PTD5. We also show that cells pretreated with enzyme-conjugated PTD5 ex vivo are able to deliver the enzyme selectively to pulmonary metastases following re-inoculation into tumor-bearing animals.
MATERIALS AND METHODS
Animals and Tumor Cell Lines
Specific pathogen-free 8–12-week-old female C57BL/6J (Thy1.2) and congenic B6.PL-Thy1aCy (Thy1.1) mice were obtained from Jackson Laboratories (Bar Harbor, ME, USA) and housed in a specific pathogen-free animal facility at the Hillman Cancer Center, University of Pittsburgh. In all experiments, the Principles of Laboratory Animal Care (National Institutes of Health publication n. 85-23, revised 1985) were followed and IACUC approval was obtained.
For establishment of lung metastases, subline B16-F10-P1 of the B16 melanoma was used (the F10-P1 subline was established in our laboratory from a B16-F10 lung metastasis). The B16 cells were maintained in RPMI1640 medium (Life Technologies, Gaithersburg, MD, USA) supplemented with 10% heat-inactivated fetal calf serum, 2 mM glutamine, 20 mM Hepes buffer, 0.8 g/l streptomycin, and 1.6 × 105 U/l penicillin. Adherent cells were detached by exposure to 0.02% EDTA for 10 min and washed three times in RPMI1640 containing 2% fetal bovine serum. The cells were re-suspended in media to a final concentration of 106 per milliliter. The cell viability was always more than 95% as judged by trypan blue dye exclusion test. Pulmonary metastases were established by tail vein injection of 0.4 × 106 B16 cells in a volume of 0.2 ml of RPMI1640 into Thy1.2+ C57BL/6 mice pretreated 1 day earlier with 25 μl antiasialo GM1 antiserum (Wako Pure Chemicals, Wako, TX, USA) by intraperitoneal injection (i.p.) to eliminate endogenous NK cells.
A-NK Cell Preparation
Spleens were removed from congenic B6.Pl-Thy-1aCy mice and a single-cell suspension was prepared in RPMI1640. Erythrocytes were lysed by incubation with ammonium chloride–potassium buffer at room temperature for 3 min and the spleen cells were subsequently washed twice in RPMI1640. Cells were transferred to T150 plastic flasks (Falcon, B&D, Franklin Lakes, NJ, USA) and cultured at 37°C in an atmosphere of 5% CO2 in 50 ml of RPMI1640 supplemented with 5% heat-inactivated fetal calf serum and 5% normal human serum, 10 ml/l non-essential amino acids (Life Technologies), 50 mM 2-mercaptoethanol, 2 mM glutamine, 20 mM Hepes buffer, 0.8 g/l streptomycin, and 1.6 × 105 U/l penicillin, hereafter referred to as complete medium (CM). Cells were stimulated with 6,000 IU/ml of human recombinant IL-2 (a kind gift from the Chiron Corporation, Emeryville, CA, USA). After 3 days of incubation, CD8-positive cells were magnetically removed following incubation of the cell culture with rat anti-CD8 antibody (ATCC, TIB-105) and subsequently with antirat-coated magnetic beads (Dynal Biotech, Lake Success, NY, USA). The CD8-depleted cells were suspended in fresh CM containing 6,000 IU/ml IL-2 to a final concentration of 1 × 105 cells per milliliter and returned to culture flasks. Fresh CM containing 6,000 IU/ml IL-2 was added every 2 to 3 days as needed. After an additional 3 days of culture, non-adherent cells were decanted and the plastic-adherent cells were harvested after a brief treatment with 0.02% EDTA and washed twice in RPMI1640 before use. Routinely, these A-NK cells were >95% Thy1.1+, >95% asGM1+, >90% NK1.1+, <2% CD8+, <2% CD4+.
Labeling of A-NK Cells
Labeling of A-NK cells with various concentrations of enzyme–PTD5 (RRQRRTSKLMKR) was done and the poorly transducing control PTD-R (ARPLEHGSDKAT) peptides were synthesized (Peptide Synthesis Facility, University of Pittsburgh, PA, USA) either with biotin or fluorescein isothiocyanate (FITC) at the N-terminal end as previously described (30,33). Enzyme–PTD complexes were made by incubation of 174 μl of 50 μM biotinylated PTDs with 200 μl of 1 mg/ml streptavidin–AP (Lot 554065, Pharmingen, San Jose, CA USA) or streptavidin beta-gal (Sigma, Lot 082K8627, St. Louis, MO 63178, USA) for 2 h at room temperature. A-NK cells (3 × 106 per 0.1 ml) were labeled for 2–3 h at 37°C under gently shacking in 1:5 dilution of the enzyme–PTD complexes. After labeling, the cells were washed three times in RPMI1640 before use.
Efficiency of PTD5 labeling of A-NK Cells
First, the labeling efficiency of various concentrations of enzyme–PTD5 and enzyme–PTD-R was evaluated by addition of substrates after labeling of A-NK cells. Briefly, the efficiency of A-NK cell labeling with the beta-gal- or AP-conjugated PTD5 was evaluated by mixing 1 × 105 labeled cells in 0.9 ml of 1% paraformaldehyde with 0.1 ml beta-gal substrate (Galacto-light plus systems, Cat. BL2500G, A&B, MA, USA) or AP substrate (alkaline phosphatase yellow, pNPP, Lot#A3469, SIGMA, MO, USA). After the indicated hours of incubation at 37°C, 5% CO2, supernatants were harvested and read at em-514-nm fluorescence of luminescence spectrometer (model LR64912C-LS55, Perkin Elmer, UK) or at em-405-nm bioluminescence of a micro-plate reader (Model 550 BIO-RAD, Hercules, CA, USA). Data are expressed as relative light units (RLUs). According to the rule of higher cell viability with higher labeling efficiency, the labeling efficiency of 12.5-μM concentration of marker- and enzyme-conjugated PTD5, including the control- and FITC-conjugated PTD-R and non-conjugated PTD5, was evaluated at different time points after labeling of A-NK cells via the luminescence spectrometer or micro-plate reader and flow cytometry. Cell viability after labeling was measured by trypan blue exclusion and proliferation of labeled cells was measured with CellTiter according to the supplier recommendations (MTT, Sigma, St. Louis, MO, USA).
Adoptive Transfer of PTD-labeled A-NK Cells into Tumor-Bearing Animals
Pulmonary metastases were induced in C57BL/6J (Thy1.2) by tail vein injection of B16 tumor cells. Twelve days later, ten million AP-PTD5- and AP-PTD-R-labeled A-NK cells, which were from congenic B6.PL-Thy1aCy (Thy1.1) mice, in 200 μl RPMI were injected i.v. To support the transferred cells, the mice received i.p. injections of 500 μl phosphate-buffered saline (PBS) containing 60,000 IU polyethylene-glycol-conjugated IL-2 (a kind gift from the Chiron Corporation, Emeryville, CA, USA) every 12 h as previously described (13). At different times after the adoptive transfer (4, 18, 24, and 48 h), organs were removed and embedded in OCT compound (Sakura Finetek USA Inc. Torrance, CA 90501, USA) and frozen in −80°C hexane.
Detection of Enzyme-Carrying A-NK Cells In Vivo
Eight to twelve μm sections were cut from three to five randomly chosen areas of each organ. The sections were stained by a one-layer immunofluorescence technique. Briefly, frozen sections were fixed in acetone for 8 min, dehydrated in chloroform for 10 min, and washed twice (2 × 10 min) in PBS buffer containing 1% fetal calf serum (pH 7.4). Sections were incubated with 1:200 dilution of FITC-conjugated anti-Thy1.1 antibody (01014D, Lot: M043589, BD Bioscience, PharMingen, San Diego, CA, USA) in a humidified chamber for 30 min at room temperature to reveal the transferred cells. FITC-conjugated rat IgG2b (A95-1, BD Bioscience) was used as control. To reveal alkaline phosphatase, serial sections were incubated with 125 μl (1 mg/ml) of the red fluorescence AP substrate Fast Red (FASTTM/TR/NAPHTHOL, F-4648, SIGMA, St. Louis, MO 63178, USA) for 60 min at room temperature and washed two times with PBS. The sections were mounted under a glass coverslip with aquamount and evaluated using a Nikon fluorescence microscope with rhodamine and FITC filters (EX535/50, DM565, BA610/75 and EX480/40, DM505, BA535/50, respectively). Images were acquired with a charge-coupled device digital camera (Sensus, Photometrics).
Estimation of Cell and Enzyme Density in Normal and Tumor Tissue
The tissue density of A-NK cells was estimated by measurement of FITC fluorescence in tumor and normal tissues using MetaMorph (Universal Imaging Corporation, West Chester, PA, USA). For each mouse, Thy1.1-positive cells were counted in areas where individual A-NK cells could easily be identified and the average number of FITC-positive pixels associated with one cell was calculated. Based on this value, the density of cells in a given area could be estimated (number of FITC-positive pixels in area of interest divided by the average number of FITC-positive pixels per cell). Results are shown as average density of cells per square millimeter tissue (four to five mice per group). Likewise, to assess the density of enzyme in tumor versus normal tissue, the average number of red fluorescence pixels per square millimeter normal and tumor tissue from four to five animals was measured. A total of at least ten different tumors and ten different normal tissue areas (each approximately 1 mm2 of size) from three to five randomly chosen areas of each organ was analyzed. The results were compared using two-sided Student’s t test.
RESULTS
Efficiency and Stability of PTD5 labeling of A-NK Carrier Cells
To evaluate the efficacy of labeling with PTDs, A-NK cells were incubated with FITC-labeled PTDs. The peptides used were the cationic PTD5 and the control PTD-R (20). Both are 12-mer peptide sequences from an M 13 phage library. PTD5 possesses a characteristic positive charge on the basis of its high content of arginine and lysine residues. Following labeling with different concentrations of beta-gal-conjugated PTD5, a dose-dependent uptake of enzyme by A-NK cells was recorded (Fig. 1). A 12.5 μM concentration of FITC– and enzymes–PTD5 was used for labeling A-NK cells. The uptake of FITC–PTD conjugates by the A-NK cells was evaluated by flow cytometry at different time points. A-NK cells were efficiently labeled after incubation with 12.5 μM of the FITC–PTD5 construct and, even though the mean fluorescence intensity fell one order of magnitude within the first 24 h, 55–70% of the cells retained the FITC label at this time. Nevertheless, the retention of the PTD-loaded FITC was fairly short-lived and less than 20% of the cells remained positive beyond 48 h (Fig. 2).
Fig. 1.
Dose-dependent uptake of beta-gal following labeling with beta-gal-PTD5. A-NK cells were incubated with various concentrations of beta-gal-PTD5 and gal-PTD-R for 2–3 h at 37°C. After washing, the cells were assayed for enzyme activity as described in “MATERIALS AND METHODS.” While the beta-gal-PTD-R peptide hardly labeled the cells, the cells showed a clear dose-dependent uptake of enzyme following labeling with the beta-gal-PTD5 peptide
Fig. 2.
Efficiency and stability of PTD5 labeling of A-NK carrier cells. A-NK cells were incubated with FITC-conjugated PTD5 (black histogram) and PTD-R peptides (black circle) at 12.5 μM for 2–3 h. Control A-NK cells (black triangle) were incubated with media alone. FITC labeling of the A-NK cells was measured by flow cytometry immediately at 0, 24, 48, and 72 h after labeling. The percentage of FITC-positive A-NK cells was analyzed from separate experiments. The data were expressed as mean ± SD
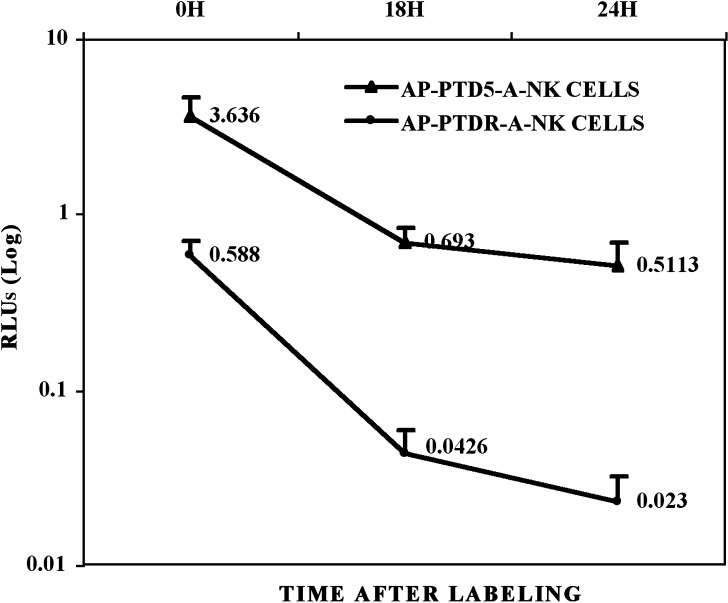
Enzyme activities associated with A-NK cells were evaluated following incubation with 12.5 μM of beta-gal- and AP-conjugated PTD5 and PTD-R peptides. As observed with the FITC conjugates, the beta-gal- and AP-conjugated PTD5 peptide resulted in a significantly better labeling of the A-NK cells than the beta-gal- and AP-conjugated PTD-R control peptide. Even though a considerable amount of the enzyme activity was lost within the initial 18–24 h after labeling, the enzyme activity of PTD5-labeled cells remained at least fivefold higher than the activity level in PTD-R-labeled cells at this time (Fig. 3). In accordance, cytospin preparations of AP-PTD5-labeled cells demonstrated very strong staining with the AP substrate Fast Red immediately after labeling. Despite a substantial reduction in the staining intensity at 18 to 24 h after incubation with the AP-PTD5, most of the cells were still clearly positive compared to control cells at this time.
Fig. 3.
Kinetics of enzyme activity elimination from enzyme PTD5-labeled A-NK cells. A-NK cells were incubated with alkaline phosphatase (AP)-conjugated PTD5 for 2–3 h at 37°C. After washing, the cells were incubated for up to 48 h and the enzyme activity of the cells was measured at various time points. While more than 75% of the enzyme is lost within 48 h after labeling, a substantial amount of enzyme is retained in the cells at 18–24 h after labeling. The data were from the separate experiments of AP-PTD5-A-NK cells and were presented as means ± SD
Effect of PTD Labeling on A-NK Cell Viability and Growth
To test whether A-NK cells labeled at the 12.5 μM concentration of AP-conjugated PTD5 peptides would negatively impact on cell viability and/or proliferative capacity, the number of the viable A-NK cells was measured—using trypan blue exclusion test—at 0, 24, and 48 h following AP-PTD5 labeling. The viability remained equally high among labeled and unlabeled cells during the 0 to 48 h observation period (Fig. 4a). The enzyme–PTD5 labeling was not toxic for the A-NK cells. In addition, the proliferation of AP-, beta-gal-, or FITC–PTD5-labeled A-NK cells was equal to that of non-labeled control cells at both 24 and 48 h (Fig. 4b). Thus, the labeling did not interfere with the proliferative capacity of the A-NK cells.
Fig. 4.
Effects on cell viability and growth. A-NK cells were incubated with AP- (FITC- and BETA-Gal-) conjugated PTD5 in 12.5 μM for 2–3 h at 37°C. After labeling, A-NK cell viability and proliferation were measured with trypan blue exclusion and CellTiter at the indicated time points, respectively. The data of AP-conjugated PTD5-A-NK cell viability was from separate experiments (Fig. 4a). The recovered proliferation of beta-gal-, AP-, and FITC-PTD5-A-NK cells was equal to that of non-labeled control cells at both 24 and 48 h (Fig. 4b). The results showed that labeling did not interfere with the proliferative capacity of the A-NK cells. All data were presented as means ± SD
Tumor Localization of Prodrug-Activating Enzyme Carried in PTD5-Labeled A-NK Cells
Having ensured that labeling of A-NK cells with conjugated PTD5 resulted in retention of sufficient amounts of label for identification for at least 24 h following labeling without any obvious deleterious effect on the cells, we injected AP-PTD5-labeled A-NK cells into animals bearing day 10 pulmonary B16 lung metastases. Analysis of sections, from lungs removed at 24 h after injection and stained with Fast Red substrate, demonstrated that AP-PTD-5-labeled A-NK cells indeed are able to localize into tumors and, importantly, to carry the alkaline phosphatase with them into the tumor (Fig. 5). However, at 48 h after injection, hardly any AP activity could be detected in the tumors even though more A-NK cells were present in the tumor tissue at this time than at 24 h. The targeting of the AP to the tumor tissues was highly specific since more than a fivefold higher concentration of AP was found in the tumor tissues compared to the surrounding normal tissues at 24 h after injection (Fig. 6a). This was paralleled by a fivefold higher number of A-NK cells found in the tumors at this time as compared to the surrounding normal lung tissues (Fig. 6b). Normal tissues such as liver, spleen, and kidneys contained very few A-NK cells and, hence, very little AP. A-NK cells that had been labeled with the AP-PTD-R peptide also localized into the tumor tissues, but, as expected, no AP activity was detected in these tumors.
Fig. 5.
Delivery of enzymes selectively to sites of tumor by AP-PTD5-loaded A-NK carrier cells. Thy1.1+ A-NK cells were incubated with AP-conjugated PTD5 at 12.5 μM for 30 min. After washing, ten million labeled cells were injected i.v. into Thy 1.2+ congenic mice bearing day 10 B16 pulmonary metastases. The mice received i.p. injections of 60,000 IU Peg-IL-2 at t = 0 and every 12 h thereafter for the duration of the experiment to support the transferred A-NK cells. At various times, animals were sacrificed and cryosections of the lungs were analyzed for the content of AP (by staining with the Fast Red, a fluorescence substrate for AP) and for their content of the A-NK cells (identified by staining with FITC anti-Thy1.1 antibody). The B16 tumors were easily recognizable by their content due to their black pigment melanin content (black pigment—left panel). The tumor area (yellow outline) contained infiltrating FITC-positive A-NK cells (center panel) and reacted strongly with the Fast Red substrate (right panel). Note the low density of A-NK cells and AP in the normal lung tissue (center and right panels). Scale bar = 100 μM
Fig. 6.
Density of AP and A-NK cells in tumor and normal lung tissue. The density of FITC anti-Thy1.1-positive A-NK cells as well as the density of AP (visualized by staining with Fast Red) in tumors and the surrounding normal lung tissue was measured by image analysis (MetaMorph software). A significance of more than fivefold higher density of AP (a) and A-NK cells (b) was found in the lung tumors compared to the normal lung tissue (p < 0.05)
These data demonstrate that PTD5 can be used for the loading of A-NK cells with prodrug-activating enzymes such as AP and that the enzyme–PTD-loaded cells are able to deliver the enzyme selectively to sites of tumor.
DISCUSSION
The aim of this study was to investigate whether prodrug-activating enzymes can be loaded into tumor-seeking lymphocytes such as A-NK cells using enzyme-conjugated PTDs and to evaluate the ability of loaded cells to deliver the enzyme to sites of tumor following adoptive transfer into tumor-bearing hosts. One of the advantages of using A-NK cells instead of antibodies as carriers of drug-activating enzymes is that the tedious and not-always-successful production of tumor-specific antibodies for ADEPT is not needed and—most importantly—that the antigen expression by the tumor is irrelevant. Furthermore, in contrast to antibodies, cells are able to traffic against osmotic and hydrostatic pressure gradients and may therefore be better able to penetrate deeply into solid tumors than antibodies, whose ability to diffuse into tissues is greatly reduced as these pressure gradients increase (7,8). Also, due to the large size of cells compared to antibodies, it is conceivable that substantial amounts of enzyme may be loaded onto each tumor-seeking lymphocyte.
While techniques for the conjugation of enzymes to antibodies are well established (1,4,5), methodologies for the loading of enzymes into lymphoid cells are not well developed. McNeil et al. (36) and others (37,38) describe “scrape loading” as a means to transfer larger molecules across the cell membrane, but “scrape loading” of adherent A-NK cells with AP leads to heterogenous uptake and a significant loss of cells. Other techniques, such as red blood cell ghost fusion (39–42) and electroporation (43–47) as a means to get enzymes across the cell membrane, also resulted in heterogeneous labeling and considerable cell loss when applied to the A-NK cells and were therefore not further pursued. In contrast, the ability of PTD to transduce A-NK cells with various molecules, such as FITC, AP, and beta-gal, was very impressive. Thus, a high percentage of the PTD-treated cells very rapidly took up these molecules when bound to the PTDs via a biotin–avidin bridge. Importantly, treatment with the PTD–enzyme or PTD–fluorochrome constructs did not seem to interfere significantly with cell viability or proliferation. Although the loaded enzymes and fluorochromes were cleared relatively quickly from the PTD-treated cells, a substantial amount of the molecules remained inside a high percentage of the treated cells within the initial 24–48 h after labeling. Since infiltration of lung tumors by adoptively transferred A-NK cells becomes significant as early as 8–12 h after injection (9,48,49), retention of the labels—enzyme or fluorochrome—for 24–48 h should allow sufficient time for the A-NK cells to reach the tumor while still containing a substantial amount of the loaded molecules.
Thus, having ensured that PTD treatment was capable of efficiently loading various enzymes onto A-NK cells, we tested whether the labeled cells would be able to bring the enzymes selectively into the tumor tissue. Indeed, this was the case in that fivefold higher density of AP was found in lung tumors compared to the surrounding normal lung tissue at 24 h after injection of PTD-AP-treated A-NK cells. This correlated with a fivefold higher density of A-NK cells in the tumors compared to the normal lung tissue. Hardly any enzyme activity was found in any other organs at this time, indicating a highly selective distribution of the PTD-treated cells into the malignant tissues. However, at 48 h after injection, no enzyme activity was detected in the tumors despite the fact that the number of intratumoral A-NK cells was higher at 48 h than at 24 h after injection. We speculate that this was due partly to clearance of label from the cells, partly to dilution of enzyme to below detection level caused by intratumoral proliferation of the A-NK cells.
In conclusion, we have successfully demonstrated that various fluorochromes and enzymes can be efficiently PTD-transduced into A-NK cells that are capable of transporting these enzymes selectively into tumor tissues. Ongoing studies in our laboratory are evaluating the antitumor efficacy of cellular-dependent enzyme prodrug therapy, i.e., cancer therapy based on A-NK cells—pretreated with enzyme-conjugated PTDs—as delivery vehicles of the enzymes. If successful, this technology may also be used to transduce other compounds with antitumor activity into the tumor-seeking lymphocytes, such as therapeutic or diagnostic radionuclides, boron-containing macromolecules for neutron capture therapy, radio synthesizers, cytokines and other biological response modifiers, antiangiogenic compounds, etc. Also, since the localizing capabilities of A-NK cells may also include infectious or inflammatory foci in general, we hypothesize that PTD-transduced A-NK cells can also be used as carriers for the delivery of antimicrobial and anti-inflammatory compounds selectively to sites of infection or inflammation. The approach shows potential for future targeted chemotherapy.
Acknowledgement
This study was supported by grants from the American Cancer Society (grant no. RPG-00-221-01-CDD) and the US-NIH (grants no. 101944-01A PO1, R01CA104560 and RO1CA87672). We thank Ms. Patricia Rice and Mrs. Lisa Bailey for excellent technical assistance.
Contributor Information
Qin Yang, Phone: +1-412-6232757, FAX: +1-412-6231119, Email: qyang@pitt.edu, Email: q-yang@northwestern.edu.
Stine K. Larsen, Email: stinekiaer@gmail.com
Zhibao Mi, Email: zmi@pitt.edu.
Paul D. Robbins, Email: probb@pitt.edu
Per H. Basse, Email: basse@imap.pitt.edu
References
- 1.Springer C. J., Niculescu-Duvaz I. I. Antibody-directed enzyme prodrug therapy (ADEPT): a review. Adv. Drug. Deliv. Rev. 1997;26:151–172. doi: 10.1016/S0169-409X(97)00032-X. [DOI] [PubMed] [Google Scholar]
- 2.Xu G., McLeod H. L. Strategies for enzyme/prodrug cancer therapy. Clin. Cancer Res. 2001;7:3314–3324. [PubMed] [Google Scholar]
- 3.Blakey D. C., Davies D. H., Dowell R. I., East S. J., Burke P. J., Sharma S. K., Springer C. J., Mauger A. B., Melton R. G. Anti-tumour effects of an antibody-carboxypeptidase G2 conjugate in combination with phenol mustard prodrugs. Br. J. Cancer. 1995;72:1083–1088. doi: 10.1038/bjc.1995.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Springer C. J., Bagshawe K. D., Sharma S. K., Searle F., Boden J. A., Antoniw P., Burke P. J., Rogers G. T., Sherwood R. F., Melton R. G. Ablation of human choriocarcinoma xenografts in nude mice by antibody-directed enzyme prodrug therapy (ADEPT) with three novel compounds. Eur. J. Cancer. 1991;27:1361–1366. doi: 10.1016/0277-5379(91)90010-B. [DOI] [PubMed] [Google Scholar]
- 5.Bagshawe K. D., Sharma S. K., Springer C. J., Antoniw P., Boden J. A., Rogers G. T., Burke P. J., Melton R. G., Sherwood R. F. Antibody directed enzyme prodrug therapy (ADEPT): clinical report. Dis. Markers. 1991;9:233–238. [PubMed] [Google Scholar]
- 6.Connors T. A., Knox R. J. Prodrugs in cancer chemotherapy. Stem Cells. 1995;13:501–511. doi: 10.1002/stem.5530130507. [DOI] [PubMed] [Google Scholar]
- 7.Jain R. K. Transport of molecules in the tumor interstitium: a review. Cancer Res. 1987;47:3039–3051. [PubMed] [Google Scholar]
- 8.Jain R. K., Baxter L. T. Mechanisms of heterogeneous distribution of monoclonal antibodies and other macromolecules in tumors: significance of elevated interstitial pressure. Cancer Res. 1988;48:7022–7032. [PubMed] [Google Scholar]
- 9.Basse P., Herberman R. B., Nannmark U., Johansson B. R., Hokland M., Wasserman K., Goldfarb R. H. Accumulation of adoptively transferred adherent, lymphokine-activated killer cells in murine metastases. J. Exp. Med. 1991;174:479–488. doi: 10.1084/jem.174.2.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Basse P. H., Nannmark U., Johansson B. R., Herberman R. B., Goldfarb R. H. Establishment of cell-to-cell contact by adoptively transferred adherent lymphokine-activated killer cells with metastatic murine melanoma cells. J. Natl. Cancer Inst. 1991;83:944–950. doi: 10.1093/jnci/83.13.944. [DOI] [PubMed] [Google Scholar]
- 11.Basse P. H., Goldfarb R. H., Herberman R. B., Hokland M. E. Accumulation of adoptively transferred A-NK cells in murine metastases: kinetics and role of interleukin-2. In Vivo. 1994;8:17–24. [PubMed] [Google Scholar]
- 12.Hokland M., Kjaergaard J., Kuppen P. J., Nannmark U., Agger R., Hokland P., Basse P. Endogenous and adoptively transferred A-NK and T-LAK cells continuously accumulate within murine metastases up to 48 h after inoculation. In Vivo. 1999;13:199–204. [PubMed] [Google Scholar]
- 13.Yang Q., Hokland M. E., Bryant J. L., Zhang Y., Nannmark U., Watkins S. C., Goldfarb R. H., Herberman R. B., Basse P. H. Tumor-localization by adoptively transferred, interleukin-2-activated NK cells leads to destruction of well-established lung metastases. Int. J. Cancer. 2003;105:512–519. doi: 10.1002/ijc.11119. [DOI] [PubMed] [Google Scholar]
- 14.He J., Landau N. R. Use of a novel human immunodeficiency virus type 1 reporter virus expressing human placental alkaline phosphatase to detect an alternative viral receptor. J. Virol. 1995;69:4587–4592. doi: 10.1128/jvi.69.7.4587-4592.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bachy M., Bonnin-Rivalland A., Tilliet V., Trannoy E. Beta galactosidase release as an alternative to chromium release in cytotoxic T-cell assays. J. Immunol. Methods. 1999;230:37–46. doi: 10.1016/S0022-1759(99)00118-0. [DOI] [PubMed] [Google Scholar]
- 16.Di Ianni M., Casciari C., Ciurnelli R., Fulvi A., Bagnis C., Sadelain M., Lucheroni F., Mannoni P., Stella C. C., Martelli M. F., Tabilio A. Retroviral transfer of herpes simplex virus-thymidine kinase and beta-galactosidase genes into U937 cells with bicistronic vector. Leuk Res. 1997;21:951–959. doi: 10.1016/S0145-2126(97)00074-X. [DOI] [PubMed] [Google Scholar]
- 17.Stockschlader M. A., Schuening F. G., Graham T. C., Storb R. Transplantation of retrovirus-transduced canine keratinocytes expressing the beta-galactosidase gene. Gene Ther. 1994;1:317–322. [PubMed] [Google Scholar]
- 18.Carrio M., Romagosa A., Merced E., Mazo A., Nadal M., Gomez-Foix A. M., Fillat C. Enhanced pancreatic tumor regression by a combination of adenovirus and retrovirus-mediated delivery of the herpes simplex virus thymidine kinase gene. Gene Ther. 1999;6:547–553. doi: 10.1038/sj.gt.3300846. [DOI] [PubMed] [Google Scholar]
- 19.Howard B. D., Boenicke L., Schniewind B., Henne-Bruns D., Kalthoff H. Transduction of human pancreatic tumor cells with vesicular stomatitis virus G-pseudotyped retroviral vectors containing a herpes simplex virus thymidine kinase mutant gene enhances bystander effects and sensitivity to ganciclovir. Cancer Gene Ther. 2000;7:927–938. doi: 10.1038/sj.cgt.7700180. [DOI] [PubMed] [Google Scholar]
- 20.Kato K., Yoshida J., Mizuno M., Sugita K., Emi N. Retroviral transfer of herpes simplex thymidine kinase gene into glioma cells causes targeting of gancyclovir cytotoxic effect. Neurol. Med. Chir. (Tokyo). 1994;34:339–344. doi: 10.2176/nmc.34.339. [DOI] [PubMed] [Google Scholar]
- 21.Ram Z., Walbridge S., Shawker T., Culver K. W., Blaese R. M., Oldfield E. H. The effect of thymidine kinase transduction and ganciclovir therapy on tumor vasculature and growth of 9 L gliomas in rats. J. Neurosurg. 1994;81:256–260. doi: 10.3171/jns.1994.81.2.0256. [DOI] [PubMed] [Google Scholar]
- 22.Rancourt C., Rogers B. E., Sosnowski B. A., Wang M., Piche A., Pierce G. F., Alvarez R. D., Siegal G. P., Douglas J. T., Curiel D. T. Basic fibroblast growth factor enhancement of adenovirus-mediated delivery of the herpes simplex virus thymidine kinase gene results in augmented therapeutic benefit in a murine model of ovarian cancer. Clin. Cancer Res. 1998;4:2455–2461. [PubMed] [Google Scholar]
- 23.Tong X. W., Agoulnik I., Blankenburg K., Contant C. F., Hasenburg A., Runnebaum L. B., Stickeler E., Kaplan A. L., Woo S. L., Kieback D. G. Human epithelial ovarian cancer xenotransplants into nude mice can be cured by adenovirus-mediated thymidine kinase gene therapy. Anticancer Res. 1997;17:811–813. [PubMed] [Google Scholar]
- 24.Wildner O., Blaese R. M., Morris J. C. Therapy of colon cancer with oncolytic adenovirus is enhanced by the addition of herpes simplex virus-thymidine kinase. Cancer Res. 1999;59:410–413. [PubMed] [Google Scholar]
- 25.Yee D., McGuire S. E., Brunner N., Kozelsky T. W., Allred D. C., Chen S. H., Woo S. L. Adenovirus-mediated gene transfer of herpes simplex virus thymidine kinase in an ascites model of human breast cancer. Hum. Gene Ther. 1996;7:1251–1257. doi: 10.1089/hum.1996.7.10-1251. [DOI] [PubMed] [Google Scholar]
- 26.Zhang R., DeGroot L. J. Gene therapy of established medullary thyroid carcinoma with herpes simplex viral thymidine kinase in a rat tumor model: relationship of bystander effect and antitumor efficacy. Thyroid. 2000;10:313–319. doi: 10.1089/thy.2000.10.313. [DOI] [PubMed] [Google Scholar]
- 27.Caron N. J., Torrente Y., Camirand G., Bujold M., Chapdelaine P., Leriche K., Bresolin N., Tremblay J. P. Intracellular delivery of a Tat-eGFP fusion protein into muscle cells. Mol. Ther. 2001;3:310–318. doi: 10.1006/mthe.2001.0279. [DOI] [PubMed] [Google Scholar]
- 28.Dietz G. P., Bahr M. Delivery of bioactive molecules into the cell: the Trojan horse approach. Mol. Cell Neurosci. 2004;27:85–131. doi: 10.1016/j.mcn.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 29.Guelen L., Paterson H., Gaken J., Meyers M., Farzaneh F., Tavassoli M. TAT-apoptin is efficiently delivered and induces apoptosis in cancer cells. Oncogene. 2004;23:1153–1165. doi: 10.1038/sj.onc.1207224. [DOI] [PubMed] [Google Scholar]
- 30.Mi Z., Mai J., Lu X., Robbins P. D. Characterization of a class of cationic peptides able to facilitate efficient protein transduction in vitro and in vivo. Mol. Ther. 2000;2:339–347. doi: 10.1006/mthe.2000.0137. [DOI] [PubMed] [Google Scholar]
- 31.Morris M. C., Depollier J., Mery J., Heitz F., Divita G. A peptide carrier for the delivery of biologically active proteins into mammalian cells. Nat. Biotechnol. 2001;19:1173–1176. doi: 10.1038/nbt1201-1173. [DOI] [PubMed] [Google Scholar]
- 32.Ribeiro M. M., Klein D., Pileggi A., Molano R. D., Fraker C., Ricordi C., Inverardi L., Pastori R. L. Heme oxygenase-1 fused to a TAT peptide transduces and protects pancreatic beta-cells. Biochem. Biophys. Res. Commun. 2003;305:876–881. doi: 10.1016/S0006-291X(03)00856-8. [DOI] [PubMed] [Google Scholar]
- 33.Shen H., Mai J. C., Qiu L., Cao S., Robbins P. D., Cheng T. Evaluation of peptide-mediated transduction in human CD34+ cells. Hum. Gene Ther. 2004;15:415–419. doi: 10.1089/104303404322959560. [DOI] [PubMed] [Google Scholar]
- 34.Wheeler D. S., Dunsmore K. E., Wong H. R. Intracellular delivery of HSP70 using HIV-1 Tat protein transduction domain. Biochem. Biophys. Res Commun. 2003;301:54–59. doi: 10.1016/S0006-291X(02)02986-8. [DOI] [PubMed] [Google Scholar]
- 35.Ziegler A., Nervi P., Durrenberger M., Seelig J. The cationic cell-penetrating peptide CPP (TAT) derived from the HIV-1 protein TAT is rapidly transported into living fibroblasts: optical, biophysical, and metabolic evidence. Biochemistry. 2005;44:138–148. doi: 10.1021/bi0491604. [DOI] [PubMed] [Google Scholar]
- 36.McNeil P. L., Murphy R. F., Lanni F., Taylor D. L. A method for incorporating macromolecules into adherent cells. J. Cell Biol. 1984;98:1556–1564. doi: 10.1083/jcb.98.4.1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Boes R., Obe G. Scrape-loading: a simple method to induce chromosomal aberrations with restriction enzymes in CHO cells. Mutat. Res. 1993;292:225–230. doi: 10.1016/0165-1161(93)90025-u. [DOI] [PubMed] [Google Scholar]
- 38.Marshall C. J., Leevers S. J. Mitogen-activated protein kinase activation by scrape loading of p21ras. Methods Enzymol. 1995;255:273–279. doi: 10.1016/S0076-6879(95)55030-5. [DOI] [PubMed] [Google Scholar]
- 39.Larson G., Pieterse A., Quick G., van der Bijl P., van Zyl J., Hawtrey A. Development of a reproducible procedure for plasmid DNA encapsulation by red blood cell ghosts. BioDrugs. 2004;18:189–198. doi: 10.2165/00063030-200418030-00005. [DOI] [PubMed] [Google Scholar]
- 40.Lee G., Delohery T. M., Ronai Z., Brandt-Rauf P. W., Pincus M. R., Murphy R. B., Weinstein I. B. A comparison of techniques for introducing macromolecules into living cells. Cytometry. 1993;14:265–270. doi: 10.1002/cyto.990140305. [DOI] [PubMed] [Google Scholar]
- 41.Mekada E., Yamaizumi M., Okada Y. An attempt to separate mononuclear cells fused with human red blood cell-ghosts from a cell mixture treated with HVJ (Sendai virus) using a fluorescence activated cell sorter (FACS II) J. Histochem. Cytochem. 1978;26:62–67. doi: 10.1177/26.1.202648. [DOI] [PubMed] [Google Scholar]
- 42.Ozawa K., Hosoi T., Tsao C. J., Urabe A., Uchida T., Takaku F. Microinjection of macromolecules into leukemic cells by cell fusion technique: search for intracellular growth-suppressive factors. Biochem. Biophys. Res. Commun. 1985;130:257–263. doi: 10.1016/0006-291X(85)90410-3. [DOI] [PubMed] [Google Scholar]
- 43.Cemazar M., Skrk J., Mitrovic B., Sersa G. Changed delivery of boron to tumours using electroporation for boron neutron capture therapy with BSH. Br. J. Radiol. 2000;73:195–200. doi: 10.1259/bjr.73.866.10884734. [DOI] [PubMed] [Google Scholar]
- 44.Gehl J. Electroporation: theory and methods, perspectives for drug delivery, gene therapy and research. Acta Physiol. Scand. 2003;177:437–447. doi: 10.1046/j.1365-201X.2003.01093.x. [DOI] [PubMed] [Google Scholar]
- 45.Jaroszeski M. J., Dang V., Pottinger C., Hickey J., Gilbert R., Heller R. Toxicity of anticancer agents mediated by electroporation in vitro. Anticancer Drugs. 2000;11:201–208. doi: 10.1097/00001813-200003000-00008. [DOI] [PubMed] [Google Scholar]
- 46.Neumann E., Kakorin S. Digression on membrane electroporation for drug and gene delivery. Technol. Cancer Res. Treat. 2002;1:329–340. doi: 10.1177/153303460200100503. [DOI] [PubMed] [Google Scholar]
- 47.Rabussay D. P., Nanda G. S., Goldfarb P. M. Enhancing the effectiveness of drug-based cancer therapy by electroporation (electropermeabilization) Technol. Cancer Res. Treat. 2002;1:71–82. doi: 10.1177/153303460200100110. [DOI] [PubMed] [Google Scholar]
- 48.Kjaergaard J., Hokland M., Nannmark U., Hokland P., Basse P. Infiltration patterns of short- and long-term cultured A-NK and T-LAK cells following adoptive immunotherapy. Scand. J. Immunol. 1998;47:532–540. doi: 10.1046/j.1365-3083.1998.00339.x. [DOI] [PubMed] [Google Scholar]
- 49.Kjaergaard J., Hokland M. E., Agger R., Skovbo A., Nannmark U., Basse P. H. Biodistribution and tumor localization of lymphokine-activated killer T cells following different routes of administration into tumor-bearing animals. Cancer Immunol. Immunother. 2000;48:550–560. doi: 10.1007/PL00006673. [DOI] [PMC free article] [PubMed] [Google Scholar]