Abstract
Model-based drug development (MBDD) has been recognized as a concept to improve the efficiency of drug development. The acceptance of MBDD from regulatory agencies, industry, and academia has been growing, yet today’s drug development practice is still distinctly distant from MBDD. This manuscript is aimed at clarifying the concept of MBDD and proposing practical approaches for implementing MBDD in the pharmaceutical industry. The following concepts are defined and distinguished: PK–PD modeling, exposure–response modeling, pharmacometrics, quantitative pharmacology, and MBDD. MBDD is viewed as a paradigm and a mindset in which models constitute the instruments and aims of drug development efforts. MBDD covers the whole spectrum of the drug development process instead of being limited to a certain type of modeling technique or application area. The implementation of MBDD requires pharmaceutical companies to foster innovation and make changes at three levels: (1) to establish mindsets that are willing to get acquainted with MBDD, (2) to align processes that are adaptive to the requirements of MBDD, and (3) to create a closely collaborating organization in which all members play a role in MBDD. Pharmaceutical companies that are able to embrace the changes MBDD poses will likely be able to improve their success rate in drug development, and the beneficiaries will ultimately be the patients in need.
Key words: drug development, modeling, pharmacodynamics, pharmacokinetics, pharmacometrics, simulation
INTRODUCTION
It is widely acknowledged that the drug development process currently employed by the pharmaceutical industry is ailing. This is illustrated by two apparent paradoxes: (1) despite an approximately eightfold increase in inflation-adjusted expenditures for research and development over the last 35 years, the number of yearly approvals for new molecular entities in the US has been stagnant (1); (2) while disciplines such as systems biology, genomics, chemistry, and biotechnology have made tremendous progress over the past few decades, the attrition rate for chemical entities in drug development remains high, with 40–50% of development programs being discontinued even in clinical Phase III (2,3). The problems leading to this inefficiency of the drug development process have drawn the attention of many different parties and stakeholders, and efforts have been brought forward to diagnose and remediate them (2–5). The Critical Path Initiative led by the US Food and Drug Administration is one of these efforts, which proposes the utilization of model-based approaches to improve drug development knowledge management and decision making (6).
The concept of model-based drug development (MBDD) originated some time ago as the application of quantitative assessments of drug disposition and drug action evolved (7–13) and was landmarked by the “learn-confirm paradigm” proposed by Lewis Sheiner in 1997 (14). The acceptance of MBDD has been growing over the last decade: Various consortiums and working groups have been formed (e.g., American Association of Pharmaceutical Scientists (AAPS) Quantitative Pharmacology (QX) Task Force), conferences and workshops are being held (e.g., American Conference on Pharmacometrics, NIH/NIGMS Quantitative and Systems Pharmacology Workshop, symposia by AAPS and the American College of Clinical Pharmacology), training programs have been established (15), and companies are actively hiring scientists that have modeling skills (15). Yet, there is still a considerable gap between today’s drug development practices and MBDD for a multitude of reasons. The purpose of this article is twofold: first, we would like to clarify the concept of MBDD and distinguish it from other related concepts, techniques, and disciplines; second, we would like to dissect the challenges in implementing MBDD in the pharmaceutical industry and propose approaches to alleviate if not to overcome them. These two objectives seem to be apart from each other: the first one focuses on general concepts, while the second one deals with practical implementation. In the following paragraphs, however, we will show how the two objectives are interrelated and why they are both important.
MODEL-BASED DRUG DEVELOPMENT: GETTING THE DEFINITION STRAIGHT
To promote a more widespread acceptance of MBDD, it is important to have an agreed-upon set of standard definitions for the terms that are often associated with MBDD, as inconsistencies in the definition and usage of these terms not only confuse the stakeholders and, thus, hinder the progress of MBDD but also hamper the presentation in communications with outside groups. In the following, we present our understanding of “pharmacokinetic (PK)–pharmacodynamic (PD) modeling,” “exposure–response modeling,” “pharmacometrics,” “quantitative pharmacology,” and “MBDD,” with the full awareness that there will be disagreement with these descriptions. Our intention is to call for consensus on the definitions of these terms rather than promoting our understanding as the standard. Different opinions on the definitions of these terms give evidence for the need for standardization. We welcome discussion and debate on this topic. The following definitions have not necessarily originated from us, but they reflect our point of view:
Modeling
Modeling in the context of drug development and MBDD is the use of mathematical means to describe the aspects of a system and/or a process, thereby focusing on the factors believed to be important. The complexity of a model is determined by its intended use. A given model involves a variety of abstract structures and may take many forms or their combinations, such as stochastic models, dynamical systems, statistical models, and differential equations. Although inexplicit, the models in “PK–PD modeling,” “exposure–response modeling,” or “pharmacometrics” refer usually to subject-matter-specific and assumption-rich modeling. This is in contrast to the more general statistical modeling that is less dependent on subject area and assumption and is frequently used for hypothesis testing.
The difference between these two types of modeling is illustrated in the following example: To demonstrate that drug exposure is proportional with respect to doses across a given range, PK modeling could be performed showing that a linear compartmental PK model adequately describes the drug concentration-time profiles for all tested doses within the range. Alternatively, statistical modeling could be done to show that the slope of the log–log linear regression of maximal concentration and dose using a power model is not different from one (16). The two approaches require different assumptions, inputs, analysis methods, and interpretation.
Pharmacokinetic–Pharmacodynamic Modeling
PK–PD modeling is the mathematical approach that links the change in drug concentration overtime to the relationship between the concentration at the effect site and the intensity of the observed response. The resulting integrated PK–PD models allow a description of the complete time course of the effect intensity in response to a given dosing regimen (17).
Exposure–Response Modeling
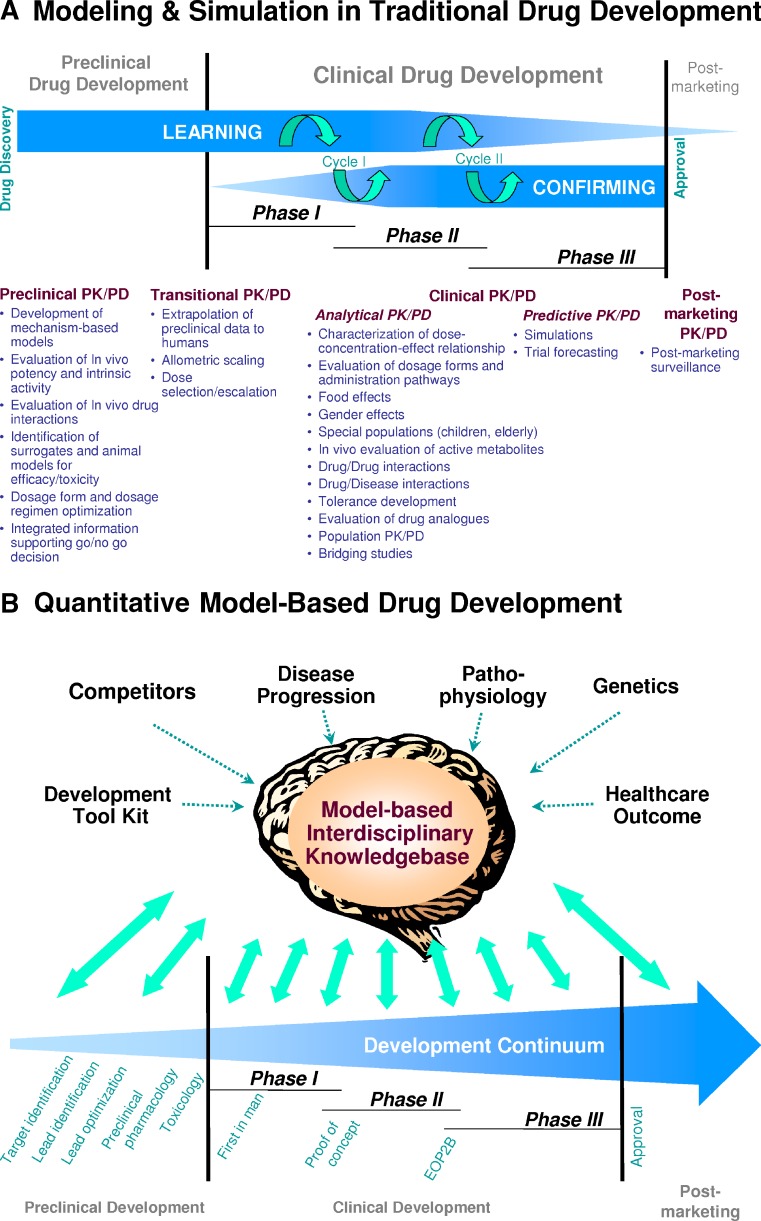
Exposure–response modeling is a similar approach as PK–PD modeling. The two terms have been used interchangeably to a large degree. “Exposure” can be the drug concentration vs. time profile or a summary metric such as area under the plasma concentration-time curve, average steady-state concentration, or the maximum concentration after application of a discrete dose. “Response” can be any type of response, such as a pharmacological marker, a physiologic parameter, an index of efficacy, or a measure of safety (18). As pharmacodynamic responses often refer to biomarker responses in clinical settings, the expression “Exposure–response modeling” has lately been favored over “PK–PD modeling.” Both expressions, however, point to the application of a modeling approach. PK–PD modeling and exposure–response modeling have previously been applied successfully throughout the drug development process, but largely in isolated settings (Fig. 1a) (8,10–12).
Fig. 1.
Model-based drug development. A Application of modeling and simulation during various preclinical and clinical phases in traditional drug development. Listed are potential applications for PK–PD concepts as well as the frequently applied two consecutive learn–confirm cycles. Model-based data analysis and simulation is usually performed in discrete isolated events throughout the development process. From (11). B Model-based drug development as a cornerstone of the drug development process. Model-based drug development is a new paradigm and mindset that embraces all aspects of drug development from drug discovery to post-marketing. By facilitating the rigorous development of a scientific knowledgebase though continuous integration of knowledge generated along the development path, it provides a data-driven model framework that serves as a key decision-making tool enabling rationale, scientifically based choices at critical decision points. The top half of Panel B represents a reservoir for the interdisciplinary knowledgebase required by MBDD. Sources of information in the top half of Panel B typically are not specific to the compound under development and are publicly available. Single arrows indicate the typically unidirectional flow of information. In contrast, information generated from the bottom half of Panel B is compound-specific, is integrated into the knowledgebase, and the knowledgebase guides how it is further being utilized to generate additional compound-specific knowledge. The double arrows indicate this bidirectional information flow
Pharmacometrics
Pharmacometrics is the scientific discipline that uses mathematical models based on biology, pharmacology, physiology, and disease for quantifying the interactions between drugs and patients. In their recent publication, Barrett and colleagues (15) emphasized the bridging character across disciplines and the Bayesian nature of pharmacometrics. Data and information from various sources are bridged together and quantitatively related to each other. The construction, refinement, and subsequent use of simulations to probe the features of the developed model are inherently Bayesian.
Models in pharmacometrics can be differentiated by their area of application, for example “Exposure–response models,” “disease models,” “trial execution model,” or any combination of these. The modeling techniques in constructing these models and obtaining parameter distributions can be differentiated by their underlying methodology, such as “nonlinear-mixed effect modeling,” “logistic regression,” or “Monte Carlo simulation.” Although commonly seen in the literature, we suggest avoiding the use of “the pharmacometrics of drug X” as this term is nonspecific and perhaps illogic. Pharmacometrics, unlike PK or PD, is not a property of a drug and does not refer to a certain technique.
Quantitative Pharmacology
Quantitative pharmacology is a multidisciplinary approach in drug development that emphasizes the integration of the relationships between diseases, drug characteristics, and individual variability across studies and development phases for rational and scientifically based decision making (19,20). It is a move away from the traditional study centric approach to a continuous quantitative integration of data across studies and development phases. By facilitating the rigorous development of a scientific knowledgebase for the drug candidate under consideration, quantitative pharmacology is intended to serve as a key decision-making tool that ultimately allows for a more efficient drug development process through informed go–no-go decisions and optimized resource allocation.
Model-Based Drug Development
MBDD is a paradigm and a mindset which promotes the use of modeling to delineate the path and focus of drug development. Models in MBDD serve as both the instruments and the aims of drug development (21). These models use available data, information, and knowledge to their maximum to improve the efficiency of the drug development process. In turn, a well-designed and implemented MBDD strategy enhances the quality of these models. Thus, these two components form an iterative cycle and provide interrelated inputs to each other.
The common features of models in MBDD are their quantitative nature, their purpose, and the way they serve in drug development rather than the type of model, estimation technique, or area of application. In MBDD, modeling is applied to a variety of aspects of drug development, such as drug design (22,23), target screening, formulation choices (24), in vitro and in vivo testing (25), exposure-biomarker response (26), disease progression (27), healthcare outcome (28), patient behavior (29), and socio-economic impact (30). Knowledge in these areas is formally summarized and reflected in these models and carried over to the next development step (Fig. 1b).
It is self-evident that the above-outlined techniques, concepts, and disciplines are interrelated. Exposure–response modeling (or PK–PD modeling) is a key application of pharmacometrics. Quantitative pharmacology is an approach that builds on pharmacometrics and emphasizes data integration and quantitative decision making. MBDD asks for a formalized summary of all available information and its full utilization in drug development. The scope and complexity of each approach, from exposure–response modeling, pharmacometrics, and quantitative pharmacology to MBDD follows an increasing order, with each approach embracing the preceding ones.
The literature provides several excellent reviews on the topic of MBDD (19,31,32). Of them, Lalonde and colleagues (31) defined the key components of MBDD and provided excellent case studies for its application. These reviews are important illustrations of MBDD. With full respect, however, we feel the scope they touched upon is largely limited to pharmacometrics, the discipline that focuses on quantitative drug-disease–patient relationships. In our view, MBDD covers the whole arena of drug development and, thus, many more aspects beyond those addressed by pharmacometrics.
As there are numerous examples in the literature that describe the methodology and application of pharmacometrics and quantitative pharmacology and how data and information could be transformed into knowledge useful for decision making, we will, in the following, focus on a strategic question: if we agree that MBDD is the right direction for drug development, why are we not there yet?
ALIGNING INDUSTRY INFRASTRUCTURE WITH MODEL-BASED DRUG DEVELOPMENT
It has been more than a decade since the “learn-confirm paradigm” was introduced (14); yet, a majority of today’s pharmaceutical industry still develops drugs at a “model-aided” level, in which models are used to support labeling and confirm decisions. While this is a progress from empirical decision making, it is still distinctly distant from MBDD. The key difference between the “model-aided” drug development and MBDD (19,31,32) is that the former uses modeling and simulation as a tool sporadically, while MBDD utilizes models as the cornerstone of the development process (Fig. 1b). Table I contrasts “model-aided” drug development to MBDD. The gap between these two approaches is not totally unexpected and cannot be attributed to the unawareness of MBDD alone. Challenges in the transition from “model-aided” to MBDD have been recognized (21,33,34). We will further analyze these challenges and propose changes to alleviate if not to overcome them.
Table I.
Comparisons Between “Model-Aided” Drug Development and Model-Based Drug Development
| “Model-aided” drug development | Model-based drug development | |
|---|---|---|
| Nature | Models are largely empirical | Both empirical and mechanistic models are developed and applied given modeling objectives |
| Model function formats are driven by the observed trend in data | Functions formats are elucidated by underlying drug, disease, and physiologic mechanisms | |
| Difficulties in linking models across experiments, response types, developmental stage, and compounds | Models include knowledge, data and scientific perspective from all relevant aspects and are constantly updated | |
| Model quality is restricted by data quantity and quality | Rich prior knowledge alleviates the dependence on data quantity and quality | |
| Limited predictability for future studies | Predictability is the key model performance requirement | |
| Content | Models are mostly developed in pharmacokinetics and pharmacodynamics in late stage clinical development, and are mainly used for quantifying | Models are developed at various stages and in different disciplines in preclinical and clinical development. Models are used for characterizing |
| Response levels in exposure, biomarkers, and endpoints | Candidate attributes | |
| Sources of variation | Disease mechanisms | |
| Covariate effects | Competitor information | |
| Trial execution patterns | ||
| Impact | Models confirm decisions, in which they | Models facilitate quantitative decisions, in which they |
| Are used at the discretion of stakeholders | Serve as instruments and aims of drug development | |
| Focus on a few attributes separately | Reflect all known attributes and call attention to important yet unknown attributes | |
| Are developed by a few scientists with “modeling expertise” and viewed skeptically by other parties | Are synergistic results from all relevant stakeholders | |
| Are not timely to influence key decisions | Are developed prospectively and are a necessity for decision making |
Traditional vs. Model-Based Drug Development
The hurdles in applying MBDD more widely are rooted in the sequential stages in which drugs have traditionally been developed. The planning, analysis, and interpretation of each stage is based on individual experiments and utilizes only limited prior knowledge gained from previous stages, other drug candidates, competitors, and experimental systems. The development pace focuses on moving the candidate to the next milestone as soon as possible and down the sequence as far as possible. Such an approach is often pursued as a performance criterion in measuring the success of a study as well as individual contributors. As a natural consequence, anything that is unconventional and requires adaptation is not welcome, as it “delays” the development timeline.
In MBDD, models are the quantitative summarization of the data, prior knowledge, and assumptions. Experiments or studies are designed to best inform and/or confirm the model. Study results are expressed in function format and parameter estimates (21). Thus, the decision makers are presented with a probability of achieving a certain goal generated from modeling and simulation, rather than a P value. Dose selections and go–no-go decisions are made based on quantitative risk or benefit assessments.
Given the differences between the design, execution, analysis, and interpretation in traditional drug development and MBDD, it is obvious that changes must be made to enable the paradigm shift from the former to the latter. It has been said that change is the result of having a desired outcome, a strategy, a tactic, and commitment (35). For pharmaceutical companies, the desired outcome for the changes is undoubtedly to provide effective treatments for unmet medical needs in an efficient manner and to meet this goal rapidly and cost-effectively. The strategy, tactic, and commitment for achieving this outcome lie within the necessary changes in mindset, process, and organization (Table II).
Table II.
Changes at Different Levels are Necessary for the Pharmaceutical Industry to Fully Embrace Model-Based Drug Development (MBDD)
| Strategy: mindset change | Invest time and resources both at the personal level and the corporate level to get acquainted with the principle of MBDD |
| Think in terms of probability instead of “yes/no” | |
| Balance drug development between “timeline-driven” and “information yield-driven” | |
| Actively involve technique modelers into development program | |
| Tactic: process change | Establish collective proprietary databases |
| Optimize development at study, compound and portfolio levels | |
| Accept flexible trial designs and timelines | |
| Automate and standardize data collection, handling, and reporting | |
| Commitment: organization change | Accept MBDD as integral part of drug development |
| Emphasize collaborations between project leaders, modelers, statisticians, and experimentalists | |
| Set up quantitative decision rules by aggregated criteria | |
| Provide sufficient manpower for implementing MBDD |
Strategy: Mindset Change
Hand waving from individuals in certain functional areas is not enough to adopt MBDD as a drug development paradigm. The support for this approach must come from a broader base. Based on their individual mindset, the people who hesitate to adopt MBDD usually belong to one of the following three categories:
People who are unfamiliar and, therefore, are uncomfortable with quantitative data summaries and decision making. These individuals usually did not receive substantial exposure to modeling principles during their training and work. They encountered scientific concepts mostly in qualitative format as usually presented in biomedical textbooks, and their training in biostatistics is mostly limited to frequentist statistics with no equivalent emphasis on Bayesian statistics. For this kind of mindset, proper education, training, and increased exposure to the principles and application of (Bayesian) modeling would be helpful.
People who feel that raw experimental data are objective and trustworthy but that the assumption-rich models in MBDD are subjective and not reliable. For this type of mindset, it is worthwhile to point out that even well-designed experiments are not assumption-free. Assumptions in modeling usually contain prior experience and knowledge and are not equal to random guessing. The strength of modeling is partly attributed to its use of assumptions: having assumptions helps bridging the missing links between existing information and elucidating the decision criteria. Examining the model robustness towards assumptions can check a model’s dependency on assumptions, and allows to identify crucial data or information that need to be collected in future experiments. For this kind of mindset, a clearly defined objective for the experiment or development stage would be helpful to increase the comfort level towards “assumption-rich” approaches. For example, the analysis objective mode can be defined as “learning” or “confirming,” each of which has a different tolerance level on assumptions and risks.
People that are content to describe data but are reluctant to interpret data. These individuals are convinced that it is easier to defend a description than an interpretation should there be a controversial opinion or the projection is proven to be wrong. As modeling itself is an interpretation of collected data and applying modeling results for decision making requires further interpretation of the model and its implications, these individuals are averse towards any application of MBDD. For this kind of mindset, a corporate culture that has leveled accountability and encourages scientists to take responsibilities would be useful. Key decision makers need to realize that MBDD, like any other development strategy, can fail in isolated instances, thereby avoiding putting blame on the shoulders of individual contributors.
Within the pharmaceutical company, the project leaders and decision makers need to invest time and resources at the personal level to get acquainted with the principles of MBDD. Instead of viewing MBDD as a subject related only to modeling scientists, the project leaders and decision makers must regard themselves as active players in adapting MBDD. They need to understand the value of implementing MBDD as an “investment in knowledge” and be comfortable with probability-based decision making (31). Progress in candidate development will be viewed as a compromise between speeding up the development timeline and maximizing the information gain at each development step. Only then can MBDD truly impact the drug development process. And only then are these stakeholders likely willing to accommodate the complexity in strategy and logistics associated with its implementation.
Modelers, the people who conduct model development and application, need to extend their attention and responsibility beyond the technical aspects of modeling. Instead of portraying themselves as service providers and act passively, modelers need to be actively engaged. They need to identify the stage-appropriate opportunities where models can contribute and need to communicate the model interpretation and implications to all stakeholders. A thorough understanding of the drug development process and good communication skills are critical in gaining acceptance of modeling from other functional areas and delivering results pertinent to the development needs.
Tactic: Process Change
Equipped with an open mindset, the drug development process needs to be aligned with MBDD. At the portfolio level, pharmaceutical companies need to establish their own collective proprietary databases to enable reuse of and learning from data accumulated throughout the development process, from past experiences, from comparators, and from knowledge around the experimental system (structure–activity relationships, disease mechanisms, target population characteristics, etc.). Rather than adhering to fixed designs and processes, project teams need to tailor a candidate’s development path, design scheme, and study conduct to its own characteristics.
At the experiment or study level, studies shall, in addition to their primary objectives, maximize the information yield and address additional compound development needs. In order to minimize the time and logistic resources, data collection needs to be standardized to enable cross-study and cross-compound data mining. This entails that the analysis framework needs to be set up prior to analysis, that the use of advanced technical tools should be promoted, and that data cleaning, dataset construction, results formatting, and reporting should be automated. Furthermore, data and knowledge gained from each study need to be deposited and integrated into collective databases to maximize knowledge retention.
Commitment: Organization Change
Organizational changes come naturally as the drug development process changes. Project leaders, modelers, statisticians, and experimentalists each carry different roles and must work collaboratively (31). Project leaders shall get comfortable with quantitatively defining clinical effects and decision criteria. Modelers need to make model assumptions and limitations explicit and transparent, develop models that are fit for their purpose, and calibrate models against data-derived statistics of interest. Statisticians need to embrace assumption-rich models, differentiate learning studies from hypothesis-testing in confirmatory studies, and strive to develop statistical methodology that allows applying innovative experimental designs and decision analysis. Experimentalists who generate data are required to understand the objectives of the modeling exercise and provide pertinent information and assumptions. Organizations that foster collaboration among these individuals, set clearly defined roles and expectations, and encourage innovations are better prepared for MBDD.
As the fields of application for modeling vary greatly, people who perform modeling tasks reside in different functional groups, for instance, a protein design group in drug discovery, a formulation group in process development, and a healthcare outcome group in marketing. The knowledge each group has may not always be applicable to the others, but a network that connects these groups under the theme of MBDD can be useful (e.g., cross-functional M&S discussion forums, modeling working subteams linked to project teams). This network could be a unique opportunity to promote and brand MBDD. It is our experience that these groups can often learn from each other for improving modeling techniques and promoting MBDD. For example, we have seen collaborations between Clinical Pharmacology and Marketing through such interactions, which would have otherwise taken a much longer time to happen if at all.
Currently, there is a shortage of people with quantitative skill sets required by MBDD. Externally, companies need to partner with academia, governmental agencies, and professional organizations to provide more training opportunities. However, like any applied science, learning on the job may always remain a great portion of the learning path for individuals applying MBDD (15). Internally, exchanging and propagating expertise within a company is essential. Thus, companies should strive to provide a platform and mentoring opportunities to develop their employees with regard to MBDD and grow an in-house talent base.
Change inevitably brings conflicts. As pharmaceutical companies go through the changes in mindset, process, and organization from MBDD, it will be helpful to learn from other industry sectors’ experience. One of the notable examples is the aerospace industry, which in the late 1960s embraced the widespread application of numerical simulation techniques in engineering new aircrafts (36). Numerous books in business also offer guidance on embracing changes within an organization (37,38). Although it is outside the scope of this work, we encourage scientists to leverage the lessons from other sectors to better serve MBDD. As an example, the book “The Necessary Revolution” by Senge et al. (38) explains why rethinking our actions and expanding our boundaries help us to see underlying limits and new forces at play. They discuss three key elements:
Rethink boundaries. Attempts to convince people that they are wrong and that they need to rethink boundaries will almost always be met with resistance. A more effective approach is to help people reflect on the assumptions that they are making.
- Seeing reality through others’ eyes. The following five steps can open our eyes to a larger reality beyond the one we usually see:
- Bring together a diverse group of people who, to the highest degree possible, represents the larger system you belong to
- Identify the different facets of the system that you will explore
- “Go there together”; travel with the entire team
- Set aside ample time to reflect and talk together about what you experience
- Pay careful attention to the intentions and commitments that arise from your reflection
Building shared commitment. Shared commitment arises through focusing first on engagement—connecting to what matters to us and the larger organization involved—and then on creating the opportunity for both focus and commitment to deepen naturally over time. Our ability to foster commitment will never be greater than our own commitment. The key in fostering shared commitment lies in connecting to what we care about and what the organization cares about and gradually knitting the two together.
The ultimate incentive for the implementation of MBDD in the drug development process are cost savings by accelerating the development process for a shortened time-to-market, by reducing the number and size of studies required to obtain regulatory approval, and/or by providing decision support to earlier terminate programs for compounds with low prospect of development and/or marketing success. Multiple case studies have been presented in the literature of instances in which pharmacometric approaches have substantially contributed throughout the drug development process (8,10,12,13,31,39–41), including drug approval (42). These reports, however, are still limited compared to the level of application of model-based approaches in drug development according to our knowledge. Moreover, there have, so far, not been any published examples of cost-benefit analyses that compare drug development processes utilizing MBDD with more traditional approaches. As these data are crucial to support the perception that implementation of MBDD adds value to the drug development process and has a positive benefit vs. cost relationship, we strongly encourage our colleagues to share their experiences with the scientific community and publish examples of proven benefits of MBDD.
Conclusions
This manuscript aims to call for a set of consented definitions on concepts that are related to MBDD. In our view, PK–PD modeling and exposure–response modeling are applications of modeling. Pharmacometrics is the scientific discipline focused on quantifying the relationship between patient, drug, and disease. Quantitative pharmacology is a multidisciplinary approach that focuses on data integration and quantitative decision making. MBDD is a paradigm and a mindset in which models are taken as the instrument and aim of the drug development. By this definition, MBDD covers the whole spectrum of the drug development process instead of being limited to a certain type of modeling technique or application area.
Having a consistent nomenclature is important for the modeling and simulation community and MBDD. The consistency will help to avoid the potential confusion, define the boundaries, establish the brand of MBDD, and facilitate its positioning in a company’s corporate strategy. We anticipate and encourage different opinions on these definitions and are eagerly looking forward to a consensus definition for MBDD by the scientific community.
While MBDD may be perceived as hindering a uniform design, implementation, and interpretation of an experiment, a study, or a development program, uniformity in these aspects is often only desirable for convenience without any scientific justification (21). The implementation of MBDD requires pharmaceutical companies to foster innovation and make changes at three levels: (1) to open mindsets that are willing to get acquainted with MBDD, (2) to align processes that are adaptive to the requirements of MBDD, and (3) to create a closely collaborating organization in which all members play a role in MBDD. Companies that are able to embrace the changes MBDD poses and requires will likely be able to improve their success rate in drug development, and the beneficiaries will ultimately be the patients in need.
Footnotes
This article is based on a symposium held jointly by the American Association of Pharmaceutical Scientists (AAPS) and the American College of Clinical Pharmacology during the 2006 AAPS Annual Meeting in San Antonio, TX, USA.
Zhang, Pfister and Meibohm contributed equally to the development of this manuscript.
References
- 1.Congressional Budget Office . A CBO Study: Research and Development in the Pharmceutical Industry. Washington, DC: The Congress of the United States; 2006. [Google Scholar]
- 2.Kola I., Landis J. Can the pharmaceutical industry reduce attrition rates. Nat. Rev. Drug Discov. 2004;3:711–715. doi: 10.1038/nrd1470. [DOI] [PubMed] [Google Scholar]
- 3.Arlington S., Barnett S., Hughes S., Palo J. Pharma 2010: The Threshold to Innovation. Somers: IBM Business Consulting Services; 2002. [Google Scholar]
- 4.Frantz S. Pipeline problems are increasing the urge to merge. Nat. Rev. Drug Discov. 2006;5:977–979. doi: 10.1038/nrd2206. [DOI] [PubMed] [Google Scholar]
- 5.Tufts Center for the Study of Drug Development . Impact Report: Fastest drug developers consistently best peers on key performance metrics. Boston: Tufts University; 2006. [Google Scholar]
- 6.Center for Drug Evaluation and Research . Innovation or Stagnation: Challenge and Opportunity on the Critical Path to New Medical Products. Rockville: US Food and Drug Administration; 2004. [Google Scholar]
- 7.Csajka C., Verotta D. Pharmacokinetic-pharmacodynamic modelling: history and perspectives. J. Pharmacokinet. Pharmacodyn. 2006;33:227–279. doi: 10.1007/s10928-005-9002-0. [DOI] [PubMed] [Google Scholar]
- 8.Bhattaram V. A., Booth B. P., Ramchandani R. P., et al. Impact of pharmacometrics on drug approval and labeling decisions: a survey of 42 new drug applications. Aaps J. 2005;7:E503–E512. doi: 10.1208/aapsj070351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Breimer D. D., Danhof M. Relevance of the application of pharmacokinetic-pharmacodynamic modelling concepts in drug development. The “wooden shoe’ paradigm. Clin. Pharmacokinet. 1997;32:259–267. doi: 10.2165/00003088-199732040-00001. [DOI] [PubMed] [Google Scholar]
- 10.Chien J. Y., Friedrich S., Heathman M. A., de Alwis D. P., Sinha V. Pharmacokinetics/Pharmacodynamics and the stages of drug development: role of modeling and simulation. Aaps J. 2005;7:E544–E559. doi: 10.1208/aapsj070355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meibohm B., Derendorf H. Pharmacokinetic/pharmacodynamic studies in drug product development. J. Pharm. Sci. 2002;91:18–31. doi: 10.1002/jps.1167. [DOI] [PubMed] [Google Scholar]
- 12.Olson S. C., Bockbrader H., Boyd R. A., Cook J., Koup J. R., Lalonde R. L., Siedlik P. H., Powell J. R. Impact of population pharmacokinetic-pharmacodynamic analyses on the drug development process: experience at Parke-Davis. Clin. Pharmacokinet. 2000;38:449–459. doi: 10.2165/00003088-200038050-00005. [DOI] [PubMed] [Google Scholar]
- 13.Reigner B. G., Williams P. E., Patel I. H., Steimer J. L., Peck C., van Brummelen P. An evaluation of the integration of pharmacokinetic and pharmacodynamic principles in clinical drug development. Experience within Hoffmann La Roche. Clin. Pharmacokinet. 1997;33:142–152. doi: 10.2165/00003088-199733020-00005. [DOI] [PubMed] [Google Scholar]
- 14.Sheiner L. B. Learning versus confirming in clinical drug development. Clin. Pharmacol. Ther. 1997;61:275–291. doi: 10.1016/S0009-9236(97)90160-0. [DOI] [PubMed] [Google Scholar]
- 15.Barrett J. S., Fossler M. J., Cadieu K. D., Gastonguay M. R. Pharmacometrics: a multidisciplinary field to facilitate critical thinking in drug development and translational research settings. J. Clin. Pharmacol. 2008;48:632–649. doi: 10.1177/0091270008315318. [DOI] [PubMed] [Google Scholar]
- 16.Gough K., Hutchison M., Keene O., Byrom B., Ellis S., Lacey L., McKellar J. Assessment of dose proportionality: Report from the statisticians in the Pharmaceutical Industry/Pharmacokinetics UK Joint Working Party. Drug Inf. J. 1995;29:1039–1048. [Google Scholar]
- 17.Derendorf H., Meibohm B. Modeling of pharmacokinetic/pharmacodynamic (PK–PD) relationships: concepts and perspectives. Pharm Res. 1999;16:176–185. doi: 10.1023/A:1011907920641. [DOI] [PubMed] [Google Scholar]
- 18.Sheiner L. B., Steimer J. L. Pharmacokinetic/pharmacodynamic modeling in drug development. Annu. Rev. Pharmacol. Toxicol. 2000;40:67–95. doi: 10.1146/annurev.pharmtox.40.1.67. [DOI] [PubMed] [Google Scholar]
- 19.Zhang L., Sinha V., Forgue S. T., Callies S., Ni L., Peck R., Allerheiligen S. R. Model-based drug development: the road to quantitative pharmacology. J. Pharmacokinet. Pharmacodyn. 2006;33:369–393. doi: 10.1007/s10928-006-9010-8. [DOI] [PubMed] [Google Scholar]
- 20.Krishna R. Quantitative clinical pharmacology: Making paradigm shifts a reality. J. Clin. Pharmacol. 2006;46:966–967. doi: 10.1177/0091270006292978. [DOI] [PubMed] [Google Scholar]
- 21.Grasela T. H., Fiedler-Kelly J., Walawander C. A., Owen J. S., Cirincione B. B., Reitz K. E., Ludwig E. A., Passarell J. A., Dement C. W. Challenges in the transition to model-based development. Aaps J. 2005;7:E488–E495. doi: 10.1208/aapsj070249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wintner E. A., Moallemi C. C. Quantized surface complementarity diversity (QSCD): a model based on small molecule-target complementarity. J. Med. Chem. 2000;43:1993–2006. doi: 10.1021/jm990504b. [DOI] [PubMed] [Google Scholar]
- 23.Schoeberl B., Nielsen U. B., Paxson R. Model-based design approaches in drug discovery, a parallel to traditional engineering approaches. IBS J. Res Dev. 2006;50:645–653. doi: 10.1147/rd.506.0645. [DOI] [Google Scholar]
- 24.Arce P., Aznar M. Modeling of phase equilibirum of binary mixtures composed by polystyrene and chlorofluorocarbons, hydrochlorofluorocarbons, hydrofluorocarbons and supercritical fluids using cubic and non-cubic equations of state. J. Supercrit. Fluids. 2008;42:134–145. doi: 10.1016/j.supflu.2007.07.019. [DOI] [Google Scholar]
- 25.Roncaglioni A., Benfenati E. In silico-aided prediction of biological properties of chemicals: oestrogen receptor-mediated effects. Chem. Soc. Rev. 2008;37:441–450. doi: 10.1039/b616276m. [DOI] [PubMed] [Google Scholar]
- 26.Pfister M., Martin N. E., Haskell L. P., Barrett J. S. Optimizing dose selection with modeling and simulation: application to the vasopeptidase inhibitor M100240. J. Clin. Pharmacol. 2004;44:621–631. doi: 10.1177/0091270004265365. [DOI] [PubMed] [Google Scholar]
- 27.Garcia-Mora B., Santamaria C., Rubio G., Luis Pontones J. Modeling the recurrence-progression process in bladder carcinoma. Comput. Math Appl. 2008;53:619–630. doi: 10.1016/j.camwa.2008.01.005. [DOI] [Google Scholar]
- 28.Larsen K., Hvass K. E., Hansen T. B., Thomsen P. B., Soballe K. Effectiveness of accelerated perioperative care and rehabilitation intervention compared to current intervention after hip and knee arthroplasty. A before-after trial of 247 patients with a 3-month follow-up. BMC Musculoskelet. Disord. 2008;9:59. doi: 10.1186/1471-2474-9-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kenna L. A., Labbe L., Barrett J. S., Pfister M. Modeling and simulation of adherence: approaches and applications in therapeutics. Aaps J. 2005;7:E390–E407. doi: 10.1208/aapsj070240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koopmanschap M. A., van Exel J. N., van den Berg B., Brouwer W. B. An overview of methods and applications to value informal care in economic evaluations of healthcare. Pharmacoeconomics. 2008;26:269–280. doi: 10.2165/00019053-200826040-00001. [DOI] [PubMed] [Google Scholar]
- 31.Lalonde R. L., Kowalski K. G., Hutmacher M. M., et al. Model-based drug development. Clin. Pharmacol. Ther. 2007;82:21–32. doi: 10.1038/sj.clpt.6100235. [DOI] [PubMed] [Google Scholar]
- 32.D. Stanski. Model-based drug development: a critical path opportunity. http://www.fda.gov/oc/initiatives/criticalpath/stanski/stanski.html (accessed 8/13/08).
- 33.Chaikin P., Rhodes G. R., Bruno R., Rohatagi S., Natarajan C. Pharmacokinetics/pharmacodynamics in drug development: an industrial perspective. J. Clin. Pharmacol. 2000;40:1428–1438. [PubMed] [Google Scholar]
- 34.Grasela T. H., Dement C. W., Kolterman O. G., Fineman M. S., Grasela D. M., Honig P., Antal E. J., Bjornsson T. D., Loh E. Pharmacometrics and the transition to model-based development. Clin. Pharmacol. Ther. 2007;82:137–142. doi: 10.1038/sj.clpt.6100270. [DOI] [PubMed] [Google Scholar]
- 35.Lesko L. J. Paving the critical path: how can clinical pharmacology help achieve the vision? Clin. Pharmacol. Ther. 2007;81:170–177. doi: 10.1038/sj.clpt.6100045. [DOI] [PubMed] [Google Scholar]
- 36.Johnson S. C. D. The role of simulation in the managememnt of research: What can the pharmaceutical industry leanr from the aerospace industry? Drug Inf. J. 1998;32:961–969. [Google Scholar]
- 37.O’Reilly C. A., Tushman M. L. Winning through innovation: a practical guide to leading organizational change and renewal. Boston: Harvard Business School Press; 2002. [Google Scholar]
- 38.Senge P. M., Smith B., Schley S., Kruschwitz N. The necessary revolution: how individuals and organisations are working together to create a sustainable world. New York: Doubleday Business; 2008. [Google Scholar]
- 39.Bhattaram V. A., Bonapace C., Chilukuri D. M., et al. Impact of pharmacometric reviews on new drug approval and labeling decisions–a survey of 31 new drug applications submitted between 2005 and 2006. Clin. Pharmacol. Ther. 2007;81:213–221. doi: 10.1038/sj.clpt.6100051. [DOI] [PubMed] [Google Scholar]
- 40.Veyrat-Follet C., Bruno R., Olivares R., Rhodes G. R., Chaikin P. Clinical trial simulation of docetaxel in patients with cancer as a tool for dosage optimization. Clin. Pharmacol. Ther. 2000;68:677–687. doi: 10.1067/mcp.2000.111948. [DOI] [PubMed] [Google Scholar]
- 41.Wang Y., Bhattaram A. V., Jadhav P. R., Lesko L. J., Madabushi R., Powell J. R., Qiu W., Sun H., Yim D. S., Zheng J. J., Gobburu J. V. Leveraging prior quantitative knowledge to guide drug development decisions and regulatory science recommendations: impact of FDA pharmacometrics during 2004–2006. J. Clin. Pharmacol. 2008;48:146–156. doi: 10.1177/0091270007311111. [DOI] [PubMed] [Google Scholar]
- 42.Miller R., Ewy W., Corrigan B. W., Ouellet D., Hermann D., Kowalski K. G., Lockwood P., Koup J. R., Donevan S., El-Kattan A., Li C. S., Werth J. L., Feltner D. E., Lalonde R. L. How modeling and simulation have enhanced decision making in new drug development. J. Pharmacokinet. Pharmacodyn. 2005;32:185–197. doi: 10.1007/s10928-005-0074-7. [DOI] [PubMed] [Google Scholar]