Abstract
Fetal tendons and skin heal regeneratively without scar formation. Cells isolated from these fetal tissues exhibit enhanced cellular migration and collagen production in comparison to cells from adult tissue. We determined whether fetal and adult fibroblasts isolated from the anterior cruciate ligament (ACL), a tissue that does not heal regeneratively, exhibit differences in cell migration rates and collagen elaboration. An in vitro migration assay showed fetal ACL fibroblasts migrated twice as fast as adult ACL fibroblasts at a rate of 38.90 ± 7.69 μm per hour compared with 18.88 ± 4.18 μm per hour, respectively. Quantification of Type I collagen elaboration by enzyme-linked immunosorbent assay showed fetal ACL fibroblasts produced four times the amount of Type I collagen compared with adult ACL fibroblasts after 7 days in culture. We observed no differences in Type III collagen with time for adult or fetal ACL fibroblasts. Our findings indicate fetal ACL fibroblasts are intrinsically different from adult ACL fibroblasts, suggesting the healing potential of the ACL may be age-dependent.
Introduction
The ACL is one of four ligaments that stabilize the knee. It is a regularly oriented, dense connective tissue whose primary function is to prevent anterior displacement of the tibia in relation to the femur. Annually, one in 3,000 individuals in the United States rupture their ACLs with 75,000 to 100,000 of these patients seeking reconstructive surgery [12, 18]. The majority of active patients seek reconstructive surgery as a result of the inability of the ACL to heal after injury [22, 44]. In contrast, the medial collateral ligament (MCL), which is also a ligamentous structure of the knee, does not require surgical intervention and often heals with nonoperative treatment (ie, an external brace) [55, 57]. Combinations of factors including the nutritional environment provided by the synovial fluid and vascular supply, mechanical loading, and intrinsic properties of the ACL may contribute to the ligament’s impaired healing response.
During the wound healing process, native tissue cells migrate to repopulate the site of injury and begin production of tissue-specific extracellular matrix (ECM) proteins [20, 42]. Studies comparing fibroblasts from the ACL and MCL suggest inferior wound healing properties of ACL fibroblasts. Specifically, fibroblasts migrating from MCL explants in vitro migrate faster in comparison to ACL fibroblasts [19, 25, 47]. Differences in migration rates and ECM gene expression and production between MCL and ACL fibroblasts have been confirmed in various species [4, 14, 15, 19, 25, 30, 37, 47, 53]. These observations support the premise that enhanced cellular properties contribute to greater healing capacity. The predominant ECM protein in the normal ACL is Type I collagen. During the ligamentous healing process, Type III collagen levels are higher early on and are replaced later by Type I collagen [2, 8, 24]. Scarlessly healing fetal tissues also have elevated levels of Type III collagen in comparison to adult tissues, suggesting specific collagen expression profiles associated with regenerative healing [11, 36, 51]. Although the secretion of Type I collagen is critical to restore tissue structure and function in the healing ACL, the presence of other collagens, such as Type III collagen, also may play a role in wound healing progression.
The rate of healing in rodents, monkeys, and humans decreases with age [6, 23, 48]. Early gestational incisional wounds of fetal skin are characterized by rapid, scarless wound repair. Fetal skin wounds at later gestational ages, however, heal with fibrosis and scarring. Subcutaneous transplantation of human fetal skin into adult athymic mice results in scarless healing with the wound site composed mainly of human fetal collagen [35]. These findings suggest fetal fibroblasts have intrinsic characteristics that influence scarless wound repair. Increased collagen synthesis and faster migration rates of fetal skin fibroblasts, in comparison to adults, are cellular properties believed to facilitate the fetal healing process [10, 11, 26]. Fetal tendon exhibits similar scarless healing characteristics as the dermis [7, 11, 33]. However, the healing response of all fetal tissues is not identical. Longaker and colleagues reported incisional wounds in the diaphragm of 100-day-old fetal lambs healed with scar formation [34]. Similarly, Meuli et al. observed dense fibrous intraabdominal adhesions in fetal and adult gastric wounds [40]. The variable regenerative capacity of fetal tissues suggests impaired healing of the ACL, which continues to be a challenge clinically, may be a function of maturation. Given intrinsic cellular properties govern the wound healing response, we presumed there would be age-dependent cellular characteristics of ACL fibroblasts.
We therefore hypothesized fetal ACL fibroblasts would exhibit increased migration rates and elaborate greater quantities of Types I and III collagen in comparison to adult ACL fibroblasts.
Materials and Methods
We conducted experiments on ACL fibroblasts harvested from the ACLs of six bovine limbs from six different animals (three adult and three fetal). The migration assay was performed on seven glass squares for each animal. Collagen quantification by an indirect enzyme-linked immunosorbent assay (ELISA) and DNA proliferation were conducted on three samples for each animal. Cellular response (migration rate, collagen production, or total DNA) was analyzed with respect to time and age (fetal verse adult). Data are presented as mean ± standard deviation. All statistical analyses were performed using Systat software (San Jose, CA).
We purchased all cell culture materials from Invitrogen (Carlsbad, CA) unless stated otherwise. The midportions of ACLs were isolated from skeletally mature 3-year-old adult and third-trimester fetal bovine hind limbs based on previous methods [25]. We harvested six ACL explants from six different animals; three adult and three fetal ligaments. Ligament specimens were minced into 0.5-cm3 pieces, rinsed with phosphate-buffered saline (PBS), and cultured in 60-mm tissue culture-treated polystyrene (TCPS) dishes containing Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 20% fetal bovine serum (FBS) (Hyclone, Logan, UT), 1% sodium bicarbonate (0.075% solution), 10% fungizone/amphotericin B (25 μg/mL), and 1% penicillin (100 U/mL)-streptomycin (100 μg/mL). We used second-passage cells grown from three adult and three fetal explants to obtain adequate cell numbers for all subsequent studies.
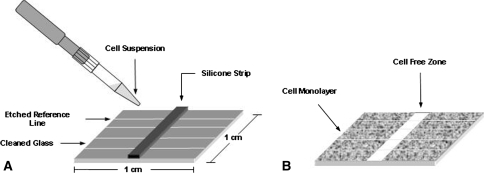
An in vitro migration assay, as described by Stenn, was adapted to a glass surface [52]. In brief, glass squares were cut (1 cm2) and etched with four parallel reference lines. We cleaned the squares by successive 5-minute treatments in hexane, acetone, ethanol, and sterile water followed by 5 minutes of sonication at room temperature. One, 1-mm wide silicone rubber strip (McMaster-Carr, New Brunswick, NJ) was placed perpendicular to the etched reference lines of each dry glass square (Fig. 1A). Individual glass squares were placed in a single well of 24-well plates. A total of 37.5 μL of a 106 cell/mL cell suspension was plated on each side of the vertical strip and allowed to adhere for 1 hour before flooding each well with DMEM containing 10% FBS and antibiotics. At 24 hours, we carefully removed the silicone strips, revealing a cell-free zone with confluent monolayers on each side (Fig. 1B). Wells were rinsed gently with PBS to remove cellular debris and the medium was replaced. Images of cells were captured (Axiovert 200 & Axiovision; Carl Zeiss, Thornwood, NY) and analyzed (Axiovision and KS300; Carl Zeiss) at 0, 1, 4, 8, 12, 16, and 24 hours to track migration during a 24-hour period. The etched reference lines ensured images were captured at uniform points along the migration width. The width of the cell-free zone was measured at each time using KS300 imaging software and compared with the initial width. The migration rate was calculated as the change in width divided by time. Seven glass square ACL fibroblast cultures were prepared for each of the six ligament explants (three adult and three fetal ligaments). A proliferation study was performed to parallel the time frame of the migration assay to determine if differences in migration were a result of greater proliferation rates by fetal ACL fibroblasts in comparison to adult ACL fibroblasts. Adult and fetal ACL cells grown from all isolated ACL explants were plated on six-well plates (10,000 cell/cm2), four wells for each ACL explant, for 72 hours and maintained in DMEM containing 10% FBS and antibiotics. At 12-hour intervals, medium was aspirated and adherent cells were scraped in 1 mL of 1% Triton X solution in PBS (Roche Diagnostics Corporation, Indianapolis, IN). After two freeze-thaw cycles, total DNA was fluorescently quantified using a PicoGreen Assay (Invitrogen, Carlsbad, CA) [38, 50]. We performed an analysis of variance (ANOVA) with repeated measures on migration data to determine the effect of time and fibroblast age. An ANOVA was conducted on proliferation data to determine the effect of time and fibroblast age on total DNA. A Tukey post hoc test was performed on DNA production for pair-wise comparisons.
Fig. 1A–B.
(A) Cleaned glass squares (1 cm2) were etched with parallel reference lines and a 1-mm wide silicone strip was placed perpendicular to the reference lines. Cells were seeded on both sides of the silicone. (B) On confluency at 24 hours, the silicone strip was removed, images were captured, and the width of the cell-free zone was analyzed to quantify cell migration.
We used ELISAs to quantify Types I and III collagen produced by adult and fetal ACL fibroblasts. Second-passage adult and fetal cells were cultured in monolayer on six-well plates (10,000 cells per/cm2), three wells for each ACL explant, and maintained in DMEM containing 10% FBS and antibiotics. The medium in each well was changed every third day and supplemented with 25 μg/mL L-ascorbic acid. On Days 3 and 7, wells were rinsed with PBS, scraped with 300 μL 0.05 N acetic acid (pH 2.8), and stored at −20°C. Samples were thawed, centrifuged, and the acetic acid supernatant was collected for analysis in subsequent steps. The resulting pellet was resuspended in 500 μL mol/L guanidine hydrochloride (GuHCl) in 0.15 mol/L Tris-HCl (pH 7.5) and rotated for 16 hours at 4°C. Afterward, the GuHCl was removed, the pellet washed twice with 800 μL cold sterile water, and resuspended in 330 μL of 1 mg/mL of pepsin in 0.05 N acetic acid. Samples were rotated gently during the pepsin digestion at 4°C for 48 hours. Excess pepsin was neutralized by the addition of 30 μL of 10X Tris-buffered saline (1.0 mol/L Tris, 2 mol/L NaCl, 50 mmol/L CaCl2) and 16.5 μL of NaOH to raise the pH to 8.0. Collected supernatants and pellet digests were plated in 96-well Nunc Maxisorp plates at a 1:1 ratio of sample to coating buffer (15 mmol/L Na2CO3, 35 mmol/L NaHCO3, 3 mmol/L NaN3, pH 9.6) and incubated overnight at 4°C. The next day, wells were washed with 0.05% (v/v) Tween 20 in PBS and blocked with 2% BSA and 0.1% NaN3 in PBS. Monoclonal antibody to Type I collagen (1:10,000; Sigma, St Louis, MO) or Type III collagen (1:3400; Chemicon, Temecula, CA), diluted in blocking solution, was applied to all wells and incubated overnight at 4°C. The next day, wells were washed and a peroxidase-based detection system using a biotinylated secondary antibody (antimouse IgG) (Vector Labs, Burlingame, CA), streptavidin-horseradish peroxidase (R&D Systems, Minneapolis, MN), and 3,3′,5,5′-tetramethylbenzidine (Vector Labs) as the substrate chromogen were used. The peroxidase reaction was stopped with 1 N H2SO4. The absorbance was read at 450 nm using a microplate reader (Synergy HT Bio-Tek Instruments, Inc, Winooski, VT). Total collagen protein was determined from standard curves of bovine Types I and III collagen isolated from bovine placenta (Type I collagen, Rockland, Gilbertsville, PA; Type III collagen, Southern Biotech, Birmingham, AL). Total collagen values were normalized to DNA measured using the PicoGreen DNA assay. Experiments were performed in duplicate.
We performed an ANOVA on ELISA data to determine the effect of time and fibroblast age on total Types I and III collagen production. A Tukey post hoc test was performed on collagen production for pair-wise comparisons.
Results
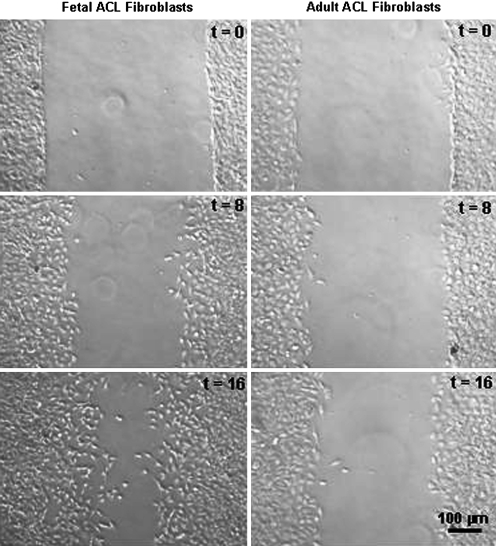
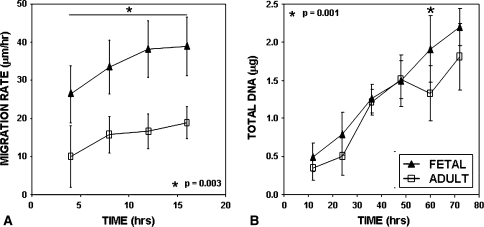
After 16 hours, fetal ACL fibroblasts closed the cell-free zone by 67%, whereas adult ACL fibroblasts closed 32% of the cell-free zone (Fig. 2). Fetal ACL fibroblasts migrated significantly faster (p = 0.003) than adult ACL fibroblasts (38.90 ± 7.69 μm/hour versus 18.88 ± 4.18 μm/hour, respectively) (Fig. 3A). The same trend was observed on tissue culture polystyrene surfaces (TCPS) (data not shown). We observed an increase (p = 0.001) in fetal fibroblast DNA content compared to adult at 60 hours, but none at 12, 24, 36, 48 or 72 hours (Fig. 3B).
Fig. 2.
Representative images of fetal and adult ACL fibroblasts migrating at 0, 8, and 16 hours are shown. Fetal ACL fibroblasts closed the cell-free zone by 67% at 16 hours in comparison to 32% by adult ACL fibroblasts.
Fig. 3A–B.
(A) Image analysis for fetal and adult ACL fibroblast cultures showed greater (p = 0.003) migration rates by fetal ACL fibroblasts in comparison to adult ACL fibroblasts. The star indicates the effect of time with age (n = 3). (B) Total DNA was quantified for fetal and adult ACL fibroblast cultures at 12-hour intervals (n = 3). Fetal ACL fibroblasts exhibited greater amounts (p = 0.001) of DNA only at 60 hours in culture when compared with adult ACL fibroblasts.
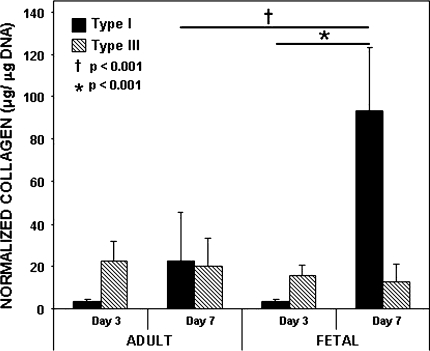
At Day 3, adult and fetal ACL fibroblasts produced similar (p = 1.00) amounts of Type I collagen (3.32 ± 1.30 μg/μg DNA versus 3.72 ± 0.89 μg/μg DNA) (Fig. 4). However, after 7 days in culture, fetal ACL fibroblasts elaborated approximately four times the amount (p < 0.001) of Type I collagen (93.12 ± 30.04 μg/μg DNA) when compared with adult ACL fibroblasts (22.59 ± 22.98 μg/μg DNA). Type III collagen levels were similar (p = 0.893) with time and age.
Fig. 4.
Types I and III collagen were quantified by an indirect ELISA and normalized to total DNA. The data represent average normalized Types I and III collagen for fetal and adult ACL fibroblast cultures (n = 3). Fetal ACL fibroblasts elaborated more (p < 0.001) Type I collagen at 7 days in comparison to adult ACL fibroblast cultures and more (p < 0.001) Type I collagen at Day 7 than Day 3. We observed no differences (p = 0.893) in Type III collagen production.
Discussion
The impaired healing capacity of the ACL limits clinical treatment options to surgical reconstruction for the majority of ACL injuries. Other mature fibrous tissues, such as tendon, heal with fibrotic scarring; however, at early gestational ages, these tissues heal scarlessly. It is possible the healing response of the ACL may not be inherently poor, but age-dependent. Scarless healing in fetal tissues, such as skin and tendon, has been attributed to the intrinsic properties of fetal cells [17, 35]. Cell migration and extracellular matrix production are believed important cellular responses facilitating tissue repair. We therefore hypothesized fetal ACL fibroblasts would exhibit increased migration rates and elaborate greater quantities of Types I and III collagen in comparison to adult ACL fibroblasts.
We note several limitations. First, the in vitro culture environment used in this study enabled us to elucidate age-dependent cellular differences of ACL fibroblasts under well-defined conditions. However, whether these differences translate into an improved wound healing response in the fetus will require further evaluation in an in vivo animal model. Second, it is difficult to identify a homogeneous population of ACL fibroblasts in fetal and adult tissues. The embryonic development of synovial joints begins with the appearance of the interzone at the site of joint formation. The interzone, which consists of closely packed mesenchymal cells connected by gap junctions, gives rise to articular cartilage, ligaments, and the synovial lining of the joint [5, 45]. How these mesenchymal cells specifically differentiate into the ligamentous structures of the knee is still unknown. Because there are no published studies documenting specific markers for fetal or adult ACL fibroblasts, we were cautious to only isolate cells from the midsubstance of each ligament, avoiding the bony insertion sites. In addition, the ACL tissues we used were harvested from anatomically developed knees of cows and their fetuses. Third, the initial quantities of primary cells isolated were insufficient for our proposed studies; therefore, second passage cells were used for all reported experiments. Nagineni et al. investigated the effect of cell passage on in vitro behavior of ACL and MCL fibroblasts, and observed a greater growth rate at passage 2 versus passage 6, with no noticeable differences in protein composition until passage 3 [43]. Fourth, when assessing Types I and III collagen levels, we quantified elaborated collagen in the cell layer and did not measure soluble collagen in the culture media. We chose to only measure elaborated collagen as it is likely a better reflection of the secreted protein that would remain localized at the wound site.
The wound healing properties of the ACL often are compared with those of the MCL, which has the ability to heal without surgical intervention. In a rabbit knee injury model, ACL wounds healed poorly as evidenced by incomplete gap closure, decreased cellular infiltration into the site of injury, and reduced collagen synthesis in comparison to MCL wounds [56]. A subsequent investigation by Amiel et al. confirmed ACL fibroblasts were intrinsically different from MCL fibroblasts in that they exhibited slower migration rates from ligament explants and slower in vitro proliferation rates [4]. The extraarticular versus intraarticular environments of the MCL and ACL, respectively, are believed partially responsible for differences in the healing capacity between these two ligaments. Others cite the enhanced migration of MCL fibroblasts, in comparison to ACL fibroblasts, as an important cellular characteristic to explain the improved wound healing response to MCL injuries [14, 19, 25]. Our findings of enhanced cellular characteristics of fetal ACL fibroblasts in comparison to those of the adult tissue may suggest the intraarticular environment alone is not responsible for the impaired healing response of the ACL. Rather, these differences may be attributable to cellular changes that occur with maturation.
In our investigation, we evaluated the directional migratory activity of adult and fetal ACL fibroblasts in the absence of specific chemotactic factors, and confirmed our hypothesis that fetal ACL fibroblasts migrate at a faster rate than adult ACL fibroblasts. Similar to the MCL, the fetal ACL might possess the ability to regenerate functional tissue, a response absent in the adult ACL. To confirm the observed differences in migration were not the result of cell division, a proliferation study was conducted over a time consistent with that of the 48-hour migration study. At 60-hours, there is a statistical difference in total DNA with age that appears resolved at 72-hours. Between 0 and 48-hours, however, adult and fetal ACL fibroblasts had similar proliferation profiles (Fig. 3B). This result suggests there is an intrinsic difference in the motility of the two cell types for the duration of the migration assay, which is independent of cell division. An in vitro wound assay of second or third passage ACL and MCL fibroblasts conducted on TCPS revealed MCL fibroblasts migrated at approximately 25 μm/hour during a 48-hour period [43]. The average migration rate for adult ACL fibroblasts that we measured was lower than that reported for MCL fibroblasts. Furthermore, fetal ACL fibroblasts migrated at a rate comparable to fetal tenocytes isolated from lateral extensor tendons of sheep (35.76 ± 6.08 μm/hour) [9], which heal regeneratively [7, 17]. Our findings also parallel those of a recent investigation comparing the motility and contractile ability of fetal and adult dermal fibroblasts [49]. Sandulache and colleagues reported elevated rates of migration and contractility in fetal fibroblasts despite external manipulations such as transitions from inert to bioactive substrates, two-dimensional to three-dimensional environments, and stimulating factors [49]. The robust motility profile of fetal fibroblasts may be the result of the presence of specific cell surface receptors. Cass et al. reported fetal and adult skin fibroblasts exhibit differential expression of the collagen-specific integrin receptors α1β1, α2β1, and α3β1 [13]. Differences in integrin receptor expression in fetal skin wounds might account for the rapid migration and reepithelialization of fetal wounds in comparison to adult wounds [41]. Further exploration into integrin expression and adhesion strength of fetal and adult ACL fibroblasts will help to determine plausible mechanisms by which fetal ACL fibroblasts migrate faster than adult ACL fibroblasts.
Ligament wound healing is characterized by three phases: an inflammatory phase, a reparative phase, and a remodeling phase. Collagen elaboration provides the structural framework for regenerating tissue, bridging ligament wound edges after injury. Type I collagen is the most abundant extracellular matrix component in the ACL and is important in all three stages of ligament healing [29]. In the absence of growth factors, collagen initiates the migration of human dermal fibroblasts, a cellular response necessary for effective cell infiltration into the injury site [28]. Extracellular collagen provides adhesion ligands for cell surface receptors, thereby allowing cell attachment and enhancing cell infiltration during the wound repair process. We hypothesized fetal ACL fibroblasts would elaborate greater amounts of Type I collagen in comparison to adult ACL fibroblasts. We found fetal ACL fibroblasts produced four times the amount of normalized Type I collagen as adult ACL fibroblasts after 7 days in culture. This decrease in collagen elaboration by ACL fibroblasts with age is consistent with results of Amiel et al. who reported collagen content and synthesis are age-dependent [3]. A similar effect of age on collagen elaboration has been observed in other connective tissues such as tendon and skin [9, 54]. In an in vivo sheep skin injury model, Lovvorn et al. observed more rapid deposition of Type I collagen and greater collagen cross-linking in early gestational fetal skin wounds in comparison to adult skin, which correlated with scarless healing [36]. The proficiency of fetal ACL fibroblasts to secrete considerably more Type I collagen than adult ACL fibroblasts supports the notion that the healing potential of the ACL may vary with age. In other in vitro systems, Types I and III collagen gene expression were elevated in fetal fibroblasts compared with adult cells [9, 54]. However, Type III collagen elaboration in our studies did not follow this trend. There was no statistical difference in Type III collagen levels between adult and fetal cultures. This was surprising given reports of increased expression of Type III collagen in fetal tissues in comparison to those of adults [16, 21, 39, 54]. It is possible Type III collagen production in ACL fibroblasts is more sensitive to the culture environment, requiring additional soluble factors to stimulate protein production. For example, exposure of fetal dermal fibroblasts to transforming growth factor-β1 (TGF- β1) up-regulated Type III collagen gene expression whereas postnatal dermal fibroblasts showed no change [46]. The absence of factors, such as TGF- β1, in our in vitro system might explain why we were unable to detect noticeable differences in elaborated Type III collagen with age. We intend to expand on the current study by investigating the effects of growth factors and cytokines on fibroblast behavior.
With maturation, the regenerative healing capacity of fetal tissues diverges toward the reparative healing process observed in adults. The difference in the cellular response of fetal and adult ACL fibroblasts suggests the healing potential of ACL fibroblasts is not intrinsically poor, but rather may be age-dependent. The fetal ACL may behave similar to other fetal tissues, such as tendon, which heal scarlessly. Considerable progress has been made in determining the mechanisms involved in scarless repair. Characterization of the fetal environment has revealed a shift toward an anti-inflammatory cytokine expression profile with a concomitant reduction in the number of localized inflammatory cells [1, 27, 31, 32]. Furthermore, fetal cells themselves promote scarless healing even outside their native environment [17, 35]. Understanding the fetal environment and the cellular characteristics of fetal cells may lead to the discovery of specific factors (ie, soluble signaling molecules) that could be used in the clinical setting to improve wound repair.
Acknowledgments
We thank Dr. Anna M. Lipski for assistance with the migration assay.
Footnotes
One or more of the authors have received funding from a Whitaker Foundation Graduate Fellowship (SSS) and National Institutes of Health Grant R21 AR051056 (SBN).
Each author certifies that his or her institution has approved the animal protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
References
- 1.Adzick NS, Harrison MR, Glick PL, Beckstead JH, Villa RL, Scheuenstuhl H, Goodson WH 3rd. Comparison of fetal, newborn, and adult wound healing by histologic, enzyme-histochemical, and hydroxyproline determinations. J Pediatr Surg. 1985;20:315–319. [DOI] [PubMed]
- 2.Amiel D, Frank CB, Harwood FL, Akeson WH, Kleiner JB. Collagen alteration in medial collateral ligament healing in a rabbit model. Connect Tissue Res. 1987;16:357–366. [DOI] [PubMed]
- 3.Amiel D, Kuiper SD, Wallace CD, Harwood FL, VandeBerg JS. Age-related properties of medial collateral ligament and anterior cruciate ligament: a morphologic and collagen maturation study in the rabbit. J Gerontol. 1991;46:B159–165. [DOI] [PubMed]
- 4.Amiel D, Nagineni CN, Choi SH, Lee JE. Intrinsic properties of ACL and MCL cells and their responses to growth factors. Med Sci Sports Exerc. 1995;27:844–851. [PubMed]
- 5.Archer CW, Dowthwaite GP, Francis-West P. Development of synovial joints. Birth Defects Res C Embryo Today. 2003;69:144–155. [DOI] [PubMed]
- 6.Baker H, Blair CP. Cell replacement in the human stratum corneum in old age. Br J Dermatol. 1968;80:367–372. [DOI] [PubMed]
- 7.Beredjiklian PK, Favata M, Cartmell JS, Flanagan CL, Crombleholme TM, Soslowsky LJ. Regenerative versus reparative healing in tendon: a study of biomechanical and histological properties in fetal sheep. Ann Biomed Eng. 2003;31:1143–1152. [DOI] [PubMed]
- 8.Boykiw R, Sciore P, Reno C, Marchuk L, Frank CB, Hart DA. Altered levels of extracellular matrix molecule mRNA in healing rabbit ligaments. Matrix Biol. 1998;17:371–378. [DOI] [PubMed]
- 9.Brink HE, Miller GJ, Beredjiklian PK, Nicoll SB. Serum-dependent effects on adult and fetal tendon fibroblast migration and collagen expression. Wound Repair Regen. 2006;14:179–186. [DOI] [PubMed]
- 10.Brink HE, Stalling SS, Nicoll SB. Influence of serum on adult and fetal dermal fibroblast migration, adhesion, and collagen expression. In vitro Cell Dev Biol Anim. 2005;41:252–257. [DOI] [PubMed]
- 11.Bullard KM, Longaker MT, Lorenz HP. Fetal wound healing: current biology. World J Surg. 2003;27:54–61. [DOI] [PubMed]
- 12.Cameron ML, Mizung Y, Cosgarea AJ. Diagnosing and managing anterior cruciate ligament injuries. J Musculoskeletal Med. 2000;17:47–53.
- 13.Cass DL, Bullard KM, Sylvester KG, Yang EY, Sheppard D, Herlyn M, Adzick NS. Epidermal integrin expression is upregulated rapidly in human fetal wound repair. J Pediatr Surg. 1998;33:312–316. [DOI] [PubMed]
- 14.Chen H, Tang Y, Li S, Shen Y, Liu X, Zhong C. Biologic characteristics of fibroblast cells cultured from the knee ligaments. Chin J Traumatol. 2002;5:92–96. [PubMed]
- 15.Chun J, Tuan TL, Han B, Vangsness CT, Nimni ME. Cultures of ligament fibroblasts in fibrin matrix gel. Connect Tissue Res. 2003;44:81–87. [DOI] [PubMed]
- 16.Cuttle L, Nataatmadja M, Fraser JF, Kempf M, Kimble RM, Hayes MT. Collagen in the scarless fetal skin wound: detection with picrosirius-polarization. Wound Repair Regen. 2005;13:198–204. [DOI] [PubMed]
- 17.Favata M, Beredjiklian PK, Zgonis MH, Beason DP, Crombleholme TM, Jawad AF, Soslowsky LJ. Regenerative properties of fetal sheep tendon are not adversely affected by transplantation into an adult environment. J Orthop Res. 2006;24:2124–2132. [DOI] [PubMed]
- 18.Frank CB, Jackson DW. Current concepts review: the science of reconstruction of the anterior cruciate ligament. J Bone Joint Surg Am. 1997;79:1556–1576. [DOI] [PubMed]
- 19.Geiger MH, Green MH, Monosov A, Akeson WH, Amiel D. An in vitro assay of anterior cruciate ligament (ACL) and medial collateral ligament (MCL) cell migration. Connect Tissue Res. 1994;30:215–224. [DOI] [PubMed]
- 20.Greiling D, Clark RA. Fibronectin provides a conduit for fibroblast transmigration from collagenous stroma into fibrin clot provisional matrix. J Cell Sci. 1997;110:861–870. [DOI] [PubMed]
- 21.Hallock GG, Merkel JR, Rice DC, DiPaolo BR. The ontogenetic transition of collagen deposition in rat skin. Ann Plast Surg. 1993;30:239–243. [DOI] [PubMed]
- 22.Hawkins RJ, Misamore GW, Merritt TR. Followup of the acute nonoperated isolated anterior cruciate ligament tear. Am J Sports Med. 1986;14:205–210. [DOI] [PubMed]
- 23.Holm-Pedersen P, Viidik A. Tensile properties and morphology of healing wounds in young and old rats. Scand J Plast Reconstr Surg. 1972;6:24–35. [DOI] [PubMed]
- 24.Jia F, Shimomura T, Niyibizi C, Woo SL. Downregulation of human type III collagen gene expression by antisense oligodeoxynucleotide. Tissue Eng. 2005;11:1429–1435. [DOI] [PubMed]
- 25.Kobayashi K, Healey RM, Sah RL, Clark JJ, Tu BP, Goomer RS, Akeson WH, Moriya H, Amiel D. Novel method for the quantitative assessment of cell migration: A study on the motility of rabbit anterior cruciate (ACL) and medial collateral ligament (MCL) cells. Tissue Eng. 2000;6:29–38. [DOI] [PubMed]
- 26.Kondo H, Yonezawa Y, Ito H. Inhibitory effects of human serum on human fetal skin fibroblast migration: migration-inhibitory activity and substances in serum, and its age-related changes. In vitro Cell Dev Biol Anim. 2000;36:256–261. [DOI] [PubMed]
- 27.Krummel TM, Nelson JM, Diegelmann RF, Lindblad WJ, Salzberg AM, Greenfield LJ, Cohen IK. Fetal response to injury in the rabbit. J Pediatr Surg. 1987;22:640–644. [DOI] [PubMed]
- 28.Li W, Fan J, Chen M, Guan S, Sawcer D, Bokoch GM, Woodley DT. Mechanism of human dermal fibroblast migration driven by type I collagen and platelet-derived growth factor-BB. Mol Biol Cell. 2004;15:294–309. [DOI] [PMC free article] [PubMed]
- 29.Liu SH, Yang RS, al-Shaikh R, Lane JM. Collagen in tendon, ligament, and bone healing: a current review. Clin Orthop Relat Res. 1995;318:265–278. [PubMed]
- 30.Lo IK, Marchuk L, Majima T, Frank CB, Hart DA. Medial collateral ligament and partial anterior cruciate ligament transection: mRNA changes in uninjured ligaments of the sheep knee. J Orthop Sci. 2003;8:707–713. [DOI] [PubMed]
- 31.Longaker MT, Adzick NS, Hall JL, Stair SE, Crombleholme TM, Duncan BW, Bradley SM, Harrison MR, Stern R. Studies in fetal wound healing VII: fetal wound healing may be modulated by hyaluronic acid stimulating activity in amniotic fluid. J Pediatr Surg. 1990;25:430–433. [DOI] [PubMed]
- 32.Longaker MT, Harrison MR, Langer JC, Crombleholme TM, Verrier ED, Spendlove R, Stern R. Studies in fetal wound healing II: a fetal environment accelerates fibroblast migration in vitro. J Pediatr Surg. 1989;24:793–797; discussion 798. [DOI] [PubMed]
- 33.Longaker MT, Moelleken BR, Cheng JC, Jennings RW, Adzick NS, Mintorovich J, Levinsohn DG, Gordon L, Harrison MR, Simmons DJ. Fetal fracture healing in a lamb model. Plast Reconstr Surg. 1992;90:161–171; discussion 172–173. [DOI] [PubMed]
- 34.Longaker MT, Whitby DJ, Jennings RW, Duncan BW, Ferguson MW, Harrison MR, Adzick NS. Fetal diaphragmatic wounds heal with scar formation. J Surg Res. 1991;50:375–385. [DOI] [PubMed]
- 35.Lorenz HP, Lin RY, Longaker MT, Whitby DJ, Adzick NS. The fetal fibroblast: the effector cell of scarless fetal skin repair. Plast Reconstr Surg. 1995;96:1251–1259; discussion 1260–1261. [DOI] [PubMed]
- 36.Lovvorn HN 3rd, Cheung DT, Nimni ME, Perelman N, Estes JM, Adzick NS. Relative distribution and crosslinking of collagen distinguish fetal from adult sheep wound repair. J Pediatr Surg. 1999;34:218–223. [DOI] [PubMed]
- 37.Lyon RM, Akeson WH, Amiel D, Kitabayashi LR, Woo SL. Ultrastructural differences between the cells of the medial collateral and the anterior cruciate ligaments. Clin Orthop Relat Res. 1991;272:279–286. [PubMed]
- 38.McGowan KB, Kurtis MS, Lottman LM, Watson D, Sah RL. Biochemical quantification of DNA in human articular and septal cartilage using PicoGreen and Hoechst 33258. Osteoarthritis Cartilage. 2002;10:580–587. [DOI] [PubMed]
- 39.Merkel JR, DiPaolo BR, Hallock GG, Rice DC. Type I and type III collagen content of healing wounds in fetal and adult rats. Proc Soc Exp Biol Med. 1988;187:493–497. [DOI] [PubMed]
- 40.Meuli M, Lorenz HP, Hedrick MH, Sullivan KM, Harrison MR, Adzick NS. Scar formation in the fetal alimentary tract. J Pediatr Surg. 1995;30:392–395. [DOI] [PubMed]
- 41.Moulin V, Plamondon M. Differential expression of collagen integrin receptor on fetal vs. adult skin fibroblasts: implication in wound contraction during healing. Br J Dermatol. 2002;147:886–892. [DOI] [PubMed]
- 42.Murray MM, Bennett R, Zhang X, Spector M. Cell outgrowth from the human ACL in vitro: regional variation and response to TGF-beta1. J Orthop Res. 2002;20:875–880. [DOI] [PubMed]
- 43.Nagineni CN, Amiel D, Green MH, Berchuck M, Akeson WH. Characterization of the intrinsic properties of the anterior cruciate and medial collateral ligament cells: an in vitro cell culture study. J Orthop Res. 1992;10:465–475. [DOI] [PubMed]
- 44.O’Donoghue DH, Frank GR, Jeter GL, Johnson W, Zeiders JW, Kenyon R. Repair and reconstruction of the anterior cruciate ligament in dogs: factors influencing long-term results. J Bone Joint Surg Am. 1971;53:710–718. [PubMed]
- 45.Pacifici M, Koyama E, Iwamoto M. Mechanisms of synovial joint and articular cartilage formation: recent advances, but many lingering mysteries. Birth Defects Res C Embryo Today. 2005;75:237–248. [DOI] [PubMed]
- 46.Rolfe KJ, Irvine LM, Grobbelaar AO, Linge C. Differential gene expression in response to transforming growth factor-beta1 by fetal and postnatal dermal fibroblasts. Wound Repair Regen. 2007;15:897–906. [DOI] [PubMed]
- 47.Ross SM, Joshi R, Frank CB. Establishment and comparison of fibroblast cell lines from the medial collateral and anterior cruciate ligaments of the rabbit. In vitro Cell Dev Biol. 1990;26:579–584. [DOI] [PubMed]
- 48.Roth GS, Kowatch MA, Hengemihle J, Ingram DK, Spangler EL, Johnson LK, Lane MA. Effect of age and caloric restriction on cutaneous wound closure in rats and monkeys. J Gerontol A Biol Sci Med Sci. 1997;52:B98–102. [DOI] [PubMed]
- 49.Sandulache VC, Parekh A, Dohar JE, Hebda PA. Fetal dermal fibroblasts retain a hyperactive migratory and contractile phenotype under 2-and 3-dimensional constraints compared to normal adult fibroblasts. Tissue Eng. 2007;13:2791–2801. [DOI] [PubMed]
- 50.Singer VL, Jones LJ, Yue ST, Haugland RP. Characterization of PicoGreen reagent and development of a fluorescence-based solution assay for double-stranded DNA quantitation. Anal Biochem. 1997;249:228–238. [DOI] [PubMed]
- 51.Smith LT, Holbrook KA, Madri JA. Collagen types I, III, and V in human embryonic and fetal skin. Am J Anat. 1986;175:507–521. [DOI] [PubMed]
- 52.Stenn KS. Quantitative assay of dissociated tissue-cell motility in vitro. In vitro. 1980;16:357–360. [DOI] [PubMed]
- 53.Sung KL, Kwan MK, Maldonado F, Akeson WH. Adhesion strength of human ligament fibroblasts. J Biomech Eng. 1994;116:237–242. [DOI] [PubMed]
- 54.Takeda K, Gosiewska AP, Peterkofsky B. Similar, but not identical, modulation of expression of extracellular matrix components during in vitro and in vivo aging of human skin fibroblasts. J Cell Physiol. 1992;153:450–459. [DOI] [PubMed]
- 55.Weiss JA, Woo SL, Ohland KJ, Horibe S, Newton PO. Evaluation of a new injury model to study medial collateral ligament healing: primary repair versus nonoperative treatment. J Orthop Res. 1991;9:516–528. [DOI] [PubMed]
- 56.Wiig ME, Amiel D, VandeBerg J, Kitabayashi LR, Harwood FL, Arfors KE. The early effect of high molecular weight hyaluronan (hyaluronic acid) on anterior cruciate ligament healing: an experimental study in rabbits. J Orthop Res. 1990;8:425–434. [DOI] [PubMed]
- 57.Woo SL, Suh JK, Parsons IM, Wang JH, Watanabe N. Biological intervention in ligament healing effect of growth factors. Sports Med Arthrosc Rev. 1998;6:74–82. [DOI]