Abstract
Selection of API phase is one of the first decision points in the formulation development process. Subsequent to phase selection, the focus shifts to the API physical properties such as particle size. Oftentimes, such properties are closely monitored throughout the drug development, as they can have a direct impact on the formulation bioperformance. The purpose of this mini-review was to describe the potential for application of absorption modeling in understanding the effect of API properties on bioavailability. Examples are provided to demonstrate how absorption modeling can be applied both early on to set the formulation strategy as well as during the development process to help with setting of specifications around the API. Limitations of the existing models and areas of possible expansion of such tools are also discussed.
Key words: absorption modeling, API properties, bioavailability, formulation, oral absorption
INTRODUCTION
In the recent years, there has been a steady increase in the number of low solubility compounds in drug development. It is estimated that up to 90% of new chemical entities would be categorized as BCS class II or IV compounds (1). When comparing this number to that of marketed drugs where more than 50% are classified as highly soluble (2–4), it becomes evident that poor compound solubility is a major hurdle for formulators today. Optimization of API chemical (e.g., salt formation) and physical (e.g., particle size reduction through milling) properties is oftentimes employed to improve oral bioavailability of insoluble compounds. However, with the increase in the number of compounds in development and the shortened timelines for formulation development, it is becoming increasingly apparent that empirical approaches typically employed to study API effect on bioavailability, such as in vivo studies in animal models, need to be complemented and potentially replaced by computational tools that allow for an efficient and more mechanistic link between API properties and bioperformance.
Absorption estimates such as the maximum absorbable dose calculation (5,6) or the absorption potential proposed by Dressman et al. (7) can be used to link the solubility of compound to the expected extent of oral absorption. Such estimates would appear suitable for early decisions on API phase selection as far as overall exposure is concerned. However, formulation development is frequently driven by specific pharmacokinetic and pharmacodynamic needs (e.g., rapid solubilization of drugs for fast onset of action), in which case more detailed models are needed to account for the rate of dissolution as well as the linkage between absorption and pharmacokinetic profile. Similarly, when trying to understand effect of API bulk properties on formulation bioperformance, models that can account for the effect of API on dissolution rate are needed to guide formulation efforts. While custom-built code can be used for such purposes as demonstrated in literature reports, the availability of more powerful commercial software packages greatly facilitates the use of absorption simulations during drug development. Such software packages include PK-Sim® from Bayer Technology Services, which is based on PB/PK modeling principles (8); the INTELLIPHARM® PKCR which combines simulation of drug dissolution and absorption and is based on the mixing tank model (5,9) linked to a one- or two-compartment pharmacokinetic model; the GastroPlus™ software from Simulations Plus, Inc. which allows for simulation of oral absorption using the advanced compartmental absorption and transit model (10) [an extension of the original CAT model (11) proposed by Yu] which is linked to systemic pharmacokinetics through standard compartmental or PB/PK modeling and the advanced dissolution absorption and metabolism model, also a compartmental absorption model, which is employed in the SIMCYP population-based pharmacokinetic modeling and simulation software (12). Utilizing such models and taking into account all the relevant biopharmaceutical properties of the compound of interest, one can assess in silico the potential advantage of various API properties in terms of improving oral bioavailability before proceeding to in vivo studies. Such computational models can be applied at different stages of formulation development process to both make formulation decisions early on prior to first-in-man studies as well as later in development to help with better understanding of observed pharmacokinetics, formulation optimization, or setting of API specification. Potential applications of absorption modeling with regards to API properties are shown in Table I.
Table I.
Potential Applications of Absorption Modeling to Assess Effect of API Properties on Bioavailability Throughout Clinical Formulation Development
| Pre-FIM formulation development efforts | Post-FIM formulation development efforts |
|---|---|
| Selection of API phase to enable FIM studies | Assess API particle size reduction or API phase change as means to improve bioperformance in response to human data (e.g., address food effect, enable fast T max, etc.) |
| Assess need for API particle size reduction for FIM studies | Assess impact of changes of API properties as a result of different synthesis or crystallization process across different API bulk lots |
| Set specifications around API particle size | |
| Assess impact of changes in API phase or polymorph during manufacturing, scale up or on stability | |
| Assess impact of API phase/API properties on bioperformance for special populations (e.g., achlorhydric patients) |
The goal of this review was to demonstrate the application of absorption modeling in driving formulation decisions by assessing the effect of API properties on bioavailability. We discuss some of the theoretical aspects of the models and provide examples of utilization of modeling to help with API phase selection or API bulk properties specification. Finally, we discuss some of the limitations of existing models.
THEORETICAL CONSIDERATIONS
Based on the Nernst–Brunner/Noyes–Whitney equation (13–15), the solid API dissolution rate is proportional to the surface area available for dissolution:
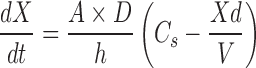 |
1 |
where dX/dt = dissolution rate, Xd = amount dissolved, A = particle surface area, D = diffusion coefficient, V = volume of fluid available for dissolution, Cs = saturation solubility, and h = effective boundary layer thickness.
From Eq. 1, it becomes apparent that the two main parameters affecting in vitro dissolution of drug compound is the solubility of the compound and the surface area. The solubility will depend on the API phase (crystalline vs. amorphous, salt vs. free form), while the surface area term directly links the dissolution rate to the bulk API properties. As described by Hintz and Johnson (9) who used a simplified form of the initial equations derived by Dressman and Fleisher (16), for a set number of monodispersed spherical API particles, the Nernst–Brunner/Noyes–Whitney equation can be rearranged to link the dissolution rate to the particle size (r0) and the density (ρ) of the API as follows:
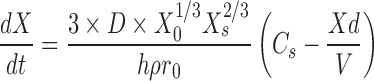 |
2 |
where X0 is the initial amount of drug, Xs is the amount of drug at time t, and ρ is the density of the API.
Equations 1 and 2 can be further modified to account for gastrointestinal transit of the particles which was the initial derivation by Dressman and Fleisher, changes in the effective boundary layer thickness over time (9), cylindrical geometry instead of spherical (17), or for the nonlinear concentration gradient across the diffusion layer of spherical particles as proposed by Wang and Flanagan model (18). Currently available commercial software packages apply the above discussed principles to simulate in vivo dissolution of drug compounds. Polydispersed powders can be simulated as a number of individual monodisperse fractions (9,17).
It is worth noting that solubility in the above models is generally considered a constant. However, as described by the Freudlich–Ostwald equation, an increase in compound saturation solubility is also expected at very small particle sizes. Specifically, the effect of particle size on solubility is given by (19):
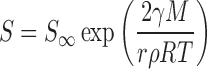 |
3 |
where S is the saturation solubility of the nano-sized API, S is saturation solubility of an infinitely large API crystal, γ is the crystal medium interfacial tension, M the compound molecular weight, r the particle radius, ρ the density, R is a gas constant, and T the temperature.
For a typical drug development candidate with a molecular weight of 500 and assuming ρ = 1 and a γ value of 0.015–0.020 N/m, Eq. 3 would predict an approximately 10–15% increase in solubility at a particle size of 100 nm compared to the solubility of large particles. However, a more significant increase in solubility appears to be possible in reality, as Muller et al. reported an increase of 50% in solubility of an insoluble antimicrobial compound when particle size was reduced from 2.4 μm to 800 or 300 nm (19). It is worth noting that the increase in solubility as a function of particle size does not appear to be accounted in the currently available software.
ASSESSMENT OF EFFECT OF API PHASE ON ORAL ABSORPTION
One of the earliest decisions in formulation development is the selection of the appropriate API form (for the purposes of this review, the terms API form and API phase will be used interchangeably referring to assessment of differences between different salts and the free form or between different polymorphs). For ionizable compounds, typically, this entails the selection between the free form or a salt of the compound. Salt formation has been one of the common approaches to improve the dissolution rate of insoluble compounds. The theoretical and practical considerations of salt behavior with respect to solubility and dissolution have been extensively reviewed in the literature (20,21). In general, salts are associated with faster dissolution rate and with achieving supersaturation. While supersaturation may be followed by precipitation, oftentimes, the duration of supersaturation can be sufficient to allow for higher absorption. It is also possible that the in vivo precipitate, due to high surface area, redissolves faster to provide the necessary absorption (22). Several examples of increased bioavailability of the salt compared to the free form are available. For non-ionizable compounds, development of the amorphous form of the drug may be desirable due to the increase in solubility compared to the crystalline API (23). The amorphous form in such cases may need to be stabilized, typically with the use of polymer, to avoid conversion to the crystalline form during storage
Similar questions as related to bioperformance of different API phases could also be encountered later in formulation development. Conversion between API phases may occur during a processing step or as a function of storage conditions (e.g., high humidity, high temperature). In such cases, it is of interest to understand the differences in in vivo behavior of the different forms to ensure the product maintains its intended pharmacokinetic profile.
While capturing all the aspects of salt or amorphous API dissolution may not be possible with the existing absorption models, absorption modeling can be useful to assess the effect of API phase on oral absorption after inputting appropriate in vitro experimental data. Two case studies using the GastroPlus™ software are presented to illustrate this approach.
Case Study 1
Compound A is a weakly basic BCS class II compound (Caco-2 permeability 78.6 × 10−6 cm/s) with a pKa value of 2.1. This compound exhibits low solubility in aqueous buffers in the physiological pH range (~4 μg/mL). Dissolution experiments in biorelevant media were conducted to assess in vivo solubility of the API following administration of solid dosage forms. In FaSSIF, the neutral form of the API achieved a concentration of 4.6 μg/mL, barely above the equilibrium solubility of the compound in aqueous buffers. The hydrochloride salt on the other hand resulted in significant supersaturation, achieving concentration of 57 μg/mL in SGF and 47 μg/mL in FaSSIF that was maintained for several hours.
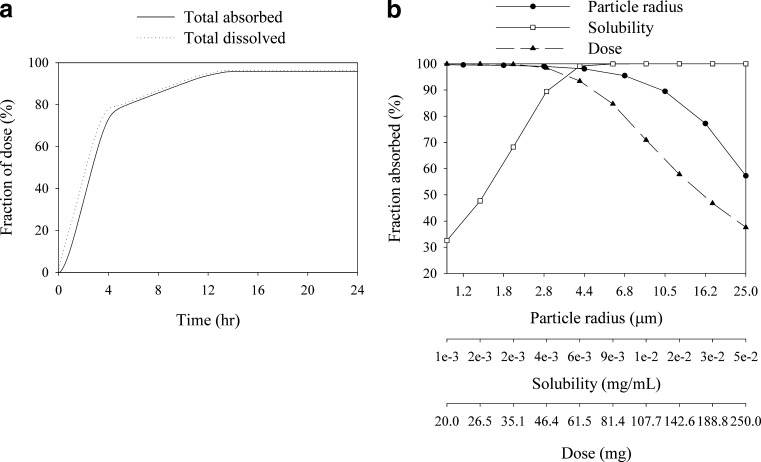
In order to assess the applicability of absorption modeling in helping with selection of solid formulations with the two API forms, absorption simulations were conducted using GastroPlus™ software (Simulations Plus, Inc). The Caco-2 permeability was converted to human permeability using an internally developed correlation equation. The kinetic solubility values from the dissolution experiments were used as input in the models. For salt simulation, as a worst case scenario, it was assumed that the salt advantage will be only limited to increased solubility in the stomach. Precipitation time was set to 3 h based on the observed supersaturation in vitro. Colonic solubility was set equal to the equilibrium solubility in buffer. Particle size was 10 μm (d50) with a density of 0.44 g/cm3. Diffusion layer thickness was set equal to the particle radius. Single administration simulations as well as a parameter sensitivity analysis for solubility and particle size were conducted for a dose of 50 mg which represented the upper end of anticipated efficacious dose.
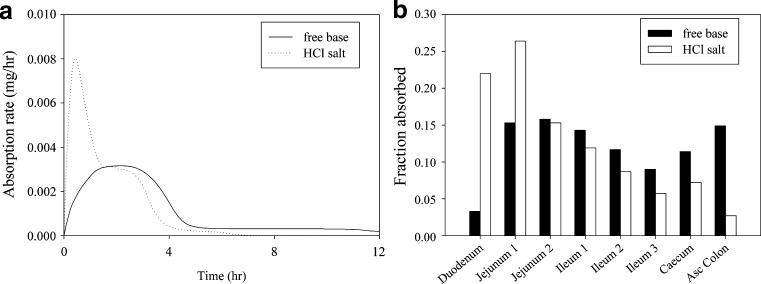
The results are shown in Fig. 1. At the 50-mg dose, despite poor solubility, the free base is expected to result in almost complete absorption (95.8% predicted) as the high permeability of the compound is expected to drive in vivo dissolution (Fig. 1a). However, it is evident from the sensitivity analysis that exposure will be sensitive to particle size and bioavailability is expected decrease as dose increases (Fig. 1b). At higher doses, solubility would also become a limiting factor (simulation outcome not shown). Figure 2 compares the anticipated absorption rates for the salt versus the free base and the regional distribution of absorption for the 50-mg dose. The salt provides a significantly faster absorption rate with more than 60% of the drug being absorbed in the duodenum and jejunum, while for the free base, significant absorption is predicted in the lower part of the intestine (25% absorption in ceacum and colon predicted). Given the uncertainties around lower gut absorption for insoluble compounds (since lumenal water volumes are limited), the faster absorption for the salt is also expected to result in improved variability compared to the free base. The results of the simulations are also compared against a pharmacokinetic study in beagle dogs at a 0.7-mg/kg dose. The salt formulation resulted in a moderate increase in AUC (1.00 ± 0.162 vs. 0.767 ± 0.174 μg h/mL). This moderate difference in AUC in the dog model may be associated with the shorter small intestinal transit time in the dog relative to humans, which could lead to a lower exposure for the free base formulation due to incomplete absorption through the large bowel. In line with the results of the simulations, a more pronounced effect was observed for Cmax (204 ± 38.2 vs. 110 ± 20.0 ng/mL) and Tmax (0.5 vs. 2.0 h).
Fig. 1.
Dissolution and absorption curves and parameter sensitivity analysis for a 50-mg dose of BCS class II compound A
Fig. 2.
Comparison of predicted absorption rates (a) and regional distribution of absorption (b) for the free base and the salt of compound A
While for compound A form selection was facilitated by in vivo data in a preclinical animal model, the absorption simulations provided a more mechanistic view of the absorption process of the two forms and allowed for identification of key parameters (such as the initial dissolution rate) that would need to be monitored to ensure optimal bioperformance. Based on the example of compound A, it becomes evident that absorption modeling can help not only with assessing API form impact on overall exposures but also with identifying potential pharmacokinetic advantages. At lower doses, for a compound with extended absorption and for which total exposure is determinant of the pharmacodynamic effect, a salt may not necessarily offer an exposure advantage. However for compounds where fast onset of action is desirable, the initial dissolution rate of the salt may be advantageous.
Case Study 2
Compound B is a weak base (pKa 3.7) and exhibits pH-dependent solubility with solubility of 0.9 mg/mL at pH 2 and 0.018 mg/mL in FaSSIF. Following initial studies with the free base of compound B, a new polymorph of the free base was identified that exhibited significantly lower solubility in the intestinal pH region (solubility in FaSSIF measured at 0.010 mg/mL). As part of assessing the risk of reduced bioavailability of formulations with the new polymorph, absorption simulations were conducted in GastroPlus™ for formulations of micronized API (12-μm diameter) using a monodisperse model with diffusion layer thickness equal to the particle radius. Simulations were conducted assuming normal fasting stomach pH (default software value) as well as at a stomach pH of 3 and 6 to capture impact of less acidic stomach conditions (either due to normal stomach pH variability or in special populations such as achlorhydric patients) on the pharmacokinetics of the two polymorphs. A human permeability estimate of 5 × 10−4 cm/s was used based on observed early clinical data that suggested rapid absorption. Large bowel absorption was minimized (ASF values set at 1/3 of default) due to anticipated lower colonic bioavailability based on a regional absorption study in dogs. The outcome of the simulations for a 100-mg dose is shown in Fig. 3.
Fig. 3.
Model predicted dissolution (a) and absorption (b) for the initial formulation and the new polymorph of compound B under different stomach pH values
Given the high solubility of compound B at low pH, in normal fasted stomach conditions, fast and complete dissolution of either API form is expected. As indicated by the shape of the dissolution curve in Fig. 3a, following initial dissolution in the stomach, precipitation was predicted upon entry into the intestine. The form that will be generated following precipitation in vivo is not known. For simplicity in simulation setup, the simulations were conducted assuming that any solid generated from precipitation would redissolve based on the same solubility of the form dosed. The goal of the models was not to provide an accurate accounting of the absorption process but rather to provide an assessment of differences of the two forms in overall exposure. The simulated curves in Fig. 3 are believed to represent a worst case scenario for the new polymorph, as precipitation and re-dissolution should be similar in vivo following initial dissolution of either form. Based on the absorption modeling, comparable bioperformance of the two API forms would be anticipated under normal fasting conditions. Some differentiation of the two forms may appear at reduced stomach acidity. As shown in Fig. 3b, at a stomach pH of 6, approximately 20% difference in the fraction absorbed of the two formulations is predicted. The effect is anticipated to be lower if dose is reduced (data not shown). The results of the standard fasted state simulations were compared against the data obtained in a study in pentagastrin-treated dogs where comparable exposures were obtained for the two forms (AUC 42.2 ± 8.63 vs. 36.2 ± 2.73 μg hr/mL, n = 3, mean ± SD).
In the case of compound B, modeling data were used, in conjunction with a small animal study (only n = 3 was used), as a risk assessment tool that allowed for formulation development to proceed without going through major composition changes to accommodate for the lower solubility of the new polymorph. Furthermore, although no preclinical or human PK data under both normal and elevated gastric pH conditions have been generated to date to confirm the modeling results in this case, such simulation approach can be used in general as a risk assessment tool during the development of the clinical formulation, especially if co-administration of a proton pump inhibitor or a H2 antagonist is common in the targeted patient population.
ASSESSMENT OF EFFECT OF API BULK PROPERTIES ON ORAL ABSORPTION
As discussed previously, API bulk properties (particle size, density, surface area) are directly related to the dissolution rate of the API and thus potentially impacting the bioperformance. Efforts to improve bioavailability can take place either early on prior to human studies or as a response to a need for improved bioavailability later on. As a result, reduction of particle size has been one of the most common ways to improve oral bioavailability of insoluble compounds. Nevertheless, there are additional benefits gained from understanding the effect of particle size besides improvement of bioperformance. Requirement of small particle size typically means additional steps during the API production (e.g., milling), resulting in an increase in the complexity of the process and the associated resources. Early assessment of the potential benefits, or lack of, allows for the appropriate allocation of these resources. In addition, it is not uncommon for different API bulk lots to be used to support various stages of clinical development. Understanding the sensitivity of bioavailability to API bulk properties can help assess bridging risks and, if necessary, take the appropriate measures to mitigate those risks. Thus, control of API properties essentially becomes an important aspect of quality by design (QbD) approaches to formulation design where the appropriate specifications around API properties are set to ensure optimal bioperformance.
Some limited reports in the literature are available to demonstrate the utility of absorption modeling on understanding the effect of particle size on bioavailability. Using a custom model in ADAPT to modify the CAT model and take into account drug particle dissolution, Yu (24) demonstrated good agreement between the predicted and observed fraction absorbed for digoxin, griseofulvin, and panadiplon (Table II). Dannenfelser et al. (25) demonstrated through absorption modeling that while particle size reduction would have a significant effect in improving exposure for the BCS class IV compound LAB687, low bioavailability would be observed even with the micronized drug. The authors further demonstrated in a dog study that a solid dispersion and a co-solvent/surfactant solution formulation resulted in tenfold higher bioavailability compared to micronized drug. Kuentz et al. (26) utilized absorption simulations in GastroPlusTM to demonstrate the lack of particle size effect on the absorption of the poorly soluble compound R1315. Tubic-Grodzanis et al. (27) used absorption modeling to highlight the potential for favorable absorption of miconazole at particle sizes below 25 μm. More recently, Brandl et al. (28) reported the use of GastroPlus™ to predict the effect of particle size across doses for the nucleoside pro-drug R1626. However, with the exception of the publication by Yu, none of the other published reports attempted to quantitatively validate the predicted particle size reduction effect. Here, we present three case studies with internal development candidate compounds using the GastroPlus™ software to demonstrate the potential application of absorption modeling based on API bulk properties to facilitate formulation development.
Table II.
Effect of Particle Size on Absorption for Digoxin, Griseofulvin, and Panadiplon as Predicted by the Integrated Absorption Model Proposed by Yu (23)
| Digoxin | ||||
| Simulated dose (mg) | 0.5 | 0.5 | 0.5 | |
| Particle size (μm) | 7 | 13 | 98 | |
| Predicted Fa (%) | 100 | 90 | 20 | |
| Observed Fb (%) | 97 | 78 | 34 | |
| Griseofulvin | ||||
| Simulated dose (mg) | 500 | 250 | 250 | |
| Particle size (μm) | 5 | 30 | 4 | |
| Predicted Fa (%) | 38 | 35 | 58 | |
| Observed Fb (%) | 45 | –a | ||
| Panadiplon | ||||
| Simulated dose (mg) | 10 | 10 | 10 | 10 |
| Particle size (μm) | 8.8 | 25 | 39 | 100 |
| Predicted Fa (%) | 89 | 79 | 73 | 45 |
| Observed Fb (%) | 81 | 74 | 71 | 24 |
aA twofold increase in griseofulvin absorption through micronization was reported
Case Study 3
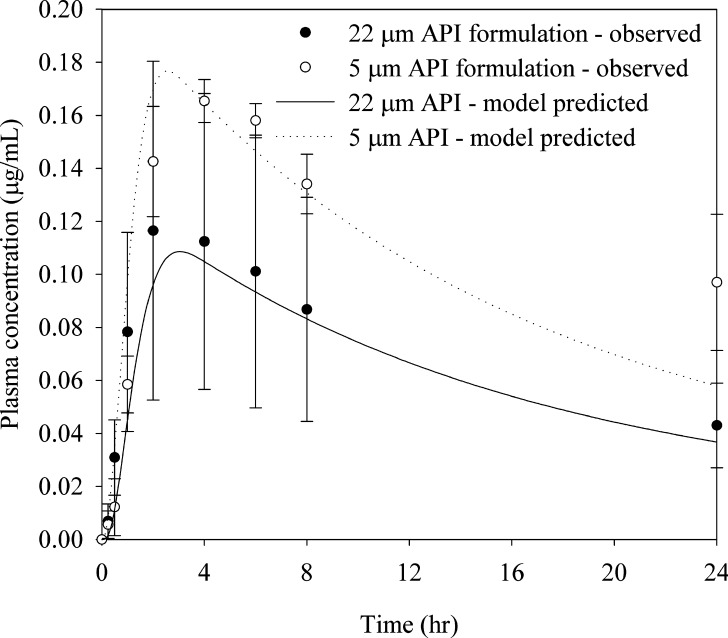
Compound C is a BCS class II compound with very low aqueous solubility (<0.5 μg/mL). Solubility in FaSSIF was measured at 6 μg/mL. While initial clinical studies for compound C were supported with a liquid-filled capsule formulation in a lipid vehicle to maximize bioavailability, subsequent reduction in dose allowed for the development of simple solid formulations. Given the BCS II classification, we were interested in assessing the sensitivity of bioperformance with respect to API particle size. A preliminary dog study was conducted to compare the exposure from milled API (5-μm diameter) to that from unmilled compound (22-μm diameter) at a dose of 3 mg (0.3 mg/kg). As seen in Fig. 4, approximately 40% difference in exposures was observed between the two treatments. Due to the uncertainty of the final clinical dose and the need to better understand the general effect of particle size on exposure, absorption simulations were conducted to probe the sensitivity of formulation performance in humans to API particle size. The analysis was conducted in GastroPlus™. Human permeability of 2.03 × 10−4 cm/s was estimated based on rat intestinal perfusion data using a correlation established between rat and human permeabilities for a set of 15 reference compounds. Colonic absorption was not accounted in the models due to the anticipated poor bioavailability of the compound in the lower bowel based on the observed pharmacokinetics in dogs and the poor compound solubility in aqueous buffers. Prior to conducting the human simulations, simulations using the dog absorption model with similar assumption accurately predicted the observed plasma profiles, validating the use of this approach (Fig. 4).
Fig. 4.
Effect of particle size on exposures for compound C following administration of a 3-mg tablet formulation to male beagle dogs (mean ± SD, n = 3). Absorption model predicted exposures are also shown
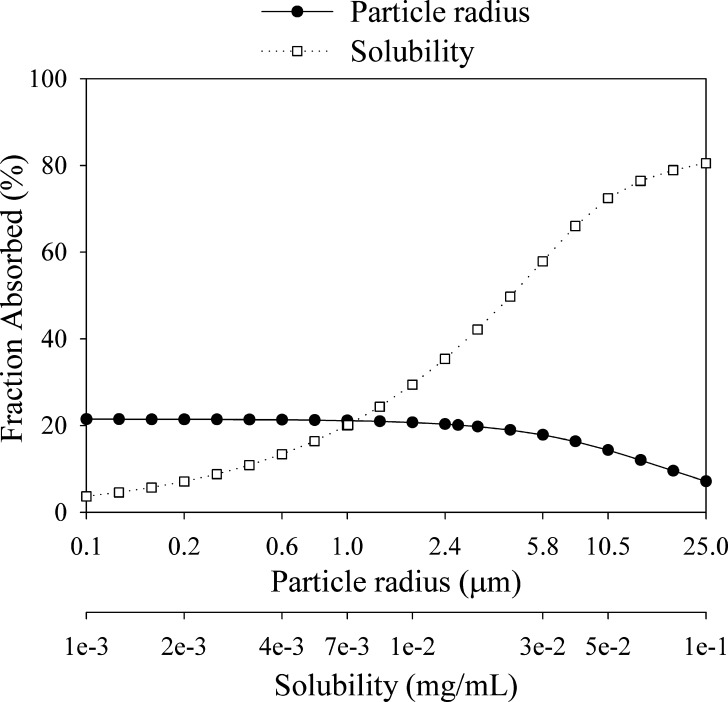
The results from a sensitivity analysis looking at the particle size effect across different doses are shown in Fig. 5. At lower doses and small particle size, almost complete absorption (>85%) is predicted. However, at the dose range of clinical interest, absorption of compound C is largely dissolution-rate-limited (although solubility limitation starts to become apparent at the 20-mg dose), and thus, micronization of the API can help optimize bioavailability of the conventional solid formulations. Dissolution limitation is apparent even at a dose of 5 mg, with particles above 25 μm projected to result in more than 15% decrease in bioavailability relative to particle sizes below 10 μm.
Fig. 5.
Fraction absorbed for compound C as a function of dose and particle size
Based on case study 3, it is evident how absorption modeling can help with quickly assessing multiple scenarios around dose/particle size combinations that would be impractical to assess through preclinical or clinical studies. Specifically for compound C, while the initial exploration of the particle size vs. exposure question was conducted through a quick animal study (n = 3 at a single dose), modeling was utilized to mechanistically assess the clinical relevance of the observation in the animal model and to help with particle size specifications. Such modeling approaches can prove invaluable during QbD efforts as related to API properties, significantly reducing the needs for multiple experimental measurements on other bioavailability surrogate approaches (e.g., dissolution or animal studies).
Case Study 4
Aprepitant (MK-0869), a potent substance P antagonist, is the active ingredient of EMEND®. Aprepitant has poor aqueous solubility (3–7 μg/mL) and moderate permeability (Caco-2 permeability reported at 7.85 × 10−6 cm/s; 29). Early clinical tablet formulations of aprepitant showed significant food effect on the rate and extent of absorption. To improve fasted state exposures, a nanoparticle (NanoCrystal®) formulation was developed which eliminated the food effect and is used in the final marketed product. Due to the role that particle size reduction played in the formulation development of aprepitant, we retrospectively assessed the applicability of absorption modeling in predicting the relationship between particle size and bioavailability. Furthermore, we were interested in comparing the predictions of the models to the observed pharmacokinetics in a dog study for four suspensions with particle sizes ranging from 0.1 to 5 μm (29).
All simulations were conducted in GastroPlus™. The FaSSIF solubility of 6 μg/mL was used for the simulations. A monodisperse model was used due to the tight particle size distribution of the API lots used in the study. The diffusion layer thickness was set equal to the radius of the particles for particle radius <30 μm. Colonic absorption was not accounted for in the model, as it was previously shown to be minimal (29). Pharmacokinetic parameters were obtained following IV administration. While there are differences in the formulation of the nanosuspension [50 mg/mL aprepitant in 4% hydroxypropyl cellulose, 0.08% sodium dodecyl sulfate (SDS), and 20% sucrose in water] compared to the larger APIs (suspension in 0.5% methylcellulose with 0.02% SDS in water at a concentration of 0.8 mg/mL), the concentration of surfactants following dilution with the dosing water volumes (total water volume of 5 mL/kg for all formulations) are not expected to significantly alter solubility of aprepitant in the bulk stomach environment. Thus, the same solubility values were used during all simulations, with the only parameter modified being the particle size of the suspension.
As seen in Fig. 6, aprepitant bioavailability in human is expected to exhibit a dependency on solubility and particle size. The solubility dependence prediction is in line with the positive food effect observed for aprepitant in the clinic and in dog studies (30). In the fasted state, the model also clearly suggests a strong influence of particle size on bioavailaibility, with micronized API predicted to provide more than twofold increase compared to non-micronized material. However, it is evident that the model predicts a plateau of exposure once micronization is utilized, which is not consistent with the observed enhanced performance of the nanosuspension.
Fig. 6.
Parameter sensitivity analysis for human fraction absorbed for aprepitant
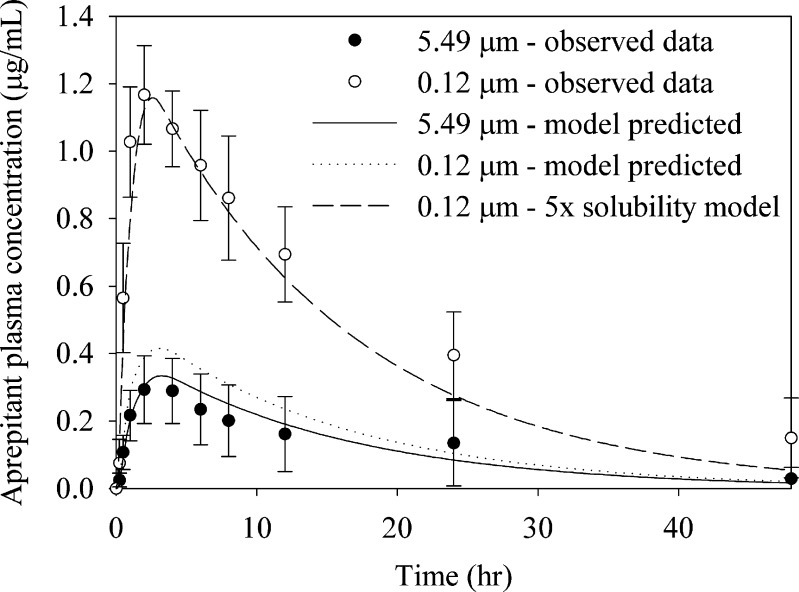
To further assess the ability of the model to quantitatively predict exposures in the lower particle size region, simulations using the dog physiology model were conducted. The dog physiology was used to capture faster absorption of the compound in dogs. Since faster absorption would further drive aprepitant dissolution, the curves describing the relationship between particle size and absorption, as shown in Fig. 6, may not completely overlap in dogs and humans. The results from those simulations and the comparison to experimental data are shown in Table III. While the trend towards particle size reduction is obvious, the simulations show significant deviation from the observed data for particle sizes below 1 μm. Specifically, while the model predicts a slight increase in exposure moving from 5.49 to 1.9 μm (19% increase predicted compared to 28% increase observed in vivo), no further increase in exposure is predicted for submicron particles, which is contrary to the in vivo data. The nanosuspension exposures could only be accurately simulated assuming a much higher solubility for the nanosuspension (Fig. 7). Thus, in the case of aprepitant, while absorption modeling correctly points to the benefit of particle size reduction in improving bioavailability, it fails to quantitatively predict the performance of the final marketed formulation technology (nanosuspension). We have noticed similar difficulties in predicting exposures of nanosuspensions with other compounds. Such limitations in the absorption modeling will be discussed in the end of this report.
Table III.
Predicted vs. Observed Aprepitant Exposure for a 2-mg/kg Suspension Administration in Dogs
| Particle size (μm) | Predicted AUC (μg h/mL) | Observed AUC (μg h/mL) |
|---|---|---|
| 5.49 | 5.85 | 5.88 ± 1.86 |
| 1.85 | 6.94 | 7.54 ± 1.86 |
| 0.48 | 7.22 | 10.5 ± 2.70 |
| 0.12 | 7.26 | 25.3 ± 3.29 |
Predictions were made across four different particle sizes
Fig. 7.
Observed and model predicted exposures for aprepitant following suspension administration to male beagle dogs at a dose of 2 mg/kg
Case Study 5
Compound D exhibits pH-dependent solubility with a solubility of 13 μg/mL at pH 1.2, less than 7 μg/mL between pH 2.5 and 6, and 120 μg/mL at pH 7.2. Solubility in FaSSIF was 81 μg/mL. It was classified as BCS class IV based on the low Caco-2 (<1 × 10−6 cm/s) and rat jejunum (0.17 × 10−4 cm/s) permeability values. Despite the low solubility, a conventional solid formulation of the free acid was developed for initial clinical studies as stability issues were identified with the attempted salts. Initial studies were conducted with an API lot of high surface area (17.4 m2/g). However, API from a second delivery to support study extensions exhibited a lower surface area of 3 m2/g, raising the question of whether comparable bioperformance would be anticipated. Below, we present the strategy of trying to answer this question through absorption modeling.
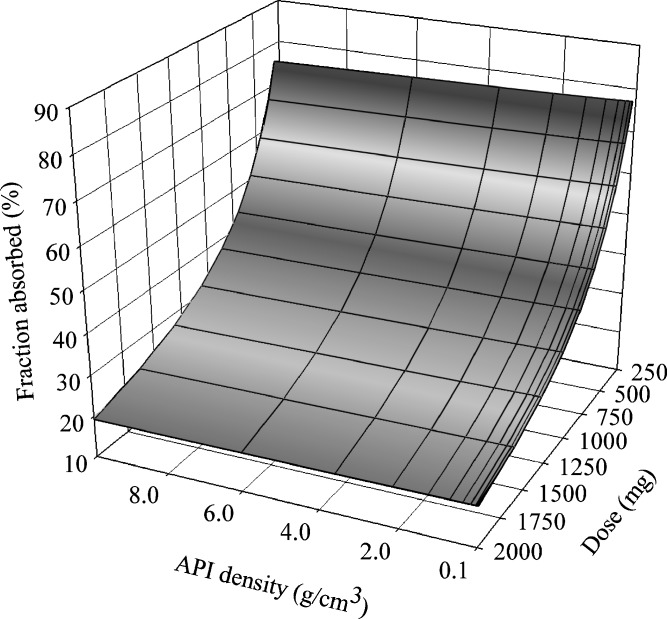
While API surface area (SA) cannot be directly input in the GastroPlus™ software, theoretical spherical particles were simulated that corresponded to the measured surface values to approximate the dissolution of the API. Since the lowest allowed density value in GastroPlus™ is 0.1 g/cm3, this value was used as the base case for the high SA API. The observed SA values of the two lots, under these assumptions, correspond to dissolution numbers (Dn) of 24 and 4.2, respectively (380 and 66 if looking only at the FaSSIF solubility), indicating that the dissolution rate of either lot is not expected to be the rate-limiting factor to bioavailability. Another contributing factor in the simulations was the level of confidence in human permeability prediction. While the human predicted permeability based on either the standard Caco-2 assay at pH 7 or rat permeability data is low, transporter involvement in intestinal absorption was suspected based on pH dependency of the Caco-2 permeability value. Thus, given the uncertainty around permeability prediction, multiple simulations were run assuming different permeability values. A representative graph at a human permeability of 2 × 10−4 cm/s (high permeability compound) looking at effect of dose and API density on exposure is shown in Fig. 8. As indicated by the favorable values of the dissolution number mentioned earlier, dissolution rate is not the rate-limiting factor to absorption, rather solubility is, as reflected in Fig. 8 by the dependence of the fraction absorbed on dose. Even if higher permeability values are assumed as the worst case scenario (as faster permeation will help increase bioavailability of the faster dissolving formulations), similar curves are generated. The results from the models are in agreement with an in vivo study conducted in dogs where following dosing of a 250-mg capsule, no significant differences in the extent or rate of exposure were observed between formulations containing API from two different lots. The predicted solubility limitation also agrees with the moderate improvement in exposure (~60%) observed with a salt formulation (data not shown).
Fig. 8.
3D parameter sensitivity analysis for compound D assessing the effect of dose and API density on fraction absorbed
Similar to other cases studies presented in this manuscript, absorption modeling served as a risk assessment tool in assessing the impact of compound D API surface area change on bioavailability and increased the confidence in the preclinical animal model data, thus facilitating the decision to use the new API lot in its existing form for future studies.
It should be noted that while for the case studies presented in this manuscript some in vivo data in an animal model that allowed for verification of simulation data were sought or were previously available, such verification may not be always needed. Depending on the quantity of experimental in vitro data and the level of confidence on translating the in vitro measurements to human parameters, simulations could be used as the primary tool in assessing potential bioperformance issues as related to API bulk properties and in vivo studies can be reserved only to test a specific hypothesis of interest that stems out from the simulation results.
LIMITATIONS OF EXISTING MODELS AND AREAS FOR FUTURE IMPROVEMENTS
The case studies described above clearly demonstrated the utility of absorption modeling in understanding the relationship between API properties and pharmacokinetics. In the examples shown in the current manuscript as well as other examples in the literature, it has been demonstrated how oral bioavailability can be successfully linked to either the form of the API or the bulk properties. However, it is worth noting that gaps still exist in predicting effects of API properties, and consequently, such limitations may represent hurdles for successful application of absorption modeling in assessment of certain formulations or for a more quantitative assessment of clinical formulation bioperformance.
Currently available models assume that the API from oral dosage forms, upon contact with the GI fluids, behaves as a uniformly dispersed powder that dissolves based on the Noyes–Whitney equation. The majority of the available software further assume a spherical form of the particles (after version 5.2, GastroPlus™ also supports cylindrical API). While these approximations would appear adequate to study directional effect of changes in API properties (and in some cases even get good agreement to experimental data), it does not truly reflect the behavior of solid dosage forms. Incorporation in the simulations of additional factors describing the behavior of the formulation (e.g., interplay between disintegration and dissolution, erosion, etc) would appear as the next step in improving the prediction of the current models. Also taking into account the effect of surfactant solubilization on diffusion and using effective diffusion coefficients in the dissolution models, as suggested by Okazaki et al. (31), is another possible improvement of the existing models. It should be noted that in some cases, this limitation can be circumvented by direct input of experimental measured in vitro dissolution/release profiles.
A second major limitation of existing models appears to be the inability to accurately predict the in vivo dissolution of nanosuspensions, especially below the 200-nm range, solely based on API bulk properties. The utilization of nanosuspensions to improve bioavailability of insoluble compounds is well documented in the literature (32). As discussed above in the case study of aprepitant, significant increases in bioavailability are oftentimes achieved with nanosuspensions. However, use of existing models appears to fail to accurately predict the solubilization processes that lead to the improved bioperformance of nanoparticles. As demonstrated above in the case of aprepitant, the decrease in particle size was not sufficient to accurately model data in the submicron region. We have made similar observations with other internal drug development candidates as well as with nanosuspension data published in the literature. The discrepancy remains even if the theoretical effect on solubility is accounted for (as discussed, previously existing software does not take this effect under consideration). While the use of in vitro dissolution/solubility measurements can still provide the means to measure such systems, there is a clear need for better models in this area to enable better in silico projections for such systems.
Finally, prediction of in vivo precipitation for a given compound remains challenging. Precipitation rates are critical when it comes to assessing bioavailability of salts or for weak bases. Until the precipitation process can be adequately modeled, generation of the appropriate in vitro data to try to approximate the in vivo behavior and use as input in the software would appear critical.
CONCLUSIONS
Questions around effect of API phase and API bulk properties on bioavailability are a common occurrence during the development of formulations for BCS class II and IV compounds. Such questions arise both early in the development process where goal is to optimize drug bioavailability as well as later in development where maintenance of bioperformance is critical. Absorption modeling offers formulators the advantage to quickly understand the interactions of API properties and bioavailability. Multiple scenarios can be explored in a much shorter timeframe than what would be required for testing in preclinical animal models or in the clinic. Efforts can focus on the lead options that emerge from the models and help justify the appropriate resource allocation (e.g., milling of the API). While gaps still exist in the ability of the models to fully mimic in vivo dissolution processes, it is clear that absorption modeling can be an invaluable tool that allows for timely and cost-effective strategic decisions and speeds up the formulation development process.
References
- 1.Benet L. Z., Wu C. Y. Using a biopharmaceutics drug disposition classification system to predict bioavailability and elimination characteristics of new molecular entities. Somerset, NJ: NJDMDG; 2006. [Google Scholar]
- 2.Lindenberg M., Kopp S., Dressman J. B. Classification of orally administered drugs on the World Health Organization model list of essential medicines according to the biopharmaceutics classification system. Eur. J. Pharm. Biopharm. 2004;58:265–278. doi: 10.1016/j.ejpb.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 3.Kasim N. A., Whitehouse M., Ramachandran C., Bermejo M., Lennernas H., Hussain A. S., Junginger H. E., Stavchansky S. A., Midha K. K., Shah V. P., Amidon G. L. Molecular properties of WHO essential drugs and provisional biopharmaceutical classification. Mol. Pharm. 2004;1:85–96. doi: 10.1021/mp034006h. [DOI] [PubMed] [Google Scholar]
- 4.Takagi T., Ramachandran C., Bermejo M., Yamashita S., Yu L. X., Amidon G. L. A provisional biopharmaceutical classification of the top 200 oral drug products in the United States, Great Britain, Spain, and Japan. Mol Pharm. 2006;3:631–643. doi: 10.1021/mp0600182. [DOI] [PubMed] [Google Scholar]
- 5.Johnson K. C., Swindell A. C. Guidance in the setting of drug particle size specifications to minimize variability in absorption. Pharm. Res. 1996;13:1795–1798. doi: 10.1023/A:1016068705255. [DOI] [PubMed] [Google Scholar]
- 6.Sun D., Yu L. X., Hussain M. A., Wall D. A., Smith R. L., Amidon G. L. In vitro testing of drug absorption for drug ‘developability’ assessment: Forming an interface between in vitro preclinical data and clinical outcome. Curr. Opin. Drug Discov. Dev. 2004;7:75–85. [PubMed] [Google Scholar]
- 7.Dressman J. B., Amidon G. L., Fleisher D. Absorption potential: Estimating the fraction absorbed for orally administered compounds. J. Pharm. Sci. 1985;74:588–589. doi: 10.1002/jps.2600740523. [DOI] [PubMed] [Google Scholar]
- 8.Willmann S., Lipper J., Sevestre M., Solodenko J., Fois F., Schmitt W. PK-Sim: A physiologically based pharmacokinetic ‘whole-body’ model. Biosilico. 2003;1:121–124. doi: 10.1016/S1478-5382(03)02342-4. [DOI] [Google Scholar]
- 9.Hintz R. J., Johnson K. C. The effect of particle size distribution on dissolution rate and oral absorption. Int. J. Pharm. 1989;51:9–17. doi: 10.1016/0378-5173(89)90069-0. [DOI] [Google Scholar]
- 10.Agoram B., Woltosz W. S., Bolger M. B. Predicting the impact of physiological and biochemical processes on oral drug bioavailability. Adv. Drug Deliv. Rev. 2001;50(Suppl 1):S41–S67. doi: 10.1016/S0169-409X(01)00179-X. [DOI] [PubMed] [Google Scholar]
- 11.Yu L. X., Lipka E., Crison J. R., Amidon G. L. Transport approaches to the biopharmaceutical design of oral drug delivery systems: Prediction of intestinal absorption. Adv. Drug Deliv. Rev. 1996;19:359–376. doi: 10.1016/0169-409X(96)00009-9. [DOI] [PubMed] [Google Scholar]
- 12.Simcyp. Absorption. Research & development—Simcyp science. http://www.simcyp.com/ResearchDevelopment/SimcypScience/Absorption/ (accessed on July 13th).
- 13.Noyes A., Whitney W. The rate of solution of solid substances in their own solutions. J. Am. Chem. Soc. 1897;19:930–934. doi: 10.1021/ja02086a003. [DOI] [Google Scholar]
- 14.Nernst W. Theorie der Reaktionsgeschwindigkeit in heterogenen Systemen. Z. Phys. Chem. 1904;47:52–55. [Google Scholar]
- 15.Brunner E. Reaktionsgeschwindigkeit in heterogenen Systemen. Z. Phys. Chem. 1904;47:56–102. [Google Scholar]
- 16.Dressman J. B., Fleisher D. Mixing-tank model for predicting dissolution rate control or oral absorption. J. Pharm. Sci. 1986;75:109–116. doi: 10.1002/jps.2600750202. [DOI] [PubMed] [Google Scholar]
- 17.Lu A. T., Frisella M. E., Johnson K. C. Dissolution modeling: Factors affecting the dissolution rates of polydisperse powders. Pharm. Res. 1993;10:1308–1314. doi: 10.1023/A:1018917729477. [DOI] [PubMed] [Google Scholar]
- 18.Wang J., Flanagan D. R. General solution for diffusion-controlled dissolution of spherical particles. 1. Theory. J. Pharm. Sci. 1999;88:731–738. doi: 10.1021/js980236p. [DOI] [PubMed] [Google Scholar]
- 19.Muller R. H., Peters K. Nanosuspensions for the formulation of poorly soluble drugs I. Preparation by a size-reduction technique. Int. J. Pharm. 1998;160:229–237. doi: 10.1016/S0378-5173(97)00311-6. [DOI] [Google Scholar]
- 20.Serajuddin A. T. M. Salt formation to improve drug solubility. Adv. Drug Deliv. Rev. 2007;59:603–616. doi: 10.1016/j.addr.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 21.Pudipeddi M., Serajuddin A. T. M., Grant D. J. W., Stahl P. H. Solubility and dissolution of weak acids, bases and salts. In: Stahl P. H., Wermuth C. G., editors. Handbook of Pharmaceutical Salts: Properties, Selection, and Use. Weinheim, Germany: Wiley-VCH; 2002. pp. 19–40. [Google Scholar]
- 22.Stahl P. H., Nakano M. Pharmaceutical aspects of the drug salt form. In: Stahl P. H., Wermuth C. G., editors. Handbook of Pharmaceutical Salts: Properties, Selection, and Use. Weinheim, Germany: Wiley-VCH; 2002. pp. 83–116. [Google Scholar]
- 23.Leuner C., Dressman J. Improving drug solubility for oral delivery using solid dispersions. Eur. J. Pharm. Biopharm. 2000;50:47–60. doi: 10.1016/S0939-6411(00)00076-X. [DOI] [PubMed] [Google Scholar]
- 24.Yu L. X. An integrated model for determining causes of poor oral drug absorption. Pharm. Res. 1999;16:1883–1887. doi: 10.1023/A:1018911728161. [DOI] [PubMed] [Google Scholar]
- 25.Dannenfelser R. M., He H., Joshi Y., Bateman S., Serajuddin A. T. Development of clinical dosage forms for a poorly water soluble drug I: Application of polyethylene glycol-polysorbate 80 solid dispersion carrier system. J. Pharm. Sci. 2004;93:1165–1175. doi: 10.1002/jps.20044. [DOI] [PubMed] [Google Scholar]
- 26.Kuentz M., Nick S., Parrott N., Rothlisberger D. A strategy for preclinical formulation development using GastroPlus as pharmacokinetic simulation tool and a statistical screening design applied to a dog study. Eur. J. Pharm. Sci. 2006;27:91–99. doi: 10.1016/j.ejps.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 27.Tubic-Grozdanis M., Bolger M. B., Langguth P. Application of gastrointestinal simulation for extensions for biowaivers of highly permeable compounds. AAPS J. 2008;10:213–226. doi: 10.1208/s12248-008-9023-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brandl M., Wu X., Holper M., Hong L., Jia Z., Birudaraj R., Reddy M., Alfredson T., Tran T., Larrabee S., Hadig X., Sarma K., Washington C., Hill G., Smith D. B. Physicochemical properties of the nucleoside prodrug r1626 leading to high oral bioavailability. Drug Dev. Ind. Pharm. 2008;34:683–691. doi: 10.1080/03639040701836636. [DOI] [PubMed] [Google Scholar]
- 29.Wu Y., Loper A., Landis E., Hettrick L., Novak L., Lynn K., Chen C., Thompson K., Higgins R., Batra U., Shelukar S., Kwei G., Storey D. The role of biopharmaceutics in the development of a clinical nanoparticle formulation of MK-0869: A Beagle dog model predicts improved bioavailability and diminished food effect on absorption in human. Int. J. Pharm. 2004;285:135–146. doi: 10.1016/j.ijpharm.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 30.F. Kesisoglou, Y. Wu, and A. Chin. Prediction of micronized aprepitant food effect through absorption modeling. The AAPS Journal.9:Abstract T3121 (2007).
- 31.Okazaki A., Mano T., Sugano K. Theoretical dissolution model of poly-disperse drug particles in biorelevant media. J. Pharm. Sci. 2008;97:1843–1852. doi: 10.1002/jps.21070. [DOI] [PubMed] [Google Scholar]
- 32.Kesisoglou F., Panmai S., Wu Y. Nanosizing-oral formulation development and biopharmaceutical evaluation. Adv. Drug Deliv. Rev. 2007;59:631–644. doi: 10.1016/j.addr.2007.05.003. [DOI] [PubMed] [Google Scholar]