INTRODUCTION
The dissolution and diffusion of molecules through porous materials is of great interest to many researchers and can be found in diverse areas of science including catalyst manufacture, food and cosmetic production, tissue engineering, and a whole range of medicines. In terms of conventional medicines, tablets are by far the most popular formulations on the market and are preferred by patients and physicians alike. However, the science underpinning the design and function of more complex formulations, specifically those with controlled release profiles, is lacking in some respect. Due to their ease of manufacture and low costs, the simplest form of controlled release tablet is the matrix tablet (1–3). Matrix-based systems are usually obtained by direct compression of an active (drug) blended with retardant material and additives to form a simple matrix (3,4), in which the nature of the retardant material, which is usually a polymer, determines the nature of the matrix formed. If hydrophobic materials such as wax and ethyl cellulose are used, they form porous matrices, which may be either inert or erodable (1,3). In theory, the retardant material in the inert matrix controls drug release by forming an inert diffusion barrier around the drug particles (4). Clearly, the liquid media must penetrate the matrix to allow the drug to be released from the matrix via diffusion and finally dissolution (3); thus, porosity is a key parameter (5,6). Production and manufacture variables encountered during the production of matrix-based materials lead to variations in performance and therefore jeopardise regulatory acceptance.
Subsequently, it would be advantageous to visualise the internal structure of such materials post-manufacture and focus on the dynamic property of pore structure and porosity during dissolution and diffusion. X-ray microtomography (XCMT) can produce 3D images of materials with a voxel size of around several micrometers cubed, allowing the visualisation of internal and microstructural details with different X-ray absorbencies. The intensity values associated with the different features of an XCMT image are determined by the X-ray transmission measured by an X-ray detection system, which is dependent on the material’s atomic mass and the energy of X-rays (7,8).
In a previous study, the investigators have focused on the use of XCMT for the study of modified release systems (Adalat® OROS, Alza Corporations OROS® technology, Alza, CA, USA). These formulations contain different excipients (swellable polyethylene oxide polymer), sodium chloride crystals, and nifedipine (active drug). All components were easily resolved by XCMT due to their inherent variations in density, giving evidence of the scope of applicability of the method (9).
For this specific investigation, the goal was to evaluate XCMT as a nondestructive imaging technique, to study porous matrix structure and the diffusion and dissolution process that occurs during drug release. Inert matrix tablets (Ferrogradumet®, Abbott Laboratories, Australia) containing ferrous sulphate in a matrix of lactose and Eudragit® were chosen as the model tablets and evaluated using the XCMT at set time points during dissolution using a custom-built sample mount.
EXPERIMENTAL
Materials and Methods
Inert matrix tablets (ferrous sulphate-Ferrogradumet®, Abbott Laboratories, Australia) were used as supplied. Each tablet contained 375 mg ± 5% of ferrous sulphate, which is approximately 105 mg of elemental iron. The matrix consisted of lactose and Eudragit®. Water was purified by reverse osmosis (MilliQ, Millipore, France).
USP Dissolution Test
The standard pharmacopeial test to determine drug release (United States Pharmacopoeia dissolution Apparatus II) was used. The test was performed with 900 mL of distilled water at 37°C with a stirring rate of 50 rpm. Samples were extracted at set time points over a 24-h period, and elemental analysis (atomic absorbance) was used to determine iron concentration. Six replicates were conducted. Samples were diluted with water to fit within the linearity of the atomic absorbance analysis (2 to 10 ppm, R2 = 0.999)
In Vitro Simulated Dissolution Apparatus for XCMT Analysis
Due to the limitation of performing the USP dissolution test in conjunction with XCMT, an ‘in vitro’ dissolution apparatus was developed to simulate the pharmacopoeia dissolution test. To insure sink conditions, a large 900-ml heated reservoir was assembled upon a magnetic stirrer. The dissolution media was equilibrated at 37°C. Tablets were glued, using hot melt glue, onto a low density Styrofoam, which in turn was mounted in an 80-ml polymer tube. The tube was manufactured in order to maximise volume but, at the same time, be easily placed into the XCMT sample holder for analysis. At specific time points (60 min intervals) during the dissolution process, the inner polymer container was removed, and the liquid volume reduced so that the tablet was not exposed to the media. The polymer container was covered by a layer of parafilm to create a microenvironment of 100% relative humidity before being placed in the XCMT for analysis.
X-Ray Computed Microtomography
Basically, XCMT uses a mathematical algorithm to reconstruct a three-dimensional structure from multiple two-dimensional X-ray images (10–13). The sample is placed on a precision turntable between a high power X-ray source and a detector array (line or area array), which is used to measure the intensities of the diverging X-ray beam transmitted through the sample (12,15). Multiple attenuation coefficient values, which correlate to the degree of attenuation of the X-rays, are obtained as the sample is rotated relative to the X-ray beam (to obtain multiple sets of attenuation coefficients from different viewing angles) (12,16,17). This raw data can be converted to pixel greyscale data, which a mathematical algorithm can translate into two-dimensional grey-scale radiograph or projection images (12,15). Furthermore, the computer system can be calibrated so that values are assigned to certain materials. For example, air may be assigned a value of zero and water a value of one thousand allowing the pixels to correspond to variation in density (5,12,13). A three-dimensional reconstruction, called a tomogram, is formed from this set of two-dimensional images using a mathematical algorithm that is based on the Beer–Lambert law of absorption (12,15). The digital unit for this picture is called a voxel. Similar to pixels, voxels can be calibrated to display apparent density (12). The XCMT scans were obtained using a Skyscan 1172 high-resolution desktop XCMT system (Skyscan, Aartselaar, Belgium) with a 100-kV X-ray source at 0.35° increments over a 180° rotation. A 0.1 mm aluminium filter was used to lower the intensity of the X-rays. Resolution was 1,024 × 1,024 pixels at 8 μm per pixel with a scan time of 20 min.
Density sensitivity is an issue that needs to be taken into consideration when performing XCMT scans. This issue has several related components that have to be assessed.
The basic sensitivity and stability of the instrument. In general, the sensitivity of desktop X-ray micro-tomography systems is relatively limited when compared to synchrotron-based systems. This is essentially due to limitations in both the X-ray source and the detectors that are used. The micro-focus X-ray sources that are used in this study produces X-rays having a range of energies (polychromatic X-rays). Subsequently, the absorption of X-rays by the specimen is detected if there is a sufficient lowering of the average X-ray energy. On the detector side, the system sensitivity is a function of the quantum efficiency and signal to noise characteristic of the imaging system. Some phosphors are more sensitive than others but are less durable. The P53 phosphor used in the Skyscan systems is a reasonable balance in this respect. Similarly, the camera used will have a large influence on sensitivity. The Skyscan systems use 12-bit air-cooled CCD cameras with moderate sensitivity that is a good match for the P53 phosphor. Other systems that use water-cooled 14-bit cameras or back-thinned cameras would have better sensitivity. Consideration also needs to be given to component stability. The X-ray source for instance will age giving rise to a shift in X-ray energy output. The phosphor will decrease in its quantum efficiency as it gets older and the CCD camera itself will degrade due to X-ray damage. So, comparison of studies done at different times and/or on different instruments needs to be done carefully. Within a single study carried out over a short period of time, however, the issue of sensitivity drift is of less concern.
The density of the materials. The basic density of the materials being imaged is clearly a consideration. If two adjacent materials have a density that is similar that they may not be differentiated unless some other factor such as different hydration levels acts to alter their base density.
The structural nature of the materials. The physical nature of the material, however, does effect its X-ray absorption over and above its bulk density. For instance, porosity either above the system resolution limit or at sub-voxel levels will result in differences in X-ray absorption that will be seen as different grey levels, and this will be in turn dependent on such variables as the distributive characteristic of the porosity, the point-spread function of the X-ray source, and the pixel size of the camera.
The complexity of material interfaces. The material interface also needs to be considered, as the degree of complexity (i.e., material interaction) will determine how detectable the boundary will be. In some instances where mixing of materials is considerable, a fairly diffuse boundary may be imaged; in other instances, the interface may be less complex giving rise to sharper delineations. In other cases, the porosity and, hence localised density, may be affected by material interaction occurring at the boundary.
Although there are limitations to this technique, previous published data (18) have demonstrated that meaningful data can be obtained for interpretation by taking into account previously described issues.
Image Acquisition and Reconstruction
The attenuation coefficients obtained from the XCMT was converted into two-dimensional radiographs using the SkyScan software (as Tiff files). NRecon cone-bream reconstruction software (Thomson Scientific Instruments, Victoria, Australia) generated a series of 8-bit axial slices, each of 1,024 × 1,024 voxels, that had Z dimensional spacing equal to the within-slice voxel spacing. These bit-mapped images were three-dimensional (3D) slices and could be subsequently stacked to form a complete 3D image of the tablet. Beam hardening was set at 30% and ring artefact correction at 10%. The 3D volume images were digitally rendered using VG Studio Max software (Volume Graphics GmbH, Heidelberg, Germany) with a 93 Gaussian filter to remove background noise. A software “segmentation” tool was used to isolate differences in density and calculate the amount of iron remaining in the tablet as a volume of the total tablet volume.
RESULTS AND DISCUSSION
Radiographs of Single Ferrous Sulphate Inert Matrix Tablet
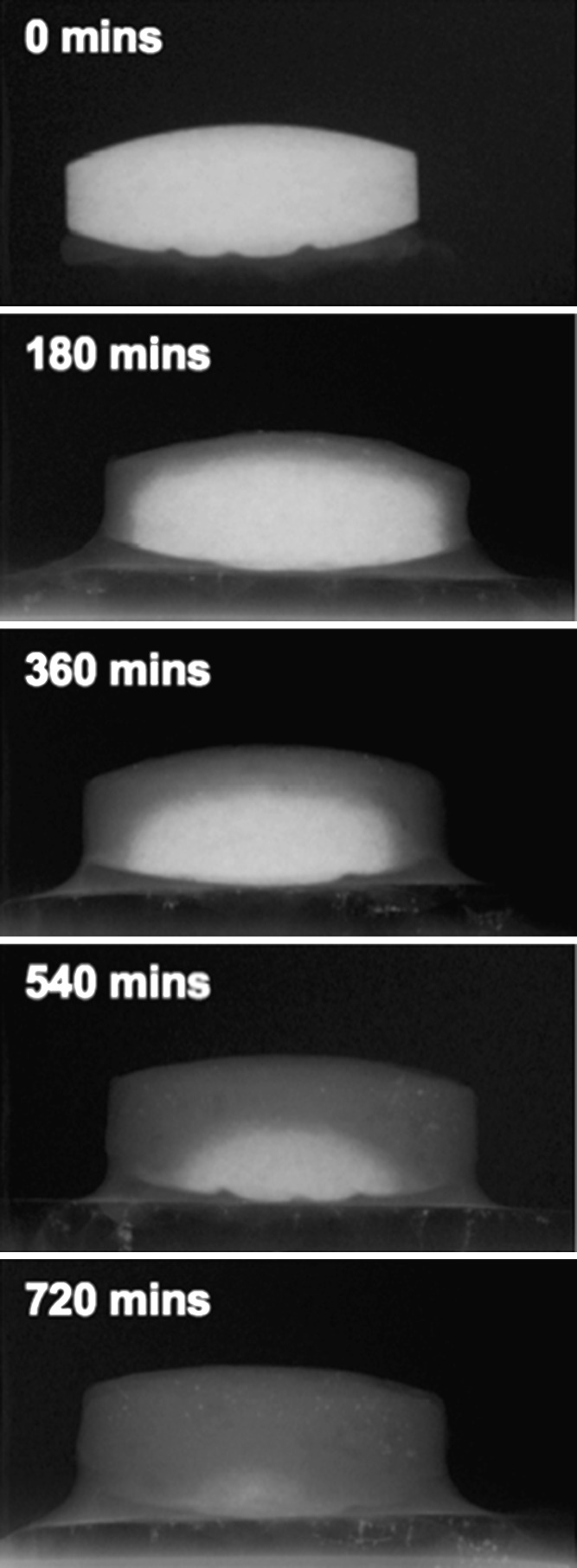
Two-dimensional radiographs of a single ferrous sulphate matrix tablet, taken at 180 min intervals, during the dissolution process are shown in Fig. 1. The large density variation between the ferrous sulphate (approximately 1.9 g.cm−3) and the other components of the matrix (typically ≤1.5 g cm−3) allow clear identification of the active component dissolution. From Fig. 1, it can be seen that the iron component decreases radially converging on a central point, which at 720 min was indicative of complete dissolution. Furthermore, the uniform nature of this process suggests an open-pore network within the inert matrix.
Fig. 1.
Two-dimensional radiographs of an inert matrix tablet imaged at 180-min intervals
In Vitro and XCMT Release Rate Comparison
Analysis of the in vitro release rate of the ferrous sulphate from the inert matrix (n = 6) suggested that the rate decreased with respect to time. This is likely due to a series of dynamic processes occurring (1,10): (1) increased liquid penetration time as the ferrous core volume is reduced (2) increased time for ferrous sulphate diffusion into the surrounding media as the core boundary to matrix boundary distance increases and (3) reduction in the core volume with respect to time. Whilst this process is logically and mathematically sound, the radiographs (Fig. 1) clearly show this dynamic process.
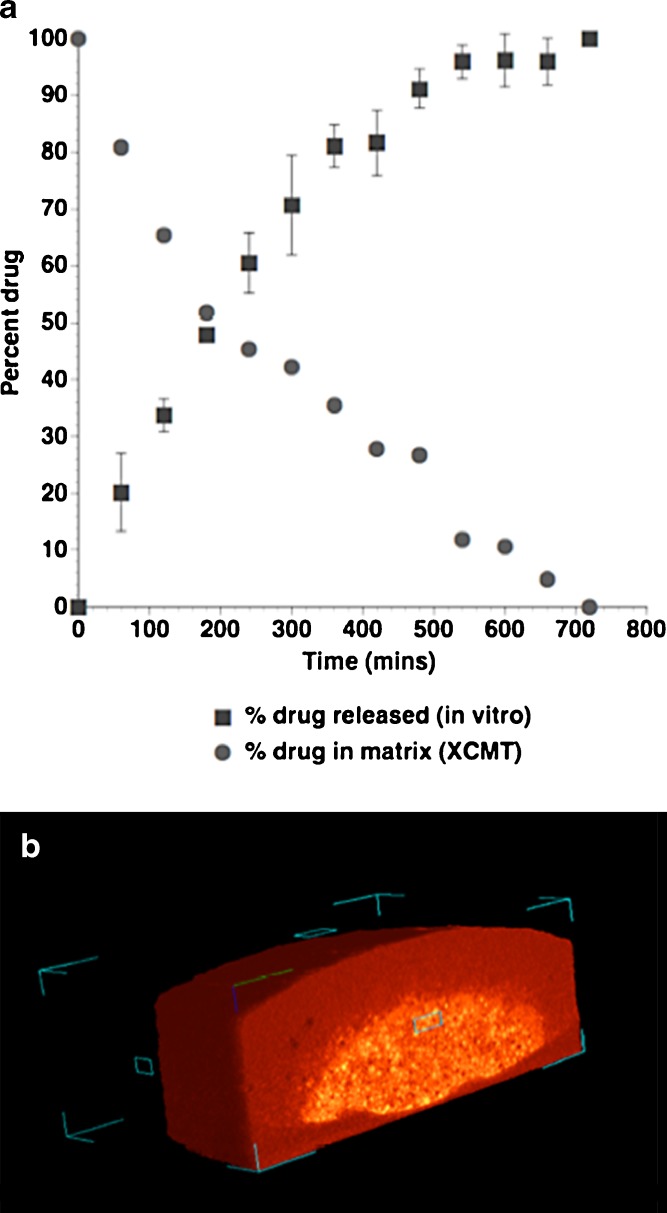
To further understand this dynamic process in a quantifiable manner, 3D tomography may be used to evaluate the volume components with respect to dissolution time. XCMT tomography images of the entire matrix were processed from images collected at 60 min time intervals, during the dissolution process. Data sets were treated to produce total matrix volume, void volume, and two “density” volumes based on a threshold value relating to the difference between the ferrous sulphate and inert matrix components. The remaining ferrous sulphate, calculated from XCMT is plotted with the in vitro dissolution drug release data in Fig. 2a, along with a representative 3D axial slice, taken after 360 min dissolution (Fig. 2b). Although the 3D images produce similar information to that obtained in the radiographs (Fig. 1), the remaining core volume can be calculated, and thus, a relationship between the in vitro release and internal structure be evaluated. In general, the amount of ferrous sulphate remaining in the matrix, calculated from XCMT data, was in good agreement with the released concentration, measured in vitro. Furthermore, calculation of theoretical ferrous sulphate released from the XCMT images (based on the total volume and ferrous sulphate volume parameters) resulted in percentage release values that were within the error (expressed as standard deviation) associated with the in vitro data sets in most cases. It is interesting to note however, that a deviation between the XCMT data and in vitro data was observed at 300, 360 and 480 min time points. It is possible that such observations are due to the specific sample preparation methods employed for XCMT, where the matrix under study was mounted on a fixed base. This procedure may result in a reduced rate of diffusion through the lower face of the tablet (as seen in Fig. 2B) and deviation from the in vitro data set (which do not have this constraint).
Fig. 2.
a Percent drug released measured by USP in vitro dissolution apparatus plotted with percent drug remaining in the tablet (obtained from the XCMT volume analysis). b Representative XCMT volume cross-section of the matrix tablet taken at 180 min dissolution time
CONCLUSION
The internal structure of Ferrogradumet® inert matrix tablets during dissolution was studied using XMCT using USP dissolution apparatus and a custom-built apparatus coupled with XMCT capabilities. Data collected with the novel integrated apparatus were in good agreement with the released drug concentration, measured with the standard in vitro dissolution test. The limitation of the XMCT has also been reported. However, this study has shown that XCMT may be a reliable and appropriate method for elucidation of physical transformations that occur within inert matrix-based systems, which to-date have only been studied via theoretical or destructive investigation. In conclusion, XMCT is a sensitive in vitro technique suitable for the study of inert matrix tablets with the potential to be used to investigate other solid dosage systems.
REFERENCES
- 1.Grassi M., Grassi G. Mathematical modelling and controlled drug delivery: matrix systems. Curr Drug Deliv. 2005;1:97–116. doi: 10.2174/1567201052772906. [DOI] [PubMed] [Google Scholar]
- 2.Varma M. V. S., Kaushal A. M., Garg A., Garg S. Factors affecting mechanism and kinetics of drug release from matrix-based oral controlled drug delivery systems. American Journal of Drug Delivery. 2004;2(1):43–57. doi: 10.2165/00137696-200402010-00003. [DOI] [Google Scholar]
- 3.Reza S., Quadir M. A., Haider S. S. Comparative evaluation of plastic, hydrophobic and hydrophilic polymers as matrices for controlled-release drug delivery. J Pharm Pharm Sci. 2003;6(2):282–91. [PubMed] [Google Scholar]
- 4.Frutos P., Pabon C., Lastres J. L., Frutos G. In vitro release of metoclopramide from hydrophobic matrix tablets: Influence of hydrodynamic conditions on kinetic release parameters. Chem Pharm Bull (Tokyo). 2001;49:1267–1271. doi: 10.1248/cpb.49.1267. [DOI] [PubMed] [Google Scholar]
- 5.Ferrero C., Bravo I., Jimenez-Castellanos M. R. Drug release kinetics and fronts movement studies from methyl methacrylate (MMA) copolymer matrix tablets: effect of copolymer type and matrix porosity. J Control Release. 2003;92(1–2):69–82. doi: 10.1016/S0168-3659(03)00301-8. [DOI] [PubMed] [Google Scholar]
- 6.Lemaire V., Belair J., Hildgen P. Structural modeling of drug release from biodegradable porous matrices based on a combined diffusion/erosion process. Int J Pharm. 2003;258(1–2):95–107. doi: 10.1016/S0378-5173(03)00165-0. [DOI] [PubMed] [Google Scholar]
- 7.Herman G. T. Image Reconstructions from Projections: The Fundamentals of Computerized Tomography. New York: Academic Press; 1980. [Google Scholar]
- 8.Stock S. R. X-ray microtomography of materials. Int Materials Reviews. 1999;44(4):141–164. doi: 10.1179/095066099101528261. [DOI] [Google Scholar]
- 9.Traini D., Loreti G., Jones A., Young P. M. X-Ray micro-computed tomography: novel application to investigate modified release systems. Microscopy and Analysis. 2008;61:15–17. [Google Scholar]
- 10.Villalobos R., Cordero S., Vidales A. M., Dominguez A. In silico study on the effects of matrix structure in controlled drug release. Physica A. 2006;367:305–18. doi: 10.1016/j.physa.2005.11.009. [DOI] [Google Scholar]
- 11.Farber L., Tardos G., Michaels J. N. Use of X-ray tomography to study the porosity and morphology of granules. Powder Technology. 2003;132:57–63. [Google Scholar]
- 12.Sinka I. C., Burch S. F., Tweed J. H., Cunningham J. C. Measurement of density variations in tablets using X-ray computed tomography. Int J Pharm. 2004;271(1–2):215–24. doi: 10.1016/j.ijpharm.2003.11.022. [DOI] [PubMed] [Google Scholar]
- 13.Ozeki Y., Watanabe Y., Inoue S., Danjo K. Comparison of the compression characteristics between new one-step dry-coated tablets (OSDRC) and dry-coated tablets (DC) Int J Pharm. 2003;259(1–2):69–77. doi: 10.1016/s0378-5173(03)00208-4. [DOI] [PubMed] [Google Scholar]
- 14.Hancock B. C., Mullarney M. P. X-ray microtomography of solid dosage forms. Pharm Technol. 2005;2:92–100. [Google Scholar]
- 15.Busignies V., Leclerc B., Porion P., Evesque P., Couarraze G., Tchoreloff P. Quantitative measurements of localized density variations in cylindrical tablets using X-ray microtomography. Eur J Pharm Biopharm. 2006;64(1):38–50. doi: 10.1016/j.ejpb.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 16.Ketcham R. A., Carlson W. D. Acquisition, optimization and interpretation of X-ray computed tomographic imagery: applications to the geosciences. Computers & Geosciences. 2001;27:381–400. doi: 10.1016/S0098-3004(00)00116-3. [DOI] [Google Scholar]
- 17.Morton E. J., Webb S., Bateman J. E., Clarke L. J., Shelton C. G. Three-dimensional x-ray microtomography for medical and biological applications. Phys Med Biol. 1990;35(7):805–20. doi: 10.1088/0031-9155/35/7/001. [DOI] [PubMed] [Google Scholar]
- 18.Huang T. T. Y., Jones A. S., Hong He L., Darendeliler M. A., Swaina M. V. Characterisation of enamel white spot lesions using X-ray micro-tomography. Journal of dentistry. 2007;35:737–743. doi: 10.1016/j.jdent.2007.06.001. [DOI] [PubMed] [Google Scholar]