Abstract
Resurfacing hemiarthroplasties were performed to treat advanced osteonecrosis of 20 femoral heads in 14 patients (median age, 19.8 years; range, 15.1–27.4 years), treated for hematologic cancer in childhood or adolescence. Seven hips in five patients were revised to total hip arthroplasties (THA) because of pain; three of these showed radiographic loosening of the femoral head resurfacing component. The median time from resurfacing to revision was 2.4 years (range, 0.9–4.8 years). Marginal Cox-regression analysis, adjusting for correlations owing to bilateral involvement, showed positive association of revision-free survival of the prosthesis with patient’s age; time from resurfacing to the end of anticancer therapy, end of glucocorticosteroid therapy; percentage of joint space at the last radiograph; and size of the lesion has a negative association with revision-free survival. Because of this study’s exploratory nature, p values were not adjusted for the number of statistical comparisons. Among 14 patients, the probability of not requiring resurfacing prosthesis revision was 66% (SE, ±15%; 95% CI, 44%–100%) at 3 years. Osteonecrosis of the femoral head in young patients treated for hematologic cancer in childhood or adolescence poses a serious challenge to the orthopaedic surgeon. The data of this preliminary study suggest that in selected patients resurfacing hemiarthroplasty may delay the need for THA for 3–7 years.
Level of Evidence: Level II, prognostic study. See the Guidelines for Authors for a complete description of levels of evidence.
Introduction
Evidence-based guidelines for treating advanced osteonecrosis of the femoral head (ONFH) in patients treated for hematologic cancer in childhood and adolescence are urgently needed. Each year in the United States, approximately 2000 patients become 5-year survivors of childhood acute lymphoblastic leukemia (ALL), which is the most common childhood cancer and accounts for 76% of pediatric leukemias [9]. The majority of these long-term survivors of childhood ALL are expected to have a normal lifespan [24]. However, ONFH has become one of the most severe long-term complications of treatment for pediatric hematologic cancers [20, 26, 29]. Osteonecrosis affects 9% to 39% of pediatric patients with ALL [20, 22]. Although other anticancer medications have been implicated in ONFH [26], high-dose glucocorticosteroid (GCS) therapy, a well-known risk factor for osteonecrosis development [10], is the most common cause in these patients.
The prognosis of ONFH depends heavily on the size of the necrotic lesion; involvement greater than 30% of the femoral head leads to progressive femoral head collapse and further joint deterioration [14]. When a large osteonecrotic lesion of the femoral head collapses, the resulting pain and deformity frequently require a major reconstructive procedure for symptom control. A THA offers good functional and symptomatic outcome; however, long life expectancy and increased physical activity in young patients puts them at increased risk for multiple revisions resulting from implant failure [15, 23]. In one study, at 16 years followup, revision rates for THA performed for patients with ONFH differed by greater than 20% between patients younger and older than 50 years at the time of THA; younger patients had survival of approximately 60% at 17 years of followup [23]. In another study of patients younger than 30 years at the time of THA, the prosthesis survival free of revision was 79% at 5 years followup [3]. If we assume life expectancy of the prosthesis before revision to be 15 to 20 years, a long-term survivor of ALL receiving the first THA in his or her twenties may need two to four revisions in later life. Revisions and rerevisions are associated with subsequent lower quality of life and patient satisfaction, higher failure rates (26%–57% at 10 years) and more early (readmission, infection, hip dislocation) and late (nonunion of the trochanter, thigh pain, heterotopic bone formation, rerevision, recurrent dislocation) complications than primary THA [8, 16, 18, 19]. Because of these considerations, bone-conserving procedures such as resurfacing provide a possible alternative for young patients with advanced ONFH [1]. Resurfacing hemiarthroplasty preserves the acetabulum and proximal femur and does not violate the femoral medullary canal. Several authors suggest performing a subsequent THA after a resurfacing procedure is not more difficult than performing a primary THA [2, 13]. However, utility of the resurfacing procedure as treatment for ONFH is a subject of disagreement. One study suggested femoral surface replacement is not a reasonable option for treatment of ONFH, because the results of this procedure are unpredictable, with an overall failure rate (based on Harris hip scores and revisions) of 64.8% at 33 months followup [27]. Others offer this procedure as a treatment option for ONFH, based on satisfactory results in 62.5% of patients at 3 years followup [1].
We addressed this controversy in a preliminary study by: (1) evaluating prosthesis survival, (2) attempting to find relationships between various clinical factors and revision, (3) describing modes of failure, and (4) ascertaining clinical outcomes of resurfacing for treatment of ONFH in patients treated for hematologic cancers in childhood and adolescence.
Materials and Methods
We retrospectively reviewed the medical records of 14 consecutive patients who underwent resurfacing hemiarthroplasties of 20 femoral heads to treat advanced ONFH (Ficat Stages III and IV) between January 1, 1998, and December 31, 2005. The group consisted of four males and 10 females whose median age at first resurfacing was 19.8 years (range, 15.1–27.4 years). Eight patients had the primary diagnosis of ALL. Four had nonHodgkin lymphoma, one had acute myeloblastic leukemia, and one had chronic lymphoblastic leukemia. All patients had intermittently disabling or severe, continuously disabling pain in the hip. Before resurfacing hemiarthroplasty, six patients were treated nonoperatively, and eight (12 hips) underwent core decompression. The median time from diagnosis of osteonecrosis until resurfacing hemiarthroplasty was 1.5 years (range, 0.6–6.5 years). The median followup after resurfacing and before revision or last clinical followup was 2.6 years (range, 0.9–7.2 years). This retrospective study was reviewed and approved by the St Jude Children’s Research Hospital Institutional Review Board. Data collection and management adhered to the Health Information Portability and Accountability Act of 1996.
These procedures were performed by one orthopaedic surgeon (MDN). The indications for resurfacing hemiarthroplasty included intractable pain in patients with advanced ONFH. Prerequisites included the absence of or minimal degenerative disease of the hip on preoperative radiographs. Contraindications included advanced degenerative disease of the acetabulum. We used the cobalt-chrome femoral head cemented component CONSERVE® (Wright Medical Technology, Inc, Arlington, TN), which is available in 1-mm increments between 36 and 57 mm. Magnetic resonance imaging was used for preoperative sizing of the implant. The posterolateral approach was used during the procedure. After surgical exposure, the hip was dislocated posteriorly and the acetabular cartilage was visually inspected. The guide pin for the resurfacing arthroplasty then was placed using a free-hand technique down the center of the femoral neck. Position of the pin was confirmed on biplane fluoroscopic images. The femoral head was reamed sequentially with progressively smaller reamers until the required head diameter was reached. The guide for the stem reamer then was placed down the femoral neck. A trial femoral head was placed onto the neck and then into the acetabulum and checked for fit. The bone was prepared for cementation with drilling of sclerotic bone and curettage of cystic areas followed by pulsatile lavage. The prosthesis then was affixed with bone cement and held until the cement dried. The hip was reduced and put through a range of motion to ensure stability. The incision was closed in layers. Immediate postoperative physical therapy with weightbearing to tolerance was started on postoperative Day 1.
We considered revision to a THA as the end point. Using pain and range of motion descriptions from the medical records, we retrospectively classified clinical symptoms similarly to the pain and motion components of the Musculoskeletal Tumor Society functional evaluation system [6]. Pain was classified into five categories: 0, no pain; 1, modest/nondisabling pain, no pain during rest, only occasional nonnarcotic analgesics needed; 2, modest/nondisabling pain that also happens at rest, nonnarcotic analgesics are needed regularly but narcotic analgesics are not needed; 3, intermittently disabling pain, intermittent narcotic analgesics needed; and 4, severe, continuously disabling pain. Range of motion was classified into four categories based on the combined degrees of active flexion, extension, abduction, adduction, and rotation in the hip: excellent, greater than 180°; good, 120° to 180°; fair, 60° to 120°; and poor, 0° to 60°.
We (EJK, MDN, SCK) used preoperative radiographs and MRI of the hips to determine the Ficat stage of osteonecrosis [7], depression of the collapsed segment [28], and percent volume of the femoral head that was necrotic [11, 14]. We also evaluated the radiographic joint space on the last postoperative radiograph. The percentage of remaining joint space was expressed as the ratio of the joint space of the affected hip divided by half of the joint space of the contralateral (normal) hip. If the hip that was contralateral to the prosthesis had radiographic evidence of ONFH, then an earlier radiograph of the patient was used to determine the normal width of the joint space [4].
We used the marginal Cox-regression model [17] to assess the association between the revision-free survival of individual hips and patients’ demographic variables and risk factors. The marginal Cox model adjusts for correlations attributable to bilateral involvement. The revision-free survival of hips is defined as the interval from the resurfacing date to the revision date or the last followup. The p values were not adjusted for the number of statistical comparisons made because of the exploratory nature of this study. We also produced a Kaplan-Meier probability curve of the revision-free period. We defined the revision-free period as the interval from the resurfacing date to the earliest revision date among the paired hips or the last followup. Analyses were performed using SAS V9 software (SAS Institute Inc, Cary, NC).
Results
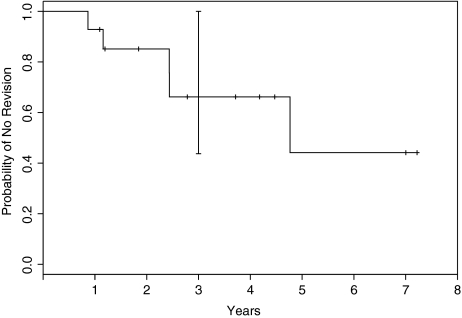
The revision-free period was 66% (se, ±15%; 95% CI, 44%–100%) at 3 years (Fig. 1). One patient died of leukemia relapse 13 months after the surgery without implant revision. Seven of the 14 hips were revised with THA during the entire study period. The median time from resurfacing to revision was 2.4 years (range, 0.9–4.8 years).
Fig. 1.
A Kaplan-Meier survival analysis with 95% confidence intervals (dotted lines) of patients remaining free of implant revision is shown. The probability of a patient not requiring revision to THA was 66% at 3 years.
The revision-free survival was positively associated with age of the patient at resurfacing (p = 0.014), time from resurfacing until the end of anticancer therapy (p = .003), end of glucocorticosteroid therapy (p = 0.023), and radiographic joint space on the last postoperative radiograph (p = 0.043). The size of the lesion was negatively associated with revision-free survival (p = 0.004).
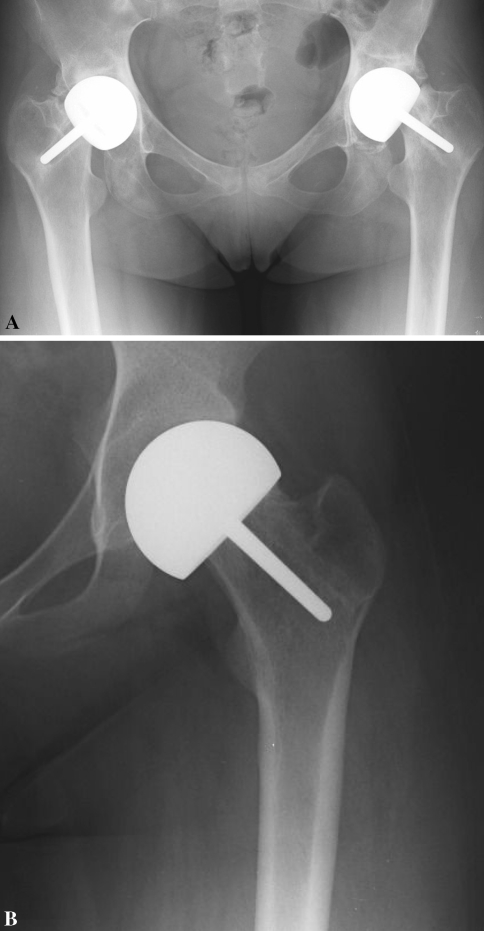
The modes of failure included component loosening and pain (Fig. 2A). Development of debilitating refractory pain without involvement of the component was the cause for revision to THA in five hips of four patients (Fig. 2B). In all eight hips that underwent revision, visual intraoperative inspection revealed wear and fibrillation of the acetabular cartilage. No perioperative complications occurred in our patient cohort. At their 1-month postoperative checkup, all patients reported better pain control and greater range of motion in the hip.
Fig. 2A–B.
(A) This radiograph of the hips of a 20-year-old female survivor of acute lymphoblastic leukemia (ALL) shows bilateral narrowing of the joint spaces, sclerotic appearance of the acetabula, and regions of lucency surrounding the implant stems, indicating loss of fixation of the implants in the proximal femurs. The implants are displaced inferiorly. (B) This radiograph of a hip of an 18-year-old female survivor of ALL shows a well-fixed implant but a narrow joint space. Both patients underwent revision to THA bilaterally.
Of the 11 surviving hips, the ranges of motion at the last followup varied: nine had an excellent range, one had a good range, and one had a fair range. The pain level at the last followup was classified as Class 1 (modest/nondisabling, with only occasional need for nonnarcotic analgesics) in eight hips, Class 2 (modest/nondisabling pain that required regular nonnarcotic analgesics) in two hips, and Class 3 (severe pain, occasionally requiring intermittent narcotic analgesia but not yet necessitating a revision to THA) in one hip. Four hips (in two patients) survived for 7 years after the resurfacing procedure and showed excellent range of motion and only occasional pain that was well controlled with nonsteroidal antiinflammatory medications.
Discussion
There is no consensus regarding the best treatment of postcollapse ONFH in childhood cancer survivors. One recent study of THA showed excellent short-term results, however because of concerns regarding long-term outcomes, THA sometimes still is delayed in young patients [12]. Bone-sparing procedures such as resurfacing hemiarthroplasty and free vascularized fibular graft are being offered for patients younger than 30 years [12]. Because resurfacing hemiarthroplasty preserves the acetabulum and proximal femur and does not violate the femoral medullary canal, it does not compromise the success of the subsequent THA, unlike the free vascularized fibular graft [5]. We therefore examined prosthesis survival, factors that may influence prosthesis survival, modes of failure, and clinical outcomes of resurfacing hemiarthroplasties in survivors of hematologic cancer in childhood or adolescence.
Our study is limited by the small number of patients, the short followup, and retrospectively collected data on function, and we consider the study preliminary. The small number of patients is unavoidable because of the relative infrequency of the indications for the procedure, but we presume the survival data would apply to larger numbers. With small numbers it is difficult to identify factors predicting outcome, although we identified several; other factors might not be identified owing to an underpowered study. We cannot say, however, whether longer followup would result in considerably more failures. We did not prospectively collect the MSTS functional scores, and there may be some errors in retrospectively classifying pain and range of motion status; we believe these would not be substantially differing interpretations.
The Kaplan-Meier estimate of the revision-free period was 66% (standard error, ±15%; 95% CI, 44%–100%) at 3 years (Fig. 1). In another study of young patients, the resurfacing implant survival rate was reportedly 75.9% at 3 years [1]. Overall, seven of 20 hips were revised with THA. The revision-free survival of hips had a negative association with lesion size. All hips with lesions occupying less than 33% of the femoral head (eight hips) survived without revision. All hips with lesions occupying greater than 66% of the femoral head (five hips) needed revision to THA (Table 1). Among hips with lesions occupying between 33% and 66%, three (60%) survived without THA and two (40%) required THA. These data suggest that small lesion size may predict better outcome of resurfacing hemiarthroplasty; however, the numbers are too small to make any definitive statement.
Table 1.
Summary of patients’ data
| Number in series | Primary diagnosis | Gender | Age (years) | Side | Preoperative Ficat stage | Lesion size volume% femoral head | Treatment with core decompression | Years between end of cancer treatment and resurfacing | Depression of the collapsed segment (mm) | Followup (years) | Reason for revision | Revision to THA |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | ALL | F | 15.1 | L | 3 | 32.7 | no | 1.0 | 11.0 | 1.2 | no | |
| 2 | ALL | M | 22.6 | R | 3 | 57.7 | no | 1.3 | 12.0 | 7.8 | no | |
| L | 4 | 62.2 | no | 1.8 | 10.0 | 7.2 | no | |||||
| 3 | NHL | F | 26.8 | L | 4 | * | no | 0.5 | 3.0 | 7.6 | no | |
| R | 4 | * | no | 1.1 | 4.0 | 7.0 | no | |||||
| 4 | ALL | F | 17.9 | R | 4 | 95.6 | yes | 0.0 | 8.0 | 3.3 | loosening | yes |
| L | 4 | 78.1 | yes | 0.3 | 6.0 | 2.4 | loosening | yes | ||||
| 5 | ALL | F | 19.3 | R | 3 | 66.3 | no | 0.0 | 2.0 | 2.4 | loosening | yes |
| 6 | CML | M | 20.7 | R | 4 | 29.3 | no | 6.5 | 15.0 | 4.5 | no | |
| 7 | ALL | M | 21.1 | L | 4 | 31.1 | yes | 1.6 | 4.5 | 5.7 | no | |
| R | 4 | 21.0 | yes | 3.0 | 4.5 | 4.2 | no | |||||
| 8 | ALL | F | 17.1 | L | 4 | 43.2 | yes | 0.1 | 5.0 | 0.9 | pain | yes |
| 9 | ALL | F | 15.8 | R | 4 | 60.2 | yes | 0.7 | 12.0 | 4.8 | pain | yes |
| L | 4 | 39.3 | yes | 0.9 | 10.0 | 5.2 | no | |||||
| 10 | ALL | F | 18.1 | L | 4 | 70.5 | yes | 0.0 | 9.0 | 1.2 | pain | yes |
| R | 4 | 78.8 | yes | 0.0 | 13.0 | 1.3 | pain | yes | ||||
| 11 | NHL | F | 19.6 | L | 4 | 21.2 | yes | 2.6 | 2.5 | 3.7 | no | |
| 12 | NHL | F | 24.2 | L | 3 | 7.7 | yes | 4.7 | 1.5 | 2.8 | no | |
| 13 | AML | F | 18.7 | L | 3 | 21.0 | no | 4.8 | 2.5 | 1.8 | no | |
| 14 | NHL | F | 23.9 | L | 4 | 14.1 | yes | 3.2 | 2.5 | 1.1 | no |
The revision-free survival of hips had a positive association with patient’s age at resurfacing. Although one previously cited study of young patients suggested an implant survival rate at 75.9% at 3 years [1], studies involving older patients had higher implant survival rates of 79% [2] and 91% [13] at 5 years. Implant survival after resurfacing may be influenced by patient age. A negative influence of young age on the outcome of THA was reported [15].
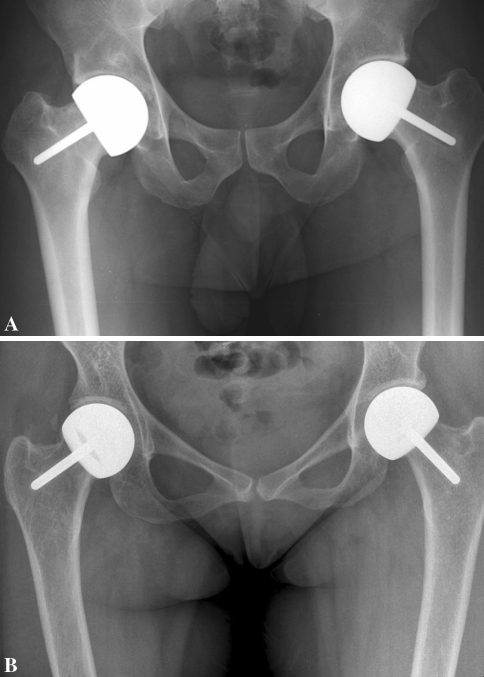
The utility of resurfacing hemiarthroplasty probably lies in delaying THA in a young patient with ONFH who otherwise would have received a THA. The important questions are, how long of a delay is meaningful and are there any factors that could extend this period? At the time of this review, most of our patients were only 2 to 3 years postresurfacing procedure. However two patients operated on at the beginning of the series have very good clinical and radiographic outcomes 7 years after surgery (Fig. 3). For these two patients, resurfacing provided lasting symptom control and succeeded in delaying THA for at least 7 years.
Fig. 3A–B.
(A) This radiograph shows intact resurfacing implants and well-preserved joint spaces in a 29-year-old male survivor of childhood acute lymphoblastic leukemia 7 years after the procedure. (B) This radiograph shows intact resurfacing implants and well-preserved joint spaces in a 34-year-old female survivor of childhood nonHodgkin lymphoma 7 years after the procedure.
We found a positive association between the revision-free survival of the prosthesis and radiographic joint space on the last postoperative radiograph. Dalldorf et al. showed loss of joint space, as observed on radiographs, accurately predicted deterioration of hyaline cartilage in patients with bipolar and unipolar hemiarthroplaties [4]. The resurfacing hemiarthroplasty creates articulation of the implanted metal femoral component with the acetabular hyaline cartilage similar to bipolar and unipolar hemiarthroplasties. Therefore, we inferred the loss of acetabular cartilage, as reflected by the loss of joint space, could play a role in the survivorship of the resurfacing implant. Hip pain presumably associated with cartilage loss was the primary cause for conversion to THA in this series. Currently only visual intraoperative inspection at the time of resurfacing is used to determine if any major damage of acetabular cartilage is present. This can lead to underestimation of damage to the cartilage and results in early failure attributable to pain associated with acetabular cartilage loss. Future research should be directed at determining the integrity of the acetabular cartilage at the time of resurfacing.
Prospective studies and careful examination of accumulated experience are needed to recommend the best treatment for ONFH in patients treated for hematologic cancer in childhood and adolescence. These patients exhibit several unique aspects of the complication: most have bilateral disease [21] and additional osteonecrotic lesions in the metaphyses, diaphyses, and epiphyses of long bones. As a result, these patients commonly experience frequent, simultaneous involvement of several joints, including the hips, shoulders, knees, ankles, and elbows. Multiple-joint osteonecrosis can be devastating to the quality of life in these patients.
The cure rate for pediatric ALL is currently 80% and is projected to increase to 90% [25]. The majority of patients with ALL are expected to have a normal lifespan [24]. Long-term outcome of multiple-joint arthroplasties in these patients has not been studied. As a result of the high activity level, long life expectancy, and possibly the complicating issue of widespread concurrent bone mineral deficits [21], the necessity of multiple future revisions is a concern for these young survivors. Therefore, in patients with compromised bone quality it is best to perform the most bone-conserving surgery possible. Another advantage is potential staging of a THA. Subsequent THAs further reduce bone stock and are associated with a lower success rate. Therefore, bone-conserving procedures that delay the need for THA are of great interest in this population.
Our preliminary results show that resurfacing hemiarthroplasty may delay the need for THA for 3 years in the majority of patients and for 7 to 8 years in select patients. Another theoretical advantage of resurfacing hemiarthroplasty is the potential to perform a total resurfacing in the future should acetabular wear occur. Hypothetically, the femoral component can be left in place and the acetabulum can be resurfaced.
We examined the outcomes of resurfacing hemiarthroplasties performed on 20 femoral heads in 14 patients treated for hematologic cancers during childhood or adolescence. The probability of a patient not requiring a revision to THA was 66% at 3 years. Several factors associated with revision-free survival of hips were identified: older age, longer time between the end of cancer treatment and resurfacing, longer time between diagnosis of osteonecrosis and resurfacing, larger percentage of joint space on the last radiograph, and smaller lesion size.
Acknowledgment
We thank Dr. James Boyett for help with statistical analysis and data interpretation in this study.
Footnotes
One or more authors (EJK, SCK, SNR, JW, LB, MDN) have received funding from the American Lebanese Syrian Associated Charities (ALSAC).
Each author certifies that his or her institution has approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research.
References
- 1.Adili A, Trousdale RT. Femoral head resurfacing for the treatment of osteonecrosis in the young patient. Clin Orthop Relat Res. 2003;417:93–101. [DOI] [PubMed]
- 2.Beaule PE, Schmalzried TP, Campbell P, Dorey F, Amstutz HC. Duration of symptoms and outcome of hemiresurfacing for hip osteonecrosis. Clin Orthop Relat Res. 2001;385:104–117. [DOI] [PubMed]
- 3.Chandler HP, Reineck FT, Wixson RL, McCarthy JC. Total hip replacement in patients younger than thirty years old: a five-year follow-up study. J Bone Joint Surg Am. 1981;63:1426–1434. [PubMed]
- 4.Dalldorf PG, Banas MP, Hicks DG, Pellegrini VD Jr. Rate of degeneration of human acetabular cartilage after hemiarthroplasty. J Bone Joint Surg Am. 1995;77:877–882. [DOI] [PubMed]
- 5.Davis ET, McKee MD, Waddell JP, Hupel T, Schemitsch EH. Total hip arthroplasty following failure of free vascularized fibular graft. J Bone Joint Surg Am. 2006;88(suppl 3):110–115. [DOI] [PubMed]
- 6.Enneking WF (ed). Modification of the system for functional evaluation in the surgical management of musculoskeletal tumors. Limb Salvage in Musculoskeletal Oncology (Bristol-Myers/Zimmer Orthopedic Symposium). New York, NY: Churchill Livingstone; 1987:626–639.
- 7.Ficat RP. Idiopathic bone necrosis of the femoral head: early diagnosis and treatment. J Bone Joint Surg Br. 1985;67:3–9. [DOI] [PubMed]
- 8.Fyda TM, Callaghan JJ, Olejniczak J, Johnston RC. Minimum ten-year follow-up of cemented total hip replacement in patients with osteonecrosis of the femoral head. Iowa Orthop J. 2002;22:8–19. [PMC free article] [PubMed]
- 9.Gurney JG, Severson RK, Davis S, Robison LL. Incidence of cancer in children in the United States: sex-, race-, and 1-year age-specific rates by histologic type. Cancer. 1995;75:2186–2195. [DOI] [PubMed]
- 10.Hernigou P, Beaujean F, Lambotte JC. Decrease in the mesenchymal stem-cell pool in the proximal femur in corticosteroid-induced osteonecrosis. J Bone Joint Surg Br. 1999;81:349–355. [DOI] [PubMed]
- 11.Hernigou P, Lambotte JC. Volumetric analysis of osteonecrosis of the femur: anatomical correlation using MRI. J Bone Joint Surg Br. 2001;83:672–675. [DOI] [PubMed]
- 12.Hungerford DS. Treatment of osteonecrosis of the femoral head: everything’s new. J Arthroplasty. 2007;22(4 suppl 1):91–94. [DOI] [PubMed]
- 13.Hungerford MW, Mont MA, Scott R, Fiore C, Hungerford DS, Krackow KA. Surface replacement hemiarthroplasty for the treatment of osteonecrosis of the femoral head. J Bone Joint Surg Am. 1998;80:1656–1664. [DOI] [PubMed]
- 14.Karimova EJ, Rai SN, Howard SC, Neel M, Britton L, Pui CH, Kaste SC. Femoral head osteonecrosis in pediatric and young adult patients with leukemia or lymphoma. J Clin Oncol. 2007;25:1525–1531. [DOI] [PubMed]
- 15.Letson GD, D’Ambrosia RD, Aguilar EA, Wagespack A. Activity relationships of total hip arthroplasty in patients with osteonecrosis and osteoarthritis. Orthopedics. 1996;19:665–668. [DOI] [PubMed]
- 16.Lie SA, Havelin LI, Furnes ON, Engesaeter LB, Vollset SE. Failure rates for 4762 revision total hip arthroplasties in the Norwegian Arthroplasty Register. J Bone Joint Surg Br. 2004;86:504–509. [PubMed]
- 17.Lin DY. Cox regression analysis of multivariate failure time data: the marginal approach. Stat Med. 1994;13:2233–2247. [DOI] [PubMed]
- 18.Lubbeke A, Katz JN, Perneger TV, Hoffmeyer P. Primary and revision hip arthroplasty: 5-year outcomes and influence of age and comorbidity. J Rheumatol. 2007;34:394–400. [PubMed]
- 19.Mahomed NN, Barrett JA, Katz JN, Phillips CB, Losina E, Lew RA, Guadagnoli E, Harris WH, Poss R, Baron JA. Rates and outcomes of primary and revision total hip replacement in the United States medicare population. J Bone Joint Surg Am. 2003;85:27–32. [DOI] [PubMed]
- 20.Mattano LA Jr, Sather HN, Trigg ME, Nachman JB. Osteonecrosis as a complication of treating acute lymphoblastic leukemia in children: a report from the Children’s Cancer Group. J Clin Oncol. 2000;18:3262–3272. [DOI] [PubMed]
- 21.Nachman JB. My aching bones: skeletal complications of acute lymphoblastic leukemia. Pediatr Blood Cancer. 2006;46:1. [DOI] [PubMed]
- 22.Ojala AE, Paakko E, Lanning FP, Lanning M. Osteonecrosis during the treatment of childhood acute lymphoblastic leukemia: a prospective MRI study. Med Pediatr Oncol. 1999;32:11–17. [DOI] [PubMed]
- 23.Ortiguera CJ, Pulliam IT, Cabanela ME. Total hip arthroplasty for osteonecrosis: matched-pair analysis of 188 hips with long-term follow-up. J Arthroplasty. 1999;14:21–28. [DOI] [PubMed]
- 24.Pui CH, Cheng C, Leung W, Rai SN, Rivera GK, Sandlund JT, Ribeiro RC, Relling MV, Kun LE, Evans WE, Hudson MM. Extended follow-up of long-term survivors of childhood acute lymphoblastic leukemia. N Engl J Med. 2003;349:640–649. [DOI] [PubMed]
- 25.Pui CH, Evans WE. Treatment of acute lymphoblastic leukemia. N Engl J Med. 2006;354:166–178. [DOI] [PubMed]
- 26.Sala A, Mattano LA Jr, Barr RD. Osteonecrosis in children and adolescents with cancer: an adverse effect of systemic therapy. Eur J Cancer. 2007;43:683–689. [DOI] [PubMed]
- 27.Squire M, Fehring TK, Odum S, Griffin WL, Bohannon MJ. Failure of femoral surface replacement for femoral head avascular necrosis. J Arthroplasty. 2005;20(7 suppl 3):108–114. [DOI] [PubMed]
- 28.Steinberg ME, Hayken GD, Steinberg DR. A quantitative system for staging avascular necrosis. J Bone Joint Surg Br. 1995;77:34–41. [PubMed]
- 29.Strauss AJ, Su JT, Dalton VM, Gelber RD, Sallan SE, Silverman LB. Bony morbidity in children treated for acute lymphoblastic leukemia. J Clin Oncol. 2001;19:3066–3072. [DOI] [PubMed]